Abstract
Recent evidence indicates there is a role for small membrane vesicles, including exosomes, as vehicles for intercellular communication. Exosomes secreted by most cell types can mediate transfer of proteins, mRNAs, and microRNAs, but their role in the transmission of infectious agents is less established. Recent studies have shown that hepatocyte-derived exosomes containing hepatitis C virus (HCV) RNA can activate innate immune cells, but the role of exosomes in the transmission of HCV between hepatocytes remains unknown. In this study, we investigated whether exosomes transfer HCV in the presence of neutralizing antibodies. Purified exosomes isolated from HCV-infected human hepatoma Huh7.5.1 cells were shown to contain full-length viral RNA, viral protein, and particles, as determined by RT-PCR, mass spectrometry, and transmission electron microscopy. Exosomes from HCV-infected cells were capable of transmitting infection to naive human hepatoma Huh7.5.1 cells and establishing a productive infection. Even with subgenomic replicons, lacking structural viral proteins, exosome-mediated transmission of HCV RNA was observed. Treatment with patient-derived IgGs showed a variable degree of neutralization of exosome-mediated infection compared with free virus. In conclusion, this study showed that hepatic exosomes can transmit productive HCV infection in vitro and are partially resistant to antibody neutralization. This discovery sheds light on neutralizing antibodies resistant to HCV transmission by exosomes as a potential immune evasion mechanism.
Most tissue and cell types produce and release exosomes, a distinct population of microvesicles ranging from about 30 to 150 nm in size. Exosomes are formed in the endocytic compartment of multivesicular bodies (1) and are secreted in various body fluids under normal and pathological conditions (2, 3). Extensive studies have now implicated exosomes in many biological processes such as tissue injury and immune responses by transfer of antigens, antigen presentation (2, 4), and the shuttling of proteins, mRNAs, and microRNAs (miRNA) between cells (5). As such, it has been postulated that exosomes play a crucial role in cell communication and in the transfer of genetic information between cells (5).
The role of exosomes and other secretory vesicles in the transfer of pathogen-derived antigens and virulence factors is emerging (6, 7). Whether release of vesicles from infected cells contributes to immune control and clearance of infection by the host is still not clear. For example, the HIV Gag protein recruits the outward vesicle-budding machinery of exosomes to form free virions (8). Recently, it has been shown that exosomes released from HIV-infected cells contain negative regulatory factor, which induces apoptosis of uninfected cells (9). Epstein-Barr virus-infected B cells also secrete exosomes that contain virally encoded miRNA (10). This study further demonstrates the delivery of naturally occurring functional genetic elements to neighboring cells via exosomes, indicating that viral particles or molecules associated with viral infection can be transmitted to adjacent uninfected cells via exosomes and become functional. More recently, the hepatitis A virus has shown to be able to escape humoral immunity by cloaking itself in cellular membranes on release from host cells. These virus-containing microvesicles, resembling exosomes, were shown to protect virions from antibody-mediated neutralization (11).
Hepatitis C virus (HCV) infection, a leading liver disease, has been shown to have multiple routes of transmission. Apart from classical transmission by free viral particles, an antibody-resistant cell-to-cell transmission route also has been described (12). Indeed, HCV is known to evade humoral immune responses, as indicated by a lack of resistance to HCV reinfection in i.v. drug users (13), HCV reinfection during liver transplantation (14), and an ongoing difficulty of developing effective vaccines. The role of exosomes in HCV infection is still largely unknown. One earlier paper reported the presence of viral RNA in exosomes isolated from plasma of HCV-infected patients (15) but did not show exosome-mediated transmission of infection. More recent studies suggest that HCV virus assembly and release in hepatocytes may be linked to the exosome secretory pathway (16) and that hepatocyte-derived exosomes can transfer viral RNA to plasmacytoid dendritic cells, triggering their activation and IFN-α production (17). However, the role of exosomes in the cell-to-cell transmission route of HCV between hepatocytes has not been demonstrated. The aim of our study was to investigate transmission of HCV infection by hepatocyte-derived exosomes in the presence of neutralizing antibodies (nAbs) in vitro that could explain the ineffectiveness of prophylactic nAbs and agents targeting the entry of HCV into a cell. We further observe that this route of infection can generate productive viral infection.
Results
Exosomes Derived from HCV-Infected Hepatoma Cells Contain Virus Particles.
