Abstract
The receptor tyrosine kinases Axl and Mer, belonging to the Tyro3, Axl and Mer (TAM) receptor family, are expressed in a number of tumor cells and have well-characterized oncogenic roles. The therapeutic targeting of these kinases is considered an anticancer strategy, and various inhibitors are currently under development. At the same time, Axl and Mer are expressed in dendritic cells and macrophages and have an essential function in limiting inflammation. Inflammation is an enabling characteristic of multiple cancer hallmarks. These contrasting oncogenic and anti-inflammatory functions of Axl and Mer posit a potential paradox in terms of anticancer therapy. Here we demonstrate that azoxymethane (AOM) and dextran sulfate sodium (DSS)-induced inflammation-associated cancer is exacerbated in mice lacking Axl and Mer. Ablation of Axl and Mer signaling is associated with increased production of proinflammatory cytokines and failure to clear apoptotic neutrophils in the intestinal lamina propria, thereby favoring a tumor-promoting environment. Interestingly, loss of these genes in the hematopoietic compartment is not associated with increased colitis. Axl and Mer are expressed in radioresistant intestinal macrophages, and the loss of these genes is associated with an increased inflammatory signature in this compartment. Our results raise the possibility of potential adverse effects of systemic anticancer therapies with Axl and Mer inhibitors, and underscore the importance of understanding their tissue and cell type-specific functions in cancer.
The proto-oncogenes AXL and MER were first cloned from chronic myelogenous and lymphoblastic leukemia cells (1–3). Increased expression of AXL has been reported in lung, breast, ovarian, gastric, pancreatic, and prostate cancers; leukemias and lymphomas; melanoma; and glioblastoma multiforme (4, 5). Increased MER expression is associated with leukemias, lymphomas, melanoma, rhabdomyosarcomas, and gastric and prostate cancers (4–6). AXL and MER expression also has been directly correlated with poor prognosis in cancer (6–9). Moreover, ectopic expression of AXL has been shown to confer resistance to EGF receptor therapy in lung cancer (10, 11). Multiple studies have demonstrated AXL and MER function in survival, invasion, and metastasis in a variety of tumors (12–15); thus, attention has focused on the pharmacologic targeting of AXL and MER in cancer. Axl and Mer share structural homology in the kinase domain with other tyrosine kinases, including conserved molecular interactions with ATP; however, several unique features of the active site allow for selective inhibition (16), and small-molecule inhibitors as well as biologics are in preclinical development (6, 16–19).
Axl and Mer are associated with another distinct feature of cancer—inflammation. Tumor-promoting inflammation has been described as an enabling characteristic that promotes the acquisition of cancer hallmarks and orchestrates tumor progression (20). Chronic inflammation increases the risk of colorectal cancer (21, 22). The relative risk of being diagnosed with colorectal cancer in the United States was ∼0.05% in 2007 (www.cdc.gov). This risk is increased dramatically in patients with inflammatory bowel disease; for example, ulcerative colitis increases the risk by ∼20-fold (23), and the reported risk in patients with pancolitis is 30% after 35 years of disease (24).
Recently, a direct role of innate immune cells and cytokines in colorectal cancer has been demonstrated using mouse models (25). One of the main physiological functions of Axl and Mer is in the inhibition of the innate immune response (26). Axl and Mer are expressed in dendritic cells and macrophages, and their activation limits toll-like receptor and cytokine receptor signaling (26–29); consequently, Axl and Mer should inhibit tumor-promoting inflammation and reduce the risk of colorectal cancer. In light of the oncogenic and anti-inflammatory functions of Axl and Mer, we sought to test the role of these receptor tyrosine kinases (RTKs) in inflammation-driven cancer.
Using a mouse model of AOM/DSS-induced colorectal cancer (30, 31), combined with genetic ablation of Axl and Mer, we found that Axl and Mer signaling prevents colitis and significantly reduces the number and size of colorectal adenomas. We detected a significant increase in the number of apoptotic neutrophils and in the production of proinflammatory cytokines in the lamina propria of Axl−/−Mer−/− mice in the context of DSS-induced colitis. Axl and Mer are expressed primarily in the hematopoietic compartment (32). Surprisingly, the loss of Axl and Mer function in radiosensitive hematopoietic cells is not associated with increased colonic inflammation. Axl and Mer are expressed in a radioresistant population of lamina propria macrophages upon inflammation, and the absence of Axl and Mer results in an increased proinflammatory profile in lamina propria macrophages. Axl−/−Mer−/− macrophages show increased production of inflammatory cytokines and reduced expression of genes associated with alternative activation—a distinct macrophage phenotype that decreases inflammation and promotes tissue repair (33). Our results demonstrate that although Axl and Mer can function as oncogenes in a number of cancers, these genes have a protective role against the development of colitis-associated cancer. Our findings underscore the potential adverse effects of systemic inhibition of Axl and Mer and highlight the importance of developing therapeutic strategies to spare these RTKs in macrophage populations relevant to the regulation of local inflammation and tissue homeostasis.
