Abstract
The activity and morphology of mitochondria are maintained by dynamic fusion and fission processes regulated by a group of proteins residing in, or attached to, their inner and outer membranes. Hypoxia-induced gene domain protein-1a (Higd-1a)/HIMP1-a/HIG1, a mitochondrial inner membrane protein, plays a role in cell survival under hypoxic conditions. In the present study, we showed that Higd-1a depletion resulted in mitochondrial fission, depletion of mtDNA, disorganization of cristae, and growth retardation. We demonstrated that Higd-1a functions by specifically binding to Optic atrophy 1 (Opa1), a key element in fusion of the inner membrane. In the absence of Higd-1a, Opa1 was cleaved, resulting in the loss of its long isoforms and accumulation of small soluble forms. The small forms of Opa1 do not interact with Higd-1a, suggesting that a part of Opa1 in or proximal to the membrane is required for that interaction. Opa1 cleavage, mitochondrial fission, and cell death induced by dissipation of the mitochondrial membrane potential were significantly inhibited by ectopic expression of Higd-1a. Furthermore, growth inhibition due to Higd-1a depletion could be overcome by overexpression of a noncleavable form of Opa1. Collectively, our observations demonstrate that Higd-1a inhibits Opa1 cleavage and is required for mitochondrial fusion by virtue of its interaction with Opa1.
Mitochondria are active in respiration, oxidative phosphorylation, calcium homeostasis, and apoptosis. They are highly dynamic organelles subject to continuous fusion and fission processes that are essential for maintaining their morphology and activity (1). Several components of the mitochondrial fusion and fission machinery have been identified. Mitofusin 1 and 2 (Mfn1 and Mfn2) are mitochondrial outer membrane GTPases (2, 3) that form homodimers and heterodimers and tether adjacent mitochondria during mitochondrial fusion (4). Optic atrophy 1 (Opa1) is a dynamin-related GTPase that resides or attaches to the mitochondrial inner membrane, facing the intermembrane space (5). It plays a role in inner membrane fusion, cooperating with Mfn1 to regulate fusion of the two mitochondrial membranes (6). Dynamin-related protein 1 (Drp1) can assemble into multimeric ring-like structures, wrap around the constriction sites of dividing mitochondria, and lead to mitochondrial fission (7). Opa1 has eight alternatively spliced isoforms (8). The Opa1 protein is further processed to produce variants of the long (L) and short (S) isoforms, both types of which are required for mitochondrial fusion (9). There are constitutive and induced sites of cleavage in Opa1. Constitutive cleavage is performed at exon 5b by the i-AAA protease Yme1L and is required for optimal fusion of mitochondria (10, 11). Inducible Opa1 cleavage can occur under various conditions, such as loss of mitochondrial membrane potential, apoptosis, and decreased ATP levels, and can completely inactivate Opa1 (10, 12, 13). Although the inducible cleavage by OMA1 protease is known to occur in exon 5 (14, 15), its precise mechanism of cleavage and role in inner membrane fusion remain to be clarified. Opa1 has additional roles in maintaining mitochondrial inner membrane structure and in the remodeling of cristae (16, 17).
Hypoxia-induced gene domain protein-1a (Higd-1a) was referred to as hypoglycemia/hypoxia inducible mitochondrial protein1-a (HIMP1-a) (18) or just hypoxia induced gene 1 (HIG1) (19-21). It is a 10.4-kDa mitochondrial inner membrane protein and is predicted to have two transmembrane domains oriented in an “N-terminal outside-C-terminal outside and loop inside” conformation (18). It is thought to promote the survival of the α and β cells of the pancreas under conditions of stress (18). Although it is induced by hypoxia and promotes survival, its modes of action remain to be clarified. We showed that Higd-1a–transfected cells underwent significantly less apoptosis, as the result of inhibition of the release of cytochrome c and reduction of caspase activities (22). In the present study, we found that Higd-1a protects the Opa1 L isoform from induced cleavage. Higd-1a silencing results in loss of the L isoform, along with mitochondrial fission, loss of mtDNA, cell growth retardation, and disorganization of the cristae. Higd-1a does not act as a homomultimer but rather interacts with Opa1 in an association that appears essential for Higd-1a function.
