Abstract
One of the most intriguing problems in developmental biology is how an organism can replace missing organs or portions of its body after injury. This capacity, known as regeneration, is conserved across different phyla. The imaginal discs of Drosophila melanogaster provide a particularly well-characterized model for analyzing regeneration. We have developed a unique method to study organ regeneration under physiological conditions using the imaginal discs of Drosophila. Using this method, we revisited different aspects of organ regeneration. The results presented in this report suggest that during the initial stages of regeneration, different processes occur, including wound healing, a temporary loss of markers of cell-fate commitment, and pattern reorganization. We present evidence indicating that all of these processes occur even when cell division has been arrested. Our data also suggested that Wingless is not required during the early stages of disc regeneration.
Keywords: epimorphic, morphallatic
Regeneration allows for the replacement of missing organs or body parts when they are damaged. In 1901, Morgan (1) proposed two different regenerative models: regeneration that allows for the reconstruction of the lost structure as a result of regenerative growth and regeneration that remodels the remaining tissues without proliferation. The first model is an add-on regeneration, known as epimorphic regeneration, which has been extensively studied in different organisms (2). In these animals, after the amputation of a portion of the limb, a group of differentiated cells near the injured region dedifferentiate and form the blastema (3). These cells then proliferate to reconstruct the amputated structure. In contrast, in the second model of regeneration, which is known as morphallactic regeneration, tissue remodeling and the redifferentiation of cells in the absence of proliferation occurs (4).
The imaginal discs of Drosophila have been used as a classical model for the study of the genetic and molecular basis of organ regeneration (review by ref. 5). These structures give rise to the adult organs of Drosophila. The imaginal wing disc is the precursor of the wing and notum in the adult. Cells that constitute these discs begin to divide during the first larval stage and continue proliferating until the end of larval development, and it is during this time that the characteristics of the adult wings are defined (6).
The standard approach used to study regeneration in Drosophila has been to grow the regenerating structures in an in vivo culture. The disc is extracted from the larva and, after removing a fragment of it, transplanted into the abdomen of an adult host where the cells proliferate but do not differentiate (7, 8). The results obtained from these experiments revealed a zone of high cell proliferation on the edges of the wound, similar to the blastema that originates during limb regeneration in amphibians (9, 10). These studies suggest that the regeneration of lost structures in Drosophila is the result of regenerative growth following an epimorphic model (11). One of the disadvantages of the in vivo culture is that regeneration does not occur under physiological conditions. In addition, disc extraction and its transplantation into the host causes severe stress to the discs, which induces cell death (12). Some of these problems have been recently resolved using the Gal4/UAS binary system in combination with Gal80ts, which allows for the transient induction of cell death in specific domains of the discs for a specific period, after which the discs can recover (5, 13). This tissue-damage technique mimics some aspects of microsurgical ablation, although there are important differences between these two methods. For instance, the overexpression of a cell-death inducer was not sufficient to kill all of the cells in the target region. Thus, dying cells could coexist with living cells.
In this study, we developed a unique method to study regeneration in the wing imaginal discs that involves the removal of a section of the disc in situ and allows regeneration to occur in its normal developmental context. Furthermore, our technique permits larval and pupal development, making it possible to study the size and pattern of adult structures after regeneration. Using this method, we addressed and revisited different aspects of the regenerative process that have thus far remained unclear, such as the role of cell proliferation during the early stages of regeneration. The results presented in this study suggest that regeneration can be divided into different phases: wound healing, dedifferentiation, and pattern and tissue reorganization. We found that although cell proliferation was increased during regeneration, most of the processes that occurred during the early stages of regeneration could proceed even in the absence of cell division. Our results indicate that disc regeneration is an experimental model, in which are involved characteristics of blastema formation and processes independent of cell division that resemble a morphallatic model of regeneration.
Results
Surgical Ablation of a Section of the Wing Disc in Situ.
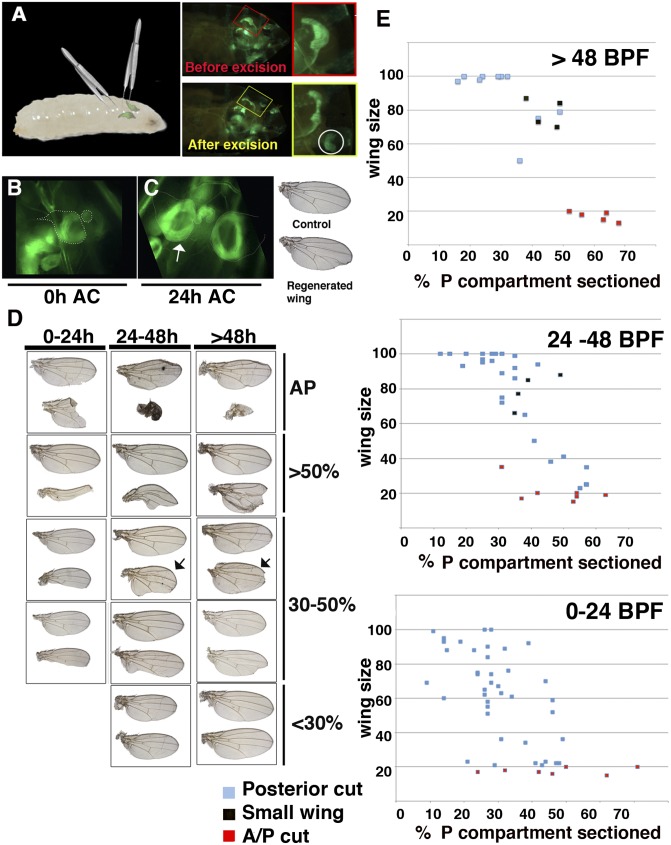
To study regeneration under physiological conditions, we modified a technique for aseptically wounding the wing discs in situ (14) to remove a section of the disc. The surgical ablation was performed in live midlate third instar [96–144 h after egg laying (AEL)] en-Gal4 UAS-GFP or Gal4-zfh2 LP30 UAS-GFP larvae by closing a pair of forceps over the wing disc without breaking the larval cuticle (Fig. 1 A–C, Fig. S1, and Movie S1). To quantify the amount of tissue removed during ablation, we imaged the amputated discs inside the larvae. We sectioned either part of the posterior compartment or a fragment that contained both the anterior and posterior compartments. We found normal adult wings derived from amputated discs, indicating that the regenerative response induced using this method was sufficiently strong to completely restore the eliminated fragment (Fig. 1).
