The signaling protein PII, highly conserved in archaea, bacteria, and plants, is an example of beauty in biology (1). PII is relatively small, a homotrimer with each subunit of just 12–13 kDa; yet it binds to several effectors and exercises its regulatory roles by interacting with its partners that can be enzymes, transcriptional factors, or membrane transport proteins. Moreover, PII can be modified posttranslationally, which may take the form of phosphorylation, uridylylation, or adenylylation. Depending on how many of the three monomers of PII bind to an effector or are modified, PII is given a large array of conformational variations that allow it to signal various metabolic states of the cells and fine-tune the metabolic activities. The primary role of PII is in the control of nitrogen metabolism; it was first reported in 1969 (2) and has been extensively studied since then. In a review published in 2013, 47 structures of the PII proteins were recorded, representing 19 proteins from 16 organisms spreading among the three domains of life (1). Thus, one may think that we know more or less all the details about the biochemical basis of PII function. Now, Radchenko et al. report in PNAS that PII has a weak but detectable activity of ATPase (3), and their studies clarify several puzzling observations previously reported on the function of PII.
Because of the importance of nitrogen metabolism, bacteria harbor complex regulatory mechanisms that allow them to adapt to the changes of nitrogen supply in their environment. These mechanisms can operate at different levels, from transcriptional control to protein–protein interaction, or transport of nitrogen compounds across the cell membrane. PII can be involved in any of these regulatory levels in different organisms so far studied (1). Three subgroups of PII proteins are defined based on phylogenetic studies: GlnB, GlnK, and NifI. With exceptions, glnB is linked to glnA encoding the glutamine synthetase or nadE encoding NAD+ synthetase; glnK is cotranscribed with amtB encoding ammonium transporter; nifI is associated with the structural genes encoding the nitrogenase complex. All PII proteins so far crystallized possess a well-conserved 3D structure. The compact, cylindrical, core structure of PII is formed by an antiparallel β-sheet of four β-strands with two α-helices at the lateral surface. A variable loop, the T-loop, extends from each core, and forms an antenna-like structure (Fig. 1). The T-loop contains the posttranslational modification site and can adopt either a flexible conformation or a rigid one. Both the binding of the allosteric effectors and posttranslational modification affect the conformation of the T-loop, which acts as a plastic site to recognize various protein partners. The binding sites for the three allosteric effectors, ATP/ADP and 2-oxoglutarate (2-OG), are highly conserved among the PII proteins. Three nucleotide-binding sites are located in the clefts at the interface between two monomers, and 2-OG binds at the vicinity of the nucleotide-binding site. ATP and 2-OG binding occur cooperatively and require the presence of Mg2+ ions (4).
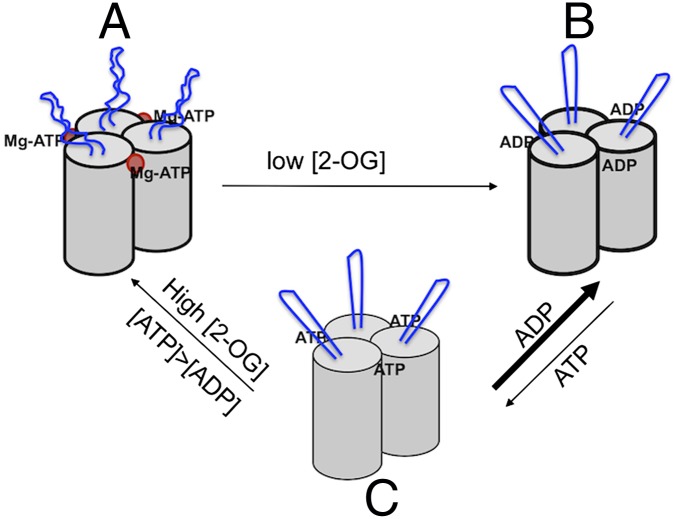
Fig. 1.
PII is a homotrimer, each subunit with a cylindrical core structure and antenna-like T-loop (in blue) extending out from the core. (A) Under nitrogen limitation, Mg-ATP and 2-OG (red dot) bind at proximity at the cleft between each subunit; the presence of 2-OG inhibits the hydrolysis of ATP, and the T-loops adopt a flexible conformation. (B) When nitrogen supply increases, 2-OG is consumed for ammonium assimilation and its level drops; Mg-ATP is hydrolyzed to ADP and the T-loops become more rigid. ADP inhibits ATP binding under low 2-OG levels. (C) High 2-OG and ATP concentrations favor the exchange of ADP to ATP, and PII is reloaded with Mg-ATP and 2-OG.
