Abstract
Alcohol-related acute pancreatitis can be mediated by a combination of alcohol and fatty acids (fatty acid ethyl esters) and is initiated by a sustained elevation of the Ca2+ concentration inside pancreatic acinar cells ([Ca2+]i), due to excessive release of Ca2+ stored inside the cells followed by Ca2+ entry from the interstitial fluid. The sustained [Ca2+]i elevation activates intracellular digestive proenzymes resulting in necrosis and inflammation. We tested the hypothesis that pharmacological blockade of store-operated or Ca2+ release-activated Ca2+ channels (CRAC) would prevent sustained elevation of [Ca2+]i and therefore protease activation and necrosis. In isolated mouse pancreatic acinar cells, CRAC channels were activated by blocking Ca2+ ATPase pumps in the endoplasmic reticulum with thapsigargin in the absence of external Ca2+. Ca2+ entry then occurred upon admission of Ca2+ to the extracellular solution. The CRAC channel blocker developed by GlaxoSmithKline, GSK-7975A, inhibited store-operated Ca2+ entry in a concentration-dependent manner within the range of 1 to 50 μM (IC50 = 3.4 μM), but had little or no effect on the physiological Ca2+ spiking evoked by acetylcholine or cholecystokinin. Palmitoleic acid ethyl ester (100 μM), an important mediator of alcohol-related pancreatitis, evoked a sustained elevation of [Ca2+]i, which was markedly reduced by CRAC blockade. Importantly, the palmitoleic acid ethyl ester-induced trypsin and protease activity as well as necrosis were almost abolished by blocking CRAC channels. There is currently no specific treatment of pancreatitis, but our data show that pharmacological CRAC blockade is highly effective against toxic [Ca2+]i elevation, necrosis, and trypsin/protease activity and therefore has potential to effectively treat pancreatitis.
Keywords: capacitative Ca2+ entry, alcohol metabolite, pancreas, hepatocyte Ca2+ entry, AR42J
Acute pancreatitis is a human disease mostly caused by alcohol abuse or complications from biliary disease. In this disease, against which there is currently no effective therapy, digestive proenzymes are prematurely activated inside the acinar cells leading to autodigestion and necrosis (1–3). Intracellular Ca2+ plays a critical role in the initiation of this disease process (2–4), but intracellular Ca2+ also plays a critical role in the physiological regulation of the normal exocytotic secretion of the digestive proenzymes (5).
The pancreatic acinar cells are capable of generating multiple patterns of cytosolic Ca2+ signals depending on the type and concentration of the stimulating agent (5). The physiological Ca2+ signals regulating secretion—evoked by the neurotransmitter acetylcholine (ACh) or the hormone cholecystokinin (CCK)—consist of repetitive short-lasting rises in the cytosolic Ca2+ concentration ([Ca2+]i). These are mostly confined to the apical area, in which the secretory (zymogen) granules (ZGs) are concentrated, by a belt of perigranular mitochondria operating as a firewall against the globalization of the Ca2+ signals (6). Rapid Ca2+ uptake into the perigranular mitochondria following local cytosolic Ca2+ signals also plays a crucial role in activating local mitochondrial ATP production, which is essential for the exocytotic secretion process (5–9). At supraphysiological concentrations of ACh or CCK, or in response to various types of pathological stimulants, sustained global elevations of [Ca2+]i occur (2, 4). Such signals initiate trypsinogen activation in the apical region of the pancreatic acinar cells as well as vacuole formation (10, 11). Both the trypsinogen activation and the intracellular vacuolization can be prevented by intracellular Ca2+ chelation or simply by removal of extracellular Ca2+ (10).
Unlike nerve and endocrine as well as muscle cells, exocrine cells do not possess voltage-gated Ca2+ channels and the cytosolic Ca2+ signals governing pancreatic acinar secretion are primarily generated by release of Ca2+ from intracellular stores, principally the endoplasmic reticulum (ER) (5, 12, 13). However, the intracellular Ca2+ stores are by definition finite. Ca2+ATPase pumps in the plasma membrane are activated to increase extrusion of Ca2+ whenever [Ca2+]i increases and therefore pancreatic acinar cells would run out of Ca2+ in the ER if there were not a mechanism of compensatory Ca2+ uptake from the external solution (5). This uptake mechanism is known as store-operated or Ca2+ release-activated Ca2+ entry (CRAC) and CRAC channels in the plasma membrane have generally been well characterized (14). The molecular nature of these channels (Orai1) is now also known (15) and the link between Ca2+ depletion of the ER and opening of the CRAC channels has been established: A reduction in the Ca2+ concentration in the ER ([Ca2+]ER) causes translocation of a Ca2+-sensing protein, called STIM1, widely distributed in the ER membrane to so-called puncta in the ER close to the plasma membrane, where it can interact with and open Orai1 channels (16–18). This mechanism also operates in the pancreatic acinar cells, because stimulant-elicited release of