Abstract
Endothelial cells (ECs) are constantly exposed to xenobiotics and endobiotics or their metabolites, which perturb EC function, as well as to shear stress, which plays a crucial role in vascular homeostasis. Pregnane X receptor (PXR) is a nuclear receptor and a key regulator of the detoxification of xeno- and endobiotics. Here we show that laminar shear stress (LSS), the atheroprotective flow, activates PXR in ECs, whereas oscillatory shear stress, the atheroprone flow, suppresses PXR. LSS activation of PXR in cultured ECs led to the increased expression of a PXR target gene, multidrug resistance 1 (MDR1). An in vivo study using rats showed that the expression of MDR1 was significantly higher in the endothelium from the descending thoracic aorta, where flow is mostly laminar, than from the inner curvature of aortic arch, where flow is disturbed. Functionally, LSS-activated PXR protects ECs from apoptosis triggered by doxorubicin via the induction of MDR1 and other detoxification genes. PXR also suppressed the expression of proinflammatory adhesion molecules and monocyte adhesion in response to TNF-α and lipopolysaccharide. Overexpression of a constitutively active PXR in rat carotid arteries potently attenuated proinflammatory responses. In addition, cDNA microarray revealed a large number of the PXR-activated endothelial genes whose products are responsible for major steps of detoxification, including phase I and II metabolizing enzymes and transporters. These detoxification genes in ECs are induced by LSS in ECs in a PXR-dependent manner. In conclusion, our results indicate that PXR represents a flow-activated detoxification system to protect ECs against damage by xeno- and endobiotics.
Keywords: hemodynamics, endothelial homeostasis, nuclear hormone receptor, gene regulation
The state of circulation plays important homeostatic roles in health and disease. It can provide a hostile milieu, with increased concentrations of xenobiotics and endobiotics resulting from drugs, chemical agents, cigarette smoking, nutritional metabolites, and pathogenic microbes or their toxins. Under physiological conditions, a detoxification system operates to protect tissues from being damaged by xeno- and endotoxins. Located at the interface of blood circulation and the vascular wall, endothelium is exposed to not only these toxins but also the wall shear stress resulting from blood flow. There is abundant evidence indicating that appropriate types and levels of shear stress are crucial for endothelial functions, including the dynamically adaptive vascular tone, well-regulated vascular permeability, and anti-inflammatory and antithrombotic status. A physiological level of laminar shear stress (LSS) potently inhibits the apoptosis and proinflammatory responses of vascular endothelial cells (ECs) to many injurious stimuli (1–3). In human atherosclerotic lesions, EC apoptosis and proinflammatory phenotypes preferentially occur at the areas with disturbed flow patterns (4). It has been well-established that shear stress may modulate several major steps of signal cascades induced by the triggering agents. It has been suggested that ECs are endowed with a detoxification system against various xeno- and endotoxins to inactivate and/or eliminate such perturbing agents and restore a structural and functional homeostasis. However, whether blood flow can modulate such a “detoxification” mechanism remains to be experimentally proven. Pregnane X receptor (PXR) belongs to the superfamily of nuclear receptors and acts as a xenosensor to protect organisms from chemical insults (5, 6). As a ligand-activated nuclear receptor and transcriptional factor, PXR dimerizes with retinoid X receptor and binds to the PXR-response elements (PXRE) in the promoter regions of the target genes, especially those encoding drug-metabolizing enzymes and transporters (7). Among the identified PXR targets are phase I cytochrome P450 genes (CYPs), phase II UDP-glucuronosyltransferases (UGTs), glutathione-S-transferases (GSTs), and sulfotransferases (SULTs), as well as drug transporters multidrug resistance 1 (MDR1) and multidrug resistance-associated protein 2 (MRP2) (8). Highly expressed in the liver and intestine (9), PXR can be activated by a variety of xenobiotics and endobiotics to transactivate the target genes. Recent studies have shown that PXR is also expressed in vascular cells, including ECs and smooth muscle cells (SMCs) (10, 11). The present study was conducted to test our hypothesis that atheroprotective shear stress may activate PXR in ECs to detoxify and eliminate xeno- and endobiotic agents, thus maintaining endothelial integrity and functions.
Results
Laminar Shear Stress Activates PXR in ECs.