Fig. 1.
Method for surgical ablation in situ. (A) Representation of the method used to surgically remove sections of the wing discs in situ. (Right) An en-Gal4 UAS-GFP wing disc through the cuticle of the larvae before and after the excision. (B) An amputated Gal4-zfh2 LP30 UAS-GFP disc after the excision (48 h BPF). (C) The discs shown in B but 24 h later (arrow indicates regenerating disc). (Right) The adult wing differentiated from these discs. (D) Different examples of adult regenerated wings and control contralateral wings (upper wings). The discs were amputated at different times during the development. Depending on the size of the fragment amputated [shown as the percentage of the P compartment eliminated (Right)] and time past BPF we observed a range of regenerated adult wings phenotype. (E) Graphs comparing the adult wing sizes (y axis) with the percent of the P compartment eliminated (x axis) at different times during disc development. The red squares indicate discs in which we removed part of both A and P compartments. The black squares represent normally patterned small wings, arrows in D.
To examine the regenerative capacity of the wing discs during development, surgical ablations were inflicted at different times. In all cases, the adult regenerated wings resulting from these discs were compared with the control contralateral wing to analyze potential differences as a result of the regeneration. Most (96%, n = 47) of the wing discs amputated late in development [140–160 h AEL, 0–24 h before puparium formation (BPF)] gave rise to adult wings that displayed a loss of wing tissues or small wings. Thus, even if the fragment amputated was relatively small [<30% of the posterior compartment (P compartment)], we found that 90% (n = 23) of the wings developed from these discs displayed nicks in the posterior or in both compartments, and their size was significantly smaller than the control wings (Fig. 1 D–E). Wings recovered from the discs in which larger sections were eliminated showed large nicks or resulted in vestigial wings (stump wing). In contrast, we found a good regenerative response when the wing discs were sectioned at earlier stages (120–140 h AEL, 24–48 h BPF). Thus, when we sectioned a fragment of the discs smaller than 30% of the P compartment in larvae of this age, all of the adult wings derived from these discs had either completed the regeneration process [normal wings (67%, n = 12)] or were slightly smaller than the control (95–99% vs. 100% control). These later wings displayed very small nicks in the posterior compartments (Fig. 1 D–E). The adult wings developed from discs in which we removed fragments between 30 and 50% of the P compartment were either smaller than the controls, but with a relatively normal vein pattern (28%, n = 14) (occasionally they have nicks), or contained large nicks (71%, n = 14). When the eliminated fragment was very large (>50% of the P compartment), these discs differentiated either into very small wings with nicks in both the anterior and posterior compartments (50%, n = 4) or stump wings (50%, n = 4). Similar stump wings always developed from the discs in which a region containing part of the posterior and anterior compartments was removed (Fig. 1 D–E). We found similar results when the wing discs were sectioned at earlier stages [96–120 h AEL (48–72 h BPF)]. Thus, all of the adult wings that differentiated from discs in which the fragments amputated were smaller than 30% of the P compartment were either identical to the controls (72%, n = 79) or have very small nicks (Fig. 1 D–E). A large section of the discs (>50%) always gave rise to stump or very small wings (Fig. 1 D–E). At this stage we also found small but normally patterned wings.
These data indicated a good correlation between the extent of the tissue removed in the disc and the phenotype of the adult regenerated wing. In addition, our results suggest that the time between the moment that the cut was inflicted and puparium formation was essential for the completion of regeneration. In our experimental conditions, regeneration could be completed when the amputation was performed during the larvae stage between 24 and 72 h BPF and if the fragment eliminated was smaller than 30% of the size of the P compartment. Although we found a range of different phenotypes in adult wings that were indicative of significant regeneration when the amputated sections were larger (30–50%), most of the adult wings either contained nicks or were smaller than the controls, indicating that regeneration was not completed. Studies using a cell-death-inducing method (13) have also reported a range of phenotypes following regeneration. Moreover our results suggest that a loss of regenerative capacity occurred at the end of the third larval instar or during early pupal development, as previously reported (15).
Interestingly, we also found small normally patterned wings, which suggested the existence of a pattern and tissue reorganization mechanism in wing disc regeneration.
Gene Expression Associated with Pattern Formation Genes Is Disrupted During Regeneration.