Ca2+ from the ER results in translocation of STIM1 to puncta close to the basolateral plasma membrane, specifically at locations where the ER is devoid of ribosomes and where interaction between STIM1 and Orai1 therefore occurs (19). Despite this, electrophysiological investigations of the currents evoked by Ca2+ store depletion have so far failed to provide evidence for the existence in pancreatic acinar cells of Ca2+-selective currents of the CRAC type (20–22) originally discovered in mast cells (23), although activation of nonselective currents were shown (21, 22). Therefore, although the linkage between agonist-evoked Ca2+ release from the ER and store-operated Ca2+ entry, through STIM1 and Orai1, is established for the pancreatic acinar cells (19), there is uncertainty about the biophysical nature of the principal Ca2+ entry channels. Endocytic Ca2+ uptake (24) could also be important and does occur in pancreatic acinar cells (25). It is also the case that there are important intracellular Ca2+ stores (acid) outside the ER (4, 26–29). Ca2+ release from acid stores—located in ZGs, endosomes, and lysosomes—plays a crucial role in intracellular trypsinogen activation elicited by alcohol and fatty acid ethyl esters in experiments on permeabilized pancreatic acinar cells (28, 29), and trypsinogen activation induced by hyperstimulation occurs in postexocytotic endocytic (and acid) structures (25). In view of the complexities of pancreatic acinar cell Ca2+ signaling in physiology and pathology and, in particular, the uncertainty about the nature of the Ca2+ entry pathways, it is unknown whether blockade of Ca2+-selective CRAC channels could in principle be effective as therapy against acute pancreatitis. With the availability of relatively specific CRAC channel blockers (30–34) there is now an opportunity to test the hypothesis that one type of Ca2+ entry channel is so important that pharmacological blockade could afford effective protection against toxic Ca2+ signal generation and its consequences in intact pancreatic acinar cells. Given the current absence of any specific therapy against pancreatitis, a demonstration that pharmacological blockade of CRAC channels could prevent the dangerous necrosis evoked by agents known to initiate pancreatitis, would be a proof of principle that a specific therapy could be developed. In this study we have therefore tested the effect of the CRAC channel blocker developed by GlaxoSmithKline, GSK-7975A (33, 34) on store-operated Ca2+ entry, induced by inhibiting specifically the Ca2+ATPase pumps in the ER, as well as on Ca2+ signal generation elicited by palmitoleic acid ethyl ester (POAEE), one of the mediators of alcohol-related pancreatitis (26, 35–38). As part of this investigation we also provide a characterization of Ca2+-selective CRAC currents in pancreatic acinar cells. We show that pharmacological CRAC channel blockade prevents the sustained toxic elevation of [Ca2+]i, which is induced by severe depletion of intracellular Ca2+ stores or by POAEE. In contrast, such a blockade has little effect on the physiological Ca2+ spiking (oscillations) evoked by ACh or CCK and has only minor or no effects on Ca2+ entry in hepatocytes and the pancreatic acinar cell line AR42J. Importantly, the CRAC blocker is also effective against the intracellular protease activation and necrosis induced by POAEE. We conclude that pharmacological CRAC blockade has potential for effective treatment of acute pancreatitis.
Results
GSK-7975A Blocks Store-Operated Ca2+ Entry in Normal Pancreatic Acinar Cells.
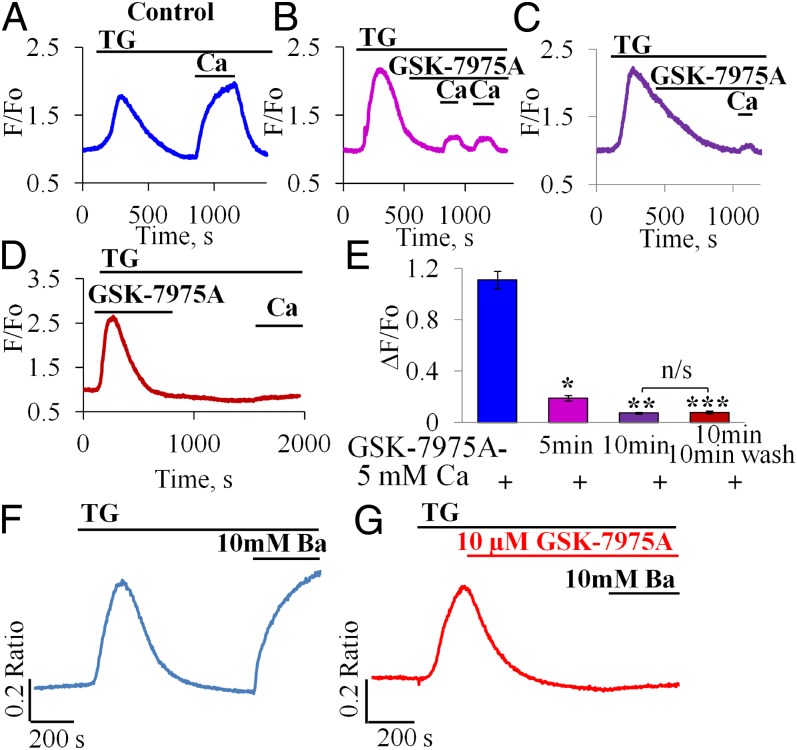
Our standard protocol for store-operated Ca2+ entry investigations consisted of first emptying the ER store of Ca2+ by applying the specific ER Ca2+ pump blocker thapsigargin (TG) in the absence of external Ca2+. This caused a transient rise in [Ca2+]i (Fig. 1A). After [Ca2+]i had returned to near its normal resting level, Ca2+ was admitted to the external solution and this resulted in a substantial rise in [Ca2+]i, which was sustained as long as Ca2+ was present outside the cell (Fig. 1A). When the CRAC blocker GSK-7975A was applied before the admission of Ca2+ to the external solution, the subsequent rise in [Ca2+]i was markedly reduced (Fig. 1 B–D). As seen in Fig. 1D, the effect of GSK-7975A was maintained after washout of the compound at least for the first 10–15 min. As summarized in Fig. 1E, the inhibitory effect was already marked 5 min after start of exposure to GSK-7975A and after 10 min exposure there was very little Ca2+ entry. Ten minutes after the start of washout of GSK-7975A, there was still no recovery of Ca2+ entry (Fig. 1E). Importantly, GSK-7975A had no influence on the thapsigargin-evoked Ca2+ mobilization from the ER store (Fig. 1D). GSK-7975A also had little or no effect on the Ca2+ spiking responses to low (physiological) concentrations of the normal stimulants of pancreatic secretion, namely ACh (50 and 100 nM) or CCK (5 pM) (Fig. S1 A–F) but reduced the late elevated [Ca2+]i plateau phase following stimulation with a high concentration of ACh (1 μM) (Fig. S1 G and H).
Fig. 1.
