Abstract
Tick-borne encephalitis (TBE) virus is the most important human pathogen transmitted by ticks in Eurasia. Inactivated vaccines are available but require multiple doses and frequent boosters to induce and maintain immunity. Thus far, the goal of developing a safe, live attenuated vaccine effective after a single dose has remained elusive. Here we used a replication-defective (single-cycle) flavivirus platform, RepliVax, to generate a safe, single-dose TBE vaccine. Several RepliVax-TBE candidates attenuated by a deletion in the capsid gene were constructed using different flavivirus backbones containing the envelope genes of TBE virus. RepliVax-TBE based on a West Nile virus backbone (RV-WN/TBE) grew more efficiently in helper cells than candidates based on Langat E5, TBE, and yellow fever 17D backbones, and was found to be highly immunogenic and efficacious in mice. Live chimeric yellow fever 17D/TBE, Dengue 2/TBE, and Langat E5/TBE candidates were also constructed but were found to be underattenuated. RV-WN/TBE was demonstrated to be highly immunogenic in Rhesus macaques after a single dose, inducing a significantly more durable humoral immune response compared with three doses of a licensed, adjuvanted human inactivated vaccine. Its immunogenicity was not significantly affected by preexisting immunity against WN. Immunized monkeys were protected from a stringent surrogate challenge. These results support the identification of a single-cycle TBE vaccine with a superior product profile to existing inactivated vaccines, which could lead to improved vaccine coverage and control of the disease.
Keywords: flavivirus vaccine, prophylaxis, preclinical, nonhuman primate
Tick-borne encephalitis (TBE) is an acute infection of the central nervous system and the most important viral disease of humans transmitted by ticks. It is endemic in Europe, Russia and parts of China and Japan, and is considered an emerging pathogen spreading to previously unaffected territories. The disease results in >10,000 hospitalizations annually, with case-fatality rates of 1–2% in Europe and up to 40% in Siberia and the Far East of Russia. Public awareness of TBE is high due to the severity of the disease and long-lasting neuropsychiatric sequelae occurring in up to ∼40% of patients (1). There are no specific antiviral drugs, and therefore prevention through vaccination remains the most effective means of countering the disease. Although highly effective inactivated vaccines (INV) produced in Europe and Russia have been available for several decades (2), overall vaccination rates remain low in most endemic countries (6–22%), with a few exceptions (Austria 88%, Latvia 38%). Among vaccinated, at least one-third of people do not adhere to the recommended vaccination schedules (three doses over 1 y for primary immunization followed by booster doses every 3–5 y) and thus may not be adequately protected. Among the unvaccinated majority, many people resort to protecting themselves by taking precautions to avoid tick bites or refraining from certain outdoor activities altogether rather than choosing vaccination (3), with the inconvenience of multiple immunizations being a major factor in such decisions.
TBE virus belongs to the Flavivirus genus of small enveloped plus-strand RNA viruses, which also includes significant pathogens transmitted by mosquitoes such as yellow fever (YF), Japanese encephalitis (JE), West Nile (WN), and dengue types 1–4 (DEN1–4) viruses (4). The flavivirus genomic RNA of ∼11,000 nt contains a single ORF which encodes the three structural proteins of the virion, capsid C, prM/M, and envelope E, followed by seven nonstructural (NS) proteins involved in virus replication (5). The E protein is the main immunogen, eliciting neutralizing (N) antibodies (Abs) which are considered to be the main correlate of protective immunity. Available INVs, live attenuated (LAV) flavivirus vaccines, and novel vaccine candidates have been reviewed (6, 7). These include the highly successful, single-dose YF 17D LAV and the recently developed ChimeriVax-JE LAV obtained by chimerization with the YF 17D virus (licensed as IMOJEV), as well as ChimeriVax-DEN and -WN candidates that are in clinical development.
A safe and effective LAV against TBE capable of eliciting durable immunity after a single dose has been sought after since the discovery of the virus in 1949. In the early 1970s, a naturally attenuated Langat (LGT) virus serologically related to TBE was used to vaccinate >600,000 people in the former Soviet Union. This experimental vaccine was abandoned because of vaccine-induced cases of encephalitis observed at the rate of 1/18,570 (8). However, long-term follow-up of the vaccinees confirmed that durable protective immunity against TBE was induced using this candidate (8). More recent attempts to develop a TBE LAV by means of various molecular modifications of the viral genome or chimerization of TBE or LGT viruses with DEN4 resulted in several promising candidates based on preclinical data. However, uncertainty remained about whether the right balance of attenuation and immunogenicity was achieved (9–13). DEN4/LGT chimera progressed to a phase I trial and was found to be overattenuated (13).
Here we used an approach to flavivirus vaccines, RepliVax, to develop a single-dose TBE vaccine. The approach is based on replication-defective flavivirus mutants produced in helper cells (14–17). RepliVax constructs are engineered to have a deletion, removing most of the capsid protein C gene. As such they are propagated in vitro in complementing cells supplying the C protein in trans. In normal cells, both in vitro and in vivo, they undergo a single round of replication only, and cannot spread to surrounding cells. Infected cells efficiently secrete empty virus-like particles (VLP, the product of the prM-E genes) devoid of the nucleocapsid (14) which represent the active immunogen. Cryoelectron microscopy of TBE VLPs has shown that they are smaller than complete virions but have similar architecture of the envelope (18) and therefore should be highly immunogenic. A direct head-to-head comparison of several RepliVax prototype vaccines (RV-WN, -JE, and -YF) to available licensed LAV and INV vaccines for safety, immunogenicity and efficacy in mice and hamsters demonstrated that they are extremely attenuated and have the potential to be as immunogenic and efficacious as LAVs after a single dose (19). Here we report the construction of several RepliVax-TBE and chimeric LAV candidates and results of their characterization in mice and nonhuman primates (NHP). The data presented for the selected RepliVax-TBE candidate demonstrate its potential as a single-dose TBE vaccine for humans superior to existing INVs.
Results
In Vitro Replication of RepliVax-TBE Variants and Live TBE Chimeras.