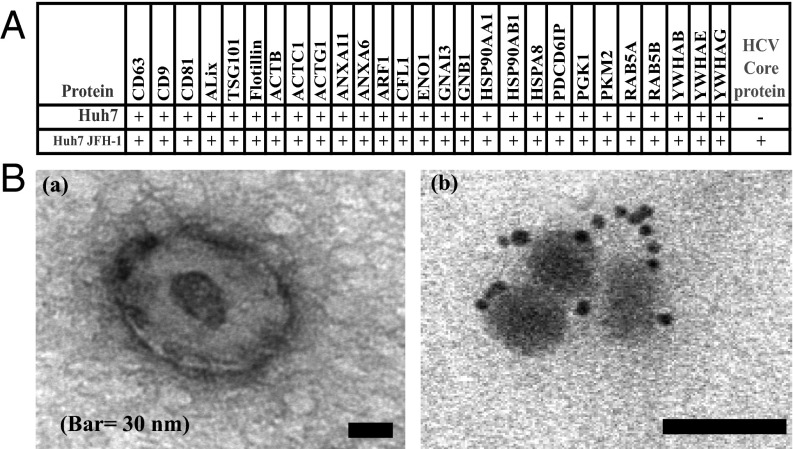
To establish the role of exosomes in shuttling HCV between cells, exosomes were isolated from Huh7.5.1 hepatoma cells using an established sucrose-gradient ultracentrifugation procedure. As shown in Fig. 1A, mass spectrometric analysis of exosomes confirmed the presence of common exosome markers (3) such as tumor susceptibility gene 101, CD63, CD9, and apoptosis-linked gene 2-interacting protein X in these preparations. Moreover, exosomes isolated from Huh7.5.1 cells infected with the chimeric HCV replicon, named Jc1, contained detectable levels of HCV core protein (Fig. 1A). Two unique HCV peptides were detected with mascot scores higher than 50 in three independent batches. As reported in a previous study, using a comprehensive proteome profiling of exosomes secreted by hepatocytes (2, 3), apolipoproteins ApoE and ApoB also were detectable. These lipoproteins were present in both control and HCV-positive exosomes, suggesting that ApoE and ApoB are associated with hepatocyte-derived exosomes rather than contamination by HCV-associated LDL particles.
Fig. 1.
Purified exosomes of HCV-infected Huh7.5.1 cells harbor viral protein and RNA. (A) Mass spectrometric analysis of Huh7.5.1 and Huh7.5.1 Jc1-derived exosomes contain exosome-related proteins and HCV core (n = 3). (B) Electron microscopic images of purified exosomes isolated from infected Huh7.5.1 cells (a), using uranyl acetate staining. Immunogold labeling of HCV E2 envelope protein shows the presence of viral protein in exosomes derived from infected Huh7.5.1 cells (b). Immunogold and uranyl acetate staining could not be combined. Shown is one representative experiment of three.
Electron microscopic imaging confirmed the purity of the exosome preparations, using negative staining, with uranyl acetate showing a lipid bilayer structure (Fig. 1B). Immunogold labeling with an anti-E2 antibody showed the presence of viral envelope proteins in exosomes isolated from HCV-infected Huh7.5.1 but not in exosomes from naive Huh7.5.1 cells. In these preparations, no electron-dense particles were observed outside the exosomes. RT-PCR analysis showed crude exosomes isolated from Jc1-infected cells, and HCV subgenomic replicon cells did contain HCV RNA (Fig. S1). Full-length PCR analysis showed that these exosomes contained complete HCV genomes. As determined by direct Sanger sequencing, the amplified products from exosomes were identical to the HCV genotype 2a of Jc1. Consistent with our earlier report (18), both control and HCV-positive exosomes contained high levels of miRNAs, including miR-122.
Exosomes Can Transmit HCV and Establish a Productive Infection.