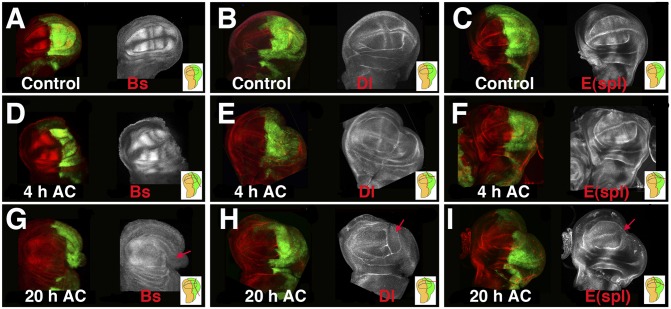
The characteristic pattern of the wing is defined during larval development by the restricted expression and/or activity of different genes and signaling pathways. Thus, in the third instar wing disc, Blistered (Bs) is specifically expressed in intervein cells (16), whereas Delta is expressed in vein cells (Fig. 2). Delta is a ligand of the Notch receptor and plays a crucial role in the formation of the wing margin and the definition of vein thickness. To study the activity of this pathway, we analyzed the expression of the E(spl)Mβ-CD2 reporter, which was expressed in the intervein tissue and at the dorsal/ventral (D/V) boundary in response to Notch signaling (ref. 17, Fig. 2C). We examined the expression patterns of these genes at different times after cut generation (AC) to assess whether cell-fate commitment was maintained during regeneration. When a fragment between 20% and 50% of the posterior compartment was amputated in en-Gal4 UAS-GFP discs, we found that the expression of Bs (n = 5), Delta (Dl) (n = 3), and E(spl)Mβ-CD2 (n = 4) was similar to the control contralateral discs from 0 to 6 h AC (Fig. 2 D–F). However, at 20 h AC, the expression of Bs (n = 6) was absent from the wound edge and was considerably reduced in both the adjacent region and in the remaining posterior compartment (Fig. 2G). The loss of vein/intervein commitment in the posterior compartment was confirmed by the absence of Dl (n = 13) and E(spl)Mβ-CD2 (n = 10) from the vein or intervein cells in the posterior compartment (Fig. 2 H and I). When the amputated fragment was large (30–50% of posterior compartment), and even if the cut had been performed only in the posterior compartment, Dl expression revealed a high proportion (62%, n = 13) of regenerating discs that lacked the vein/intervein pattern in the anterior compartment (Fig. 2H). Similar alterations were observed in cases of Bs and E(spl)Mβ-CD2 (Fig. 2 G and I). Because disc patterning is dynamic and changes rapidly during development, the developmental time of the regenerating discs was inferred by individual comparisons between the regenerating and the contralateral uncut discs.
Fig. 2.
Regeneration induces changes in cell-fate commitment and patterns. Third instar wing imaginal discs stained with anti-Bs (A, D, and G), anti-Delta (B, E, and H), and anti-CD2 (C, F, and I) to visualize the expression of the E(spl)mβ-CD2 reporter in the control (A–C) and regenerating discs (D–I). (D, E, and F) At 4 h AC, the expression of Bs, Dl, and the E(spl)mβ reporter were similar to the controls compared with A–C. However, at 20 h AC, the expression of Bs (G), Dl (H), and E(spl)mβ-CD2 (I) defining the vein/intervein pattern was no longer evident. Near the wound edge, we observed a down-regulation of these markers (red arrows). Interestingly, the vein/intervein pattern also disappeared in the anterior compartment, although the cuts were only in the P compartment. Here and in the rest of the figures, Insets indicate the cutting lines and the region eliminated in each disc. Here, as in all of the disc figures, posterior is to the right, and it is marked in green by the expression of UAS-GFP driven by en-Gal4.
Our data suggest that during regeneration, vein/intervein cells lose commitment to their cell fate at the wound edge. A similar loss of cell-fate commitment has been proposed to occur after genetic ablation (13). Unexpectedly, we found that this effect could also be extended to regions far away from the wound edge.
Wingless Expression Was Down-Regulated at the D/V Boundary During Regeneration.
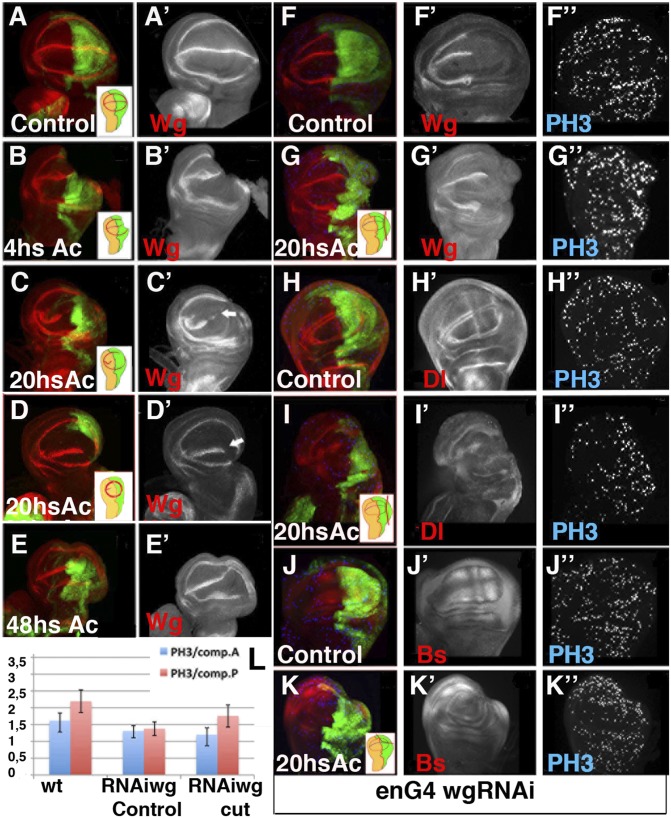
Previous studies have suggested that Wingless (Wg) is required during regeneration (13). Using our method we analyzed the requirement for Wg during regeneration and examined the evolution of its expression in regenerating discs. At the third instar stage, Wg is expressed along the D/V boundary and in two concentric circles with the internal ring at the border of the wing pouch (Fig. 3A). When a portion between 20% and 50% of the posterior compartment was severed, we found that 0–6 h AC, the expression of Wg was similar in the experimental and control discs (Fig. 3B). However, between 18 and 24 h AC, Wg expression disappeared from part of the D/V boundary in all wing discs tested (n = 25) (Fig. 3 C–D). Moreover, in 48% of these discs, the expression of Wg at the D/V boundary of the anterior compartment was down-regulated, even though we had only removed a fragment of the posterior compartment (Fig. 3 C–D). At this time, the expression of the internal ring of Wg was either completely (40%, n = 25) or partially restored in all of the discs analyzed (Fig. 3 C–D). Interestingly, when the amputated section was between 30% and 50% of the P compartment, the reestablishment of the internal ring of Wg redefined a new wing pouch that was significantly smaller than the control wing pouch (Fig. 3D and Fig. S2). In all of the discs examined at 48 h AC, the expression of Wg in the internal ring was completely reestablished, whereas at the D/V boundary, it was completely restored in 66% (n = 6) of the discs (Fig. 3E). The changes in Wg protein expression were also detected using a wg-LacZ reporter (Fig. S2 D–F′), indicating that transcriptional activation of Wg at the D/V boundary was transiently lost during regeneration.