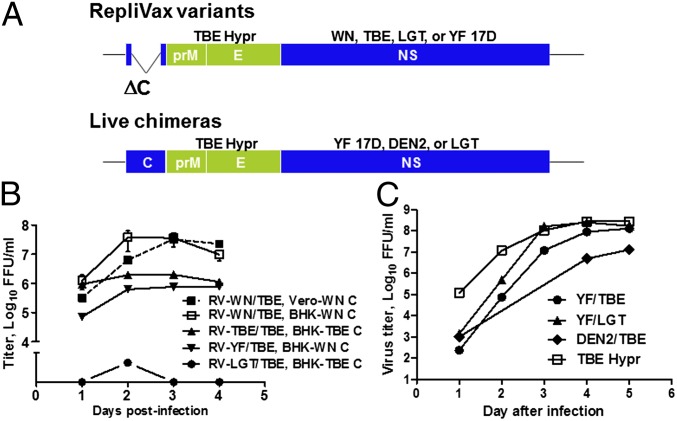
Several single-cycle RepliVax-TBE variants containing the prM-E genes from TBE strain Hypr were constructed based on the WN, TBE Hypr, LGT E5, and YF 17D backbones with a ΔC deletion (RV-WN/TBE, RV-TBE/TBE, RV-YF/TBE, and RV-LGT/TBE, respectively). Live chimeras with TBE Hypr prM-E and backbones of attenuated YF 17D, DEN2 PDK-53 (used in the development of other chimeric flavivirus vaccines; refs. 20 and 21), and LGT E5 (an additionally attenuated derivative of LGT TP21) viruses were also generated (YF/TBE, DEN2/TBE and LGT/TBE) (Fig. 1A). One additional chimera, YF/LGT, contained the prM-E genes from LGT E5.
Fig. 1.
RepliVax-TBE variants and live TBE chimeras constructed and their replication in vitro. (A) RepliVax and chimeric virus genomes. RepliVax constructs, with a large deletion in the C gene, are produced in helper cells supplying the C protein in trans. (B) Growth curves of RepliVax variants in helper BHK or Vero cells supplying the indicated C proteins in trans, MOI 0.1. (C) Growth curves of live chimeras, and wild-type TBE Hypr parent in Vero cells, MOI 0.001. LGT/TBE chimera and the DEN2 PDK-53 virus were also examined in Vero cells, with peak titers of 7 log10 FFU/mL.
The RV-WN/TBE variant (with the TBE specific prM signal) grew very efficiently in both BHK and Vero helper cells containing a VEE replicon expressing the WN C protein and a puromycin resistance selection marker. Peak titers were in excess of 7.5 log10 focus forming units (FFU)/mL (Fig. 1B). Equally high titers were observed at a broad range of multiplicities of infection (MOIs 0.001, 0.01, and 0.1 FFU per cell). Other RepliVax constructs, RV-TBE/TBE, RV-YF/TBE, and RV-LGT/TBE, replicated to significantly lower titers than RV-WN/TBE, with peak titers not higher than 6 log10 FFU/mL (Fig. 1B). RV-LGT/TBE replicated particularly poorly. Live chimeras of, YF/TBE, YF/LGT, LGT/TBE, and DEN2/TBE, were also constructed and found to replicate efficiently in Vero cells with peak titers of ∼7–8 log10 FFU/mL (Fig. 1C).
To demonstrate genetic stability, RV-WN/TBE was serially passaged >10 times in two independent passage series in BHK WN C helper cells at an MOI of 0.01 FFU per cell. At passage 11, the full genomes were sequenced by consensus sequencing revealing only a small number of mutations (E122Q in the E protein in the first passage series, and K3M in the prM signal and R122L in NS2A in the second passage series). Importantly, no recombination restoring replication competent virus was observed during these passages, as shown by titration in Vero cells (starting sample dilution 10−1), with staining at a late time postinfection (day 3–5 for immunostaining and day 5–7 using crystal violet) to detect foci/plaques of any replication competent virus. Subsequently, a substantial database was generated demonstrating the absence of recombination which included results of analysis of numerous RepliVax-TBE samples and preparations (e.g., dozens of independent small scale samples and four large scale stocks that were tested specifically for replication competent virus in vitro and in vivo, and hundreds of growth curve aliquots, etc., examined by regular titration using earlier immunostaining), as well as mouse brain homogenates harvested after intraccerebral (i.c.) inoculation, including after serial brain-to-brain-passages, or mouse spleen samples after i.p. inoculation. The absence of recombination was in accord with previous data on RepliVax prototypes (19), and our ongoing studies on several other RepliVax vaccines. In addition, no adverse effects were observed in animal experiments following immunization.
Attenuation in Mice.
Outbred Institute for Cancer Research (ICR) mice (3.5 wk old) were inoculated with graded doses of RepliVax-TBE constructs and chimeric viruses by the i.c. route to measure neurovirulence or by the i.p. route to measure neuroinvasiveness. All animals that received doses up to 5 log10 FFU of RV-WN/TBE by both routes survived without any signs of illness. As expected, YF 17D vaccine was neurovirulent (i.c. LD50 of 1 log10 FFU) but not neuroinvasive (Table 1). In addition to adult mice, neurovirulence of all RepliVax-TBE variants was also examined in more sensitive 8-d-old ICR mice, and 100% survival without illness was observed at doses up to 5 log10 FFU, whereas i.c. LD50 values in these suckling mice of YF 17D and YF/JE LAVs, both licensed vaccines for humans, were 0.5 and 316 FFU, respectively. Thus, as expected, RepliVax-TBE constructs are highly attenuated.
Table 1.
Neurovirulence and neuroinvasiveness of RepliVax variants and live chimeras in 3.5-wk-old ICR mice
| Construct | i.c. LD50 (log10 FFU) | i.p. LD50 (log10 FFU) | |
| Live chimeras | YF/TBE | 0.63 | 3.5 |
| YF/LGT | <2* | >5† | |
| DEN2/TBE | <0‡ | >6‡ | |
| LGT/TBE | <2* | n.c.§ | |
| RepliVax | RV-WN/TBE | >5† | >5† |
| RV-YF/TBE | >5†¶ | >5† | |
| RV-TBE/TBE | >5†¶ | >5† | |
| RV-LGT/TBE | >5†¶ | >3† | |
| Control viruses | YF 17D | 1 | >5† |
| TBE Hypr | −0.83 | 0 | |
| LGT TP21 | 0.38 | >5‡ | |
| DEN2 PDK-53 | >7† | >6† |
n.c., not calculated.