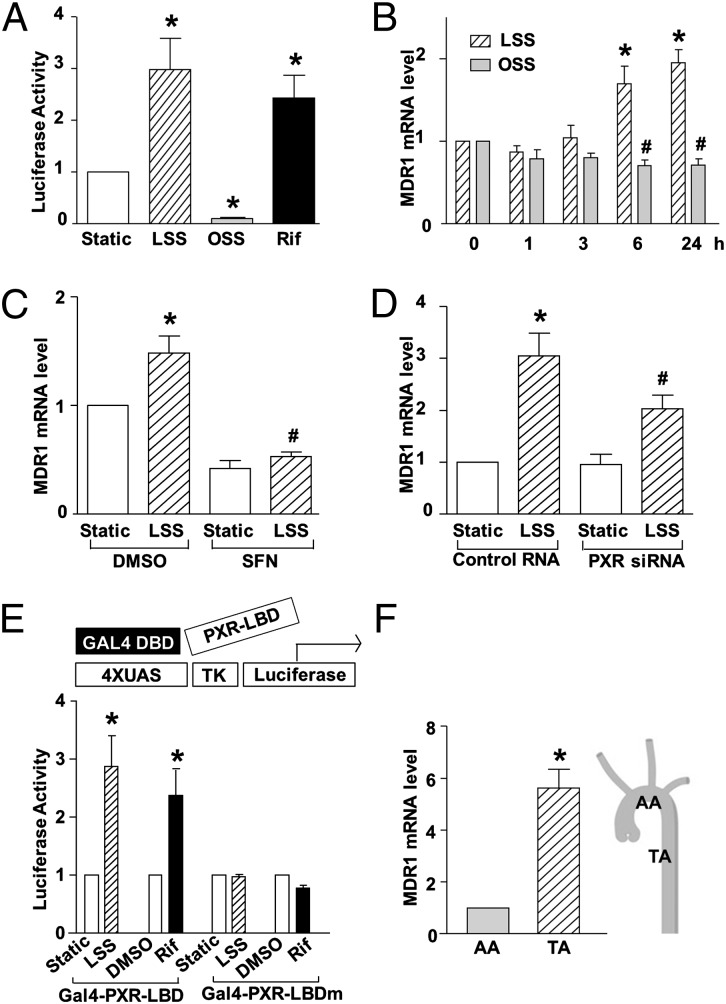
To examine whether shear stress affects the transactivational activity of PXR, plasmids expressing a human PXR and a luciferase reporter driven by PXRE (pCYP3A4XREM-362/+53) were cotransfected into bovine aortic ECs (BAECs), which were used to achieve a better transfection efficiency, and the cells were exposed to a physiological level of laminar shear stress (LSS; 12 dyn/cm2), oscillatory shear stress (OSS; 0.5 ± 4 dyn/cm2, 1 Hz), or static condition for 18 h. Luciferase assay showed that PXR activity was significantly increased by LSS but decreased by OSS compared with static control (Fig. 1A). Next, we asked whether shear stress increased the expression of an endogenous PXR target gene MDR1, which is a known PXR target encoding p-glycoprotein or ATP-binding cassette B1 (ABCB1), a transporter responsible for drug efflux (12, 13). Quantitative reverse-transcriptase PCR (qRT-PCR) showed that MDR1 expression was induced by LSS, but not OSS, in human umbilical vein ECs (HUVECs) (Fig. 1B; see also Table S1 for primer sequences). Next, the role of PXR in mediating the induction of MDR1 by LSS was examined, using sulforaphane (SFN), an effective in vitro antagonist of ligand activation of human PXR (14) and small interfering RNA (siRNA) specific for PXR (Fig. S1 A and B). As shown in Fig. 1C, the LSS induction of MDR1 was diminished by SFN. Consistently, PXR knockdown with PXR siRNA also attenuated the LSS-induction of MDR1 (Fig. 1D). PXR is known to be a ligand-activated transcription factor. To investigate whether shear stress activates PXR through a ligand-dependent mechanism, we used the yeast transcription factor GAL4-reporter with a plasmid expressing a fusion protein GAL4-PXR-LBD (ligand-binding domain) or Gal4-PXR-LBDmutant, a mutant deficient in its ligand-dependent activation domain (AF-2) (15). As shown in Fig. 1E, LSS activated GAL4-PXR-LBD, similar to the effect of the known PXR agonist rifampicin. The LSS-induced activity was diminished when Gal4-PXR-LBDmutant was cotransfected, indicating that the activating effect of LSS might involve the production of PXR ligands.
Fig. 1.
Shear stress regulates PXR activity in ECs. (A) BAECs were cotransfected with the PXR overexpression plasmid together with pCYP3A4XREM-362/+53, which is the plasmid for PXRE-driven luciferase reporter. Transfected cells were exposed to LSS or OSS or kept static for 18 h. Rifampicin (Rif, 20 μM) was used as a positive control. (B) MDR1 expression was analyzed by qRT-PCR in HUVECs exposed to LSS or OSS for 0, 1, 3, 6, and 24 h. (C and D) HUVECs were pretreated with SFN or DMSO for 24 h (C) or transfected with PXR siRNA or control siRNA for 48 h (D) and then exposed to laminar flow for another 6 h (C) or 24 h (D), mRNA levels of MDR1 were analyzed by qRT-PCR. (E) BAECs were transfected with the GAL4 reporter plasmid together with Gal4-PXR-LBD or Gal4-PXR-LBDmutant. Cells were then exposed to LSS or kept under static condition for 24 h. Rifampicin was used as a positive control. The results are expressed as fold change in luciferase activities compared with static control. Luciferase activity was assayed, normalized, and expressed as fold induction compared with static control. (F) MDR1 mRNA levels from intima at TA and AA were analyzed by qRT-PCR. Data are shown as mean ± SEM of three independent experiments. *P < 0.05 vs. control. #P < 0.05 vs. static (B) or control and with LSS treatment (C and D).
To relate the PXR activity to local hemodynamic flow patterns, we examined the gene expression level of MDR1 in rat arteries in vivo. qRT-PCR demonstrated that the mRNA level of MDR1 was significantly higher in endothelium from the rat descending thoracic aorta (TA), where flow is mostly laminar, than those from the inner curvature of aortic arch (AA), where flow is disturbed (16) (Fig. 1F).
PXR Attenuates the Proapoptotic Effect of Xenobiotics in ECs.