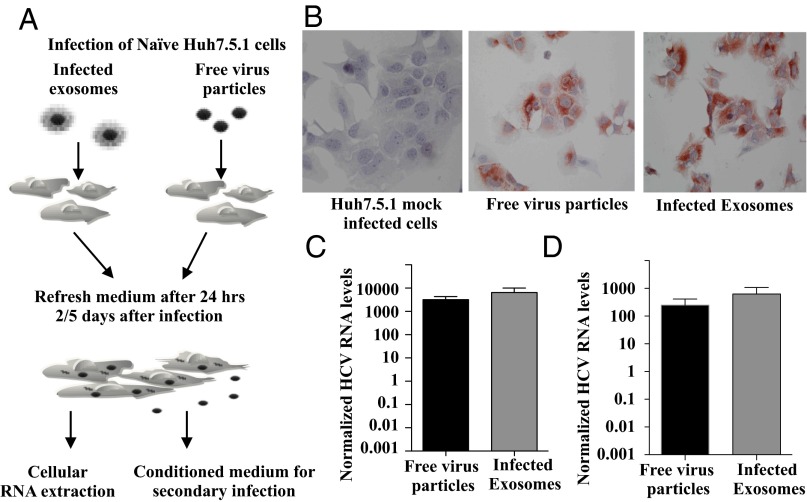
To investigate the functional role of exosomes in the transmission of infection, exosomes were isolated from Jc1-infected cells and incubated with naive Huh7.5.1 cells, as outlined in Fig. 2A. As shown in Fig. 2B, 2 d after exposure to HCV-positive exosomes, Huh7.5.1 cells stained highly positive for HCV core protein by immunohistochemistry. The level of HCV core staining was comparable to that of cells infected with free virus particles. Huh7.5.1-uninfected cells were used for mock infection. The percentage of HCV-positive cells when infected with free virus was a mean of 93.0 ± 3.7 SD compared with 91.0 ± 5.4 in exosome-treated cells, with no significant differences among the treatments. As HCV RNA input was normalized, exosomes appear as efficient as free virus in transmitting infection. As shown in Fig. 2C, cellular RNA of exosome-treated cells contained high levels of HCV viral RNA, which is comparable to levels seen in cells infected by free infectious particles. Importantly, conditioned medium of cells infected by HCV-positive exosomes was able to establish a secondary infection of naive cells, confirming that the exosome pathway results in productive infection (Fig. 2D). The level of this secondary infection was comparable to that established by free infectious particles. The uptake of exosomes by Huh7.5.1 cells was confirmed by real-time confocal microscopy, using fluorescently labeled exosomes. As shown in Fig. S2, during a period of 30 min, cells gradually take up exosomes. Paraformaldehyde-fixed cells remained negative over that time, confirming that exosome uptake is an active process and excluding passive transfer of fluorescent label.
Fig. 2.
Infected exosomes transmit infection to naive Huh7.5.1 cells and cause productive infection. (A) Schematic representation of infection experiments. (B) Immunocytochemical staining of HCV core protein Huh7.5.1 cells infected with infectious virus or exosomes (n = 4). (C) Quantitative RT-PCR analysis of HCV RNA after primary infection and (D) secondary infection. The levels are normalized HCV RNA multiplied by 1,000. Shown are the mean ± SEM of four independent experiments in triplicates.
Exosome-Mediated Transmission Is Partly Resistant to Neutralizing Antibodies and Independent of Structural Viral Proteins.
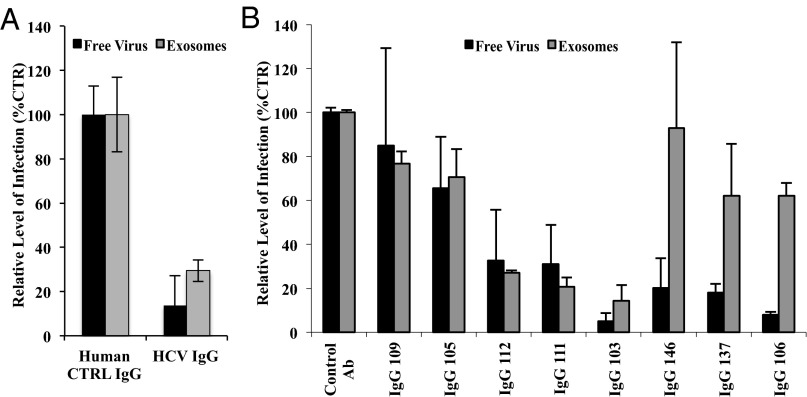
Next, we investigated exosome-mediated transmission of infection in the presence of nAbs. For this, HCV-specific immunoglobulins (IgGs) were purified from serum from 10 patients with chronic HCV, as reported earlier (19). Infectivity levels of Jc1 virus containing the luciferase reporter gene were tested in the presence of a pool of these nAbs. As shown in Fig. 3A, both the infection by free virus and HCV-positive exosomes were significantly inhibited by pooled nAbs. Of these 10 patients, nAbs were present in eight (Table S1). As shown in Fig. 3B, a comparative analysis of neutralization by HCV-specific IgGs from these eight patients showed variable levels of neutralization of both free virus and exosomes. For five patients, IgGs showed similar neutralization of exosome- and free virus-mediated infection (P > 0.05). In three patients, however, IgGs showed limited inhibition of exosome-mediated infection compared with free virus (mean % inhibition ± SEM of exosomes, 27.6 ± 13.5 vs. free virus, 84.6 ± 4.4; P = 0.002 Mann–Whitney test). No link was found between patient’s characteristics or virus genotypes and the inability of IgGs to inhibit exosome-mediated infection (Table S1).
Fig. 3.