CRAC channel blocker inhibits Ca2+ and Ba2+ influx. (A–C) Effects of different periods of incubation [5 min, n = 5 (B) and 10 min, n = 14 (C)] of pancreatic acinar cells with GSK-7975A (10 μM) compared with control (A, n = 5) (15 min incubation did not significantly further increase the degree of blockade, n = 3). (D) Washout of GSK-7975A did not result in recovery of the Ca2+ signal within ∼10 min. (E) Summary of results shown in A–D. Mean [Ca2+]i amplitude change (ΔF/F0) due to Ca2+ influx (n = 5, example trace in A) was dramatically reduced after 5 min with GSK-7975A (n = 14, *P < 10−6, example trace in B) and reduced further after 10 min with GSK-7975A (n = 14, **P < 10−11, example in C). Inhibition was effectively irreversible (after washing out for 10 min) as amplitude remained very low (n = 7, ***P < 10−8, example trace in D). As seen in E, there was no significant difference in averaged amplitudes from experiments of the type shown in C and D (P > 0.69); whereas the averaged amplitudes from the type of experiments shown in B and C are significantly different (P < 10−6). Data presented as mean ± SEM (F and G) GSK-7975A inhibits Ba2+ influx in pancreatic acinar cells. Representative traces of changes in [Ba2+]i (using Fura-2) due to Ba2+ influx in cells exposed to GSK-7975A (10 μM) for 10 min (G) compared with control untreated cells (F).
Inhibition of Unidirectional Ba2+ Influx.
Net Ca2+ transport across the plasma membrane depends not only on Ca2+ influx, but also on Ca2+ extrusion and in the pancreatic acinar cells, extrusion is mediated exclusively by the plasma membrane Ca2+ pump, activated by a rise in [Ca2+]i (39). To assess changes in unidirectional Ca2+ influx, we used measurements of [Ba2+]i, because Ba2+ can easily pass through CRAC channels (40), but cannot be extruded by the plasma membrane Ca2+ pump (39, 40). As seen in Fig. 1 F and G, preincubation with GSK-7975A for 10 min markedly reduced store-operated Ba2+ entry into the pancreatic acinar cells. Our data on the inhibition of Ba2+ entry by the CRAC blocker are summarized in Fig. S1 I–K.
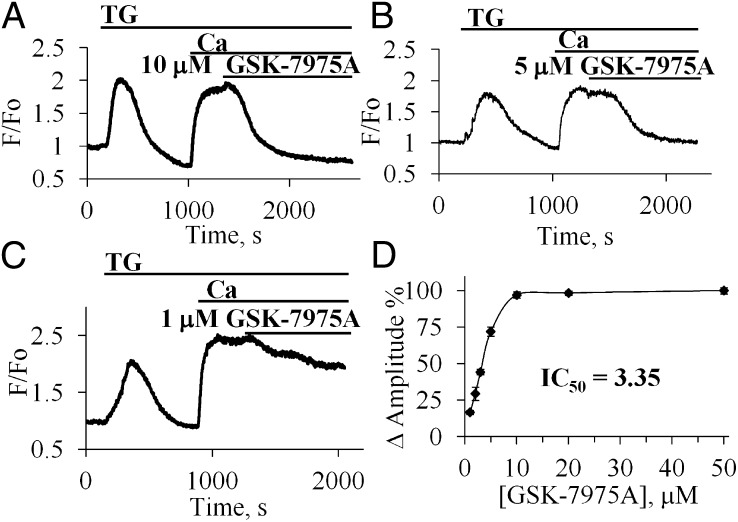
Concentration Dependence of the Acute Effects of GSK-7975A on Ca2+ Entry.
We also investigated the acute effect of the CRAC blocking agent. As seen in Fig. 2A, application of 10 μM GSK-7975A soon after admission of Ca2+ to the external solution resulted, after a delay of a few minutes, in a sharp reduction of [Ca2+]i which, in the continued presence of external Ca2+, fell to near the normal resting level within 5–10 min. The relatively slow time course of the GSK-7975A–induced reduction in [Ca2+]i is not necessarily a reflection of a slow action of the CRAC blocker, but may reflect the time it takes for the plasma membrane Ca2+ pump to extrude the excess of Ca2+ in the cytosol. We also tested the effects of different CRAC blocker concentrations. As seen in Fig. 2 A and B, the effects of 5 and 10 μM GSK-7975A were very similar, indicating that the maximal effect had already been attained at 5 μM. A small reduction in [Ca2+]i was seen at 1 μM (Fig. 2C). Fig. 2D shows the relationship between CRAC blocker concentration and the degree of reduction in [Ca2+]i. IC50 was calculated to be 3.4 μM. The estimated t1/2 was 292.8 ± 88.3 s. Even the highest concentration of GSK-7975A tested (50 μM) did not abolish Ca2+ entry into pancreatic acinar cells, at the external [Ca2+] ([Ca2+]o) of 5 mM, but dramatically inhibited it by 87.6 ± 1.9% (n = 10).
Fig. 2.
Concentration dependence of the inhibitory effect of GSK-7975A on the elevated [Ca2+]i following readmission of external Ca2+ after thapsigargin treatment. (A–C) Effects of acute application of GSK-7975A in different concentrations [10 μM (A), 5 μM (B), 1 μM (C)] on the elevated [Ca2+]i plateau in the presence of 5 mM CaCl2. (D) Summary of the results of the experiments on the concentration dependence of the inhibitory effect of GSK-7975A normalized to the inhibitory effect of 50 μM (100%).
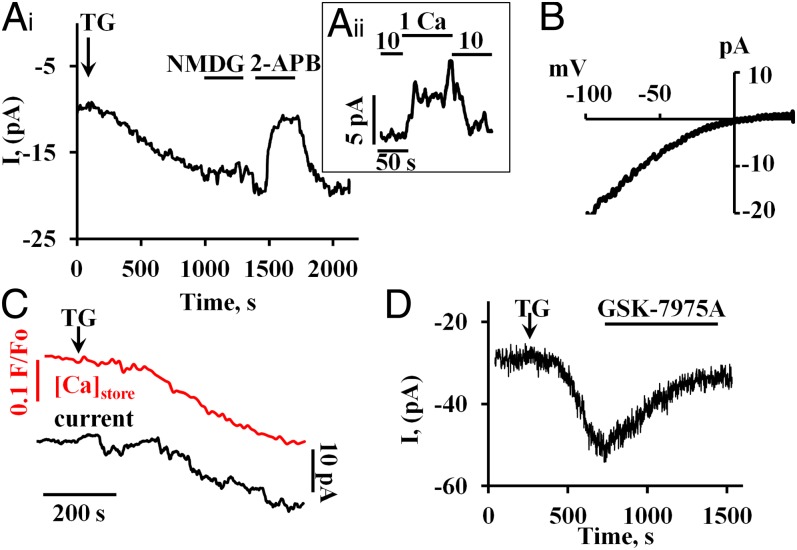
Inhibition of Store-Operated Ca2+ Current.