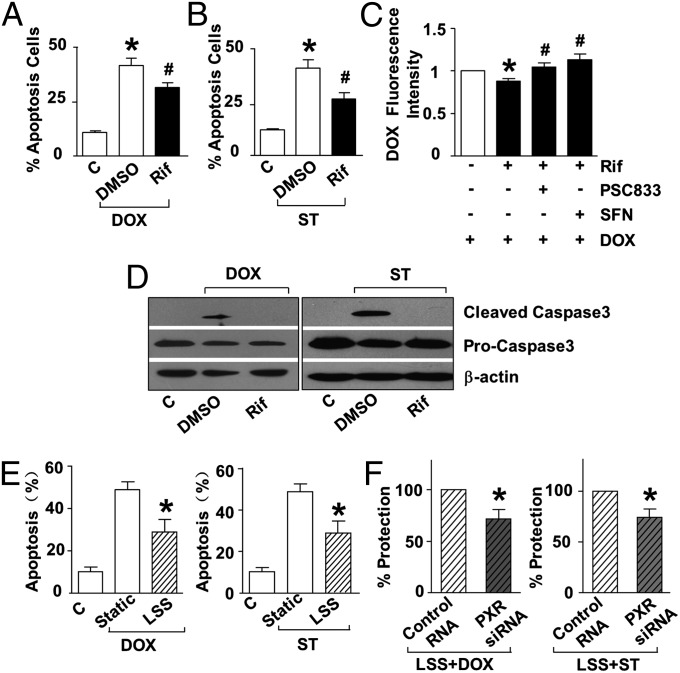
To examine the functional role of PXR in ECs, we investigated the effect of the PXR agonist rifampicin on EC apoptosis triggered by doxorubicin and staurosporine, the known xenobiotics that cause endothelial apoptosis (17, 18). As assessed with flow cytometry, PXR activation by rifampicin significantly decreased doxorubicin- or staurosporine-induced EC apoptosis (Fig. 2 A and B and Fig. S2 A and B). Intracellular accumulation of doxorubicin was decreased in ECs pretreated with rifampicin but increased in the presence of the PXR antagonist sulforaphane (SFN) or 6-[(2S,4R,6E)-4-methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-L-valine-cyclosporin A (PSC833), an inhibitor of MDR (19), suggesting the involvement of the PXR target MDR1 (Fig. 2C). Western blotting showed that rifampicin decreased the caspase 3 cleavage induced by doxorubicin or staurosporine (Fig. 2D).
Fig. 2.
PXR activation attenuates EC apoptosis. HUVECs were pretreated by rifampicin (20 μM) or vehicle (DMSO) for 24 h before exposed to doxorubicin (A) (5 μM, 24 h) or staurosporine (B) (500 nM, 6 h). Apoptosis was assessed by using flow cytometry for annexin V-FITC. (C) Intracellular level of doxorubicin was measured by using flow cytometry for fluorescence intensity in HUVECs pretreated with rifampicin (with or without SFN) in the presence or absence of PSC833 before exposure to doxorubicin. (D) Caspase 3 cleavage was detected with Western blotting. (E) HUVECs were treated with LSS or kept static for 12 h before exposure to doxorubicin for 12 h or staurosporine for 6 h. (F) HUVECs were transfected with PXR or control siRNA for 48 h and exposed to LSS or kept static for another 12 h. Apoptosis was detected after exposure to doxorubicin or staurosporine. Data shown are as mean ± SEM of three independent experiments. *P < 0.05 vs. control. #P < 0.05 vs. DMSO (A and B) or Rif (C).
Exposure to LSS significantly reduced doxorubicin- or staurosporine-induced apoptosis (Fig. 2E and Fig. S2 C and D). To examine the role of PXR in the detoxification effect of LSS, PXR expression was silenced with the use of siRNA. The protective effect of LSS was diminished in ECs transfected with PXR siRNA compared with those treated with scrambled siRNA (Fig. 2F and Fig. S2 E and F). Taken together, these results indicated a role of the LSS-activated PXR in protecting ECs against xenobiotic injuries.
PXR Suppresses Proinflammatory Response in ECs.