Results
Genetic Ablation of Axl and Mer Promotes Colitis-Associated Colon Cancer.
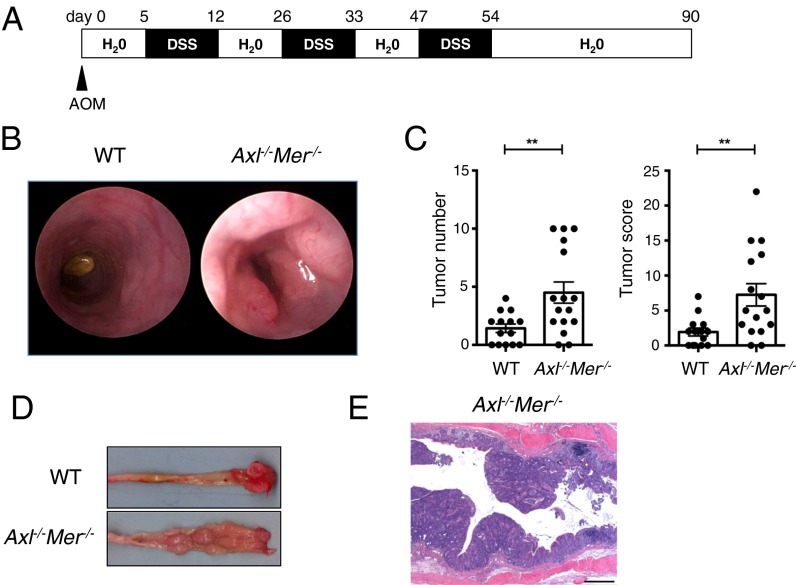
We compared the development of colitis-associated colorectal cancer in WT and Axl−/−Mer−/− mice. Colorectal cancer was induced by the administration of a single i.p. dose of the procarcinogen AOM, followed by three cycles of DSS in the drinking water separated by treatment-free intervals (Fig. 1A). Endoscopic analysis revealed that the Axl−/−Mer−/− mice developed significantly more polyps compared with WT mice (Fig. 1B). Moreover, the polyps were more numerous and significantly larger in the Axl−/−Mer−/− mice, contributing to a higher tumor score in these mice (Fig. 1C). Dissection of the gut at the end of treatment confirmed the presence of macroscopic polyps in the distal colon and rectum of Axl−/−Mer−/− mice (Fig. 1D). Histopathological analyses revealed the presence of adenomas in these mice (Fig. 1E).
Fig. 1.
Genetic ablation of Axl and Mer leads to increased susceptibility to AOM/DSS-induced colon cancer. (A) Schematic representation of AOM/DSS-induced colon cancer treatment. In brief, WT and Axl−/−Mer−/− mice were injected with the DNA-methylating agent AOM and were subsequently treated with the indicated cycles of DSS. (B) Representative colonoscopy images after 90 d of treatment in WT and Axl−/−Mer−/− mice. (C) Tumor number and tumor score in WT and Axl−/−Mer−/− mice after 90 d of treatment as determined by colonoscopy. (D) Representative luminal views of WT and Axl−/−Mer−/− colons treated as indicated in A. Tumors in the rectum and distal colon of Axl−/−Mer−/− can be seen. (E) Representative image of colon sections stained with H&E, showing adenomas in Axl−/−Mer−/− mice. (Scale bar: 500 μm.) Data are presented as representative individual samples or as the mean of 14–16 independent samples per group. **P < 0.01.