Exosome-mediated transmission of HCV in the presence of primary neutralizing Igs (IgGs) varies between patients. (A) Huh7.5.1 cells were infected with Luc-Jc1 particles or purified exosomes from Luc-Jc1–infected cells in the presence of pooled patient-derived anti–HCV-specific IgGs from ten patients with HCV. Shown are the mean ± SEM of four (control IgG) or five (HCV IgGs) independent experiments in duplicates. (B) Infectious Jc1 particles or exosomes were incubated with Huh7.5.1 cells in the presence of patient-derived anti–HCV-specific IgGs (n = 8; Table S1) or control IgGs pooled from three healthy controls. Shown are the mean ± SEM of four independent experiments in duplicates.
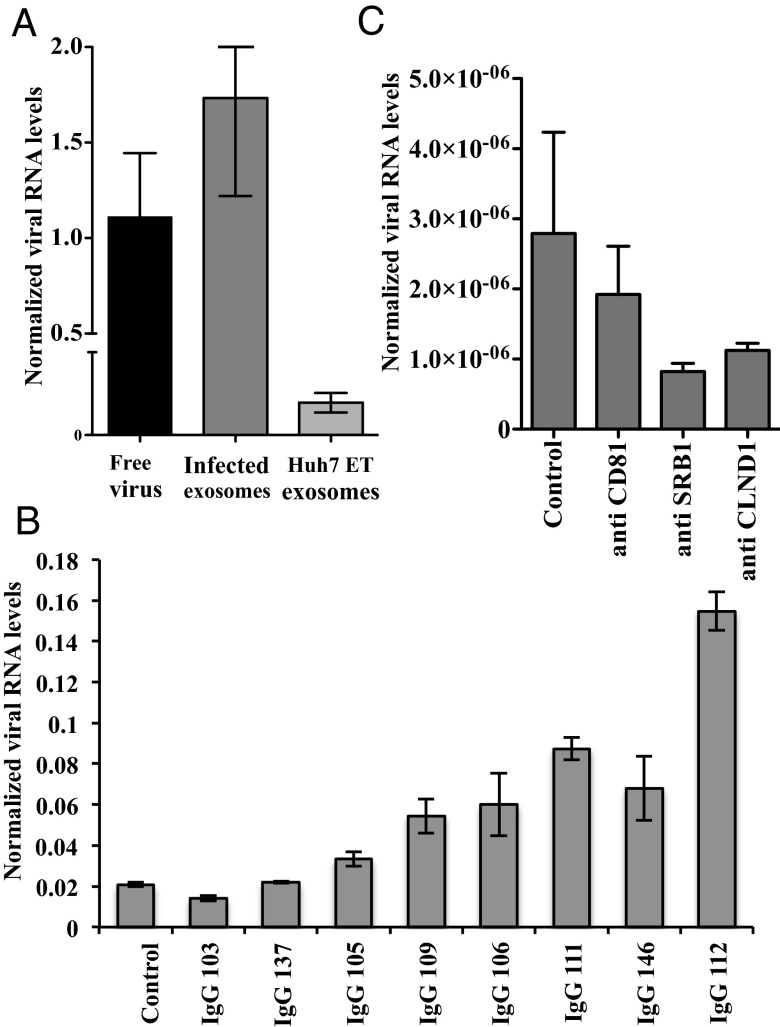
To determine the involvement of the structural proteins in the transmission of viral particles through exosomes, we exposed naive Huh7.5.1 cells to exosomes isolated from HCV subgenomic replicon cells (Huh7-ET). This bistronic HCV replication model lacks the coding sequence for the structural viral proteins E1, E2, and core, which are replaced by the luciferase reporter gene and therefore cannot result in the assembly of infectious virus particles. As shown in Fig. 4A, exosomes from subgenomic replicon cells can transmit viral RNA to naive cells. However, transmission was less efficient than that observed with exosomes derived from Jc1-infected Huh7.5.1 cells. These results indicate that virus could be transmitted by exosomes independent of structural proteins, albeit at a lower level of infection. As shown in Fig. 4B, no neutralization of subgenomic replicon-derived exosomes by HCV-specific IgGs from the eight patients was observed.
Fig. 4.
Transfer of HCV RNA via exosomes independent of structural proteins. (A) Naive Huh7.5.1 cells were incubated with Jc1 containing medium or with exosomes isolated from HCV subgenomic replicon cells (Huh7-ET) or exosomes isolated from Jc1-infected Huh7.5.1 cells. Naive cells exposed to Huh7-ET exosomes have a clearly detectable amount of HCV RNA, but at a lower level than exosomes from Jc1-infected cells. Shown are the mean ± SEM of three independent experiments in triplicates. (B) Huh7-ET–derived exosomes were incubated with Huh7.5.1 cells in the presence of patient-derived anti–HCV-specific IgGs (n = 8; Table S1) or control IgG from healthy controls. No neutralization was observed; rather, enhanced exosome-mediate transmission was seen with some IgGs. Shown is the mean of triplicates ± SEM of one representative experiment of three. (C) Naive Huh7.5.1 cells were blocked with anti-CD81, anti-SRBI, and anti–Caudin-1 antibody and infected with Huh7-ET–derived exosomes. Some inhibition of HCV RNA transfer was observed for all three entry receptors compared with control antibody, but it did not reach statistical significance. Shown is the mean ± SEM of three independent experiments in duplicates.