2-OG is a key intermediate of the Krebs cycle and a signal of nitrogen status (1, 5, 6). PII has been considered as a signaling protein that integrates the status of carbon and nitrogen availability, as well as the energy charge. Although the fact that PII acts as a sensor of 2-OG is generally well accepted, the role of binding of ATP/ADP is much less obvious (1). ATP and ADP compete for binding to PII, leading to the proposition that PII may be a sensor of energy charge. This hypothesis has yet to be confirmed in vivo, and raises several questions. First, a large number of PII proteins from different organisms have been crystallized, some of them with ADP bound on them and others with ATP. In some cases, ADP was even found on PII when ATP was initially added during the experimental process (1). Second, at least in Escherichia coli cells, the concentration of ATP is 6- to 10-fold higher than that of ADP (7, 8); thus, it is difficult to imagine that ATP drops to a level where ADP competes efficiently for PII binding. Finally, the energy charge [(ATP + 0.5× ADP)/(ATP+ADP+AMP)] is relatively stable and goes around 0.9 in E. coli; cells become nonviable when it goes down to less than 0.5 (8). Thus, the significance of the binding of the two nucleotides on PII remains unclear.
Radchenko et al. (3) use GlnK from E. coli as a model to test the ATPase activity of PII. The target of GlnK in E. coli is AmtB, a transmembrane ammonium channel (9). Under nitrogen limitation, the level of 2-OG remains relatively high; under such conditions, GlnK is cytoplasmically located, urydylylated at the T-loop, and expected to contain ATP, Mg2+, and 2-OG. When nitrogen-limited cells get a sudden ammonium supply, the level of 2-OG drops rapidly and PII becomes deurydylylated; the T-loop adopts a rigid conformation protruding into the AmtB channel, forming a complex in a 1:1 stoichiometry to block further ammonium entry (9). The idea that PII may have an intrinsic ATPase activity was first proposed by Gruswitz et al. in their publication about the AmtB-GlnK complex (9). ATP enhances the binding of GlnK to AmtB, but the complex incubated initially with ATP contained ADP instead. Based on the proximity of the ADP terminal phosphate to side chains of several positively charged residues (Arg and Lys) and a highly-coordinated buried water in GlnK, Gruswitz et al. suggested that this nucleotide binding pocket may serve to catalyze ATP hydrolysis. It took a few years for a laboratory to test this hypothesis experimentally, and one of the reasons may be the weak activity of ATP hydrolysis of GlnK. Under standard experimental procedures—that is, using a mixture of cold ATP molecules with radioactively labeled ones—ATP hydrolysis was hardly seen. However, this procedure was possible when GlnK was incubated with hot ATP molecules only (3). Radchenko et al. offer two lines of evidence to rule out the possibility of a contaminating activity from cell extract. First, this low ATP hydrolysis activity is no longer present in several GlnK mutants, each replacing K90, R101, or R105, to an Ala residue. These three positively charged residues have been previously suggested by Gruswitz et al. as being possibly involved in ATP hydrolysis (9). All three mutants displayed a much weaker ATPase activity compared with the wild-type. Second, the physiological effector, 2-OG, inhibits ATP hydrolysis of GlnK, and this inhibitory effect requires Mg2+ ions. The involvement of Mg2+ can be explained by the role of this ion in coordinating ATP and 2-OG binding in PII proteins (4); each 2-OG binds to Mg2+ ion, which is in turn coordinated by three phosphate groups of ATP. As expected, those mutant variants, K90A, R101A, and R105A, although displaying a weak activity of ATP hydrolysis compared with the wild-type, still retain the ability to respond to the presence of 2-OG. In contrast, mutation of either residue Q39 or K58, both involved in 2-OG binding, showed either little ATP hydrolysis or had a weak ATPase activity that is no longer influenced by the presence of 2-OG (3). The ATP hydrolysis activity of GlnK in E. coli can further be extended to GlnZ, a GlnK homolog in Azospirillum brazilense, and the PII protein from the plant Arabidopsis thaliana, suggesting that it is a general phenomenon among PII proteins (3).
The ATPase activity of PII acts as a switch, much as many small GTPases, in driving protein conformational change in signal transduction.