Results
Silencing of Higd-1a Affects Mitochondrial Fusion.
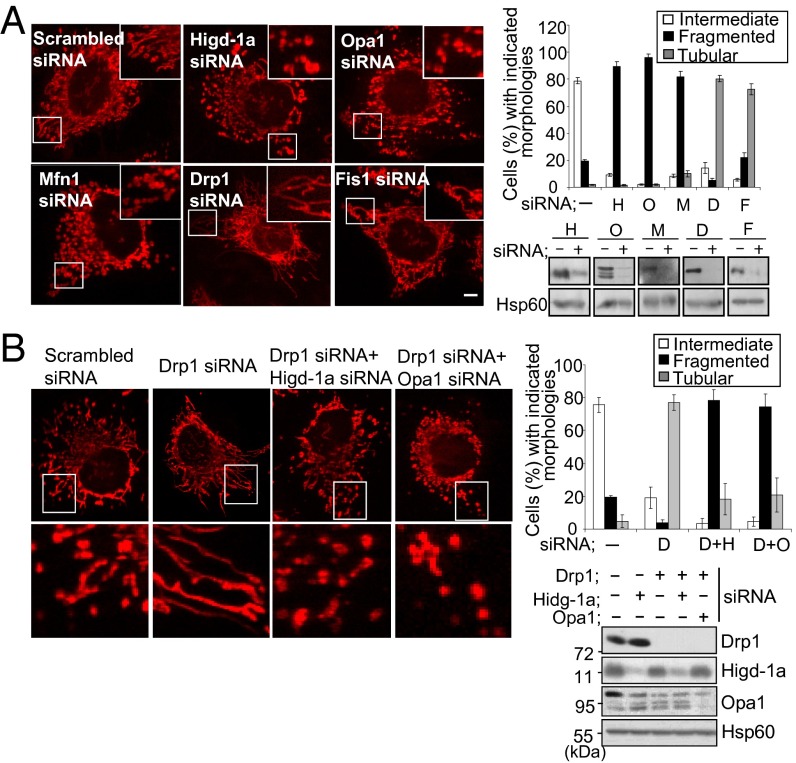
To study the role of Higd-1a, we assessed the effect of Higd-1a knockdown by transfecting siRNA into HEK293T and HeLa cells. The reduced expression of Higd-1a after siRNA transfection was confirmed by immunoblotting with polyclonal anti–Higd-1a (Fig. 1A). Because Higd-1a is an inner mitochondrial membrane protein, we first examined the morphology of mitochondria by staining with MitoTracker. Transfection with Opa1 and Mfn1 siRNA resulted in mitochondrial fragmentation, as reported (Fig. 1A) (10, 23). Silencing of the fission-related proteins Drp1 and Fis1 resulted in mainly elongated mitochondria, and silencing of Drp1 gave rise to the highest proportion of cells with tubular mitochondria (80.4 ± 2.5% of 200 cells observed and counted). We found that silencing of Higd-1a in HeLa cells resulted in highly fragmented mitochondria in 89.3 ± 3.5% of the cells examined; the mitochondria resembled the dot-like fragmented mitochondria seen in cells transfected with Opa1 siRNA (96.1 ± 2.3%) or Mfn1 siRNA (81.5 ± 4.3%). It was shown that when HeLa cells were transfected with both Drp1 siRNA and Opa1 siRNA, the mitochondria lost their elongated shape (24). This result suggested that optimal expression of Opa1 was required to generate the fused and interconnected shape of mitochondria. Therefore, we attempted to silence Drp1 and Higd-1a together (Fig. 1B). Simultaneous silencing of Drp1 and Higd-1a gave rise to fragmented mitochondria (78.5 ± 6.2%) resembling the dot-shaped mitochondria of Drp1- and Opa1-silenced cells (74.7 ± 7.5%). Therefore, Higd-1a depletion prevented the formation of the tubular-shaped mitochondria characteristic of Drp1-silenced cells, indicating that Higd-1a is required for the optimal fusion of mitochondria in Drp1-silenced cells.