To study the role of viral entry receptors, CD81, scavenger receptor class B member 1 (SR-BI), and Claudin 1 on exosome-mediated HCV transmission, naive Huh7.5.1 cells were treated with specific rat antisera before adding the Huh7-ET–derived exosomes. After 48 h, RNA levels were detected, as shown in Fig. 4C. Some inhibition of HCV RNA transfer was observed for all three entry receptors, but none reached statistical significance. This suggests that entry receptors may also partly contribute to E1/E2 envelope-independent, exosome uptake. Further research is required to specifically address this situation.
Discussion
Exosomes are established vehicles for the shuttling of proteins, mRNAs, and miRNA between cells (5), and as such, they play an important role in many biological processes (2, 4). Although a role of exosomes in the shuttling of infectious agents between cells has been postulated, this has still not been extensively demonstrated. For HCV, a recent study showed that hepatocyte-derived exosomes containing viral RNA can elicit IFN-α production in plasmacytoid dendritic cells (17). In the current study, we showed that HCV infection can be transmitted by exosomes between hepatocyte-like cells and can establish a productive infection (Fig. 2).
Electron microscopic analysis confirmed the purity of exosomes and indicated the presence of intact E2 envelope protein-positive viral particles inside the vesicles (Fig. 1). This is consistent with the earlier observation that ∼1%–2% of viral particles released from infected cells are actually associated with multivesicular particles that may represent exosomes (20). Moreover, we found that exosome-mediated transmission of HCV was partly resistant to neutralization by antibodies (Fig. 3). This latter finding suggests that the exosome route of HCV transmission is variably inhibited by nAbs. The exosomes derived from Huh7-ET cells were not inhibited by neutralization (Fig. 4B). This is consistent with the fact that neutralizing antibodies are directed against virus envelope proteins, which are lacking in the subgenomic replication model. Surprisingly, several of the IgGs enhanced the transmission of Huh7-ET derived exosomes, but the reason for this effect is unknown. In general, the mechanism of uptake of exosomes by cells is not fully understood. Several uptake pathways have been postulated, including receptor-mediated endocytosis, pinocytosis, and plasma membrane fusion, but none of these pathways has been convincingly established for hepatocytes or other epithelial cells. It is unknown whether HCV entry receptors, SR-BI, CD81, claudin-1, and occludin, are involved in exosome uptake by hepatocytes. The experiments shown in Fig. 4C, however, suggest that entry receptors may partly contribute to exosome uptake even in the absence of viral envelope or core proteins. Indeed, a very recent study on endocytosis of exosomes identified an important role for lipid rafts and caveolins as important factors for uptake (21). Both these pathways are also known to be involved in virus uptake, and lipid rafts support HCV replication (22, 23). As a matter of fact, HCV entry receptors CD81 and SR-BI are known to localize in lipid rafts (24, 25), supporting a hypothetical role of these receptors in exosome uptake by hepatocytes. Further research is required to determine different pathways involved in the uptake of exosomes and HCV virions.
There are several possible reasons why the exosome-mediated transmission of HCV was not completely resistant to neutralizing antibodies. First, although free virus particles were not observed under EM, this does not fully rule out the presence of free virus particles in the isolated exosome samples. The density of HCV in sucrose gradients has been measured at between 1.08 and 1.11 g/mL, which is very close to the buoyant density of exosomes (between 1.11 and 1.21 g/mL) (2, 5). Second, viral envelope proteins may be packaged into the outer membrane of exosomes, making the exosomes susceptible to neutralization. No evidence for this scenario was found in our immuno EM (Fig. 1), and it is unlikely, as it would require different posttranslational modifications of envelope between free virus and exosomes. Mass spectrometric analysis of exosomes only detected HCV core protein, but it may lack the sensitivity necessary to detect low quantities of envelope proteins. This analysis did confirm the presence of various proteins known to be exosome markers in exosome preparations of infected or uninfected Huh7.5.1 cells (Fig. 1).
In conclusion, although two previous studies have shown the association of HCV virus with exosomes, in the present study we demonstrate that exosomes can shuttle virus to hepatocyte-like cells and establish a productive infection. Indeed, Sanger sequencing confirmed that hepatocyte-derived exosomes contained full-length HCV genomes. Taken together, these data suggest that viral transmission through exosomes contributes to the known immune evasive properties of the virus.