Application of 2 μM TG activated a slowly developing inward membrane current (Fig. 3 A, i) with a maximal amplitude of 8.4 ± 0.6 рА (n = 35, or 0.349 pA/pF; Cm = 22.3 ± 1.7 pF) at a membrane potential of −50 mV and [Ca2+]o = 10 mM. Replacement of extracellular Na+ by N-methyl-D-glucamine (NMDG+) had little or no effect on the current (Fig. 3 A, i, n = 8), whereas a reduction in [Ca2+]o from 10 to 1 mM caused a marked decrease in the maximally developed inward current (n = 7) (Fig. 3 A, ii). The store-operated inward current was blocked by 100 μM 2-Aminoethoxydiphenyl borate (2-APB) (n = 7) (Fig. 3 A, i), a well-known (but not specific) blocker of CRAC channels (14). The current–voltage relationship showed clear inward rectification (Fig. 3B). We recorded the store-operated Ca2+ current in experiments in which we also monitored changes in the Ca2+ concentration inside the stores (41). The TG-induced inward current developed with a delay after the reduction in the store Ca2+ concentration (Fig. 3C) (n = 9). GSK-7975 (10 μM) evoked a marked reduction (by 83.0 ± 4.1%) in the TG-induced inward current in each of the six experiments carried out (Fig. 3D).
Fig. 3.
Store-operated ionic currents, developing after thapsigargin treatment, recorded with the whole cell patch clamp configuration. The individual traces shown were all recorded at a holding potential of −50 mV and with an external [Ca2+] of 10 mM. To prevent activation of the large Ca2+-dependent ion currents in acinar cells (13), patch clamp pipettes were filled with a solution containing a mixture of 10 mM BAPTA and 2 mM Ca2+. (A, i) Inward current induced by bath application of 2 μM TG to empty the ER Ca2+ content. Replacing Na+ with NMDG+ had little effect on the inward current, but 100 μM of 2-APB practically abolished the current. This effect was rapidly reversible. (A, ii) Reducing the external Ca2+ concentration from 10 to 1 mM (replacement of CaCl2 by MgCl2) reduced reversibly the stable maximal plateau amplitude of the inward current during TG exposure. (B) Representative I/V curve obtained using a voltage ramp protocol (0.4 V/s) from −100 mV to 40 mV (difference between ramp registration before and after 2-APB application). (C) Simultaneous measurements of changes in the intrastore [Ca2+] and the membrane current following TG application. The Upper red trace shows the gradual reduction of the intrastore Ca2+ concentration recorded by changes of Fluo-5N fluorescence. The Lower black trace shows the development of the inward current. (D) GSK-7975 (10 μM) inhibits markedly the inward current evoked by application of 2 μM TG.
The Effect of Blocking CRAC Channels on [Ca2+]i Changes Evoked by Palmitoleic Acid Ethyl Ester.
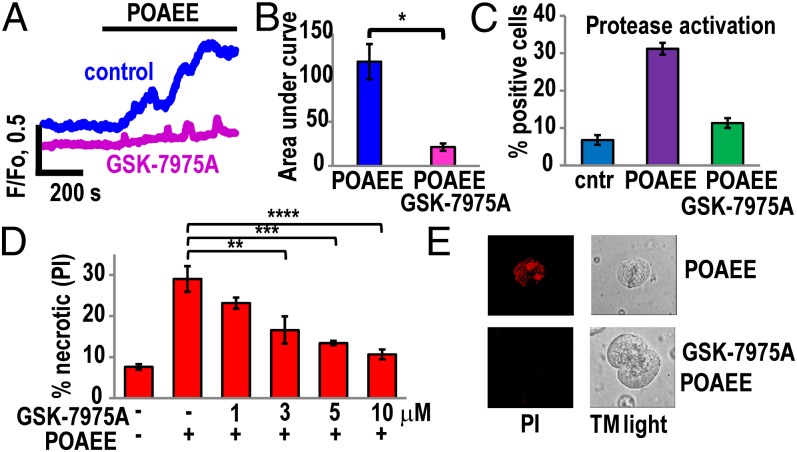
Elevation of [Ca2+]i in pancreatic acinar cells can be induced by either alcohol or fatty acids (42, 43), but in combination—as fatty acid ethyl esters—they are much more powerful Ca2+ releasers (37) and are recognized as important mediators of alcohol-induced pancreatitis (35–38, 42). We therefore tested the effects of blocking CRAC channels on the changes in [Ca2+]i induced by palmitoleic acid ethyl ester (POAEE). As previously shown, POAEE evokes substantial and sustained elevations of [Ca2+]i in normal isolated pancreatic acinar cells, initiated by release of Ca2+ from the ER (37, 42) as well as acid stores (28). Fig. 4A shows the substantial and sustained rise in [Ca2+]i elicited by 100 μM POAEE as well as the much diminished cytosolic Ca2+ signal evoked by the same concentration of POAEE in the presence of GSK-7975A. Fig. 4B shows the mean values of all of the data from this series of experiments. It is clear that GSK-7975A very markedly inhibits the [Ca2+]i elevation normally evoked by 100 μM POAEE.
Fig. 4.