Fig. 1.
Depletion of Higd-1a induces mitochondrial fragmentation. (A) HeLa cells were transfected with scrambled (-), Higd-1a (H), Opa1 (O), Mfn1 (M), Drp1 (D), and Fis1 (F) siRNAs for 3 d. To visualize mitochondria, cells were stained with MitoTracker Red, fixed, and mounted for microscopy. Images were captured with a confocal microscope (Left). Insets are enlargements of the boxed areas. (Scale bar: 10 μm.) In each case, >200 cells in several fields were counted to determine the percentages of the cell populations with tubular (highly interconnected), intermediate, and fragmented mitochondria (Right). Cells were immunoblotted after 3 d of transfection with the indicated siRNAs, using antibodies against the corresponding proteins. Hsp60 was used as an internal standard. Data are representative of more than three independent experiments. (B) Cells were transfected with scrambled siRNA (-) and siRNAs for Drp1 (D), Drp1 plus Higd-1a (D+H), and Drp1 plus Opa1 (D+O) for 3 d. The cells were stained and counted as described in A. Lower shows enlargements of the boxed areas (Left). Cells were immunoblotted with antibodies as indicated.
The Opa1 L Isoforms Are Further Cleaved in the Absence of Higd-1a.
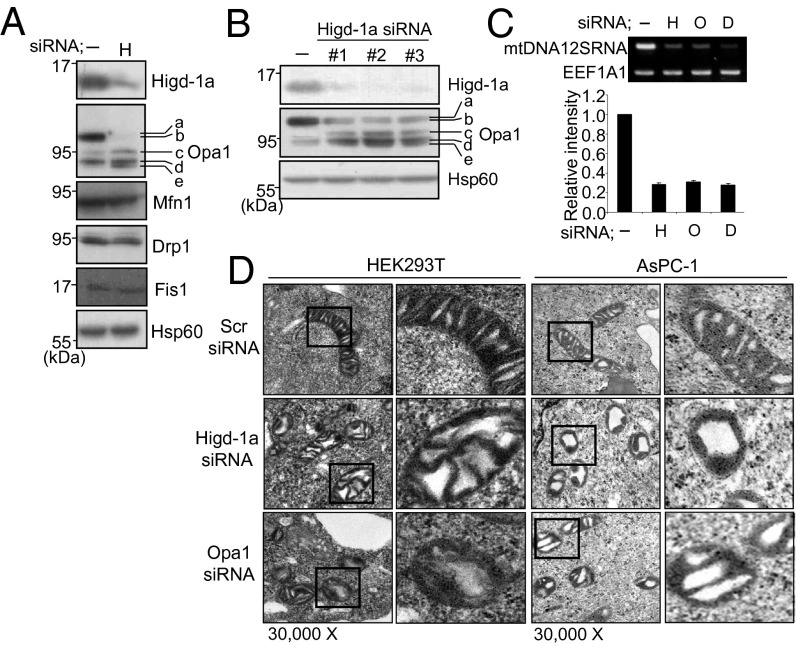
From Fig. 1B it can be seen that Higd-1a depletion affects the ratio of L and S forms of Opa1 as does silencing of Drp1. Möpert et al. reported that persistent loss of Drp1 led to processing of Opa1 to shorter forms (24). We therefore examined the levels of fusion- and fission-related proteins in Higd-1a–depleted cells. In HEK293T cells depleted of Higd-1a, levels of Mfn1, Fis1, Drp1, and Hsp60 were unchanged. However, the expression of L and S Opa1 isoforms was altered (Fig. 2A). Opa1 protein produced from eight different alternatively spliced forms of mRNA are further cleaved by proteases (8, 9). Therefore, five different bands, labeled a–e, of Opa1 are observed by immunoblot analysis. In control cells transfected with scrambled siRNA, b and d are the major bands. In Higd-1a–silenced HEK 293T cells, the intensity of band b is greatly decreased and those of bands c and e increased. Three siRNA oligonucleotides targeting different regions of Higd-1a RNA gave similar results of depleting Higd-1a with enhanced appearance of the shorter bands of Opa1 (Fig. 2B). These results suggest that the fragmented shape of mitochondria in Higd-1a–silenced cells is probably caused by the changes in the Opa1 isoforms.