Materials and Methods
Cell Culture and Isolation of Exosomes.
The following human hepatoma cell lines were used: Huh7.5.1 and Huh7-ET, containing an HCV subgenomic replicon linked to a luciferase reporter gene. Cells were cultured in Dulbecco’s Eagle’s medium (Invitrogen-Gibco) supplemented with 10% (vol/vol) FCS (HyClone), 100 IU/mL penicillin, 100 mg/mL streptomycin, and 2 mM l-glutamine (Invitrogen-Gibco) to 80% confluency, as previously described (26). Huh7.5.1 cells were primarily infected with HCV strain Jc1 genotype 2a or cell culture-derived HCV Luc-Jc1, and medium was refreshed after 24 h after inoculation. Before exosome isolation, Huh 7-ET or infected Huh7.5.1 cells were cultured for 48 h. Exosomes were isolated as described before (27). In brief, cell culture supernatants were subjected to successive centrifugations of 3,042 × g (20 min) and 10,000 × g (30 min). Exosomes were then pelleted at 64,000 × g for 110 min, using an SW28 rotor (Beckman Coulter Instruments). Exosome pellets were resuspended in 0.32 M sucrose and centrifuged at 100,000 × g for 1 h (SW60Ti rotor; Beckman Coulter Instruments). The exosome pellet was then resuspended in PBS.
Electron Microscopy of Isolated Exosomes.
Exosomes from Huh7.5.1 and Huh7.5.1 Jc1 obtained after ultracentrifugation of cell culture supernatants were resuspended in 10 µL PBS and spotted onto Formvarcoated grids (200 mesh). Adsorbed exosomes were fixed in 2% (vol/vol) paraformaldehyde for 5 min at room temperature. After fixation, the exosomes were either directly negatively stained using uranyl acetate or immunolabeled with antibody C1 (Scripps Research Institute) against E2 protein of HCV. Antigen–antibody complexes were visualized with protein A conjugated with 6-nm colloidal gold particles (1:20 dilution; Aurion). Omitting the primary antibody tested the specificity of the labeling procedure. Grids were examined by a Philips CM100 electron microscope at 80 kV.
Mass Spectrometry Data Analysis.
One-dimensional SDS/PAGE gel lanes were cut into 2-mm slices, using an automatic gel slicer and subjected to in-gel reduction with DTT, alkylation with iodoacetamide, and digestion with trypsin (Promega, sequencing grade), essentially as described by Wilm et al. (28). Nanoflow LC-MS/MS was performed on an 1100 series capillary liquid chromatography system (Agilent Technologies) coupled to an LTQ-Orbitrap mass spectrometer (Thermo) operating in positive mode and equipped with a nanospray source. Peptide mixtures were trapped on a ReproSil C18 reversed phase column (Dr. Maisch GmbH; column dimensions 1.5 cm × 100 µm, packed in-house) at a flow rate of 8 µL/min. Peptide separation was performed on ReproSil C18 reversed phase column (Dr. Maisch GmbH; column dimensions 15 cm × 50 µm, packed in-house), using a linear gradient from 0% to 80% B [A = 0.1% formic acid; B = 80% (vol/vol) acetonitrile, 0.1% formic acid] in 70 min and at a constant flow rate of 200 nL/min, using a splitter. The column eluent was directly sprayed into the electrospray ionization source of the mass spectrometer. Mass spectra were acquired in continuum mode; fragmentation of the peptides was performed in data-dependent mode. Peak lists were automatically created from raw data files, using the Mascot Distiller software (version 2.3; MatrixScience). The Mascot search algorithm (version 2.2, MatrixScience) was used for searching against a customized database containing all IPI_human protein sequences (release 2010_09) plus all HCV virus protein sequences from Uniprot (release 2010_09). The peptide tolerance was typically set to 10 ppm, and the fragment ion tolerance was set to 0.8 Da. A maximum number of 2 missed cleavages by trypsin were allowed, and carbamidomethylated cysteine and oxidized methionine were set as fixed and variable modifications, respectively. The Mascot score cutoff value for a positive protein hit was set to 65. Typical contaminants were omitted from analyses and individual peptide MS/MS spectra with Mascot scores below 40 were checked manually and either interpreted as valid identifications or discarded.
Real-Time Confocal Imaging.
For imaging the cellular uptake of labeled exosomes, Huh7.5.1 cells were seeded on 24-mm-diameter coverslips. Imaging was performed using a confocal laser-scanning microscope (LSM510; Carl Zeiss MicroImaging, Inc.) equipped with a Plan-Neofluar 40×/1.3 NA oil objective (Carl Zeiss MicroImaging, Inc.) and a heated stage. A 543-nm HeNe laser was used for the excitation of rhodamine. At the same time, a transmitted light image was made. The cells were kept at 37 °C and scanned continuously (zoom, 2×, averaging 8×) for 30 min.