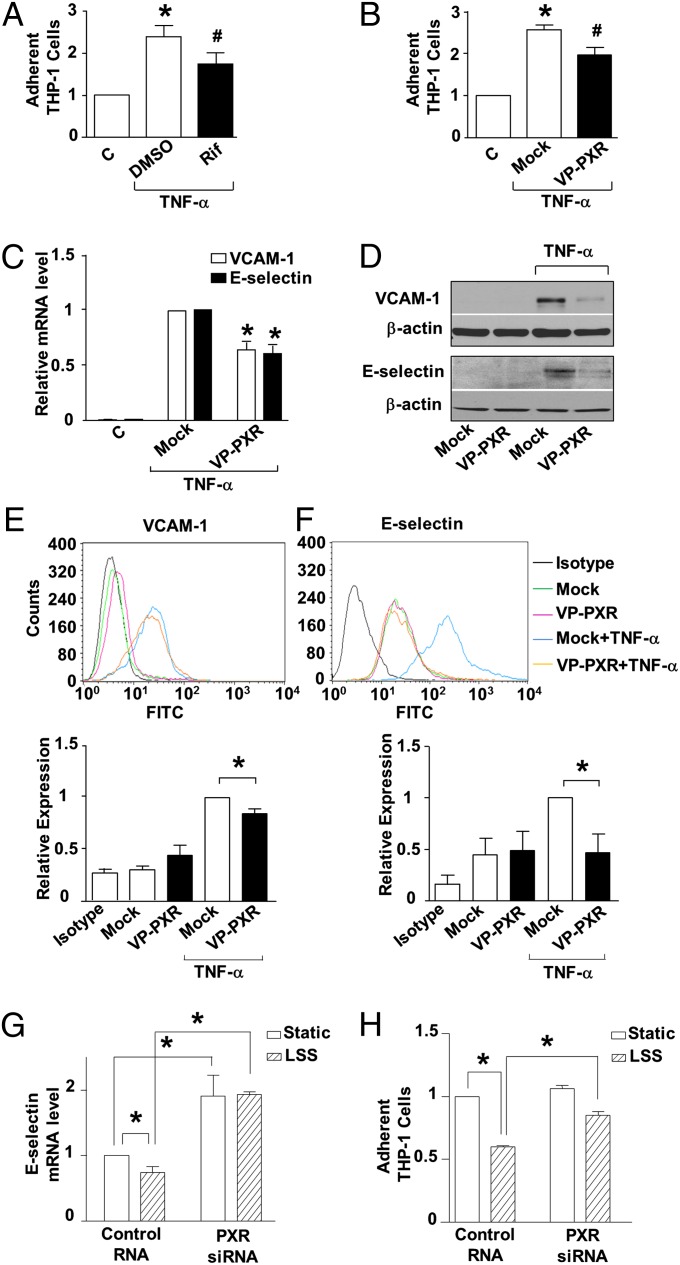
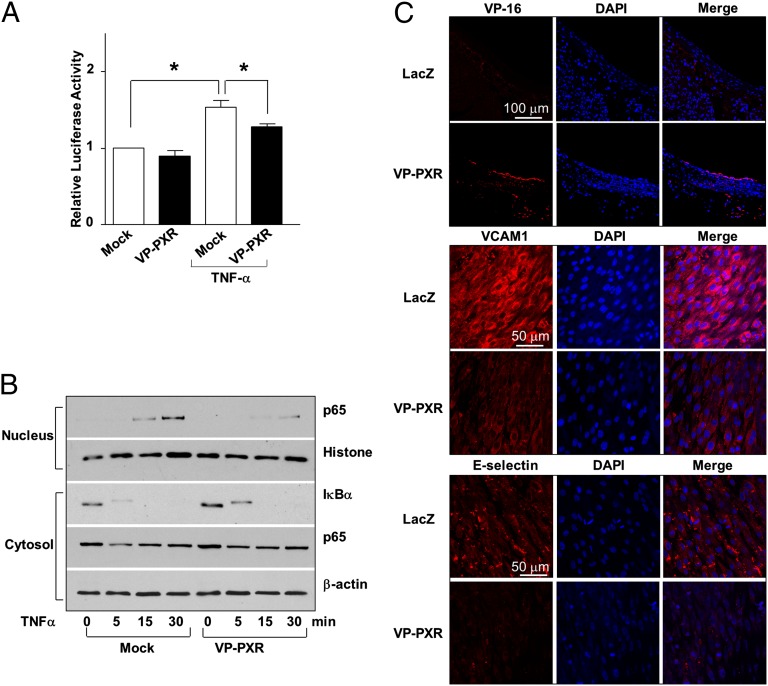
We further examined the effects of PXR on endothelial response to endobiotics, exemplified by TNF-α. As shown in Fig. 3A, pretreatment of ECs with rifampicin inhibited the adhesion of THP-1, a human acute monocytic leukemia cell line, induced by TNF-α. To rule out the potential PXR-independent anti-inflammatory effects of rifampicin (20), we constructed an adenovirus expressing a constitutively active PXR (Ad-VP-PXR), which was a human PXR fused to the minimal transactivator domain of the herpes virus transcription factor VP16 (Fig. S3). Overexpression of VP-PXR inhibited the endothelial recruitment of monocytes (Fig. 3B). VP-PXR also suppressed the induction of vascular adhesion molecule 1 (VCAM-1) and E-selectin at mRNA and protein levels (Fig. 3C–F). Importantly, siRNA-mediated PXR knockdown abrogated the suppressive effects of LSS on E-selectin as well as the recruitment of monocytes (Fig. 3 G and H). NF-κB plays a central role in the transcriptional regulation of adhesion molecule genes. A reporter assay showed that VP-PXR significantly decreased the NF-κB activity induced by TNF-α (Fig. 4A). In addition, VP-PXR inhibited the degradation of IκBα and the ensuing nuclear translocation of p65 NF-κB subunit (Fig. 4B).
Fig. 3.
PXR activation ameliorates proinflammatory response in ECs. (A) HUVECs were pretreated by rifampicin (20 μM) or DMSO for 24 h before exposure to TNF-α (10 ng/mL, 4 h). Then ECs were incubated with fluorescence-labeled THP-1 cells (5 × 105 cells per mL) for 30 min. THP-1 adhesions were counted under a fluorescence microscope. (B) Cells were infected with Ad-VP-PXR or mock infection before TNF-α stimulation. (C) VCAM-1 and E-selectin mRNA level was analyzed by using qRT-PCR in VP-PXR-infected or mock-infected ECs. (D) Protein levels of VCAM-1 and E-selectin were detected using Western blotting. (E and F) Surface expressions of VCAM-1 and E-selectin were examined using flow cytometry, and relative levels were quantified and expressed as bar graphs (Lower). (G) HUVECs were transfected with PXR or control siRNA and exposed 48 h later to LSS or static condition for 24 h. After stimulation with TNF-α for 2 h, RNA was extracted and analyzed by qRT-PCR. (H) Cells were stimulated with TNF-α for 4 h and incubated with THP-1 cells. THP-1 adhesions were counted; representative images are shown. Data are shown as mean ± SEM of three independent experiments. *P < 0.05 vs. control. #P < 0.05, Rif vs. DMSO (A) or VP-PXR vs. mock (B).