Fig. 2.
Higd-1a is needed for maintaining the L isoform of Opa1, mitochondrial morphology, and mtDNA. (A) Expression of Higd-1a, Opa1, Mfn1, Drp1, Fis1, and Hsp60 proteins in HEK293T cells was analyzed by immunoblotting after 3 d of transfection with scrambled (-) or Higd-1a siRNAs (H). Higd-1a, Opa1, Mfn1, Fis1, and Hsp60 were analyzed by using extracts of mitochondrial fractions and Drp1 using total cell extracts. Five isoforms of Opa1 are labeled a–e (9). (B) Depletion of Higd-1a and changes of Opa-1 isoforms were examined by transfection with three different Higd-1a siRNA oligonucleotides. Hsp60 protein levels are shown as a loading control. -, scrambled siRNA. (C) HEK293T cells were transiently transfected with scrambled (-), Higd-1a (H), Opa1 (O), or Drp1 (D) siRNAs and cultured for 3 d. Total DNA was analyzed by PCR for the 12S ribosomal RNA small subunit mitochondrial gene (Upper) and by quantitative real-time PCR (Lower). As a nuclear DNA control, the Elongation translation factor 1 gene (EEF1A1) was used. (D) HEK293T or AsPc-1 cells were transfected with siRNAs of scrambled (Scr), Higd-1a or Opa1 for 3 d and fixed. Thin sections of cells were visualized by transmission electron microscopy. Right for each cell line shows an enlargement of the boxed area. (Magnification: 30,000x.)
Higd-1a Depletion Causes Cristae Disorganization and mtDNA Loss.
Opa1 has been shown to be involved in cristae remodeling, and Opa1 depletion caused disorganization of the structure of the mitochondrial inner membrane (16, 17). Therefore, we observed the mitochondria of Higd-1a–depleted cells under the electron microscope. The mitochondria were found to be fragmented with hollow-shaped cristae, like those of Opa1-depleted cells (Fig. 2D). Thus, Higd-1a also plays a role in the maintenance of cristae organization. We also checked mtDNA content because optimum levels of mitochondrial fusion and fission are required to preserve the integrity of mtDNA (25–28). mtDNA content was significantly reduced in the Higd-1a–depleted cells, as in Opa1- and Drp1-depleted cells (Fig. 2C).
Silencing of Higd-1a Leads to Growth Retardation.
Next we sought to identify the cellular changes resulting from Higd-1a silencing. Higd-1a protein levels were reduced by 80∼90% in the cell lines tested for up to 6 d after siRNA transfection (Fig. 3A). Higd-1a silencing led to growth inhibition in both cell lines tested using Trypan blue exclusion. After 6 d, cell numbers in the Higd-1a–depleted HEK293T and HeLa cultures were only 38.2 ± 0.5% and 41.1 ± 2.6%, respectively, of those in control siRNA-transfected cells (Fig. 3B). Using crystal violet staining, we consistently stained far fewer viable cells in the Higd-1a–depleted cultures than in those transfected with scrambled siRNA (Fig. 3C). We examined whether the growth retardation was caused by increased apoptosis or reduced proliferation. Flow cytometry analysis showed that knockdown of Higd-1a reduced the number of cells in S phase of the cell cycle (from 20.2 ± 0.8 to 12.1 ± 0.3%; Fig. 3D). Concomitantly, culture in the presence of BrdU for 24 h on the third day after transfection resulted in significantly less BrdU incorporation into replicating DNA in the knocked-down Higd-1a cells than in those transfected with scrambled siRNA (Fig. 3E). There was no sign of apoptosis in the cultures depleted of Higd-1a, because the percentages of Annexin V–/propidium iodide– cells were unchanged (Fig. 3F). In addition, no membrane permeabilization was evident during the course of the experiment (Fig. 3G). Levels of mitochondrial ATP were minimally changed in the Higd-1a–depleted cells. However, total cellular and cytoplasm ATP levels were ∼20% higher than in cells transfected with scrambled siRNA (Fig. S1). This increase was probably caused by the significant slowing of ATP-consuming processes, such as cell proliferation and DNA replication, in cells depleted of Higd-1a (Fig. 3 B–E). Collectively, our observations demonstrate that Higd-1a silencing does not induce cell death but inhibits proliferation. In addition, it does not have a serious impact on mitochondrial membrane potential or ATP production.