100% mortality at the lowest dose tested.
No mortality observed at the highest dose tested.
Partial mortality at the highest or lowest dose tested.
Could not be calculated because of partial mortalities observed in all tested doses.
Determined only in 8-d-old suckling mice rather than 3.5-wk-old mice.
In contrast, all live chimeras with prM-E genes from TBE or LGT virus (YF/TBE, YF/LGT, LGT/TBE, and DEN2/TBE) were found to be highly neurovirulent for 3.5-wk-old ICR mice, with i.c. LD50 values below 2 log10 FFU. Additionally, YF/TBE, LGT/TBE, and DEN2/TBE chimeras, but not YF/LGT, exhibited at least some degree of neuroinvasiveness. YF/TBE had an LD50 of 3.5 log10 FFU by the i.p. route. Partial mortality was observed for LGT/TBE across all doses tested (1–6 log10 FFU), and DEN2/TBE caused partial mortality at the highest tested dose of 6 log10 FFU. Thus, live chimeras, particularly with the TBE envelope, are underattenuated. It should be noted however that chimerization resulted in some attenuation, particularly for neuroinvasiveness, compared with TBE Hypr virus (i.p. LD50 1 FFU), with most chimeras exhibiting neurovirulence/neuroinvasiveness not higher than that of the naturally attenuated LGT TP21 virus (Table 1). We also tried to further attenuate the YF/TBE virus by introducing a small (3 aa) nonlethal deletion into its C protein, or various combinations of E protein mutations shown previously to attenuate LGT E5 virus (22), with little success.
Immunogenicity and Protective Efficacy in Mice.
RV-WN/TBE and RV-TBE/TBE variants given to 3.5-wk-old ICR mice by the i.p. route at a single dose of 5 log10 FFU per dose elicited high plaque reduction neutralization (PRNT50) titers measured on day 20 or 30 [reciprocal geometric mean titers (GMTs) 1,778 and 3,237, respectively]. Titers were comparable to mice surviving a 5 log10 FFU dose of YF/TBE chimera (GMT 6,615). The animals were protected from a severe i.p. challenge with TBE Hypr virus (500 LD50), whereas mice in the negative control groups (mock, YF 17D, DEN2 PDK-53) died (Table 2). RV-YF/TBE was poorly immunogenic and only 50% efficacious. Lower immunogenicity and efficacy were observed for DEN2/TBE and particularly YF/LGT chimeras. A single dose of the human INV control resulted in low N Ab titers (GMT 13) and 50% protection, which significantly increased after the second dose, as expected (GMT 1,824; 100% protection).
Table 2.
Immunogenicity and protective efficacy of RepliVax variants and live chimeras in mice
| Immunogen | Dose log FFU | TBE PRNT50 GMT ± SD | Postchallenge mortality (%) |
| RV-WN/TBE | 5 | 1,778 ± 469 | 0/8 (0) |
| RV-YF/TBE | 5 | 126 ± 101 | 4/8 (50) |
| RV-TBE/TBE | 5 | 3,237 ± 749 | 0/8 (0) |
| YF/TBE | 5 | 6615 ± 2,595* | 0/2 (0) |
| YF/LGT | 5 | 74 ± 21* | 3/8 (37.5) |
| DEN2/TBE | 5 | 885 ± 370 | 1/7 (14.3) |
| INV, one dose† | — | 13 ± 2* | 4/8 (50) |
| INV, two doses† | — | 1,824 ± 812* | 0/6 (0) |
| YF 17D | 5 | <10* | 8/8 (100) |
| DEN2 PDK-53 | 6 | <10 | 8/8 (100) |
| LGT TP21 | 5 | 1,177 ± 412* | 0/3 (100) |
| Mock | — | <10 | 8/8 (100) |
All immunizations were by the i.p. route. TBE specific N Ab titers were determined in individual sera or pools from animals on day 20 (*) or 30, followed by i.p. challenge the next day with 500 LD50 of wild-type TBE Hypr.
INV was given at 1/20th of a human dose; in the two-dose group, the second dose was on day 14.
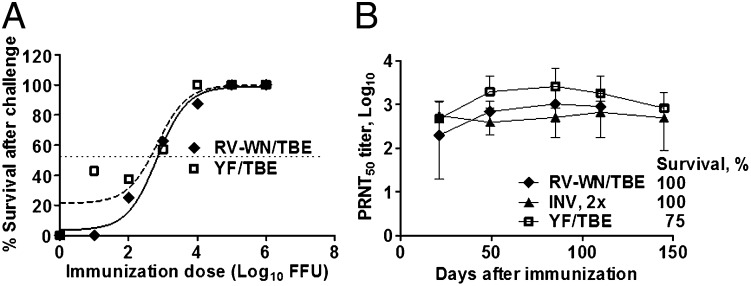
Immunization of groups of mice with graded doses of RV-WN/TBE and live YF/TBE followed by challenge on day 21 indicated that both candidates had the same 50%-protective dose (PD50) values of ∼3 log10 FFU (P = 0.5; F test) (Fig. 2A). Durability of immunity was examined by inoculating mice with doses of RV-WN/TBE, YF/TBE, and INV equivalent to 10 PD50 (two doses for INV) followed by monitoring N Ab titers for 4–5 mo, and challenge. High level N Ab titers were induced and maintained throughout the duration of the study in the three groups, and most animals were protected from challenge (100% protection for RV-WN/TBE and 2× INV) (Fig. 2B). Taken together, RV-WN/TBE, which is the preferred RepliVax-TBE variant because of its efficient replication in vitro, is essentially as immunogenic and efficacious in mice as the insufficiently attenuated (neuroinvasive) YF/TBE virus (Table 1). In additional tests, we demonstrated that the PD50 values for RV-WN/TBE and RV-TBE/TBE are also equivalent (2.4 and 2.83 log10 FFU, respectively).