Transmission of Infection via Exosomes.
Naive Huh7.5.1 cells were infected with exosomes isolated from Huh7.5.1 cells infected with HCVcc strain Jc1 genotype 2a or from Huh7-ET. Conditioned medium (CM) containing free virus from the same cell culture was used as positive control for Jc1 virus. The exosomes and free virus batches were normalized on the basis of the HCV viral genome content, as determined by quantitative RT-PCR. Twenty-four hours after the addition of samples to naive cells, the medium was refreshed. The cells were analyzed at day 5 postinfection by quantitative RT-PCR and at day 2 for immunohistochemistry. Huh7.5.1 cells exposed to Huh7-ET exosomes were analyzed at day 2 by quantitative RT-PCR.
Antibody-Mediated Neutralization of HCV.
Naive Huh7.5.1 cells were infected with exosomes isolated from Huh7.5.1 cells infected with HCV strain Jc1 and HCV Luc-Jc1. CM containing free virus from the same cell culture was used as positive control. Naïve Huh7.5.1 cells were also exposed to Huh7-ET exosomes. Nine different purified IgGs (100 μg/mL) from serum from chronically infected patients with HCV (approval from the Strasbourg University Hospital institutional review board (ClinicalTrial.gov identifier NCT00638144) were obtained. Three purified control IgGs derived from anti–HCV-negative individuals were used as control. nAbs were added to the Jc1/Luc-Jc1–infected exosomes and Jc1/Luc-Jc1 CM 1 to 2 h before infection. nAbs were also added to exosomes isolated from Huh7-ET cells. The cells infected with HCV Jc1 were lysed 5 d after infection and quantified for viral infection by real-time RT-PCR. For cells infected with HCV Luc-Jc1, luciferase activity was measured 3 d after infection. For cells exposed to Huh7-ET exosomes, cells were lysed 2 d later and quantified for viral RNA by real-time RT-PCR. Informed consent was obtained from the patients involved in research.
Inhibition of HCV Entry Receptors CD81, SR-BI, Claudin-1 for Huh7-ET Exosome Transmission.
To study the involvement of HCV entry receptors in exosome route of infection, naive Huh7.5.1 cells were incubated with rat anti-CD81 (1/100), rat anti–Claudin-1 (1/100), rat anti–SR-BI (1/100) serum, and control rat serum (1/100) for 2 h at 37 °C (29). Exosomes isolated from Huh7-ET cultured cells were added to these cells and analyzed after 48 h for viral transmission by RT-PCR.
Immunocytochemistry.
The infectivity assay was performed in an 8-well chamber slide. After treatment with exosomes, the cells were permeabilized, and endogenous peroxidase was blocked with PBS containing 0.3% hydrogen peroxide. The cells were then labeled with an HCV-Core antibody (Affinity Bioreagents; MA1-080; Clone C7-50). Secondary antibody binding and amplification of signal was accomplished with Envision horseradish peroxidize (DAKO Corporation) and then visualized with 3′-amino-ethyl-carbozole (Sigma). Blinded scoring of nine optical fields by two independent observers was performed for quantification at 200-fold magnification.
Viral RNA Isolation and Direct Sanger Sequencing.
Viral RNA was extracted from 140 µL sucrose purified exosome suspension, using the QIAamp viral RNA mini kit (Qiagen), and RNA was eluted with 40 µL buffer AVE, according to the manufacturer's instructions. Ten microliters of this RNA were reverse transcribed with the superscript III first-strand synthesis system (Invitrogen Corp), using HCV specific primer HCV 2a cDNA (5′-GCTCTACCGAGCGGGGAG-3′). Partial NS5b sequences and partial core sequences spanning the region from positions 426–904 and 8322–8713 of the HCV genome (GenBank accession no. AB047639) were amplified by PCR, using HotStart Hifidelity Taq DNA polymerase (Qiagen) with the 2a Core primers (forward, 5′-AGATCGTTGGCGGAGTATAC-3′ and reverse 5′-CGGAACGGTGATGCAGGACA-3′) and 2aNS5b primers (5′-ATGATACCCGATGCTTCGAC-3′ and 5′-AGGGGCAGAGTACCTGGTCA-3′). The PCR cycling conditions were as follows: 95 °C for 5 min, followed by 35 cycles of 95 °C for 30 s, 48 °C for 30 s, 72 °C for 40 s, and a final extension of 72 °C for 10 min. The PCR-amplified products were gel purified using a gel extraction kit (Qiagen) and directly sequenced on both strands, using the BigDye Terminator version 3.1 Cycle sequencing kit on an ABI PRISM 3100 genetic analyzer (Applied Biosystems). The obtained sequences were compared with the reference HCV 2a sequence, and the analysis was performed using the CLC genomics work bench (CLC bio).