Fig. 3.
Evolution of Wg expression during wing disc regeneration. (A–G′) Expression of Wg, shown by anti-Wg (red in A–G) in en-Gal4; UAS-GFP third instar control discs (A–A′), regenerating discs at different times AC (B–E′) and in the en-Gal4 UAS-GFP /UAS-wg RNAi control (F–F′) and regenerating discs (G–G′). (H–K″) The en-Gal4 UAS-GFP /UAS-wg RNAi wing discs stained for anti-Dl (red in H and I) and anti-Bs (red in J and K). Mitotic cells were marked with Phospho-Histone H3 (blue in F–K). (B–B′) The expression of Wg was not altered at 0–4 h AC. (C–D′) At 20 h AC the expression of Wg disappears at the D/V boundary near the wound edge as well as in a section of the anterior wing margin (white arrows in C′ and D′). At this time the expression of the internal ring of Wg was partially (C–C′) or totally (D –D′) restored. (E–E′) At 48 h AC the expression of Wg was restored. (F–G′) Wing discs of en-Gal4 UAS-wg RNAi larvae displayed a strong down-regulation of Wg in the P compartment. (G–K″) Cell proliferation increase in the posterior compartment of regenerating discs with undetectable levels of Wg; see also L. The vein/intervein pattern defined by Dl (H–H′) and Bs (J–J′) in the control discs was disrupted in the en-Gal4 UAS-wg RNAi regenerating discs 20 h AC (I–I′ and K–K′). (L) Bar chart showing the average mitotic index in the posterior and anterior compartments of en-Gal4 UAS-GFP (wt control); en-Gal4 UAS-wg RNAi larvae raised at 17 °C and shifted to 25 °C for 40 h before being dissected (RNAiWg control); and regenerating discs of en-Gal4 UAS-wg RNAi larvae treated as in F–G (RNAiWg cut).
To assess whether Wg was required for regeneration, we inhibited its function by overexpressing UAS-wg RNAi under the regulation of en-Gal4. When these larvae were raised at 17°C, they always gave rise to normal adult wings. However, when these larvae where shifted to 25°C for 20 h during the third instar period, their wing discs displayed a strong down-regulation of Wg in the posterior compartment (Fig. 3F). To block the function of Wg during regeneration, en-Gal4 UAS-wg RNAi larvae were shifted to 25 °C for 20 h before the ablation and were maintained at that temperature during the regeneration process (Fig. S3). The adult wings develop from these discs displayed large nicks in the posterior compartment. We next investigated whether the down-regulation of Wg might affect the wound-healing process. As previously described in the control discs (12), we found that in discs in which the function of Wg was eliminated, an actin cable appeared at the wound edge 1 h AC, and the epithelial continuity was reestablished (Fig. S3).
Similar to previous reports using other methods, we found that the density of mitotic cells steadily increased during regeneration at the wound edges and in nearby regions (Fig. S4). Using our method, we found that cell proliferation peaked at ∼20 h AC (3.2 ± 0.3 in regenerating discs vs. 2.1 ± 0.3 in the control, n = 11, P < 0.01) (Fig. S4). These results suggested that the blastema was already formed at this time. We examined whether blastema generation was altered in en-Gal4 UAS-wg RNAi discs. As expected, considering the growth-promoting function of Wg, its down-regulation reduced the mitotic index in both the anterior and posterior compartments of these discs. This reduction was also evident in the regenerating discs at 20 h AC (Fig. 3L). However, the mitotic index increased in the posterior compartment of these regenerating mutant discs compared with nonregenerating discs (1.34 ± 0.21 control vs. 1.76 ± 0.33 regenerating discs, n = 13, P < 00.1) (Fig. 3L). As previously observed in the controls, we found that at 20 h AC, the vein/intervein pattern defined by Dl and Bs was no longer evident when Wg was depleted (Fig. 3 H–K″).
Taken together, our results suggested that the down-regulation of Wg did not perturb either wound healing or blastema generation. Moreover, these results indicated that a loss of vein/intervein cell-fate commitment and pattern reorganization observed during regeneration of the control discs also occurred in discs with undetectable levels of Wg in the wound edge.