GSK-7975A dramatically reduces Ca2+ overload and necrosis induced by the fatty acid ethyl ester POAEE. (A) Representative traces of changes in [Ca2+]i in response to 100 μM POAEE in the absence (n = 8) and presence of GSK-7975A (10 μM) (n = 12). (B) Quantitative analysis of experiments of the type shown in A by comparing the integrated [Ca2+]i elevation above the baseline (area under the curve) recorded during the first 10 min of POAEE application. Blue bar represents the control (without GSK-7975A), whereas the red bar represents the test results (cells pretreated with 10 μM GSK-7975A for 10 min before application of POAEE). The mean values (±SEM) are significantly different, P < 0.0002. (C) Pretreatment of cells with 10 μM GSK-7975A for 10 min inhibited by 64% (green bar) protease activation induced by POAEE (100 μM) (purple bar) as measured with generic protease substrate bis-l-aspartic acid amide rhodamine 110 (D2-R110). Blue bar represents the results from control (not exposed to POAEE) cells. The mean values (±SEM) in control and GSK-7975A plus POAEE treatment are not significantly different, P = 0.05; n = 4, >200 cells in each group. (D) POAEE (100 μM)-induced necrosis was dramatically reduced in cells treated with 1 μM, 3 μM, 5 μM, and 10 μM GSK-7975A for 10 min. In the control series of experiments (no POAEE treatment), the level of necrosis was low (n = 3 series of experiments with number of tested cells in each group >350). Inhibition of necrosis was significant for 3 μM GSK-7975A (**P < 0.02) and highly significant for 5 and 10 μM GSK-7975A (***P < 0.003 and ****P < 0.001). (E) Necrosis was visualized by staining cells with propidium iodide (PI). All experiments were performed in the presence of 1 mM CaCl2.
Inhibition of CRAC Channels Protects Against POAEE-Induced Trypsin and Protease Activation.
It has been shown previously that hyperstimulation-induced trypsinogen activation and vacuolization can be prevented by intracellular Ca2+ chelation or simply by removal of extracellular Ca2+ (10). Preincubation (1 h) of pancreatic acinar cells with POAEE (100 μM) induced a substantial increase of protease activity (from 6.8 ± 1.3% of cells in control to 31.2 ± 1.6% of cells, Fig. 4C). Pretreatment of cells with 10 μM GSK-7975A for 10 min before POAEE application reduced protease activity to levels relatively close to control (Fig. 4C). Similar levels of inhibition by GSK-7975A were observed in experiments assessing trypsin activity, using the specific trypsin substrate BZIPAR (10, 28, 29). Preincubation of pancreatic acinar cells with POAEE (100 μM for 1 h) induced substantial increase of trypsin activity (from 3.4 ± 0.5% of cells in control to 15 ± 1.1% in the POAEE-treated groups). Pretreatment of cells with GSK-7975A (10 μM for 10 min) before POAEE application substantially reduced trypsinogen activation (6.9 ± 0.7% of cells, P < 0.0009, n = 4 series of experiments with number of cells >150 in each group).
Blockade of CRAC Channels Protects Against POAEE-Elicited Necrosis.
It has previously been shown that POAEE elicits Ca2+-dependent necrosis of pancreatic acinar cells (37, 42). Because GSK-7975A protects against the POAEE-induced [Ca2+]i rise, as well as against protease activation, we tested whether it would also protect against necrosis. As seen in Fig. 4 D and E, GSK-7975A did indeed provide a concentration-dependent protection against the development of POAEE-induced necrosis. There was a protective effect already at 3 μM and at 10 μM the percentage of necrotic cells was only slightly higher than the control value (without POAEE) (Fig. 4D).
GSK-7975A Protects Against Necrosis Solely by Inhibiting Ca2+ Entry.
Although GSK-7975A inhibits markedly both Ca2+ entry and necrosis, it is theoretically possible that the protection against necrosis could be due to an effect other than on Ca2+ entry. To test this point, we exploited the ability of 2-APB—a well-known CRAC blocker (14) (Fig. 3)—to also elicit cytosolic Ca2+ signals in pancreatic acinar cells (44). At 100 μM, 2-APB induced substantial rises in [Ca2+]i and also induced marked necrosis (Fig. S2), but in this situation (where the CRAC channels are already blocked by 2-APB) GSK-7975A was unable to inhibit necrosis (Fig. S2B). We also carried out an experiment in which we counteracted the inhibitory effect of GSK-7975A by increasing [Ca2+]o (Fig. S3) and tested the effect on necrosis. At [Ca2+]o = 1 mM, POAEE evokes a significant increase in [Ca2+]i (Fig. 4A) and a substantial increase in the percentage of necrotic cells (Fig. 4D), which is markedly inhibited by GSK-7975A (Fig. 4D). Raising [Ca2+]o to 10 mM in the presence of inhibitor restored the POAEE-induced [Ca2+]i elevation (Fig. S3A) and increased the POAEE-induced rise in the percentage of necrotic cells to the level seen without GSK-7975A at [Ca2+]o = 1 mM (Fig. S3B). These experiments (Figs. S2 and S3) indicate that the protective effect of GSK-7975A against necrosis is solely due to inhibition of Ca2+ entry.
The Effects of Blocking CRAC Channels on [Ca2+]i Changes Evoked by Acetylcholine.
We tested whether CRAC blockade would also prevent the normal cytosolic Ca2+ spiking, elicited by ACh or CCK. As seen in Fig. S1, a low ACh concentration (50 or 100 nM) evoked repetitive [Ca2+]i spikes and this spike generation was hardly affected, at least for the first ∼15 min, by application of 10 μM GSK-7975A (Fig. S1 A, B, and D–F). Likewise, the Ca2+ spiking evoked by 5 pM CCK was only affected to a minor degree by CRAC blockade (Fig. S1C). A high ACh concentration (1 μM) evoked a large initial rise in [Ca2+]i, followed by a slow decline toward a sustained quasiplateau at a slightly elevated [Ca2+]i (Fig. S1G). In this case, blockade of CRAC channels markedly reduced or even eliminated the sustained plateau phase and during continued exposure to the high (1 μM) ACh concentration, [Ca2+]i returned to the prestimulation resting level (Fig. S1 G and H). Because CRAC blockade markedly reduced the sustained phase of Ca2+ entry, during prolonged supramaximal stimulation, we tested to what an extent this would have an effect on the intracellular Ca2+ stores. A 1-h preincubation of the acinar cells with 10 μM GSK-7975A had very little effect on the rise in [Ca2+]i in response to a supramaximal ACh stimulus (10 μM) in the presence of thapsigargin (Fig. S4 A and B). To assess the potential effect of CRAC blockade on Ca2+ store reloading, we compared the magnitudes of the [Ca2+]i response to a second stimulation with a high concentration of ACh (10 μM), after a long period of rest, under three different conditions, namely control, absence of external Ca2+, and in the presence of both Ca2+ and the CRAC blocker (Fig. S4C). In the pancreatic acinar cells, full reloading of virtually empty ER stores is a very slow process (45, 46) and, as seen by comparing Fig. S4 A with C, a second supramaximal ACh stimulus, even after a substantial interval during control conditions, only evoked a much diminished response. Even in the absence of external Ca2+, there is a tiny rise in [Ca2+]i following the second ACh application, most likely due to a small reuptake of Ca2+ into the ER before all of the released Ca2+ had been extruded across the cell membrane. In the presence of 10 μM GSK-7975A with external Ca2+, there is a significant reduction in the amplitude of the second response to ACh compared with the control situation, but this reduced response is nevertheless significantly larger than the one seen under Ca2+-free conditions (Fig. S4 C and D). This is consistent with our finding that store-operated Ca2+ entry is not abolished by 10 μM GSK-7975A, although very markedly diminished (Fig. 1).