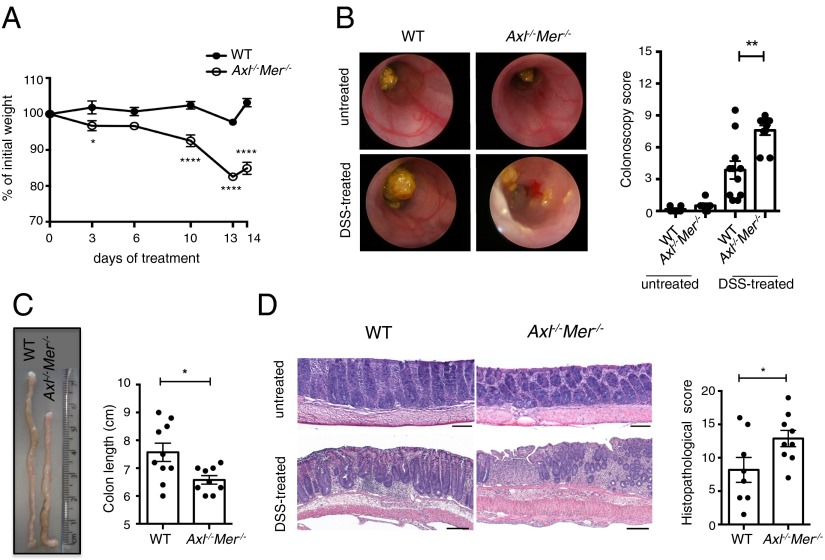
Distinct mechanisms accounting for an increased incidence of tumors have been reported in this model. Although cancer is commonly caused by colitis, inflammation-independent mechanisms have been reported (34). Thus, we directly tested whether the loss of Axl and Mer results in increased colitis. WT and Axl−/−Mer−/− mice were acutely treated with DSS in the drinking water. The DSS dose was adjusted to induce minimal inflammation in WT mice as judged by colonoscopy (Fig. 2B). Axl−/−Mer−/− mice showed a significant reduction in body weight after DSS treatment compared with WT mice (Fig. 2A). Colonoscopy in the Axl−/−Mer−/− mice revealed enhanced colitis as demonstrated by increased granularity, loss of apparent vasculature, decreased translucency, and looser stool consistency (Fig. 2B). Consistent with increased inflammation after DSS administration, colon length was significantly reduced in the Axl−/−Mer−/− mice (Fig. 2C). Histopathological analysis following established methods (35) confirmed the increased severity of colitis (i.e., mucosal ulceration, crypt loss, crypt hyperplasia, inflammatory cell infiltration, and edema) in the Axl−/−Mer−/− mice compared with WT mice after DSS administration (Fig. 2D). Colons from untreated Axl−/−Mer−/− mice were unremarkable and within normal limits by both colonoscopy and histopathological analysis (Fig. 2 B and D). Taken together, these results demonstrate that the increased severity of colitis and tissue injury in the Axl−/−Mer−/− mice is associated with an increased risk of colorectal cancer.
Fig. 2.
Genetic ablation of Axl and Mer leads to increased susceptibility to DSS-induced colitis. (A and B) Body weight (A) and representative colonoscopy images and colonoscopy scores (B) in WT and Axl−/−Mer−/− mice in the steady-state condition (untreated) and after DSS treatment (DSS-treated). (C) Representative images and colon length in WT and Axl−/−Mer−/− mice after DSS treatment. (D) Representative images of H&E-stained colon sections showing normal colon morphology in untreated mice and increased colitis in Axl−/−Mer−/− mice compared with WT after DSS treatment. Histological scoring of colitis is reported. (Scale bars: 500 μm.) Data are representative images or mean ± SEM of at least six independent samples per group. *P < 0.05; **P < 0.01; ****P < 0.0001.
Axl- and Mer-Dependent Phagocytosis of Apoptotic Neutrophils and Inhibition of Cytokine Production Prevent Colitis.
We next evaluated additional features of intestinal inflammation in WT and Axl−/−Mer−/− mice. The percentages of Ly6G+ neutrophils and F4/80+CD11b+ macrophages were not altered in WT and Axl−/−Mer−/− mice at steady state (Fig. 3A and Fig. S1). The induction of colitis was associated with a significant increase in Ly6G+ cells or neutrophils in both WT and Axl−/−Mer−/− mice (Fig. 3A). Interestingly, immunohistochemical and FACS analyses revealed a significant increase in the number of TUNEL+/Ly6G+ apoptotic neutrophils in the lamina propria of Axl−/−Mer−/− mice compared with WT mice (Fig. 3 B and C). Clearance of apoptotic neutrophils is essential for the resolution of inflammation and tissue repair (36).
Fig. 3.