Fig. 2.
Analysis of immunogenicity in mice. (A) Dose–responses. Mice were immunized with graded i.p. doses of RV-WN/TBE or YF/TBE chimera, and challenged on day 21 with 500 LD50 of TBE Hypr to determine 50% protective dose values (PD50). PD50 of INV given in two doses on days 0 and 14 was also determined (0.061 µg; whereas complete human dose is 2.4 μg). (B) Durability of immunity. Mice were immunized with 10 PD50 doses of RV-WN/TBE (5,500 FFU), YF/TBE (10,420 FFU), or 2× INV (0.61 µg each) and N Ab titers in sera were monitored for up to ∼5 mo. % survival after challenge with 500 LD50 of TBE Hypr is indicated.
To obtain evidence that a RepliVax-TBE and a TBE LAV both demonstrate the same T-helper (Th) skewing in vivo, concentrations of IgG isotypes were determined in pooled sera from mice immunized with RV-WN/TBE, YF/TBE and INV by IgG type specific ELISA. The IgG2a/IgG1 Ab ratios were found to be 4:1 and 16:1 for RV-WN/TBE and YF/TBE, respectively, consistent with a Th1-biased immune response. For the INV, the ratio was 1:4, consistent with a Th2 bias.
Previous data indicate that anti-vector (YF) preimmunity is not of concern for ChimeriVax vaccines in humans (23, 24). However, this aspect has not been examined in mice. To compare the effect of anti-vector preimmunity on immunogenicity of live chimeric viruses and RepliVax-TBE, mice were preimmunized with YF 17D and then immunized 3 wk (short interval) or 6 mo (long interval) later with YF/JE and YF/TBE. Mice were similarly preimmunized with RV-WN to induce WN-specific preimmunity and then given RV-WN/TBE. All doses were 5 log10 FFU by the i.p. route. Preimmunization was confirmed by measuring YF and WN N Ab titers. JE or TBE N Ab titers in these groups were determined 21 d after vaccinations and compared with parallel groups of naïve animals vaccinated with YF/JE, YF/TBE, or RV-WN/TBE. For RV-WN/TBE, preimmunization resulted in some reduction of TBE specific response compared with that in naïve animals, which was not statistically significant (P > 0.30; Mann–Whitney test). Importantly, the reduction appeared to be less pronounced compared with the effect of YF 17D preimmunization on immunogenicity of YF/JE and YF/TBE viruses (Fig. S1), suggesting that anti-vector immunity should not be a concern for RepliVax-TBE in humans.
We also investigated in mice whether RV-WN/TBE and INV vaccines could be interchangeable in prime-boost vaccination regimens (Table S1). In this experiment, a single dose of INV induced low TBE-specific N Ab titers and provided only 60% protection from TBE challenge, whereas a second dose of INV significantly increased the titers (PRNT50 GMT 1,019 on day 42) resulting in 100% protection. RV-WN/TBE was efficient in prime-boost vaccination regimens when used alone or in combination with INV. The highest PRNT50 titers were observed in INV prime–RV-WN/TBE boost, RV-WN/TBE prime–RV-WN/TBE boost, and RV-WN/TBE prime–INV boost groups (GMTs 3,287, 6,291, and 14,205, respectively). Animals in all groups primed or boosted with RV-WN/TBE were protected from challenge (Table S1). In this experiment, responses in the RV-WN/TBE prime-INV boost group were significantly higher than in INV prime–RV-WN/TBE boost group (P < 0.001; T test). This finding requires further evaluation in NHP.
In these experiments, we also determined that TBE challenge resulted in a several-fold increase of TBE-specific N Ab titers in sera of immunized mice, indicative of an anamnestic response.
Attenuation, Immunogenicity, and Efficacy in NHP.
Schedules of studies in Rhesus monkeys are illustrated in Figs. S2–S4. Because monkeys do not develop clinical disease following peripheral TBE virus infection, a surrogate challenge model was first established. Animals were inoculated by the s.c. route with 6 log10 FFU of LGT T1674, LGT TP21 or YF/TBE viruses followed by measuring viremia on days 1–8. Only animals inoculated with the LGT T1674 virus developed readily detectable, uniform viremia with GMTs of 3.2 and 2.1 log10 FFU/mL on days 1 and 2, respectively (Fig. S5A), and therefore this virus was selected for challenge. LGT TP21 caused no detectable viremia, and YF/TBE induced a low-level viremia in some animals, ≤1.6 log10 FFU/mL, on days 5–6. We have observed previously for ChimeriVax-JE that a reduction in inoculation dose delays the onset of viremia in Rhesus monkeys without affecting the peak titer (25). Therefore, subsequently, during challenge of vaccinated animals, LGT T1674 virus was given at a lower s.c. dose of 5 log10 FFU, which indeed allowed for a better resolution of viremia in nonimmunized control animals, with a peak on day 2 (Fig. S5 B–D). This is considered a stringent challenge model for TBE vaccines because it is based on use of a heterologous, genetically and serologically distant virus.
No health abnormalities were observed in monkeys inoculated with any of the constructs shown in Table 3 based on monitoring body temperature and weight, food and water consumption and general appearance and behavior, with the exception of some animals that received the YF/TBE chimera. Specifically, two of four monkeys inoculated with a lower, 5 log10 FFU s.c. dose of YF/TBE developed neurological signs (miscoordination, imbalance). One animal started showing signs on day 14 but recovered by day 20. The second animal started showing signs on day 21 and appeared to be deteriorating, therefore it was removed from the study on day 28.
Table 3.