Effect of CRAC Blockade in Other Cell Types.
We have also tested whether CRAC channel blockade in other cell types would have effects on store-operated Ca2+ entry. In freshly isolated mouse hepatocytes, using the classical thapsigargin protocol for examining store-operated Ca2+ entry, we only found a relatively modest inhibitory effect of GSK-7975A (P < 0.042, Fig. S5 A–C) compared with what was observed in pancreatic acinar cells (Fig. 1). We also tested the pancreatic acinar cell line AR42J, which has a neuronal phenotype (47). As seen in Fig. S6, GSK-7975A had little effect on store-operated Ca2+ entry in these cells, in marked contrast to what we show for the real pancreatic acinar cells (Fig. 1).
Discussion
The results presented here indicate that blockade of store-operated Ca2+- selective entry channels effectively prevents an important mediator of alcohol-related pancreatitis, POAEE, from evoking sustained elevation of [Ca2+]i, protease activation, and pancreatic acinar cell necrosis. This indicates that pharmacological CRAC blockade has therapeutic potential as a unique rational treatment against severe acute pancreatitis, a life-threatening human disease that at the moment is untreatable. Our result, showing that a CRAC channel blocker markedly reduces the sustained [Ca2+]i elevation that normally follows depletion of the Ca2+ store in the ER (Figs. 1 and 2), provides fresh evidence for the importance of store-operated Ca2+ channels in this phase of Ca2+ signaling, which has already been identified as crucial for the initiation of acinar cell injury (10). Although it has been known for 40 years that cytosolic Ca2+ signals in pancreatic acinar cells are initiated by release from internal stores (5), it has also been recognized for a long time that following the initial release from the intracellular stores there is an important Ca2+ entry phase that is essential for refilling the stores and indeed for stimulant-evoked enzyme secretion (48–50). Previous investigations have demonstrated that the most important mediators of acinar cell damage, namely alcohol, fatty acids, fatty acid ethyl esters, and bile acids all primarily release Ca2+ from various internal stores (28, 29, 37, 42, 43, 51), but that this initial phase is followed by store-operated Ca2+ entry, which plays a crucial role in the destruction of the cells (2, 4, 5, 10). Our electrophysiological data (Fig. 3) show that in the pancreatic acinar cells this store-operated inward current is relatively insensitive to removal of external Na+, but sensitive to changes in the external Ca2+ concentration. It is therefore not a Transient receptor potential (TRP)-type nonselective cation current, but a Ca2+-selective CRAC-type current, consistent with the very marked current inhibition evoked by GSK-7975A (Fig. 3D), a relatively selective CRAC channel blocker with almost no inhibitory effect on TRP-channel currents (with the exception of those mediated by TRPV6) (33). This agent has recently been shown to block Ca2+ currents through CRAC channels in human lung mast cells, T cells, and platelets (31–34). The CRAC channel is emerging as a potentially important therapeutic target in a number of human diseases (52, 53) and could also be important for pancreatitis (54). We have therefore used GSK-7975A (33, 34), to inhibit store-operated Ca2+ entry in pancreatic acinar cells. GSK-7975A markedly inhibited Ca2+ and Ba2+ entry elicited by releasing Ca2+ from the ER (Figs. 1–3) as well as the late phase Ca2+ entry in response to stimulation with a high ACh concentration (Fig. S1). These results indicate therapeutic potential for curbing excessive Ca2+ entry in the early phase of pancreatitis.
We have specifically investigated one pathophysiologically relevant situation, namely the acinar cell injury initiated by POAEE, one of the known mediators of alcohol-related pancreatitis (35–37, 42). These data (Fig. 4) show not only that GSK-7975A markedly reduces the POAEE-evoked [Ca2+]i elevation, but—even more importantly—also markedly inhibits the extent of the protease activation and necrosis induced by this fatty acid ethyl ester. The GSK-7975A CRAC channel blocker did not inhibit completely the unidirectional Ba2+ inflow evoked by arresting the ER Ca2+ pump (Fig. S1 I–K) and this inability to abolish store-operated Ca2+ entry (also reflected in Fig. 1 A–E) may be the reason that GSK-7975A has so little effect on the repetitive Ca2+ spiking elicited by a low ACh concentration or a physiological CCK concentration (Fig. S1). Although Ca2+ spikes are due to release of Ca2+ from intracellular stores, the cell should eventually run out of Ca2+ in the absence of Ca2+ entry because every time [Ca2+]i goes up there will be activation of the plasma membrane Ca2+ pump and therefore loss of Ca2+ to the external environment (5). However, because the loss of Ca2+ during short spikes of the type shown in Fig. S1 is relatively small (5), even a severely reduced level of Ca2+ entry may be sufficient to prevent depletion of the intracellular stores. This point is also relevant with regard to another potential concern, namely the apparent irreversibility of the effect of GSK-7975A, at least for ∼10 min following start of washout (Fig. 1). Because of the incompleteness of the CRAC blockade in the acinar cells (Fig. 1 and Fig. S1 I and J), this may turn out not to be a serious problem for potential therapeutic use. Resting pancreatic acinar cells appeared to have unchanged levels of Ca2+ in the ER store after 1 h of incubation with GSK-7975A (Fig. S4 A and B). Although reloading of the intracellular stores after strong stimulation with ACh was reduced by the CRAC blocker, there was still a significant level of store refilling, compared with conditions when there was no Ca2+ entry due to absence of external Ca2+ (Fig. S4 C and D).