Supplementary Material
Acknowledgments
We thank Prof. Takaji Wakita for providing JFH1 and Thomas van Wollegem for helpful discussions. The Erasmus MC Translational Research Fund financially supported this study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221899110/-/DCSupplemental.
References
- 1.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6(3):267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 2.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 3.Conde-Vancells J, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7(12):5157–5166. doi: 10.1021/pr8004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duijvesz D, Luider T, Bangma CH, Jenster G. Exosomes as biomarker treasure chests for prostate cancer. Eur Urol. 2011;59(5):823–831. doi: 10.1016/j.eururo.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Silverman JM, Reiner NE. Exosomes and other microvesicles in infection biology: Organelles with unanticipated phenotypes. Cell Microbiol. 2011;13(1):1–9. doi: 10.1111/j.1462-5822.2010.01537.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110(9):3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth AM, et al. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172(6):923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenassi M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11(1):110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pegtel DM, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107(14):6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496(7445):367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timpe JM, et al. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47(1):17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- 13.Bruggmann P, et al. Swiss Hepatitis C Cohort Study Active intravenous drug use during chronic hepatitis C therapy does not reduce sustained virological response rates in adherent patients. J Viral Hepat. 2008;15(10):747–752. doi: 10.1111/j.1365-2893.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- 14.Fafi-Kremer S, et al. Viral entry and escape from antibody-mediated neutralization influence hepatitis C virus reinfection in liver transplantation. J Exp Med. 2010;207(9):2019–2031. doi: 10.1084/jem.20090766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masciopinto F, et al. Association of hepatitis C virus envelope proteins with exosomes. Eur J Immunol. 2004;34(10):2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- 16.Tamai K, et al. Regulation of hepatitis C virus secretion by the Hrs-dependent exosomal pathway. Virology. 2012;422(2):377–385. doi: 10.1016/j.virol.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Dreux M, et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Q, et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2012;61(9):1330–1339. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 19.Fofana I, et al. Mutations that alter use of hepatitis C virus cell entry factors mediate escape from neutralizing antibodies. Gastroenterology. 2012;143(1):223–233.e9. doi: 10.1053/j.gastro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gastaminza P, et al. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 2010;84(21):10999–11009. doi: 10.1128/JVI.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svensson KJ, et al. Exosome Uptake Depends on ERK1/2-Heat Shock Protein 27 Signaling and Lipid Raft-mediated Endocytosis Negatively Regulated by Caveolin-1. J Biol Chem. 2013;288(24):17713–17724. doi: 10.1074/jbc.M112.445403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matto M, Rice CM, Aroeti B, Glenn JS. Hepatitis C virus core protein associates with detergent-resistant membranes distinct from classical plasma membrane rafts. J Virol. 2004;78(21):12047–12053. doi: 10.1128/JVI.78.21.12047-12053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi ST, Lee KJ, Aizaki H, Hwang SB, Lai MM. Hepatitis C virus RNA replication occurs on a detergent-resistant membrane that cofractionates with caveolin-2. J Virol. 2003;77(7):4160–4168. doi: 10.1128/JVI.77.7.4160-4168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soldaini E, et al. T cell costimulation by the hepatitis C virus envelope protein E2 binding to CD81 is mediated by Lck. Eur J Immunol. 2003;33(2):455–464. doi: 10.1002/immu.200310021. [DOI] [PubMed] [Google Scholar]
- 25.Rhainds D, et al. Localization and regulation of SR-BI in membrane rafts of HepG2 cells. J Cell Sci. 2004;117(Pt 15):3095–3105. doi: 10.1242/jcs.01182. [DOI] [PubMed] [Google Scholar]
- 26.Pan Q, et al. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55(6):1673–1683. doi: 10.1002/hep.25562. [DOI] [PubMed] [Google Scholar]
- 27.Jansen FH, et al. Exosomal secretion of cytoplasmic prostate cancer xenograft-derived proteins. Mol Cell Proteomics. 2009;8(6):1192–1205. doi: 10.1074/mcp.M800443-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilm M, et al. Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature. 1996;379(6564):466–469. doi: 10.1038/379466a0. [DOI] [PubMed] [Google Scholar]
- 29.Krieger SE, et al. Inhibition of hepatitis C virus infection by anti-claudin-1 antibodies is mediated by neutralization of E2-CD81-claudin-1 associations. Hepatology. 2010;51(4):1144–1157. doi: 10.1002/hep.23445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.