Fig. 3.
Silencing of Higd-1a expression inhibits cell proliferation. HEK293T and HeLa cells were transiently transfected with scrambled (-) or Higd-1a (H) siRNAs. (A) Total cell extracts were immunoblotted with anti–Higd-1a polyclonal antibody. β-Actin was used as a loading control. (B) Total viable cells were counted by Trypan blue exclusion at the indicated times after transfection. (C) Images of representative cell culture dishes stained with crystal violet were obtained 2 wk after transfection. (D) Flow cytometry cell cycle analysis of HeLa cells 4 d after transfection. (E) Three days after transfection, BrdU was added for an additional 24 h and BrdU incorporation was analyzed by flow cytometry. (F) Annexin V/propidium iodide staining was performed 4 d after transfection. The percentages of annexin V and propidium iodide negative cells (viable cells) were quantified by flow cytometry. (G) Three days after transfection, HeLa cells were stained with rhodamine-123 and relative fluorescence intensity was analyzed by flow cytometry to measure mitochondrial membrane potential. As a positive control for membrane potential changes, cells were treated with 10 μM CCCP for 30 min. Results shown are representative of three independent experiments. Data are means ± SE. **P < 0.01 and ***P < 0.001.
Higd-1a Does Not Form Homomultimers but Binds to Opa1.
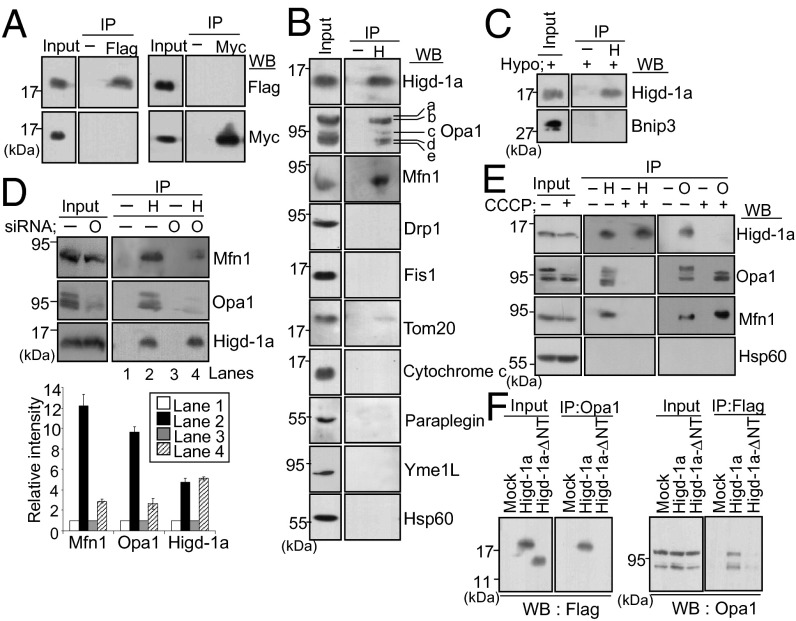
Higd-1a is a rather small membrane protein with two transmembrane domains that are highly conserved. It was suggested that these domains may interact with each other or with other components to form oligomeric complexes (18). Therefore, we first examined whether Higd-1a acted as a homomultimer, by testing for coimmunoprecipitation of Flag- and myc-tagged proteins. Immunoblotting of a lysate of HEK293T cells cotransfected with Higd-1a–Flag and Higd-1a–myc yielded two Higd-1a–tagged bands in the input lanes (Fig. 4A). However, when the lysate was immunoprecipitated with anti-Flag, no band corresponding to myc-tagged Higd-1a was seen and vice versa, demonstrating that Higd-1a does not bind to itself.
Fig. 4.