CRAC channels are widely distributed in many different cell types and effective CRAC blockade might therefore also have effects on other organ systems. However, the effects are likely to be strongest in those cell types (like the pancreatic acinar cells) in which other types of Ca2+ entry pathways are not quantitatively important, unlike the many types of electrically excitable cells. Mast cells and T cells, for example, will be affected by CRAC blockade (31, 34, 52, 53), but this will be advantageous in the treatment of acute pancreatitis, where the initial damage of the acinar cells leading to necrosis is followed by a strong inflammatory response, which is known to cause very significant further damage (1). CRAC blockade would have the most pronounced effect on cells with the most strongly activated Ca2+ entry channels, which, in cases of life-threatening severe pancreatitis, would be the pancreatic acinar cells. With regard to organ selectivity, it is interesting that CRAC channel blockade was much less effective in inhibiting store-operated Ca2+ entry in hepatocytes and in the neuronal-like AR42J cell line (compare Fig. 1 with Figs. S5 and S6), probably reflecting a more complex situation in these cells (47, 55).
Although CRAC channel inhibition will very effectively reduce cytosolic Ca2+ overload, it will not prevent the depletion of intracellular Ca2+ stores evoked by POAEE. It could therefore be potentially advantageous to combine CRAC blockade with inhibition of Ca2+ release from the internal stores using a synthetic membrane permeable calmodulin activator (29). Overall, our data provide a unique proof of principle that pharmacological CRAC channel inhibition could become an effective tool for reducing the cellular Ca2+ overload that is such an important feature of the changes occurring in the initial phase of acute pancreatitis. Specifically, our finding that GSK-7975A markedly inhibits not only the excessive [Ca2+]i elevation evoked by POAEE, an important mediator of alcohol-related pancreatitis, but also markedly reduces the extent of the associated protease activation and necrosis—although it has little or no effect on the repetitive short-lasting Ca2+ spikes evoked by the physiological neurotransmitter ACh—is promising.
Materials and Methods
Full methods are provided in SI Materials and Methods. Details about isolation of pancreatic acinar cells were described previously (56). GSK-7975A was provided by GlaxoSmithKline (31). Fluorescent measurements were performed with Fluo-4 or Fura-2 (39), whereas necrosis was assessed with propidium iodide (39) and trypsinogen activation with trypsin fluorescent substrate (10, 29). Hepatocytes were isolated according to a protocol described previously (57). AR42J cell protocols are described in refs. 39, 47.
Supplementary Material
Acknowledgments
This work was supported by Medical Research Council Programme Grant MR/J002771/1. O.H.P. is a Medical Research Council Professor (G19/22/2).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300910110/-/DCSupplemental.
References
- 1.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: Bench to the bedside. Gastroenterology. 2007;132(3):1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: Effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27(2):113–120. doi: 10.1016/j.tips.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Hegyi P, Pandol S, Venglovecz V, Rakonczay Z., Jr The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut. 2011;60(4):544–552. doi: 10.1136/gut.2010.218461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen OH, et al. Fatty acids, alcohol and fatty acid ethyl esters: Toxic Ca2+ signal generation and pancreatitis. Cell Calcium. 2009;45(6):634–642. doi: 10.1016/j.ceca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 6.Tinel H, et al. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca(2+) signals. EMBO J. 1999;18(18):4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen OH. Specific mitochondrial functions in separate sub-cellular domains of pancreatic acinar cells. Pflϋgers Arch. 2012;464:77–87. doi: 10.1007/s00424-012-1099-6. [DOI] [PubMed] [Google Scholar]
- 8.Pizzo P, Drago I, Filadi R, Pozzan T. Mitochondrial Ca2+ homeostasis: Mechanism, role, and tissue specificities. Pflϋgers Arch. 2012;464:3–17. doi: 10.1007/s00424-012-1122-y. [DOI] [PubMed] [Google Scholar]
- 9.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86(1):369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 10.Raraty M, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA. 2000;97(24):13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157(1):43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 13.Petersen OH. Stimulus-secretion coupling: Cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 15.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 16.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CY, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136(5):876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan JP, et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11(3):337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lur G, et al. Ribosome-free terminals of rough ER allow formation of STIM1 puncta and segregation of STIM1 from IP(3) receptors. Curr Biol. 2009;19(19):1648–1653. doi: 10.1016/j.cub.2009.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahnson TD, Pandol SJ, Dionne VE. Cyclic GMP modulates depletion-activated Ca2+ entry in pancreatic acinar cells. J Biol Chem. 1993;268(15):10808–10812. [PubMed] [Google Scholar]
- 21.Krause E, Pfeiffer F, Schmid A, Schulz I. Depletion of intracellular calcium stores activates a calcium conducting nonselective cation current in mouse pancreatic acinar cells. J Biol Chem. 1996;271(51):32523–32528. doi: 10.1074/jbc.271.51.32523. [DOI] [PubMed] [Google Scholar]
- 22.Kim MS, et al. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137(4):1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 24.Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol. 1998;8(24):1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood MW, et al. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA. 2007;104(13):5674–5679. doi: 10.1073/pnas.0700951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci. 2006;119(Pt 2):226–238. doi: 10.1242/jcs.02721. [DOI] [PubMed] [Google Scholar]