Fig. 4.
PXR inhibits NF-κB and vascular inflammation. (A) BAECs were cotransfected with a reporter plasmid NF-κB×5-luc together with a VP-PXR expression plasmid or vector control (pcDNA3.1). Data are shown as mean ± SEM of three independent experiments. *P < 0.05 vs. control. (B) Protein levels of p65 and IκBα in the nucleus and cytosol were analyzed using Western blotting in VP-PXR or mock-infected HUVECs after exposed to TNF-α. Histone and β-actin were used as internal controls for nuclear and cytosolic protein, respectively. (C) Rat carotid arteries were infected with Ad-VP-PXR or Ad-LacZ. Immunofluorescence staining shows overexpression of VP-PXR in endothelium 24 h after the infection. Expression of VCAM-1 and E-selectin after LPS challenge for 24 h was detected with en face staining. Nuclei were counterstained with DAPI. Microphotographs are representative of 3 rats in each group.
To validate the anti-inflammatory effect of PXR in vivo, VP-PXR was adenovirally delivered into rat carotid arteries before i.p. injection of LPS. Immunofluorescence staining showed that the LPS-induced expressions of VCAM-1 and E-selectin were markedly suppressed in ECs overexpressing VP-PXR compared with the control virus expressing Lac Z (Fig. 4C).
Endothelial PXR Is a Master Regulator of Detoxification Genes.
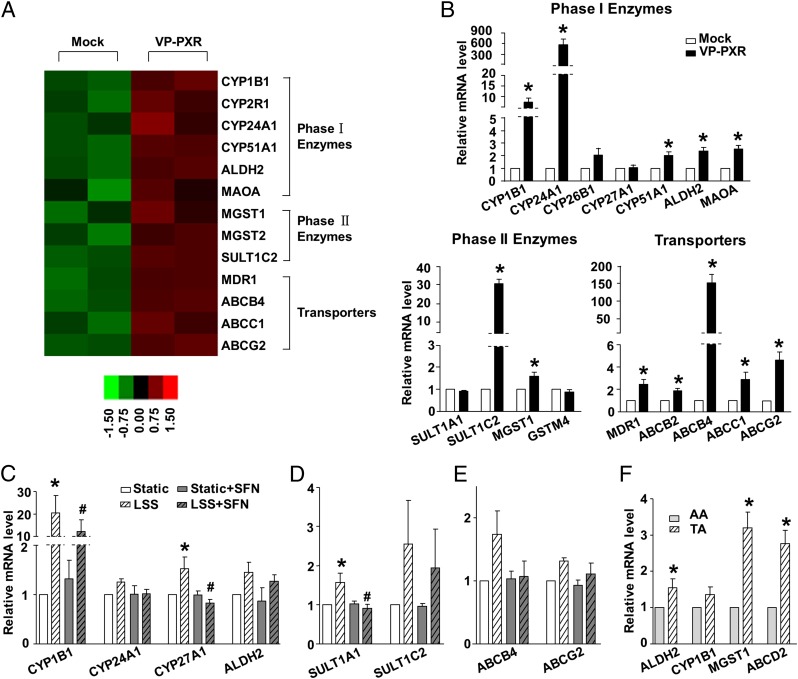
To elucidate the mechanisms by which LSS-activated PXR mediates vascular protection, we profiled the repertoire of PXR-regulated genes in ECs. Transcriptomes were compared between the Ad-VP-PXR-infected and mock-infected ECs, using the Affymetrix Human Genome U133 Plus 2.0 Array (Fig. 5A). Using gene ontology terms, we identified three major groups of the detoxification genes among the PXR regulated transcripts, including those responsible for phase I oxidative enzymes (CYP1B1, CYP24A1, CYP51A1, ALDH2, and MAOA), phase II conjugating enzymes (MGST1, MGST2, and SULT1C2), and transporters (ABCB1/MDR1, ABCB4, ABCC1, and ABCG2). Induction of these detoxification genes in ECs was validated by using qRT-PCR (Fig. 5B). In addition, exposure to LSS induced a detoxification gene profile in ECs, mainly in a PXR-dependent manner (Figs. 1 C and D and 5 C–E and Fig. S4). Furthermore, we found that several detoxification genes including ALDH2, MGST1, ABCD2, and CYP1B1 were expressed at a higher level in mouse descending TA than the inner curvature of AA (Fig. 5F). These results suggest that PXR is a master regulator of the LSS-activated endothelial detoxification program.
Fig. 5.
Gene profile of flow-induced PXR targets in ECs. (A) Heat map of the detoxification-related genes in VP-PXR-infected or mock-infected ECs. (B) PXR up-regulated genes were validated with the use of qRT-PCR in HUVECs. (C–E) Relative mRNA levels of selected PXR targets in ECs exposed to LSS for 6 h with the pretreatment of SFN or DMSO. (F) Relative mRNA levels of the representative PXR targets in mouse AA and TA. Data are shown as mean ± SEM of three independent experiments. *P < 0.05 vs. control. #P < 0.05 vs. DMSO and with LSS treatment.