Immunogenicity and efficacy of RV-WN/TBE vaccine candidate in Rhesus macaques
| Group | No. of doses, dose (log10 FFU), route | No. of monkeys | PRNT50 GMT ± SD | Protection from challenge* (%) | |
| Day 29 | Day 50 | ||||
| RV-WN/TBE | 1 × 7 log, i.d. | 8 | 1,954 ± 2,200 | 1,677 ± 1,352 | 4/4 (100) |
| 1 × 6 log, i.d. | 4 | 122 ± 252 | 133 ± 284 | 3/4 (75) | |
| 1 × 5 log, i.d. | 4 | 45 ± 19 | 44 ± 11 | 3/4 (75) | |
| 1 × 7 log, i.m. | 8 | 955 ± 1,757 | 722 ± 737 | 4/4 (100) | |
| 1 × 7 log, s.c. | 4 | 184 ± 186 | 232 ± 329 | 4/4 (100) | |
| 2 × 7 log, s.c.† | 4 | 382 ± 252 (1 dose) | 2,832 ± 2,455 (2 doses) | 4/4 (100) | |
| 1 × 7 log, i.d. (WN pos.)‡ | 4 | 178 ± 403 | 306 ± 496 | 4/4 (100) | |
| INV | 3 × i.m.§ | 8 | 3,313 ± 5,547 (2 doses) | 4,111 ± 8,200 (3 doses) | 4/4 (100) |
| YF/TBE | 1 × 6 log, s.c. | 4 | 9,202 ± 4,598 | 7,279 ± 8,977 | 4/4 (100) |
| 1 × 5 log, s.c. | 3 | 7,982 ± 4,311 | 10,018 ± 14,469 | n.a.¶ | |
| LGT T1674 | 1 × 6 log, s.c. | 3 | 4,860 ± 4,803 | 3,308 ± 2,800 | 3/3 (100) |
| LGT TP21 | 1 × 6 log, s.c. | 3 | 84 ± 227 | 95 ± 193 | 3/3(100) |
| Mock | Diluent, s.c. | 8 | <10 | <10 | 0/8 (0) |
Combined data from the first and second (the short-term part, and immunogenicity values for days 29 and 50 of the long-term part) NHP studies are shown. A standard serum sample with a known N Ab titer was included in PRNT50 titrations for accuracy. n.a., not applicable.
Challenge was s.c. with 5 log10 FFU of LGT T1674 virus on day 59 (the first study) or day 51 (the short-term part of the second study); animals were considered protected if no viremia was detected by immunofocus assay during 8 d postchallenge.
Second s.c. dose was given on day 30.
Two WN-positive monkey were given RV-WN/TBE on day 0 (day 29 PRNT50 is shown for these animals only), additional two monkeys were preimmunized with RV-WN on day 0 and given RV-WN/TBE on day 30. Day 50 PRNT50 is for all four animals.
The three complete human INV doses were given on days 0, 14, and 30.
These animals were challenged at 6 mo postimmunization and were found protected (in the long-term part of second study).
Monkeys immunized with a single 7 log10 FFU dose of RV-WN/TBE by the intradermal (i.d.) route developed high TBE-specific N Ab titers measured on days 29 and 50 postimmunization (GMTs 1,954 and 1,677, respectively). These titers were only a few fold lower compared with the 5 and 6 log10 FFU doses of the underattenuated YF/TBE chimera administered s.c., the route used for ChimeriVax vaccines in humans (e.g., GMTs 7,279 and 10,018 on day 50, respectively; P < 0.05, Mann–Whitney test), and two and three complete i.m. (the route used in humans) doses of the INV control (GMT 3,313 on day 29 after two doses and 4,111 on day 50 after three doses) (Table 3). A single 7 log10 dose of RV-WN/TBE by the i.m. route was also highly effective (GMTs 955 and 722 on days 29 and 50). However, a single s.c. 7 log10 dose of RV-WN/TBE induced a lower Ab response (e.g., GMT 232 on day 50; P = 0.004, Mann–Whitney test), albeit substantially boosted by a second s.c. dose (GMT 2,832 on day 50). We also tested the effectiveness of RV-WN/TBE at lower i.d. doses of 6 and 5 log10 FFU. These doses also induced appreciable N Ab responses, although significantly lower compared with a 7 log10 i.d. dose.
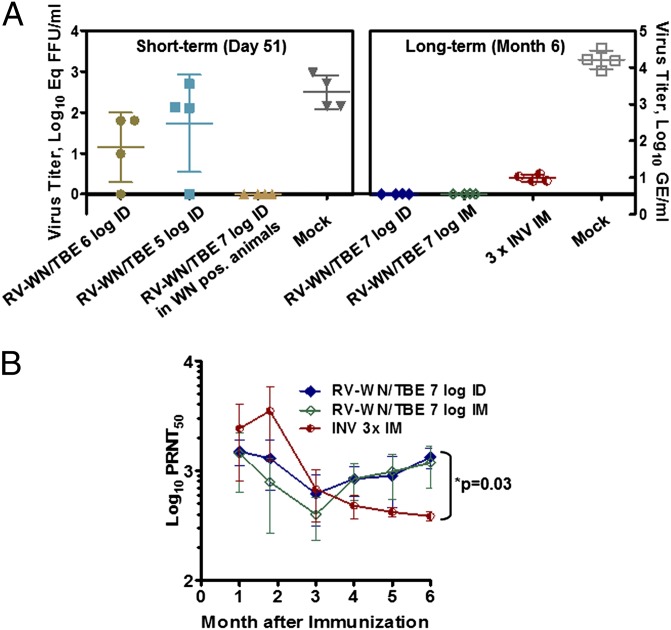
Protective efficacy was first demonstrated by challenging vaccinated animals with LGT T1674 virus (5 log10 FFU, s.c.) on day 51 or 59 after vaccination, and measuring postchallenge viremia. Standard titration of virus in monkey sera revealed that all mock immunized control animals had detectable viremia with mean peak titers of up to ∼3 log10 FFU/mL on day 2 (Fig. S5 B–D). When examined by a more sensitive quantitative (q)RT-PCR method (detection limit 0.32 log10 Eq FFU/mL), viremia in these control monkeys was detectable for three days with a mean peak titer of 29,648 (±255) GE/mL on day 2 (Fig. 3A). Among immunized animals in all treatment groups none had detectable viremia on days 1–8 after challenge by immunofocus assay, with the exception of one animal in each of the 5- and 6-log10 i.d. RV-WN/TBE groups, in agreement with prechallenge PRNT50 titers (Table 3; Fig. S5 B–D). The qRT-PCR method detected a postchallenge viremia in three out of four monkeys in the 5- and 6-log10 i.d. RV-WN/TBE groups (Fig. 3A).