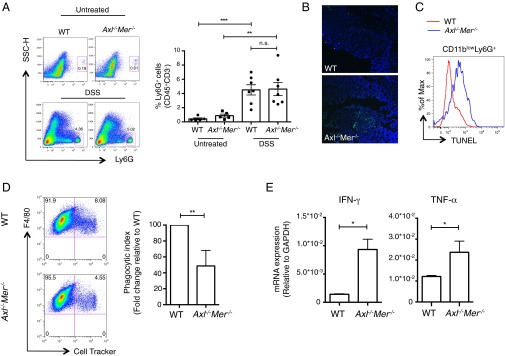
Increased number of apoptotic neutrophils and production of proinflammatory cytokines in the lamina propria of Axl−/−Mer−/− mice. (A) Percentage of neutrophils (CD45+CD3−Ly6G+) in the lamina propria of WT and Axl−/−Mer−/− before (untreated) and after 7 d of DSS treatment (DSS) as detected by FACS analysis. Representative FACS plots and independent data are shown. (B) Representative immunofluorescence staining for TUNEL in colon sections from WT and Axl−/−Mer−/− mice after 7 d of DSS treatment. Nuclei are counterstained with DAPI. (Scale bar: 75 μm.) (C) TUNEL staining in CD11blowLy6G+ cells in the lamina propria leukocytes of WT and Axl−/−Mer−/− mice as detected by FACS analysis. (D) Rate of phagocytosis of apoptotic neutrophils, labeled with CellTracker and incubated with WT or Axl−/−Mer−/− BM macrophages for 60 min. FACS plots show WT or Axl−/−Mer−/− F4/80+ BM macrophages that have engulfed CellTracker-labeled apoptotic neutrophils. The phagocytic index normalized to WT macrophages is shown on the right. (E) Expression of IFN-γ and TNF-α in the lamina propria leukocytes from WT and Axl−/−Mer−/− mice after 7 d of DSS treatment as detected by qPCR. Data are presented as representative images or as mean ± SEM of at least five samples per group. n.s., nonsignificant. *P < 0.05; **P < 0.01; ***P < 0.001.
Tyro3, Axl and Mer (TAM) receptors function in the removal of apoptotic membranes of photoreceptors and apoptotic germ cells in the testis (32); thus, we directly compared the ability of bone marrow (BM)-derived macrophages from WT and Axl−/−Mer−/− mice to phagocytose apoptotic neutrophils in in vitro assays. Apoptotic neutrophils were labeled with CellTracker dye and cocultured with BM-derived macrophages at a ratio of 5:1. Axl−/−Mer−/− macrophages demonstrated a significant deficit in the ability to phagocytose apoptotic neutrophils compared with WT macrophages (Fig. 3D). The loss of Axl and Mer also was associated with increased inflammatory cytokine production by lamina propria leukocytes. CD45+ cells were sorted from the lamina propria of WT or Axl−/−Mer−/− mice after 7 d of DSS treatment. Quantitative real-time PCR (qPCR) analyses demonstrated an increase in the expression of IFN-γ and TNF-α in Axl−/−Mer−/− leukocytes (Fig. 3E). Taken together, our results indicate that Axl and Mer function in limiting inflammation in the intestinal lamina propria through the phagocytosis of apoptotic neutrophils and regulation of proinflammatory cytokine production.
Hematopoietic Function of Axl and Mer Does Not Confer Increased Susceptibility to Colitis.
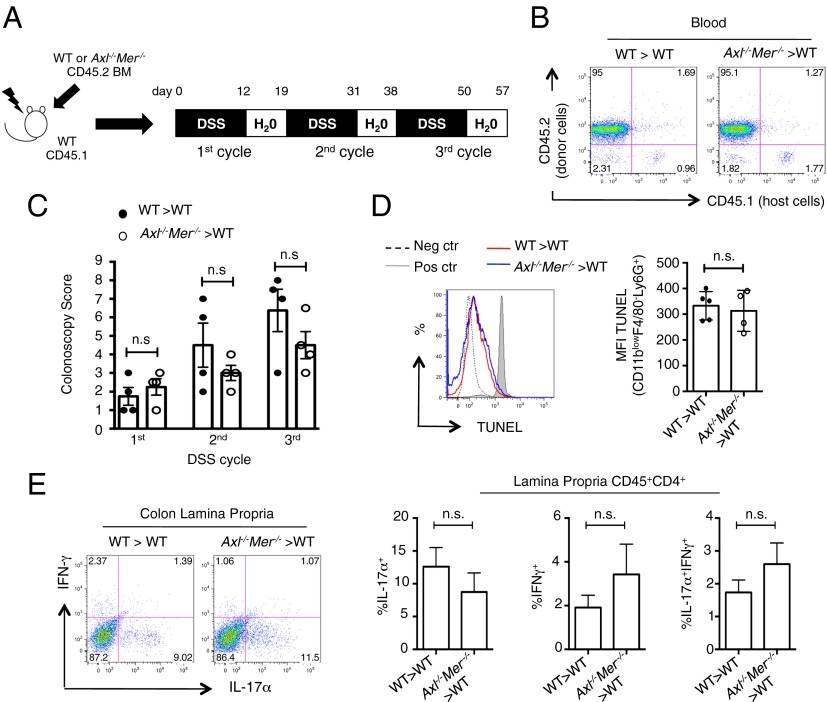