Discussion
In this study, we provide evidence that the atheroprotective flow pattern of LSS activates PXR in ECs. The activation of PXR protected ECs from xenobiotics-triggered apoptosis and suppressed a proinflammatory response evoked by cytokines and bacterial endotoxin in vitro and in vivo. LSS induction of PXR revealed a flow-induced detoxification system to protect the vessel wall from being damaged by the xeno- and endotoxins. Through transcriptional induction of phase I and phase II detoxification enzymes and efflux proteins, the LSS-induced PXR facilitates endothelial detoxification, and thus maintains a healthy vasculature.
Flow activation of PXR is supported by several lines of evidence. These include the increased activity of the reporter gene driven by PXRE and the induction of the PXR target gene MDR1. Specifically, shear activation of PXR is flow-pattern dependent. PXR is activated by LSS, the flow pattern with a net forward component. In contrast, OSS decreases PXR activity. This observation is notable in that the focal distribution of atherosclerotic lesions is related to the local flow patterns. Although the endothelium throughout the vascular tree is exposed to the same level of proatherosclerotic humoral stimuli, such as hypercholesterolemic, hyperglycemic, and hyperhomocysteinemic environments, the vascular areas that experience laminar flow are less prone to lesion development. Consistent with the in vitro finding, PXR target genes such as MDR1 are also expressed at a higher level in the endothelium, in areas where the flow is mostly laminar, than in areas with disturbed flow.
As previously shown, LSS provides a number of atheroprotective effects on endothelium, including anti-apoptotic and anti-inflammatory properties. These protections are predominantly achieved via the blockade of pathologic signaling pathways initiated by the atherogenic stimuli. The mediation of the protective effects of LSS by PXR, as described here, appears to involve two distinct mechanisms. The anti-inflammatory role of PXR against TNF-α– and LPS-elicited inflammatory responses involves the blockade of the NF-κB–driven pathway. However, protection against endothelial apoptosis caused by doxorubicin clearly involves the efflux of the uptaken xenobiotics. P-glycoprotein, a member of the ABC superfamily, is encoded by the MDR l gene and has been demonstrated to act as a very efficient toxin efflux molecule and to confer resistance to a wide range of insults (21). Previous studies have shown that LSS could induce the endothelial expression of drug transporters and metabolic properties that allow the blood–brain barrier to shield the central nerve system from potentially harmful substances (22). The LSS-induced MDR1 and other exporters (such as ABCB4, ABCD2, and ABCG2) (23, 24), together with other metabolizing enzymes (such as CYP1B1, CYP24A1, CYP27A1, and SULT1A1), may orchestrate a multistep detoxification program composed of metabolization and efflux. In fact, genes encoding these enzymes and transporters were identified as being regulated by PXR in ECs (Fig. 5). Most important, many of them are induced by laminar flow in vitro and expressed at a higher level in atheroresistant areas within the vasculature in vivo.
Recently, Swales et al. reported that rifampicin induced MDR1, which mediated efflux of fluorescence substrate rhodamine 123 in human aortic ECs (11). Our finding that the PXR-regulated genes that are involved in the metabolism and clearance of the xenobiotic and endobiotic agents are expressed at a higher level at the atheroresistant parts in vasculature provides unique insight into the atheroprotective actions of local hemodynamics. In a more general context, flow induction of the PXR-centered detoxification system may also play a role in the molecular mechanism underlying the maintenance of endothelial integrity against high levels of various xeno- and endobiotic injurious milieus in the circulation. It is worth noting that other transcription factors may also participate in the flow-mediated detoxification system. For instance, nuclear factor-like 2 activation by LSS induced the expression of several antioxidative genes, such as heme oxygenase 1 and NAD(P)H quinone oxidoreductase 1, involving phase II detoxification (25, 26). In contrast, OSS inhibited nuclear factor-like 2 activity by deacetylation, resulting in decreased expression of heme oxygenase 1 (27). In addition to PXR, constitutive androstane receptor, another xenobiotic nuclear receptor, is also expressed in ECs (28). Flow regulation of constitutive androstane receptor in vascular endothelium remains to be studied.
The mechanism by which LSS activates PXR in ECs remains to be elucidated. Several types of nuclear hormone receptors can be activated by shear stress and might be responsible for the modulatory effects of flow on lipid metabolism and trafficking (29, 30), inflammation (31), and phenotypic switch of vascular ECs and SMCs (32). Previously, we found that peroxisome proliferator-activated receptor γ (PPARγ) was activated by shear stress via the induction of endogenous ligands (31). In this study, the mutations in PXR ligand-binding domain diminished the flow effect, indicating that shear stress activation of PXR may also result from an increase in its ligands or their affinity to the receptor. It was also previously reported that in mouse hepatocytes, PXR can be cytoplasmic and undergo nuclear translocation on ligand activation (33). However, we found that PXR is exclusively localized in the nuclei in human ECs, and LSS did not affect PXR localization (Fig. S1 C and D). In addition, PXR activity can also be regulated by a number of posttranscriptional modifications such as phosphorylation, sumoylation, and acetylation (34–37). It will be intriguing to examine whether these modifications also contribute to the flow regulation of PXR activity in ECs.