Fig. 3.
Immunogenicity and efficacy of RepliVax-TBE (single dose; doses and routes are as indicated) compared with 3 complete human doses of INV in Rhesus monkeys (NHP study 2). INV was given on days 0, 14, and 30. (A) Postchallenge LGT 1674 viremia (peak titers on day 2 postchallenge) determined by a sensitive RT-qPCR method. Animals were challenged on day 51 or 6 mo after immunization. (B) Durability of N Ab responses during the 6 mo of the study.
To evaluate the effect of WN vector preimmunity on RV-WN/TBE, four monkeys with WN N Abs from natural immunity or preimmunized with RV-WN were inoculated with a 7 log10 FFU dose of RV-WN/TBE by the i.d. route. The WN preimmunity reduced the TBE specific N titers by approximately fivefold (GMT 306 on day 50) compared with the 7-log10 i.d. group of immunized WN-naïve animals (GMT 1,677), although the difference was not statistically significant (P < 0.05, Mann–Whitney test) (Table 3). Importantly, no postchallenge viremia was detected in this group, and thus the animals were completely protected (Fig. 3A). In this experiment, the effect of WN preimmunity was less pronounced compared with the effect of YF preimmunity on ChimeriVax-DEN2 vaccine in a NHP study described previously (26).
Durability of immunity elicited by a single dose of RV-WN/TBE was also evaluated. High-level N Ab titers were maintained during the 6 mo of monitoring in monkeys that received a 7 log10 FFU dose of RV-WN/TBE by both the i.d. and i.m. routes. In contrast, after three doses of the INV control, PRNT50 titers peaked on day 50 and then progressively declined (Fig. 3B). The difference in titers between the RV-WN/TBE groups and the 3× INV group became statistically significant at 5 and 6 mo (P < 0.03; Mann–Whitney test). The RV-WN/TBE vaccinated animals were protected from challenge performed at 6 mo, whereas a detectable postchallenge viremia was found in all monkeys in the 3× INV group (Fig. 3A). Thus, a single 7-log10 dose of RV-WN/TBE is superior to three doses of a human INV in terms of inducing a robust, long-lasting immunity in NHP.
Discussion
In this study, we constructed several RepliVax-TBE vaccine variants in the pursuit of a single-dose vaccine against TBE. The variant based on the WN backbone (RV-WN/TBE) replicated the most efficiently in helper cells with peak titers of up 8 log10 FFU/mL and was demonstrated to be genetically stable. It was shown to be highly attenuated in mice and Rhesus macaques, and highly immunogenic and protective in the two animal models, inducing a durable humoral response after a single shot. Therefore, it was selected as the lead vaccine candidate.
Live chimeras based on attenuated flavivirus backbones (YF 17D, LGT E5, and DEN2 PDK-53) were also constructed but found to be underattenuated. They remained highly neurovirulent in mice, including the DEN2/TBE virus constructed with a nonneurovirulent DEN2 PDK-53 backbone. The YF/TBE chimera was neuroinvasive, and LGT/TBE and DEN2/TBE viruses also retained some degree of neuroinvasiveness. This finding underscores the fact that TBE virus is difficult to rationally attenuate to obtain an acceptable LAV. Although highly immunogenic, the chimeras are not considered promising vaccine candidates.
In NHP experiments, the selected RepliVax-TBE candidate was shown to be highly immunogenic and fully protective at a 7 log10 FFU dose administered either by the i.d. or i.m. routes. Lower i.d. doses (6 and 5 log10 FFU) resulted in lower N Ab titers and incomplete protection from a stringent surrogate challenge with a heterologous LGT virus. The latter finding was in contrast to the dose–response data in mice which showed equal efficacy of RV-WN/TBE and the live YF/TBE chimera, with a PD50 of ∼3 log10 FFU for both candidates. An effective dose is determined by several parameters, including how efficiently a vaccine virus replicates in infected cells in vivo in a given model. For instance, the live ChimeriVax-WN vaccine was more immunogenic in humans than in mice and monkeys (27, 28). Based on recent epidemiological data for WN virus in North America, the WN backbone of RV-WN/TBE should enable efficient (single-cycle) replication in humans, and therefore an appropriate immunizing dose will need to be determined in clinical trials. Also it should be noted that based on evidence for INVs, N Ab titer of 1:10 is considered protective in humans (2) and thus all doses we tested here could be effective.
Durability of immunity elicited by both RepliVax-TBE and a human INV was found to be equally high in mice. However, as we concluded previously with RepliVax prototype vaccines (19), mice are not an optimal model for such a comparison because they are exceptionally responsive to flavivirus INVs. It is known that TBE immunity wanes relatively quickly after INV immunization of humans (29). Thus, a key finding in this study was that high N Ab titers were maintained in monkey sera after a single i.d. or i.m. dose of RepliVax-TBE during 6 mo of observation, and animals challenged at 6 mo were protected. In contrast, after three complete doses of INV, N titers peaked at 2 mo and then progressively declined, and animals were not completely protected as evidenced by detectable (albeit low-level) postchallenge viremia. The three-dose INV immunization schedule in our NHP experiments resembled a rapid immunization schedule used for one of the available INVs, Encepur (days 0, 7, and 21), which was shown to be as effective in humans as the conventional schedule (30, 31). A significant decline in seroconversion 6 mo after administration of the INV was observed independently of the schedule used (31). This result is in accord with our results in monkeys, which indicate that RepliVax-TBE is a superior vaccine over INVs with respect to magnitude and longevity of immunity, as well as schedule of immunization.