Because Axl and Mer are expressed primarily in BM-derived dendritic cells and macrophages (32), we tested whether the loss of Axl and Mer in the hematopoietic compartment accounted for the increased susceptibility to induced colitis. For this, CD45.1 C57B6 WT mice were lethally irradiated and their hematopoietic compartments reconstituted with CD45.2 WT or Axl−/−Mer−/− hematopoietic progenitors (Fig. 4A). Efficient reconstitution of the hematopoietic compartment was confirmed by the analysis of CD45.1+ and CD45.2+ leukocytes in the peripheral blood at 2 mo after BM transfer (Fig. 4B). Treatment with DSS did not cause enhanced colitis in recipients of Axl−/−Mer−/− hematopoietic progenitors compared with recipients of WT hematopoietic progenitors, as confirmed by colonoscopy (Fig. 4C). We continued the DSS treatment for two additional cycles. Surprisingly, even chronic DSS treatment failed to demonstrate a significant difference in colonic inflammation between recipients of Axl−/−Mer−/− hematopoietic progenitors and recipients of WT hematopoietic progenitors (Fig. 4C). In line with these results, we did not detect a significant increase in the number of TUNEL+/Ly6G+ apoptotic neutrophils in the lamina propria of recipients of Axl−/−Mer−/− BM compared with recipients of WT BM after 7 d of DSS treatment (Fig. 4D). Furthermore, recipients of WT and Axl−/−Mer−/− hematopoietic progenitors demonstrated similar induction of IL-17+, IFN-γ+, and IL-17+IFN-γ+ colon lamina propria CD4+ T cells (Fig. 4E). These results demonstrate that loss of Axl and Mer in the radiosensitive hematopoietic compartment is not responsible for the increased susceptibility to induced colitis observed in Axl−/−Mer−/− mice.
Fig. 4.
Loss of Axl and Mer in the hematopoietic compartment does not confer susceptibility to colitis. (A) Schematic representation of BM chimeras and chronic DSS treatment. (B) FACS analysis of chimerism of blood leukocytes in BM chimeras as indicated in A. (C) Colonoscopy score determined at the end of each DSS cycle in the indicated BM chimeras. (D) TUNEL staining in CD11blowF4/80−Ly6G+ cells in the lamina propria leukocytes of WT > WT and Axl−/−Mer−/− >WT BM chimera mice, as detected by FACS, after 7 d of DSS treatment. Representative histograms (Left) and bar graphs (Right) indicating mean fluorescence intensity (MFI) of TUNEL expression are shown. (E) Representative FACS plots (Left) and bar graphs (Right) showing the frequency of IL17-α+, IFN-γ+, and IL17-α+IFN-γ+ CD4+ T cells in the lamina propria of BM chimeras at the end of the chronic DSS treatment. Data are presented as representative images or as mean ± SEM of at least four independent samples per group. n.s., nonsignificant.
Loss of Axl and Mer Is Associated with Increased Inflammatory Profile of a Radioresistant Macrophage Population.
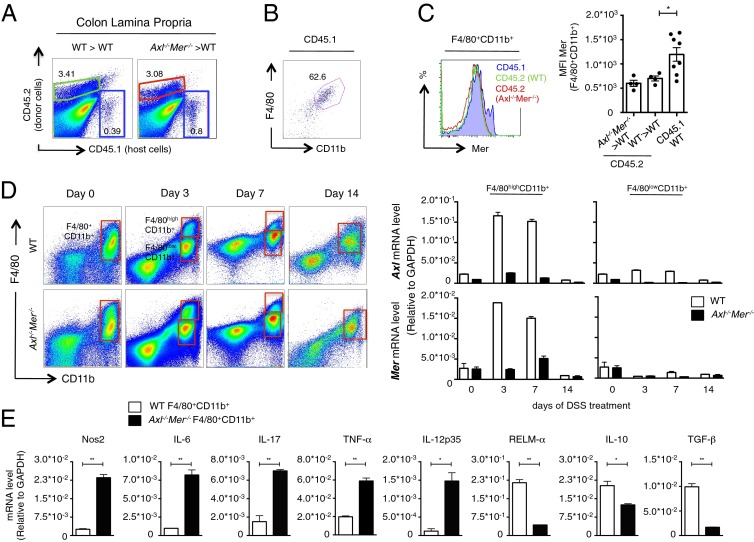
The foregoing surprising findings suggest that an Axl- and Mer-expressing radioresistant population inhibits intestinal inflammation and inflammation-associated colon cancer. Analyses of colonic lamina propria isolates from BM recipients of WT or Axl−/−Mer−/− hematopoietic progenitors after DSS treatment revealed the presence of a small but significant fraction of radioresistant CD45.1+ cells (Fig. 5A). Approximately 70% of these cells were positive for the macrophage markers F4/80 and CD11b (Fig. 5B). Interestingly, Mer-expressing cells were identified within this radioresistant macrophage population (Fig. 5C), suggesting Mer as a marker distinctly associated with mature intestinal tissue resident macrophages.