In addition to the effects of PXR on the efflux of xenobiotics and apoptosis, PXR was also activated by 5β-dihydroprogesterone, a progesterone metabolite, to mediate endothelium-dependent relaxation during pregnancy in mice (10). Importantly, the PXR agonist rifampicin or pregnenolone 16α-carbonitrile (PCN) induced several cytochrome P450 enzymes (CYP3A4, CYP2B6, and CYP2C8) in vascular SMCs and ECs (11). In this study, we found that CYP1B1, CYP27A, and CYP24A1 were induced by LSS in a PXR-dependent manner (Fig. 5C and Fig. S4). Given that CYP enzymes are involved in the generation of endothelium-derived hyperpolarizing factor, which contributes to the shear stress-induced endothelium-dependent vasodilation (38, 39), it will be important to elucidate the important role of LSS-activated PXR in the production of endothelium-derived hyperpolarizing factor. Nevertheless, physiological significance of flow-activated PXR awaits further investigation with the use of animal models with an EC-specific PXR overexpression or knockout approach.
In conclusion, our study demonstrated that atheroprotective flow activates an important nuclear hormone receptor, PXR. The PXR-mediated endothelial detoxification program may play an essential role in protecting ECs from injuries by xeno- and endobiotics, and hence maintaining vascular homeostasis.
Materials and Methods
Shear Experiments.
ECs were seeded on collagen-coated glass slides, subjected to laminar shear stress (12 dyn/cm2) or oscillatory shear stress (0.5 ± 4 dyn/cm2, 1 Hz), and kept in a constant temperature-controlled enclosure, with pH maintained at 7.4 by continuous gassing with a humidified mixture of 5% (vol/vol) CO2 in air. The cells harvested from concurrent unsheared samples were used as the static control.
Western Blotting.
Cytoplasmic proteins were extracted with use of hypotonic lysis buffer (10 mM Tris⋅HCl at pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 0.5% Nonidet P-40). Nuclear proteins were extracted with use of high-salt buffer (20 mM Tris⋅HCl, 1.5 mM MgCl2, 420 mM NaCl, 10% (vol/vol) glycerol, 0.2 mM EGTA). Protein concentration was measured by BCA protein assay kit (Pierce). The samples were resolved on SDS/PAGE and blotted to nitrocellulose membranes. Blots were reacted with primary antibodies, detected with use of horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL chemiluminescence system (Amersham Biosciences).
Adenoviral Vectors and Infection.
To generate Ad-VP-PXR, VP-PXR cDNA was subcloned into a tetracycline (tet)-off expression cassette and recombined with an E1- and E3-deleted ψ5 viral DNA in packaging cell line CRE8, as previously described. Ad-tTA, the adenovirus expressing a tetracycline-responsive transactivator, and Ad-LacZ, expressing β-galactosidase, were as previously reported (40). The adenoviruses were plaque-purified, expanded, and purified by cesium chloride methods. Confluent HUVECs were coinfected with Ad-tTA and Ad-VP-PXR in the presence or absence of tetracycline.
Statistical Analysis.
Data are expressed as mean ± SEM from at least three independent experiments. Student t test (paired groups) or one-way ANOVA followed by Newman–Keuls test (multigroup comparisons) were used to analyze the statistical significance of differences. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Wen Xie (University of Pittsburgh) and Dr. Yonggong Zhai (Beijing Normal University) for providing VP-PXR and GAL4-PXR-LBD plasmids. This study was supported in part by grants from the National Science Foundation of China (30890041 and 81220108005), Ministry of Science and Technology of China (2010CB912500), and the National Institutes of Health (HL108735-01A1).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312065110/-/DCSupplemental.
References
- 1.Dimmeler S, Haendeler J, Rippmann V, Nehls M, Zeiher AM. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399(1-2):71–74. doi: 10.1016/s0014-5793(96)01289-6. [DOI] [PubMed] [Google Scholar]
- 2.Dimmeler S, Hermann C, Galle J, Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol. 1999;19(3):656–664. doi: 10.1161/01.atv.19.3.656. [DOI] [PubMed] [Google Scholar]
- 3.Hermann C, Zeiher AM, Dimmeler S. Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1997;17(12):3588–3592. doi: 10.1161/01.atv.17.12.3588. [DOI] [PubMed] [Google Scholar]
- 4.Tricot O, et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101(21):2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 5.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7(5):584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 6.Xie W, et al. Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev. 2000;14(23):3014–3023. doi: 10.1101/gad.846800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascussi JM, et al. Evidence for the presence of a functional pregnane X receptor response element in the CYP3A7 promoter gene. Biochem Biophys Res Commun. 1999;260(2):377–381. doi: 10.1006/bbrc.1999.0745. [DOI] [PubMed] [Google Scholar]
- 8.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr Rev. 2002;23(5):687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg B, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12(20):3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagedorn KA, Cooke CL, Falck JR, Mitchell BF, Davidge ST. Regulation of vascular tone during pregnancy: A novel role for the pregnane X receptor. Hypertension. 2007;49(2):328–333. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- 11.Swales KE, et al. Pregnane X receptor regulates drug metabolism and transport in the vasculature and protects from oxidative stress. Cardiovasc Res. 2012;93(4):674–681. doi: 10.1093/cvr/cvr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderle P, et al. P-Glycoprotein (P-gp) mediated efflux in Caco-2 cell monolayers: The influence of culturing conditions and drug exposure on P-gp expression levels. J Pharm Sci. 1998;87(6):757–762. doi: 10.1021/js970372e. [DOI] [PubMed] [Google Scholar]