Because both RepliVax and LAV vaccines infect cells in vivo (single-cycle for RepliVax), they should share common mechanisms of induction of the immune response, e.g., infection of antigen-presenting cells, antigen production in draining lymph nodes, and stimulation of TLRs and similar innate pathways considered to be the prerequisites for a robust and durable immunity. One piece of evidence that this is the case was that both RepliVax-TBE and YF/TBE chimera elicited a Th1 biased immune response in mice (as opposed to Th2 for INV) as evidenced by a high IgG2a/IgG1 Ab ratio. The latter is also significant because the Th1-associated IgG1 isotype in humans (analog of mouse IgG2a) has been shown to be superior to other isotypes in terms of virus N activity (32). Further studies of innate immune responses in NHP using transcriptomic profiling and T-cell responses in mice are in progress and will be reported elsewhere.
Initial preclinical data on ChimeriVax vaccines in NHP suggested that anti-vector (YF) preimmunity could represent a concern (26), which, however, was completely dispelled in subsequent clinical trials (23, 24). Here we demonstrated that the effect of WN preimmunity on the effectiveness of RV-WN/TBE was less pronounced compared with the effect of YF preimmunity on ChimeriVax controls in mice. In NHP studies, WN-preimmune monkeys mounted a robust immune response following RV-WN/TBE vaccination and were completely protected from challenge. Hence, WN preimmunity should not represent a concern for use of RepliVax-TBE in humans. We also provide data in mice that RepliVax-TBE and INV vaccines are interchangeable in prime-boost vaccination regimens, suggesting that RepliVax-TBE can be used in persons already vaccinated with an INV and vice versa.
There are three subtypes of TBE virus represented by European, Siberian, and Far Eastern strains. Because of a high degree of homology in the E protein (∼95%), they comprise a single serotype, and a vaccine based on a TBE strain from one subtype is effective against all virus subtypes as evidenced by numerous previous studies using INVs (33–35). The fact that RepliVax-TBE protected monkeys from a surrogate challenge with a genetically distant LGT virus indicates that this vaccine will be broadly protective. This aspect is being confirmed in a collaborative study with the Chumakov Institute of Poliomyelitis and Viral Encephalitides (Moscow).
TBE is a growing health problem in Eurasia due to the continuing spread of the disease to new endemic foci (3, 36). Some endemic countries have policies aimed at increasing vaccination rates (Austria, Finland, Germany, Hungary, Latvia, Russia, Slovenia, Switzerland, and Italy), whereas the other 17 countries don’t (37), with the overall vaccine coverage remaining low. Therefore, an improved vaccine, effective after fewer doses compared with INVs can be expected to increase attractiveness of vaccination and policy compliance, thus broadening coverage of people at risk. The preclinical data presented here indicates that RepliVax-TBE could be such a vaccine.
Materials and Methods
Details on Vero and BHK cell cultures, including RepliVax packaging cells, methods, and viruses used are provided in SI Materials and Methods. Construction of RepliVax and chimeric virus vaccines was done using prM-E genes from a Central European TBE strain Hypr. NHP studies in Rhesus monkeys were performed at Bioqual, Inc. Statistical assays were performed using GraphPad Prism 5.
Supplementary Material
Acknowledgments
We thank Dr. Robert Tesh (World Reference Center for Emerging Viruses and Arboviruses collection, University of Texas Medical Branch, Galveston, TX) for providing TBE Hypr and LGT viruses, Bradley Finneyfrock and Mark Lewis (Bioqual) for performing in-life steps of NHP experiments, Kristl Edwards and Svetlana Pougatcheva for prescreening monkeys, Thomas Monath and Michael Vaine for critically reading the manuscript, Mark Parrington for assistance in organizational matters, Alfred Harvey (Becton Dickinson Technologies) for providing training on i.d. inoculation, and the staff of Animal Research Center for assistance with mice.
Footnotes
Conflict of interest statement: Sanofi Pasteur is developing RepliVax-TBE vaccine with the intent to sell. RepliVax is currently a registered trademark of the Board of Regents of the University of Texas System.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306245110/-/DCSupplemental.
References
- 1.World Health Organization Vaccines against tick-borne encephalitis: WHO position paper. Wkly Epidemiol Rec. 2011;86(24):241–256. [PubMed] [Google Scholar]
- 2.Barrett PN, Plotkin SA, Ehrlich HJ. In: Vaccines. 5th Ed. Plotkin SA, Orenstein WA, editors. Offit, PA: Elsevier Saunders; 2008. pp. 841–856. [Google Scholar]
- 3.Kunze U. International Scientific Working Group on Tick-Borne Encephalitis Tick-borne encephalitis: New paradigms in a changing vaccination environment. Wien Med Wochenschr. 2011;161(13-14):361–364. doi: 10.1007/s10354-011-0005-8. [DOI] [PubMed] [Google Scholar]
- 4. Burke DS, Monath TP (2001) Flaviviruses. Fields Virology, eds Knipe DM, et al. (Lippincott Williams and Wilkins, Philadelphia), 4th Ed, pp 1043–1126.
- 5. Lindenbach BD, Thiel H-J, Rice CM (2007) Flaviviridae: The viruses and their replication. Fields Virology, eds Knipe DM, et al. (Lippincott Williams and Wilkins, Philadelphia), 5th Ed, pp 1101–1152.