Pattern and Tissue Reorganization During Regeneration Was Independent of Cell Division.
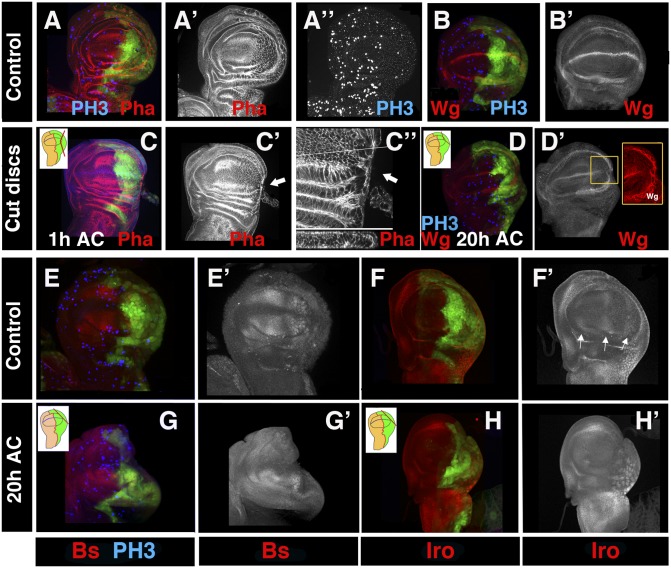
During disc regeneration, cell proliferation increased at the blastema and in regions near to the wound (refs. 9–10 and Fig. S4), suggesting that intercalary cell division played an important role for the completion of regeneration. However, our results also suggest that during regeneration, tissue and pattern reorganization processes have occurred. To investigate the contribution of cell division to early stages of regeneration, we blocked mitosis by overexpressing the CycA inhibitor roughex (rux) (18) under the control of en-Gal4 and Gal80ts. The en-Gal4 UAS-rux UAS-GFP/ Tub-Gal80ts larvae were raised at 17 °C until 240 h AEL and then shifted to 29 °C for 24 h before the amputation. The PH3 marker revealed that cell division was eliminated in the posterior compartments of these discs (Fig. 4A). After amputation, the larvae were maintained at 29 °C during the regeneration process. As observed in the control discs, we found an actin cable at the wound edge 60 min AC (Fig. 4C). Moreover, the epithelial continuity was reestablished in these discs (Fig. 4C″).
Fig. 4.
Regeneration was initiated in the absence of cell division. (A–H′) Third instar wing discs of en-Gal4 UAS-GFP/ UAS-Rux larvae shifted from 17 to 29 °C for 24 h before the surgical ablation and maintained at 29 °C during regeneration. The discs were stained with phalloidin (red in A and C); anti-Wg (red in B–D); anti-Bs (red in E and G); anti-Iro (blue in F and H); and anti-PH3 (blue in A, B, E, and G). (A–A′) Note the large size of the cells expressing rux. (C–C″) We found an actin cable (arrows) (phalloidin staining in red) in the wound edges 1 h AC. Optical z-sections below C″ showed a cross-section at the position of the white line. (D –D′) As seen in the control regenerating discs, 20 h AC the expression of Wg disappeared in a fragment of the D/V boundary (high magnification in the yellow square in D′), but was restored in the internal ring. (E–H′) The vein/intervein pattern defined by Bs (E–E′) and Iro (arrows in F–F′) observed in the control discs was disrupted in the regenerating discs at 20 h AC (G–H′).
Finally, we investigated the effect of cell division arrest on pattern and tissue reorganization during disc regeneration. Specifically, we examined the expression of Wg as well as the vein/intervein markers Iroquois (Iro) and Bs. In 77% of the discs analyzed (n = 9), the expression of Wg was restored in the internal ring by 20–22 h AC, redefining a presumptive new wing blade. As observed in the control discs, the expression of Wg disappeared from fragments of the D/V boundary (Fig. 4D). Furthermore, similar to the control regenerating discs, we found that at 20 h AC, the expression of Bs was absent at the wound edge and was considerably reduced in the posterior compartment of mutant discs (Fig. 4G). The loss of vein/intervein commitment was confirmed by the absence of Iro from the vein cells (Fig. 4H). These results suggest that the loss of cell-fate commitment, wound-healing process, and pattern and tissue reorganization occurred in the absence of cell division.
Tissue Reorganization During Regeneration.
Our results suggest that the proximal regions of the wing discs were specified before the distal regions, as indicated by the lack of expression of Wg in the wing margin when its expression was already restored in the internal ring (Fig. 3). Furthermore, when we amputated fragments between 30% and 50% of the P compartment of the discs, the expressions of the proximal markers, zfh2 and Homothorax (Hth), were restored in 64% (n = 20) and 40% (n = 5), respectively, of the regenerating discs analyzed at 20 h AC, although the expression of Wg was still undetectable in part of the wing margin (Fig. S5). As previously described, we found that the specification of the proximal region could redefine a new wing pouch that was smaller than the controls (Fig. S5). One plausible explanation regarding the origin of the small regenerated adult wings identified in the previous analysis (Fig. 1) was that they were derived from these small discs. To test this idea, we amputated fragments between 30% and 50% of zfh2-Gal4 UAS-GFP discs and documented the regeneration process of individual discs by imaging the amputated discs inside the larvae after the excision and at 20 h later (SI). We found that the expression of zfh2 was totally restored in 73% (n = 13) of the wings discs analyzed 20 h AC. The size of the newly redefined wing pouches was always smaller than the control contralateral discs, varying from less than 10% when the fragment eliminated was small (<30% P compartment) to more than 50% when we amputated larger sections (>30% P compartment) (Fig. S6). The adult wings derived from discs that, at 20 h after amputation, displayed a wing pouch that was significantly smaller than the control (>20% smaller); were either smaller than the controls, but with a normal pattern (28%, n = 7); or have nicks in the posterior or both compartments (57%, n = 7) (Fig. 5 and Fig. S6). The discs in which the expression of zfh2 was not restored at 20 h AC always differentiate stump wings (Fig. S6). These results indicate that the small but relatively patterned regenerated wings derive from amputated discs in which the specification of the proximal region has redefined a small wing pouch.