Higd-1a interacts with Opa1, and the N-terminal tail of Higd-1a participates in binding. (A) HEK293T cells were transfected with Higd-1a–myc and Higd-1a–Flag vectors for 2 d. Total cell lysates were immunoprecipitated (IP), with anti-Flag or anti-myc antibodies, and immunoblotted (WB). The amount of input loaded was approximately 5% of the lysate used for immunoprecipitation. (B) Endogenous Higd-1a was immunoprecipitated from the mitochondrial lysate with anti–Higd-1a antibody and immunoblotted with the indicated antibodies. For Drp1 immunoblotting, the total lysate was used. (C) HEK293T cells were incubated under hypoxia (0.1% O2) for 24 h. Mitochondrial lysates were immunoprecipitated with anti–Higd-1a and immunoblotted with anti–Higd-1a or anti-BNIP3 antibodies. (D) HEK293T cells were transfected with scrambled (-) or Opa1 (O) siRNA for 3 d. Mitochondrial lysates were immunoprecipitated with (H) or without (-) anti–Higd-1a and immunoblotted with the indicated antibodies (Upper). The relative intensities of the Western blot bands of lanes 1–4 in the autoradiogram were analyzed with TINA 2.0 (Lower). Data represent the mean ± SE of three independent experiments. (E) HEK293T cells were incubated with or without 10 µM CCCP for 30 min. Mitochondrial lysates were immunoprecipitated (IP) with anti–Higd-1a (H) or anti-Opa1 (O) antibodies and immunoblotted (WB) with the indicated antibodies. (F) HEK293T cells were transiently transfected with mock, Higd-1a–Flag or Higd-1a–ΔNT-Flag vectors. Forty-eight hours after transfection, total cell lysates were immunoprecipitated (IP) with anti-Opa1 (Left) or anti-Flag (Right) and immunoblotted (WB) with anti-Flag or anti-Opa1 antibodies, respectively.
To identify factors interacting with Higd-1a, we first performed a pulldown assay of cells overexpressing Higd-1a-Flag, followed by MALDI-TOF analysis of the pulled down proteins. In this way, we isolated four proteins: dynamin-1–like protein, Drp1 (NM_012062, 81 kDa, 14 peptide matches, mascot score of 49), mitochondrial ribosomal protein L38 (NM_032478, 44 kDa, 10 peptide matches, mascot score of 26), dynamin-like 120 kDa protein, Opa1 (NM_015560, 111 kDa, 18 peptide matches, mascot score of 37), and mitochondrial solute carrier family 25, SLC25A25 (NM_052901, 53 kDa, 9 peptide matches, mascot score of 33). However, none of these fell within the 95% confidence interval. Next we performed blue native gel electrophoresis (BN-PAGE) and coimmunoprecipitation with immunoblotting. On BN-PAGE, Higd-1a appeared in several high molecular mass complexes above the position of ∼300 kDa (Fig. S2). Because there were clear morphological changes of miotchondria in Higd-1a–depleted cells, our aim was to examine whether Higd-1a comigrated with some of the fusion or fission components including Opa1 and Drp1 described above. Opa1 formed multiple bands above ∼300 kDa, corresponding to oligomeric complexes of its S and L forms. Other mitochondrial proteins such as Drp1, presenilin-associated rhomboid-like protease (PARL), apoptosis-inducing factor (AIF), and Hsp60 appeared as clear single bands. Next, immunoprecipitation of HEK293T cell lysates with anti–Higd-1a showed that endogenous Higd-1a clearly pulled down Opa1 and Mfn1 (Fig. 4B). However, Higd-1a did not physically interact either with the fission-related proteins Drp1 and Fis1 or with other transmembrane or soluble proteins such as Tom20, cytochrome c, or Hsp60. Likewise, there was no evidence of interaction between Higd-1a and proteases involved in Opa1 cleavage (m-AAA protease paraplegin and i-AAA protease Yme1L). BNIP3, a mitochondrial proapoptotic BH3-only protein of the Bcl-2 family, is an outer mitochondrial membrane protein involved in mitochondrial fission and apoptosis (29). Higd-1a did not interact with BNIP3 in hypoxia-treated cells either (Fig. 4C). Because the basal level of BNIP3 is low, and its expression is strongly induced by hypoxia, cells exposed to 0.1% oxygen were used for the BNIP3 immunoprecipitation.