- 27.Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela JM. Generation of specific Ca(2+) signals from Ca(2+) stores and endocytosis by differential coupling to messengers. Curr Biol. 2006;16(19):1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 28.Gerasimenko JV, et al. Pancreatic protease activation by alcohol metabolite depends on Ca2+ release via acid store IP3 receptors. Proc Natl Acad Sci USA. 2009;106(26):10758–10763. doi: 10.1073/pnas.0904818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerasimenko JV, et al. Calmodulin protects against alcohol-induced pancreatic trypsinogen activation elicited via Ca2+ release through IP3 receptors. Proc Natl Acad Sci USA. 2011;108(14):5873–5878. doi: 10.1073/pnas.1016534108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng SW, di Capite J, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J Biol Chem. 2008;283(46):31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 31.Ashmole I, et al. CRACM/Orai ion channel expression and function in human lung mast cells. J Allergy Clin Immunol. 2012;129(6):1628–1635, e2. doi: 10.1016/j.jaci.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Kruchten R, et al. Antithrombotic potential of blockers of store-operated calcium channels in platelets. Arterioscler Thromb Vasc Biol. 2012;32(7):1717–1723. doi: 10.1161/ATVBAHA.111.243907. [DOI] [PubMed] [Google Scholar]
- 33.Derler I, et al. The action of selective CRAC channel blockers is affected by the Orai pore geometry. Cell Calcium. 2013;53(2):139–151. doi: 10.1016/j.ceca.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice LV, et al. Characterization of selective Calcium-Release Activated Calcium channel blockers in mast cells and T-cells from human, rat, mouse and guinea-pig preparations. Eur J Pharmacol. 2013;704(1-3):49–57. doi: 10.1016/j.ejphar.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231(4737):497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- 36.Werner J, et al. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113(1):286–294. doi: 10.1016/s0016-5085(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 37.Criddle DN, et al. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130(3):781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Dolai S, et al. Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology. 2012;143(3):832–843, e1–e7. doi: 10.1053/j.gastro.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Ferdek PE, et al. A novel role for Bcl-2 in regulation of cellular calcium extrusion. Curr Biol. 2012;22(13):1241–1246. doi: 10.1016/j.cub.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakowski D, Parekh AB. Voltage-dependent Ba2+ permeation through store-operated CRAC channels: Implications for channel selectivity. Cell Calcium. 2007;42(3):333–339. doi: 10.1016/j.ceca.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Hofer AM, Fasolato C, Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: A study using simultaneous measurements of ICRAC and intraluminal [Ca2+] J Cell Biol. 1998;140(2):325–334. doi: 10.1083/jcb.140.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Criddle DN, et al. Ethanol toxicity in pancreatic acinar cells: Mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci USA. 2004;101(29):10738–10743. doi: 10.1073/pnas.0403431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58(3):422–430. doi: 10.1136/gut.2007.146258. [DOI] [PubMed] [Google Scholar]
- 44.Park M, Lee K, Uhm D-Y. Slow depletion of endoplasmic reticulum Ca2+ stores and block of store-operated Ca2+ channels by 2-aminoethoxydiphenyl borate in mouse pancreatic acinar cells. Arch Pharmacol. 2002;365:399–405. doi: 10.1007/s00210-002-0535-0. [DOI] [PubMed] [Google Scholar]
- 45.Mogami H, Tepikin AV, Petersen OH. Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J. 1998;17(2):435–442. doi: 10.1093/emboj/17.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park MK, Tepikin AV, Petersen OH. The relationship between acetylcholine-evoked Ca(2+)-dependent current and the Ca2+ concentrations in the cytosol and the lumen of the endoplasmic reticulum in pancreatic acinar cells. Pflugers Arch. 1999;438(6):760–765. doi: 10.1007/s004249900128. [DOI] [PubMed] [Google Scholar]
- 47.Gallacher DV, et al. Substance P and bombesin elevate cytosolic Ca2+ by different molecular mechanisms in a rat pancreatic acinar cell line. J Physiol. 1990;426:193–207. doi: 10.1113/jphysiol.1990.sp018133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondo S, Schulz I. Calcium ion uptake in isolated pancreas cells induced by secretagogues. Biochim Biophys Acta. 1976;419(1):76–92. doi: 10.1016/0005-2736(76)90373-4. [DOI] [PubMed] [Google Scholar]
- 49.Petersen OH, Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol. 1976;254(3):583–606. doi: 10.1113/jphysiol.1976.sp011248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mogami H, Nakano K, Tepikin AV, Petersen OH. Ca2+ flow via tunnels in polarized cells: Recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell. 1997;88(1):49–55. doi: 10.1016/s0092-8674(00)81857-7. [DOI] [PubMed] [Google Scholar]
- 51.Gerasimenko JV, et al. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem. 2006;281(52):40154–40163. doi: 10.1074/jbc.M606402200. [DOI] [PubMed] [Google Scholar]
- 52.Parekh AB. Store-operated CRAC channels: Function in health and disease. Nat Rev Drug Discov. 2010;9(5):399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]
- 53.Di Capite JL, Bates GJ, Parekh AB. Mast cell CRAC channel as a novel therapeutic target in allergy. Curr Opin Allergy Clin Immunol. 2011;11(1):33–38. doi: 10.1097/ACI.0b013e32834232b0. [DOI] [PubMed] [Google Scholar]
- 54.Parekh AB. Calcium signaling and acute pancreatitis: Specific response to a promiscuous messenger. Proc Natl Acad Sci USA. 2000;97(24):12933–12934. doi: 10.1073/pnas.97.24.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barritt GJ, Chen J, Rychkov GY. Ca(2+) -permeable channels in the hepatocyte plasma membrane and their roles in hepatocyte physiology. Biochim Biophys Acta. 2008;1783(5):651–672. doi: 10.1016/j.bbamcr.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 56.Gerasimenko JV, et al. Menadione-induced apoptosis: Roles of cytosolic Ca(2+) elevations and the mitochondrial permeability transition pore. J Cell Sci. 2002;115(Pt 3):485–497. doi: 10.1242/jcs.115.3.485. [DOI] [PubMed] [Google Scholar]
- 57.Li W-C, Ralphs KL, Tosh D. Isolation and culture of adult mouse hepatocytes. Methods Mol Biol. 2010;633:185–196. doi: 10.1007/978-1-59745-019-5_13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.