Fig. 5.
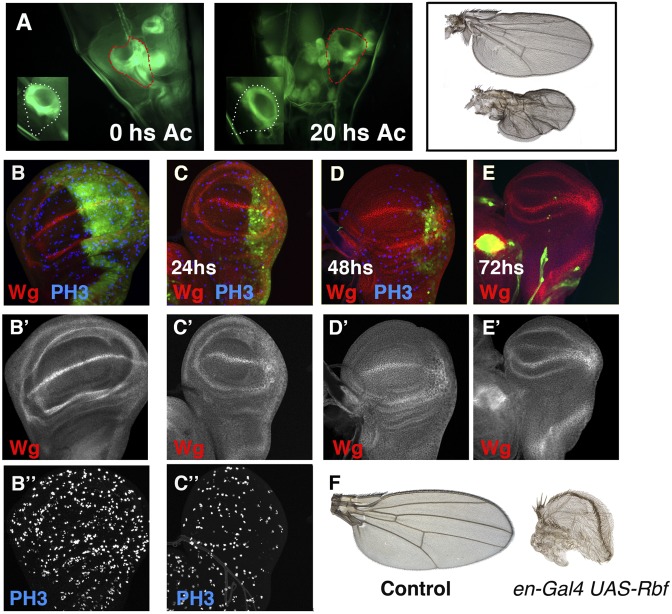
Evolution of disc regeneration in situ. (A) Image of a Gal4-zfh2 LP30 UAS-GFP amputated disc through the cuticle of the larvae after the excision, compared with the control discs (Inset). The disc was sectioned 48 h BPF. (Right) An image of the same disc but 20 h later. Note the smaller size of the amputated disc compared with the contralateral disc (35% smaller). The amputated disc is outlined in red, and the control disc is outlined in white. The adult wing differentiated from these discs are shown on the Right. Control (B–B″) and en-Gal4 UAS-Rbf280 UAS-GFP /Tub-Gal80ts (C–C″) third instar wing discs, stained for Wg (red in B–E) and PH3 (blue in B–D). Larvae were raised at 25 °C until 96 h AEL and shifted to 29 °C to block cell proliferation. Note the strong reduction of the number of mitotic cells in the P compartment, as assayed with PH3. (C–E) The en-Gal4 UAS-Rbf280 UAS-GFP /Tub-Gal80ts wing discs after 24 h (C), 48 h (D), and 72 h (E) at 29 °C. (F) Control wing and adult wing differentiated from the en-Gal4 UAS-Rbf280 UAS-GFP /Tub-Gal80ts larvae that were shifted to 29 °C at 96 h AEL and maintained at that temperature until the end of development.
Taken together, our data suggested that during regeneration, in addition to regenerative growth, tissue reorganization must also occur. We investigated whether this effect also occurred when a large region of the wing disc was genetically eliminated. To achieve this, we blocked cell division in the P compartment by overexpressing a constitutively active form of the Retinoblastome factor (rbf) (19) under the control of en-Gal4 and the Gal80ts. The en-Gal4 UAS-Rbf280 UAS-GFP /Tub-Gal80ts larvae were raised at 25 °C until 96 h AEL and then shifted to 29 °C to block cell division. The size of the P compartment was strongly reduced compared with the anterior compartment after 48 h at 29 °C (Fig. 5D). Twenty-four hours later (72 h at 29 °C), the P compartment of most of these discs was completely eliminated, and only a residual group of posterior cells were found (Fig. 5E). The cells from the P compartment were steadily eliminated by cell death, as assayed using the apoptotic marker Caspase 3. Interestingly, we found discs in which the expression of the internal ring of Wg defined a new small wing blade (>50% smaller than the control) that was formed by anterior cells. The adult wings that differentiated from these discs were approximately half of the size of a normal wing, and their pattern elements were characteristic of the anterior compartment (Fig. 5F). These results suggest that, as seen during regeneration, in these discs tissue reorganization has occurred.
Discussion
A number of techniques have been used to analyze regeneration in Drosophila, but they each pose limitations (see the Introduction) that have complicated the analysis of processes that occur during regeneration. Our approach also exhibited technical difficulties, specifically in the reproducibility of precise cuts. However, our method has several advantages over previous approaches; for example, regeneration could occur in the normal larval environment, and our technique enabled the use of the contralateral wing discs as internal controls. Capitalizing on these strengths, we were able to identify several aspects of wing disc regeneration that had previously gone unnoticed.