Mfn1 is an outer membrane protein that cooperates with Opa1 in mitochondrial fusion, and it is known to interact with both L and S Opa1 isoforms (30). Therefore, we tested whether Higd-1a interacted with either Opa1 or Mfn1. After cells were transfected with Opa1 siRNA to knockdown Opa1, mitochondrial lysates were immunoprecipitated with anti–Higd-1a, and the amount of Opa1and Mfn1 pulled down by anti–Higd-1a was compared with the amount pulled down in mock-transfected cells (Fig. 4D, lanes 2 and 4). Levels of both Opa1 and Mfn1 pulled down were decreased, although only Opa1 expression was lower in the input lane after transfection of siRNA, suggesting that Mfn1 is pulled down indirectly by Higd-1a after it (Mfn1) forms a complex with Opa1 (Fig. 4D). The specific interaction of Higd-1a with Opa1 was confirmed by treating cells with carbonyl cyanide m-chlorophenyl hydrazone (CCCP) for 30 min. In response to CCCP treatment, the L form of Opa1 was completely cleaved into its small forms, as previously reported (9), and anti–Higd-1a could not pull down these small forms (Fig. 4E). This result demonstrates that the N-terminal domain of Opa1, the transmembrane domain and the region proximal to the transmembrane domain, are required for the interaction between Opa1 and Higd-1a. Although Mfn1 levels were unchanged after CCCP treatment, Mfn1 pulled down by anti–Higd-1a completely disappeared, showing again that Mfn1 binding to Higd-1a depends on its interaction with Opa1. The interaction was further confirmed by reversal of immunoprecipitation with anti-Opa1: Anti-Opa1 pulled down endogenous Higd-1a and Mfn1 in lysates in the absence of CCCP treatment; when cells were exposed to CCCP, the interaction between Opa1 and Mfn1 was observed but not that between Opa1 and Higd-1a (Fig. 4E).
The N-Terminal Tail of Higd-1a Participates in the Binding with Opa1.
We constructed a mutant form of Higd-1a, designated Higd-1a-ΔNT (27–95 amino acids), with 26 N-terminal amino acids deleted. We showed that the missing region was necessary for the survival effect of Higd-1a (22). We used this mutant in the present study to examine whether the same region was required for its interaction with Opa1. The N-terminal deletion did not alter the mitochondrial localization of the protein: Higd-1a–ΔNT-Flag colocalized with MitoTracker, like wild-type Higd-1a–Flag (Fig. S3A). To determine the sublocalization of the mutant protein within the mitochondria, cells were treated with varying concentrations of digitonin plus proteinase K. Tom20 is an outer mitochondrial membrane protein that is immediately digested by proteinase K in the absence of digitonin treatment (Fig. S3B). In our experiment, Higd-1a–Flag and Higd-1a–ΔNT-Flag were resistant to proteinase K in the absence of digitonin, but were digested by proteinase K after treatment with 0.2 mg/mL digitonin, which selectively permeabilizes the outer mitochondrial membrane and enables digestion of the proteins in the intermembrane space (31). Although complete digestion required higher concentrations of digitonin, we were able to conclude that both wild and mutant Higd-1a showed similar protease sensitivity and both were located in the inner mitochondrial membrane. Immunoelectron microscopy yielded results consistent with this data of sublocalization: Most of the gold-labeled Higd-1a–Flag and Higd-1a–ΔNT-Flag proteins were found inside the mitochondria but not in the mitochondrial matrix (Fig. S3C). Reciprocal co-IP experiments using Higd-1a–Flag, Higd-1a–ΔNT-Flag, and Opa1 showed that only Higd-1a–Flag interacted with Opa1, and that deletion of the N-terminal tail almost completely inhibited the interaction (Fig. 4F). This result demonstrates that the N-terminal tail, which is exposed to the intermembrane space, is necessary for the interaction of Higd-1a with Opa1.