Fig. 5.
Axl and Mer are expressed in radioresistant intestinal macrophages, limiting the inflammatory response to DSS-induced colitis. (A) FACS analysis of chimerism of lamina propria leukocytes in the indicated BM chimeras, showing the persistence of a radioresistant CD45.1+ population. (B) Percentage of F4/80+CD11b+ cells in the radioresistant CD45.1+ host population, as shown by FACS analysis. (C) Representative histogram showing Mer expression in host CD45.1+ (blue), donor CD45.2+ WT (green), and donor CD45.2+ Axl−/−Mer−/− (red) F4/80+CD11b+ lamina propria leukocytes and the MFI of Mer in the indicated F4/80+CD11b+ populations isolated at 2 d after the completion of DSS treatment (day 14). MFI of Mer expression in each population is represented on the right. (D) Expression of Axl and Mer in indicated cell-sorted macrophages from the lamina propria of WT (open bar) and Axl−/−Mer−/− (black bar) mice before (day 0), during the course of DSS treatment (days 3 and 7), and 2 d after the completion of DSS treatment (day 14) as detected by qPCR. Lamina propria macrophages isolated from Axl−/−Mer−/− mice were used as internal negative controls. (E) Expression of the indicated genes in sorted macrophages from the lamina propria of WT and Axl−/−Mer−/− mice during DSS treatment, as detected by qPCR. Shown are mRNA levels for Nos2, IL-17, TGF-β, and IL-10 in the F4/80highCD11b+ population after 7 d of DSS treatment, along with mRNA levels for IL-6, TNF-α, IL-12, and RELM-α on F480+CD11b+ population at 2 d after the completion of DSS treatment (day 14). Data are presented as representative images or as mean ± SEM of at least four independent samples per group. *P < 0.05; **P < 0.01.
We next investigated the expression of Axl and Mer in lamina propria macrophages during inflammation. Compared with day 0 (untreated), at day 3 we detected two distinct subpopulations, F4/80highCD11b+ and F4/80lowCD11b+. We found a similar pattern after 7 d of DSS administration, whereas at day 14 (at 2 d after completion of the DSS regimen), the macrophage population pattern resembled that at day 0. These distinct subpopulations of macrophages were cell-sorted, and qPCR analyses of Axl and Mer during the course of DSS treatment revealed robust induction on expression of Axl and Mer mRNA at days 3 and 7 of DSS treatment. Axl and Mer expression was significantly higher in the F4/80highCD11b+ subpopulation compared with the F4/80lowCD11b+ subpopulation (Fig. 5D). Expression of Mer was confirmed at the protein level by FACS analysis (Fig. S2). In addition, Mer was selectively expressed by F4/80highCD11b+ macrophages in the lamina propria, as shown by FACS analysis performed on different cell populations of the colon after 7 d of DSS treatment (Fig. S3A) as well as by immunofluorescence analysis (Fig. S4). Upon inflammation, Axl expression was enriched in lamina propria macrophages compared with CD45+CD11b−CD11c− and CD45− cells sorted from the lamina propria, as detected by qPCR (Fig S3B).
We previously described the essential functions of TAM receptors in limiting cytokine production and in regulating the magnitude of the overall immune response (26). Thus, we compared the levels of activation of lamina propria macrophages in DSS-treated WT and Axl−/−Mer−/− mice. Our results show that loss of Axl and Mer signaling led to a significant increase in the production of multiple proinflammatory mediators (i.e., Nos2, IL-6, IL-17α, TNF-α, and IL-12p35), along with a reduction of negative regulators of inflammation associated with the alternative activation of macrophages (i.e., RELM-α, IL-10, and TGF-β) by lamina propria macrophages (Fig. 5E). These results suggest that the increased proinflammatory response and a lack of alternative activation in radioresistant lamina propria macrophages account for the increased colitis in Axl−/−Mer−/− mice.
Discussion
Here we identify a paradoxical function of the proto-oncogenes Axl and Mer RTKs in preventing excessive colonic inflammation and inhibiting inflammation-associated colorectal cancer. These RTKs are well-defined proto-oncogenes in various types of cancer, with established molecular functions in promoting cancer hallmarks. Based on the association of Axl and Mer with cancer and their suitability as RTKs for therapeutic targeting with small-molecule ATP-competitive inhibitors and biologics, various Axl and Mer inhibitors are currently in preclinical development (4, 5, 16). Our results indicate that the systemic targeting of Axl and Mer entails potential pitfalls and may actually increase the risk of inflammation-associated cancer.