Our data indicated that the behavior of the disc during regeneration was influenced by the method used to injure the disc. Previous studies (13) have shown that after the cell-death-induced regeneration, Wg was initially up-regulated; however, after 48 h of recovery, the expression of this protein was down-regulated. Although we also found that Wg was down-regulated during regeneration, we did not observe the initial up-regulation of this protein. These discrepancies are likely due to the different methods used. Previous reports have shown that Wg was up-regulated when the cells underwent apoptosis (20). Thus, it is possible that part of the initial up-regulation of Wg observed by Smith-Bolton et al. (13) could be a consequence of the massive induction of cell death. Another important difference between these two methods was that we completely eliminated a fragment of the disc, whereas the induction of apoptosis caused tissue damage that allowed healthy cells to coexist with dying cells from which they could still receive signals. Cellular responses may also operate differently depending on whether the tissue was only damaged or completely eliminated. Indeed, we found that when the wing discs were only damaged with forceps, but the fragment was not eliminated, Wg expression was frequently up-regulated (Fig. S2 I–I′). The fact that Smith-Bolton et al. (13) also found that during the recovery period many discs lacked Wg expression suggests that once the initial up-regulation of Wg concluded, this gene was down-regulated during regeneration, similar to our observations.
Using the classical method of disc transplantation, other studies (21, 22) have found Wg up-regulation during leg-disc regeneration. These experiments were performed using the prothoracic leg (L1) disc. These discs have unique regulatory potential because cells of the anterior compartment could directly convert to a posterior identity during regeneration (23). Other discs lack this ability. Therefore, it has been proposed that after L1 fragmentation, the hh-expressing peripodial cells present in these discs are fused with columnar cells at the wound site and induce the expression of engrailed, which promoted the expression of Wg in adjacent cells (21). Thus, the ectopic activation of Wg in the wound edge of the regenerating L1 leg is likely a consequence of the unique regulatory potential of this leg.
Our results suggest that down-regulation of Wg did not perturb the early stages of regeneration. However, we cannot rule out a function of this gene during later stages of regeneration. Because Wg is required for the proper development of the wing disc, when its function is eliminated during regeneration, the resulting adult wings (both control and regenerated) displayed numerous nicks. Thus, we could not determine whether the nicks displayed by the regenerated wings were due to the requirement of wg for complete organ regeneration or were caused by the insufficiency of Wg during development.
Wing Pattern and Size After Regeneration.
We have found that the small but normally patterned regenerated wings developed from amputated discs in which the specification of the proximal region has redefined a small wing pouch. The normal vein pattern of these wings might be established because at the time that the tissue restructuring occurs, there is a process of pattern reorganization. In this scenario, the vein pattern might be readjusted to the new size of the wing pouch. The small size of these wings suggests that to define the final size of the wing and therefore complete regeneration, cell proliferation is necessary. Accordingly, if there was not enough time BPF to finish this process, then the wing will differentiate smaller than the control, but with a normal pattern.
Our data indicated that in our experimental conditions, the discs in which we removed a region containing part of the posterior and anterior compartments never regenerated. However, other studies have shown that wing discs in which ablation affected this boundary could still regenerate (13). This difference is likely due to the time when the ablation was produced. The earliest time in which we could amputate the discs was at 96–120 h AEL, whereas the ablation using the cell-death-inducing approach was produced much earlier.
Disc Regeneration Phases.
Our results suggest that disc regeneration could be divided into different phases: wound healing, dedifferentiation and pattern reorganization, specification of the proximal regions of the wing discs, and redevelopment. After the amputation of a disc fragment, the epithelial integrity was reestablished by wound healing (24). After or during this process, different signals were activated to induce the generation of the blastema and to cause the temporary loss of markers of cell-fate commitment at the wound edge and adjacent regions, including the cells at the wing margin, as indicated by the lack of expression of Wg. The expression of this gene was down-regulated along the wing margin in regions far away from the wound edge. This effect could explain the presence of nicks in the anterior compartment of some regenerated wings derived from discs in which we only amputated part of the P compartment. Once these processes have concluded (or at the same time), respecification of the proximal regions of the wing discs occurs, as indicated by the expression of proximal markers. Despite the fact that cell proliferation increased during the early stages of regeneration, our evidence indicated that these processes could proceed in the absence of cell division. Finally, the redevelopment phase would occur, which mimicked normal development. Thus, once the proximal regions were specified, the definition of the distal regions of the wing discs may develop via normal mechanisms. Our data suggested that there was no modification in expression of the dorsal selector ap during regeneration (Fig. S7); thus, the interaction between ap-expressing and non-ap-expressing cells could restore the wing margin and distal region of the wing discs. During this time the vein/intervein pattern begins to be reestablished, as indicated by the expression of Dl in the presumptive veins 48 h AC (Fig. S4J).
Various studies have suggested that wing discs regenerate by intercalary proliferation following an epimorphic model (11). During limb regeneration in amphibians, which is a paradigm model for epimorphic regeneration, it has been suggested that a rearrangement of positional information may occur through intercalary events, as observed in organisms that follow a morphallactic model (25). On the basis of these observations, Agata et al. (26) proposed that epimorphosis and morphallaxis should no longer be used to classify regeneration phenomena and that classical categories of regeneration must be reconsidered. After evaluating our data, we proposed that the classification of disc regeneration as an epimorphic model must also be reconsidered, as we found features of both regenerative models.
Materials and Methods
All fly strains used are described at http://flybase.bio.indiana.edu/ unless otherwise indicated (SI Materials and Methods). Histology and immunostainings have been performed following standard protocols. See SI Materials and Methods for details.