What is the major insight into the signaling mechanism of PII? Under nitrogen limitation, 2-OG levels are high in the cells, and that favors the binding of Mg-ATP on each of the three pockets of PII; the T-loop adopts a flexible conformation (Fig. 1). When the 2-OG level drops as nitrogen supply increases, less 2-OG occupancy triggers Mg-ATP hydrolysis into ADP, and this activity acts to translate the drop of 2-OG signal into a change in the T-loop conformation, which becomes rigid and extended. Thus, the ATPase activity of PII acts as a switch, much as many small GTPases, in driving protein conformational change in signal transduction. Although the change of the PII form with 2-OG and Mg-ATP to that with ADP can be well described based on the extensive structural information of PII and the recently described data by Radchenko et al. (3), how ADP is kicked out of PII by ATP is much less obvious to imagine, because ADP and ATP compete for binding to PII (1). We suggest the following scenario: under low 2-OG level, PII hydrolyzes ATP into ADP, which occupies the nucleotide-binding pocket and inhibits ATP binding (1, 3); thus, as long as the 2-OG levels remain relatively low, ADP-bound PII should be favored over ATP-bound one, or the ATP-bound form cannot be stabilized (Fig. 1). However, in E. coli cells, the ATP concentration is 6- to 10-times higher than that of ADP (7, 8), which should help PII to reload ATP when 2-OG level increases as the later molecule stabilizes Mg-ATP at its binding pocket (Fig. 1). In essence, the PII function is to sense the level of 2-OG, a signal of nitrogen availability, and ATP hydrolysis serves as a switch for conformational change of PII as 2-OG level varies.
Although the data of Radchenko et al. (3) simplify our view on the function of PII in the control of nitrogen metabolism, the finding raises more questions, and some debate would even be expected. Why is the activity of ATP hydrolysis by GlnK so low, and how to explain the rapid response of PII to nitrogen availability in view of such a low activity? Is there an activator for the PII ATPase activity? How to confirm this finding in vivo? Obviously, the more we understand on the function of PII, the more questions are raised. This aspect may be why PII is so fascinating and has been studied ever since its discovery in 1969 (2).
Footnotes
The authors declare no conflict of interest.
See companion article on page 12948.
References
- 1.Huergo LF, Chandra G, Merrick M. P(II) signal transduction proteins: Nitrogen regulation and beyond. FEMS Microbiol Rev. 2013;37(2):251–283. doi: 10.1111/j.1574-6976.2012.00351.x. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro BM. The glutamine synthetase deadenylylating enzyme system from Escherichia coli. Resolution into two components, specific nucleotide stimulation, and cofactor requirements. Biochemistry. 1969;8(2):659–670. doi: 10.1021/bi00830a030. [DOI] [PubMed] [Google Scholar]
- 3.Radchenko MV, Thornton J, Merrick M. PII signal transduction proteins are ATPases whose activity is regulated by 2-oxoglutarate. Proc Natl Acad Sci USA. 2013;110:12948–12953. doi: 10.1073/pnas.1304386110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fokina O, Chellamuthu V-R, Forchhammer K, Zeth K. Mechanism of 2-oxoglutarate signaling by the Synechococcus elongatus PII signal transduction protein. Proc Natl Acad Sci USA. 2010;107(46):19760–19765. doi: 10.1073/pnas.1007653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent S, et al. Nonmetabolizable analogue of 2-oxoglutarate elicits heterocyst differentiation under repressive conditions in Anabaena sp. PCC 7120. Proc Natl Acad Sci USA. 2005;102(28):9907–9912. doi: 10.1073/pnas.0502337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao M-X, et al. Structural basis for the allosteric control of the global transcription factor NtcA by the nitrogen starvation signal 2-oxoglutarate. Proc Natl Acad Sci USA. 2010;107(28):12487–12492. doi: 10.1073/pnas.1001556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett BD, et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5(8):593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman AG, Fall L, Atkinson DE. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruswitz F, O’Connell J, 3rd, Stroud RM. Inhibitory complex of the transmembrane ammonia channel, AmtB, and the cytosolic regulatory protein, GlnK, at 1.96 A. Proc Natl Acad Sci USA. 2007;104(1):42–47. doi: 10.1073/pnas.0609796104. [DOI] [PMC free article] [PubMed] [Google Scholar]