The role of Axl and Mer in preventing inflammation-associated colorectal cancer is consistent with their role in limiting inflammation (26). Other genes are known to exert paradoxical effects in tumorigenesis and tumor progression; for example, the deubiquitylation and ubiquitin editing enzyme A20 functions as an oncogene in a number of solid tumors, yet A20 loss of function is associated with increased incidence of lymphoid malignancies (37). The potent capacity of A20 to inhibit NF-κB signaling has been suggested to account for its function as a tumor suppressor (37). On the other hand, the function of A20 as an antiapoptotic gene promotes tumorigenesis (37).
Axl and Mer function in a radioresistant macrophage population in the colon appears to be essential in preventing enhanced colitis. Remarkably, Mer has been recently defined as a distinctive and universal marker of mature, tissue-resident macrophages (38). It would be interesting to explore whether the immunoregulatory properties of TAM signaling described here for lamina propria macrophages extend to other tissue-resident macrophage populations. Similar to intestinal Axl+Mer+ macrophages, tissue macrophages in the liver, epidermis, and microglia have been shown to express high levels of F4/80 (38). In addition, F4/80high tissue macrophages originate from the yolk sac and arise independently of the monocytic lineage (39, 40). Recent data reported by Merad et al. (41) show a substantial turnover of tissue-resident macrophages in the lung, peritoneum, and BM, suggesting that these macrophages locally self-maintain in the steady state with only a minimal contribution from monocytes. Furthermore, expansion of pleural resident macrophages in situ, rather than the recruitment from the blood, has been proposed as a signature of innate mechanisms of inflammation in pathological settings, such as Litomosoides sigmodontis infection (42). The investigation of whether Axl+Mer+ lamina propria macrophages originate from yolk sac precursors and the identification of mechanisms that control their induction represent exciting areas of future research.
Our data also suggest that Axl and Mer are not required for the differentiation or proliferation of intestinal macrophages, as demonstrated by the lack of significant differences in the frequency of lamina propria macrophages or the expression of F4/80 and CD11b between WT and Axl−/−Mer−/− mice (Fig. 5D). How Axl and Mer are activated in this macrophage population in the context of an inflammatory response remains to be determined, however. The TAM agonists Pros1 and Gas6 are expressed by murine and human macrophages (43, 44); thus, it is possible that TAM signaling in macrophages is triggered in an autocrine manner. In addition, we have recently demonstrated that T-cell–derived Pros1 constitutes a relevant in vivo source of this anti-inflammatory TAM ligand (45). In this context, T cell–macrophage interaction could possibly provide a mechanistic model for the activation of TAM signaling in intestinal tissue homeostasis. This local function of Axl and Mer, along with their well-described role in circulating innate immune cells, underscores the complexity of Axl and Mer anti-inflammatory signaling in vivo. Furthermore, a similar anti-inflammatory function of Axl and Mer in other radioresistant cell types of the colon also may play a role in preventing colitis. The conditional ablation of Axl and Mer in specific cell types will further reveal the function of these genes in intestinal mucosal homeostasis.
Targeting of RTKs may be associated with “on-target toxicity.” For example, the anticancer use of the ERBB2 inhibitor trastuzumab can result in cardiotoxicity (46). However, recently described approaches allow active targeting of kinase inhibitors to specific organs and cells by conjugating these molecules to targeting ligands. For example, conjugation of the VEGF inhibitor to a cyclic arginine-glycine-aspartic acid peptide targets this compound to the tumor vasculature (47). Emphasis on developing therapeutic approaches that specifically target Axl and Mer signaling within tumor cells and spare the protective anti-inflammatory function of these RTKs may improve Axl and Mer efficacy as an anticancer target. Future studies focused on unraveling the divergent molecular functions of Axl and Mer signaling pathways in specific cell types are of fundamental importance for determining the suitability of therapeutic targeting of these kinases in cancer models.
Materials and Methods
Experimental Animals.
Axl−/− and Mer−/− mice have been described previously (48). B6.SJL-PtprcaPepcb/BoyJ (CD45.1) mice were obtained from Jackson Laboratory. All mice were bred at Yale University’s animal facility, were specific pathogen-free, maintained under a strict 12-h light cycle (lights on at 7:00 AM and off at 7:00 PM), and given a regular chow diet. In all of the experiments, age-matched WT and KO mice at age 4–6 wk were cohoused for a minimum of 2 wk. All experimental procedures were approved by Yale University’s Institutional Animal Use and Care Committee.
AOM/DSS-Induced Tumorigenesis.