Supplementary Material
Acknowledgments
We thank Antonio Garcia-Bellido and Jose Felis de Celis for providing reagents and useful discussion, J. Pastor-Pareja for helping us to develop this method and for providing Fig. 1B, I. Andrade for providing Fig. 5F, L. A. Baena and Willis Li for help in improving this manuscript; and we are very grateful to Gines Morata, Barbara Thomas, Wei Du, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for providing fly strains and antibodies. This work was supported by grants obtained from the Ministerio de Educación y Ciencia (BFU2011-23224/BMC) and Consolider (20072D9110). S.D.G. was supported by a Formacion de Personal Investigador Fellowship from the Ministerio de Investigación, Ciencia, e Innovación.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220543110/-/DCSupplemental.
References
- 1.Morgan TH. Regeneration and liability to injury. Science. 1901;14(346):235–248. doi: 10.1126/science.14.346.235. [DOI] [PubMed] [Google Scholar]
- 2.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 3.Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez Alvarado A, Tsonis PA. Bridging the regeneration gap: Genetic insights from diverse animal models. Nat Rev Genet. 2006;7(11):873–884. doi: 10.1038/nrg1923. [DOI] [PubMed] [Google Scholar]
- 5.Bergantiños C, Corominas M, Serras F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development. 2010;137(7):1169–1179. doi: 10.1242/dev.045559. [DOI] [PubMed] [Google Scholar]
- 6.Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117(2):597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- 7.Hadorn E, Anders G, Ursprung H. Combination derived from partial dissociated imaginal disks of various mutants and types of Drosophila. J Exp Zool. 1959;142:159–175. doi: 10.1002/jez.1401420107. [DOI] [PubMed] [Google Scholar]
- 8. Bodenstein D (1943) Hormones and tissue competence in the development of Drosophila. Biol Bull 84(1):34–58.
- 9.Bryant PJ. Regeneration and duplication following operations in situ on the imaginal discs of Drosophila melanogaster. Dev Biol. 1971;26(4):637–651. doi: 10.1016/0012-1606(71)90146-1. [DOI] [PubMed] [Google Scholar]
- 10.Hadorn E, Hürlimann R, Mindek G, Schubiger G, Staub M. Developmental capacity of embryonal blastema in Drosophila following cultivation in an adult host. Rev Suisse Zool. 1968;75(3):557–569. [PubMed] [Google Scholar]
- 11.French VP, Bryant PJ, Bryant SV. Pattern regulation in epimorphic fields. Science. 1976;193(4257):969–981. doi: 10.1126/science.948762. [DOI] [PubMed] [Google Scholar]
- 12.Bosch M, Serras F, Martín-Blanco E, Baguñà J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol. 2005;280(1):73–86. doi: 10.1016/j.ydbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell. 2009;16(6):797–809. doi: 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastor-Pareja JC, Wu M, Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis Model Mech. 2008;1(2–3):144–154, discussion 153. doi: 10.1242/dmm.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halme A, Cheng M, Hariharan IK. Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol. 2010;20(5):458–463. doi: 10.1016/j.cub.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roch F, Baonza A, Martín-Blanco E, García-Bellido A. Genetic interactions and cell behaviour in blistered mutants during proliferation and differentiation of the Drosophila wing. Development. 1998;125(10):1823–1832. doi: 10.1242/dev.125.10.1823. [DOI] [PubMed] [Google Scholar]
- 17.de Celis JF, Tyler DM, de Celis J, Bray SJ. Notch signalling mediates segmentation of the Drosophila leg. Development. 1998;125(23):4617–4626. doi: 10.1242/dev.125.23.4617. [DOI] [PubMed] [Google Scholar]
- 18.Thomas BJ, et al. roughex down-regulates G2 cyclins in G1. Genes Dev. 1997;11(10):1289–1298. doi: 10.1101/gad.11.10.1289. [DOI] [PubMed] [Google Scholar]
- 19.Duman-Scheel M, Weng L, Xin S, Du W. Hedgehog regulates cell growth and proliferation by inducing Cyclin D and Cyclin E. Nature. 2002;417(6886):299–304. doi: 10.1038/417299a. [DOI] [PubMed] [Google Scholar]
- 20.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell. 2004;7(4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Gibson MC, Schubiger G. Hedgehog is required for activation of engrailed during regeneration of fragmented Drosophila imaginal discs. Development. 1999;126(8):1591–1599. doi: 10.1242/dev.126.8.1591. [DOI] [PubMed] [Google Scholar]
- 22.McClure KD, Sustar A, Schubiger G. Three genes control the timing, the site and the size of blastema formation in Drosophila. Dev Biol. 2008;319(1):68–77. doi: 10.1016/j.ydbio.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott LC, Karpen GH, Schubiger G. Compartmental restrictions and blastema formation during pattern regulation in Drosophila imaginal leg discs. Dev Biol. 1981;87(1):64–75. doi: 10.1016/0012-1606(81)90061-0. [DOI] [PubMed] [Google Scholar]
- 24.Reinhardt CA, Hodgkin NM, Bryant PJ. Wound healing in the imaginal discs of Drosophila. I. Scanning electron microscopy of normal and healing wing discs. Dev Biol. 1977;60(1):238–257. doi: 10.1016/0012-1606(77)90122-1. [DOI] [PubMed] [Google Scholar]
- 25.Nye HL, Cameron JA, Chernoff EA, Stocum DL. Regeneration of the urodele limb: A review. Dev Dyn. 2003;226(2):280–294. doi: 10.1002/dvdy.10236. [DOI] [PubMed] [Google Scholar]
- 26.Agata K, Saito Y, Nakajima E. Unifying principles of regeneration I: Epimorphosis versus morphallaxis. Dev Growth Differ. 2007;49(2):73–78. doi: 10.1111/j.1440-169X.2007.00919.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.