- 13.Mills JB, Rose KA, Sadagopan N, Sahi J, de Morais SM. Induction of drug metabolism enzymes and MDR1 using a novel human hepatocyte cell line. J Pharmacol Exp Ther. 2004;309(1):303–309. doi: 10.1124/jpet.103.061713. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, et al. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71(1):220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]
- 15.Wentworth JM, Agostini M, Love J, Schwabe JW, Chatterjee VK. St John’s wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166(3):R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 16.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107(30):13450–13455. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu S, et al. Adriamycin-induced cardiomyocyte and endothelial cell apoptosis: In vitro and in vivo studies. J Mol Cell Cardiol. 2002;34(12):1595–1607. doi: 10.1006/jmcc.2002.2110. [DOI] [PubMed] [Google Scholar]
- 18.Yue TL, et al. Staurosporine-induced apoptosis in cardiomyocytes: A potential role of caspase-3. J Mol Cell Cardiol. 1998;30(3):495–507. doi: 10.1006/jmcc.1997.0614. [DOI] [PubMed] [Google Scholar]
- 19.Kusunoki N, et al. Inhibitory effects of a cyclosporin derivative, SDZ PSC 833, on transport of doxorubicin and vinblastine via human P-glycoprotein. Jpn J Cancer Res. 1998;89(11):1220–1228. doi: 10.1111/j.1349-7006.1998.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calleja C, Pascussi JM, Mani JC, Maurel P, Vilarem MJ. The antibiotic rifampicin is a nonsteroidal ligand and activator of the human glucocorticoid receptor. Nat Med. 1998;4(1):92–96. doi: 10.1038/nm0198-092. [DOI] [PubMed] [Google Scholar]
- 21.Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci USA. 1987;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011;12:40. doi: 10.1186/1471-2202-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elkind NB, et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib) Cancer Res. 2005;65(5):1770–1777. doi: 10.1158/0008-5472.CAN-04-3303. [DOI] [PubMed] [Google Scholar]
- 24.Lu JF, et al. The role of peroxisomal ABC transporters in the mouse adrenal gland: The loss of Abcd2 (ALDR), Not Abcd1 (ALD), causes oxidative damage. Lab Invest. 2007;87(3):261–272. doi: 10.1038/labinvest.3700512. [DOI] [PubMed] [Google Scholar]
- 25.Chen XL, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278(2):703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 26.Dai G, et al. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101(7):723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 27.Lee DY, et al. Role of histone deacetylases in transcription factor regulation and cell cycle modulation in endothelial cells in response to disturbed flow. Proc Natl Acad Sci USA. 2012;109(6):1967–1972. doi: 10.1073/pnas.1121214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertrand-Thiebault C, Masson C, Siest G, Batt AM, Visvikis-Siest S. Effect of HMGCoA reductase inhibitors on cytochrome P450 expression in endothelial cell line. J Cardiovasc Pharmacol. 2007;49(5):306–315. doi: 10.1097/FJC.0b013e31803e8756. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M, et al. Laminar shear stress regulates liver X receptor in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(3):527–533. doi: 10.1161/ATVBAHA.107.143487. [DOI] [PubMed] [Google Scholar]
- 30.Qin X, et al. Laminar shear stress up-regulates the expression of stearoyl-CoA desaturase-1 in vascular endothelial cells. Cardiovasc Res. 2007;74(3):506–514. doi: 10.1016/j.cardiores.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, et al. Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells. Circulation. 2004;110(9):1128–1133. doi: 10.1161/01.CIR.0000139850.08365.EC. [DOI] [PubMed] [Google Scholar]
- 32.Tsai MC, et al. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ Res. 2009;105(5):471–480. doi: 10.1161/CIRCRESAHA.109.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawana K, et al. Molecular mechanism of nuclear translocation of an orphan nuclear receptor, SXR. Mol Pharmacol. 2003;63(3):524–531. doi: 10.1124/mol.63.3.524. [DOI] [PubMed] [Google Scholar]
- 34.Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol. 2005;69(5):867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Ding X, Staudinger JL. Induction of drug metabolism by forskolin: The role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther. 2005;312(2):849–856. doi: 10.1124/jpet.104.076331. [DOI] [PubMed] [Google Scholar]
- 36.Biswas A, Pasquel D, Tyagi RK, Mani S. Acetylation of pregnane X receptor protein determines selective function independent of ligand activation. Biochem Biophys Res Commun. 2011;406(3):371–376. doi: 10.1016/j.bbrc.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu G, Xu C, Staudinger JL. Pregnane X receptor is SUMOylated to repress the inflammatory response. J Pharmacol Exp Ther. 2010;335(2):342–350. doi: 10.1124/jpet.110.171744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busse R, et al. EDHF: Bringing the concepts together. Trends Pharmacol Sci. 2002;23(8):374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 39.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459(6):881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang N, et al. Adenovirus-mediated overexpression of dominant-negative mutant of c-Jun prevents intercellular adhesion molecule-1 induction by LDL: A critical role for activator protein-1 in endothelial activation. Arterioscler Thromb Vasc Biol. 2001;21(9):1414–1420. doi: 10.1161/hq0901.095549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.