Age- and sex-matched, cohoused, 8- to 12-wk-old mice were i.p. injected with AOM (Sigma-Aldrich) at a dose of 12.5 mg/kg body weight. After 5 d, the mice were treated with 1.5% DSS (MP Biomedicals) in the drinking water for 7 d, followed by 14 d of regular water. This cycle was repeated three times.
DSS-Induced Colitis.
To induce acute colitis, 8- to 12-wk-old mice were treated with 1.5% DSS in the drinking water for 12 d, followed by regular access to water for 2 d. In the chronic colitis experiments, DSS was administered for 12 d, followed by normal water for 7 d; this treatment was repeated three times.
Detailed descriptions of the materials and methods used in this study are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (Grants R01 AI089824, to C.V.R. and S.G.; CA95060, to S.G.; and T32 AI007019, to E.T.S.), the Crohn’s and Colitis Foundation (C.V.R. and S.G.), a Pilot Grant from the Yale Comprehensive Cancer Center (to C.V.R.), and the National Science Foundation (Graduate Research Fellowship DGE-1122492, to E.T.S.). Colon histology was performed by Mouse Research Pathology, Section of Comparative Medicine, Yale University School of Medicine.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302507110/-/DCSupplemental.
References
- 1.Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994;5(6):647–657. [PubMed] [Google Scholar]
- 2.O’Bryan JP, et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11(10):5016–5031. doi: 10.1128/mcb.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neubauer A, et al. Axl, a novel receptor tyrosine kinase isolated from chronic myelogenous leukemia. Semin Hematol. 1993;30(3) Suppl 3:34. [PubMed] [Google Scholar]
- 4.Linger RM, Keating AK, Earp HS, Graham DK. Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin Ther Targets. 2010;14(10):1073–1090. doi: 10.1517/14728222.2010.515980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: Biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlegel J, et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J Clin Invest. 2013;123(5):2257–2267. doi: 10.1172/JCI67816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, et al. Differential expression of Axl and correlation with invasion and multidrug resistance in cancer cells. Cancer Invest. 2012;30(4):287–294. doi: 10.3109/07357907.2012.657816. [DOI] [PubMed] [Google Scholar]
- 8.Hutterer M, et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(1):130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 9.Hector A, et al. The Axl receptor tyrosine kinase is an adverse prognostic factor and a therapeutic target in esophageal adenocarcinoma. Cancer Biol Ther. 2010;10(10):1009–1018. doi: 10.4161/cbt.10.10.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byers LA, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44(8):852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paccez JD, et al. The receptor tyrosine kinase Axl is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene. 2013;32(6):689–698. doi: 10.1038/onc.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ou WB, et al. AXL regulates mesothelioma proliferation and invasiveness. Oncogene. 2011;30(14):1643–1652. doi: 10.1038/onc.2010.555. [DOI] [PubMed] [Google Scholar]
- 14.Song X, et al. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer. 2011;117(4):734–743. doi: 10.1002/cncr.25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating AK, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298–1307. doi: 10.1158/1535-7163.MCT-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10(10):1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 17.Mollard A, et al. Design, synthesis and biological evaluation of a series of novel Axl kinase inhibitors. ACS Med Chem Lett. 2011;2(12):907–912. doi: 10.1021/ml200198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland SJ, et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70(4):1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 19.Ye X, et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29(38):5254–5264. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 22.Grivennikov SI, Karin M. Inflammation and oncogenesis: A vicious connection. Curr Opin Genet Dev. 2010;20(1):65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soderlund S, et al. Decreasing time trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136(5):1561–1567. doi: 10.1053/j.gastro.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 24.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer: A population-based study. N Engl J Med. 1990;323(18):1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 25.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 27.Sharif MN, et al. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203(8):1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen P, et al. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109(2):653–660. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tibrewal N, et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem. 2008;283(6):3618–3627. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 30.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2(3):541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 31.De Robertis M, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: An immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 34.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010;107(50):21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor W, Jr, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harhaj EW, Dixit VM. Regulation of NF-κB by deubiquitinases. Immunol Rev. 2012;246(1):107–124. doi: 10.1111/j.1600-065X.2012.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautier EL, et al. Immunological Genome Consortium Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13(11):1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 40.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22(6):740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189(7):3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrera A, et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity. 2013 doi: 10.1016/j.immuni.2013.06.010. 10.1016/j.immuni.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen MH, Kerkelä R, Force T. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118(1):84–95. doi: 10.1161/CIRCULATIONAHA.108.776831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Temming K, Fretz MM, Kok RJ. Organ- and cell-type specific delivery of kinase inhibitors: A novel approach in the development of targeted drugs. Curr Mol Pharmacol. 2008;1(1):1–12. [PubMed] [Google Scholar]
- 48.Lu Q, et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398(6729):723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.