- 6.Pugachev KV, Guirakhoo F, Trent DW, Monath TP. In: New generation vaccines. 4th Ed. Levine MM, et al., editors. New York: Informa Healthcare; 2010. pp. 557–569. [Google Scholar]
- 7.Guy B, et al. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28(3):632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 8.Smorodincev AA, Dubov AV. In: Tick-Borne Encephalitis and Its Vaccine Prophylaxis. Smorodincev AA, editor. Leningrad: Meditsina; 1986. pp. 190–211. [Google Scholar]
- 9.Mandl CW, et al. Spontaneous and engineered deletions in the 3′ noncoding region of tick-borne encephalitis virus: Construction of highly attenuated mutants of a flavivirus. J Virol. 1998;72(3):2132–2140. doi: 10.1128/jvi.72.3.2132-2140.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kofler RM, Heinz FX, Mandl CW. A novel principle of attenuation for the development of new generation live flavivirus vaccines. Arch Virol Suppl. 2004;18(18):191–200. doi: 10.1007/978-3-7091-0572-6_17. [DOI] [PubMed] [Google Scholar]
- 11.Rumyantsev AA, Chanock RM, Murphy BR, Pletnev AG. Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys. Vaccine. 2006;24(2):133–143. doi: 10.1016/j.vaccine.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 12.Maximova OA, et al. Comparative neuropathogenesis and neurovirulence of attenuated flaviviruses in nonhuman primates. J Virol. 2008;82(11):5255–5268. doi: 10.1128/JVI.00172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright PF, et al. Evaluation of the Langat/dengue 4 chimeric virus as a live attenuated tick-borne encephalitis vaccine for safety and immunogenicity in healthy adult volunteers. Vaccine. 2008;26(7):882–890. doi: 10.1016/j.vaccine.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason PW, Shustov AV, Frolov I. Production and characterization of vaccines based on flaviviruses defective in replication. Virology. 2006;351(2):432–443. doi: 10.1016/j.virol.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa T, Widman DG, Bourne N, Konishi E, Mason PW. Construction and evaluation of a chimeric pseudoinfectious virus vaccine to prevent Japanese encephalitis. Vaccine. 2008;26(22):2772–2781. doi: 10.1016/j.vaccine.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki R, Fayzulin R, Frolov I, Mason PW. Identification of mutated cyclization sequences that permit efficient replication of West Nile virus genomes: Use in safer propagation of a novel vaccine candidate. J Virol. 2008;82(14):6942–6951. doi: 10.1128/JVI.00662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widman DG, Frolov I, Mason PW. Third-generation flavivirus vaccines based on single-cycle, encapsidation-defective viruses. Adv Virus Res. 2008;72:77–126. doi: 10.1016/S0065-3527(08)00402-8. [DOI] [PubMed] [Google Scholar]
- 18.Ferlenghi I, et al. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol Cell. 2001;7(3):593–602. doi: 10.1016/s1097-2765(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 19.Rumyantsev AA, et al. Characterization of the RepliVax platform for replication-defective flavivirus vaccines. Vaccine. 2011;29(32):5184–5194. doi: 10.1016/j.vaccine.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Huang CY, et al. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77(21):11436–11447. doi: 10.1128/JVI.77.21.11436-11447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CY, Silengo SJ, Whiteman MC, Kinney RM. Chimeric dengue 2 PDK-53/West Nile NY99 viruses retain the phenotypic attenuation markers of the candidate PDK-53 vaccine virus and protect mice against lethal challenge with West Nile virus. J Virol. 2005;79(12):7300–7310. doi: 10.1128/JVI.79.12.7300-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rumyantsev AA, Murphy BR, Pletnev AG. A tick-borne Langat virus mutant that is temperature sensitive and host range restricted in neuroblastoma cells and lacks neuroinvasiveness for immunodeficient mice. J Virol. 2006;80(3):1427–1439. doi: 10.1128/JVI.80.3.1427-1439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monath TP, et al. Clinical proof of principle for ChimeriVax: Recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20(7-8):1004–1018. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 24.Guirakhoo F, et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: Phase I clinical trial for safety and immunogenicity: Effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vaccin. 2006;2(2):60–67. doi: 10.4161/hv.2.2.2555. [DOI] [PubMed] [Google Scholar]
- 25.Monath TP, et al. Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: Dose-response effectiveness and extended safety testing in rhesus monkeys. J Virol. 2000;74(4):1742–1751. doi: 10.1128/jvi.74.4.1742-1751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guirakhoo F, et al. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol. 2000;74(12):5477–5485. doi: 10.1128/jvi.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arroyo J, et al. ChimeriVax-West Nile virus live-attenuated vaccine: Preclinical evaluation of safety, immunogenicity, and efficacy. J Virol. 2004;78(22):12497–12507. doi: 10.1128/JVI.78.22.12497-12507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monath TP, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci USA. 2006;103(17):6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loew-Baselli A, et al. Prevention of tick-borne encephalitis by FSME-IMMUN vaccines: Review of a clinical development programme. Vaccine. 2011;29(43):7307–7319. doi: 10.1016/j.vaccine.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 30.Schoendorf I, et al. Tick-born encephalitis (TBE) vaccination in children: Advantage of the rapid immunization schedule (i.e., days 0, 7, 21) Hum Vaccin. 2007;3(2):42–47. doi: 10.4161/hv.3.2.3747. [DOI] [PubMed] [Google Scholar]
- 31.Schöndorf I, et al. Tick-borne encephalitis (TBE) vaccination: Applying the most suitable vaccination schedule. Vaccine. 2007;25(8):1470–1475. doi: 10.1016/j.vaccine.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Hofmeister Y, et al. Human IgG subclasses: In vitro neutralization of and in vivo protection against West Nile virus. J Virol. 2011;85(4):1896–1899. doi: 10.1128/JVI.02155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holzmann H, et al. Molecular epidemiology of tick-borne encephalitis virus: Cross-protection between European and Far Eastern subtypes. Vaccine. 1992;10(5):345–349. doi: 10.1016/0264-410x(92)90376-u. [DOI] [PubMed] [Google Scholar]
- 34.Orlinger KK, et al. A tick-borne encephalitis virus vaccine based on the European prototype strain induces broadly reactive cross-neutralizing antibodies in humans. J Infect Dis. 2011;203(11):1556–1564. doi: 10.1093/infdis/jir122. [DOI] [PubMed] [Google Scholar]
- 35.Fritz R, et al. Quantitative comparison of the cross-protection induced by tick-borne encephalitis virus vaccines based on European and Far Eastern virus subtypes. Vaccine. 2012;30(6):1165–1169. doi: 10.1016/j.vaccine.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Kunze U. ISW-TBE Tick-borne encephalitis (TBE): An underestimated risk…still: Report of the 14th annual meeting of the International Scientific Working Group on Tick-Borne Encephalitis (ISW-TBE) Ticks Tick Borne Dis. 2012;3:197–201. doi: 10.1016/j.ttbdis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Donoso Mantke O, Escadafal C, Niedrig M, Pfeffer M. Working Group For Tick-Borne Encephalitis Virus C Tick-borne encephalitis in Europe, 2007 to 2009. Euro Surveill. 2011;16(39):19976. doi: 10.2807/ese.16.39.19976-en. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.