Higd-1a Overexpression Delays Cleavage of the L Opa1 Isoform.
To confirm the effect of Higd-1a on Opa1 cleavage, we tested whether Higd-1a overexpression prevented cleavage. We placed HEK293T cells under hypoxic conditions (0.1% O2) for 12 h. Cleavage of the L Opa1 isoform was observed immediately in mock-transfected cells, whereas overexpression of Higd-1a delayed cleavage for up to 6 h (Fig. 5A). Opa1 processing was also examined by using CCCP, which dissipates the mitochondrial membrane potential resulting in Opa1 cleavage and mitochondrial fragmentation. When we treated HEK293T cells with CCCP, L Opa1 quickly disappeared from the cells transfected with mock vector, and the S isoforms accumulated (Fig. 5B). However, overexpression of Higd-1a–Flag stabilized the L isoform for up to 9 min. Thus, although Higd-1a overexpression did not block cleavage of L Opa1 completely, it delayed it. The effect of Higd-1a overexpression was also reflected in the morphology of the mitochondria and in cell survival. HeLa cells were treated with CCCP for 15 min after transient transfection with the Higd-1a–Flag and Higd-1a–ΔNT-Flag vectors. Confocal fluorescence microscopy clearly showed that overexpression of Higd-1a–Flag inhibited CCCP-induced mitochondrial fragmentation (Fig. 5C). In contrast to wild-type Higd-1a, overexpression of Higd-1a–ΔNT-Flag did not prevent the CCCP-induced mitochondrial fragmentation, indicating that the N-terminal region of Higd-1a, which is responsible for the interaction with Opa1, is also essential for its function. Higd-1a also affected overall cell survival: Whereas approximately 40% of the mock-transfected cells were round, and detached from the surface of the culture dish after 30 min of CCCP treatment, more than 90% of the Higd-1a–Flag-transfected cells retained their original morphology (Fig. S4). It should be noted, however, that at this point both mock- and Higd-1a–Flag-transfected cells yielded similar amounts of viable cells, and their mitochondria reverted to their filamentous morphology after CCCP washout. Therefore, we examined the effect of extended CCCP treatment. Cells were treated with 10–50 µM CCCP for 1 h, and after 24 h of washout, total viable cells were counted by using Trypan blue exclusion. Following treatment with 10–15 µM, there were two- to threefold more viable Higd-1a–Flag-transfected cells than viable mock-transfected cells (Fig. 5D). Based on these observations, we conclude that Higd-1a at least partially inhibits cleavage of Opa1, thus conserving mitochondrial morphology and prolonging cell survival.
Fig. 5.
Higd-1a overexpression delays cleavage of the Opa1 L isomer. (A and B) HEK293T cells were transfected with mock or Higd-1a–Flag vector for 2 d and incubated under hypoxia (0.1% O2) (A) or with 10 µM CCCP (B) for the indicated times. Total cell lysates were subjected to Western blotting by using anti-Opa1 or anti-Flag antibodies. Hsp60 protein levels are shown as loading controls. (C) HeLa cells were transfected with Higd-1a–Flag or Higd-1a–ΔNT-Flag vectors for 3 d and treated with 10 µM CCCP for 15 min. Cells were visualized under a confocal fluorescence microscope after staining with MitoTracker (red) and FITC-tagged anti-Flag (green). Merged confocal fluorescence images are shown in Right, and enlargements of boxed areas a-d are shown in Left Bottom. (Scale bar: 10 µm.) Approximately 100 FITC-negative (FITC−) and 100 FITC-positive (FITC+) cells were counted, and the percentages of each having more than 50% tubular, less than 50% tubular, and fragmented mitochondria were calculated (Right). (D) HEK293T cells were transfected with mock or Higd-1a–Flag vectors for 2 d and treated with 10–50 µM CCCP for 1 h. Twenty-four hours after CCCP washout, total viable cells were counted by Trypan blue exclusion. (E) The Opa1 isoform 1 and its Opa1-ΔS1 mutant were overexpressed in HEK293T with or without Higd-1a siRNA for 4 d, and cell growth was assessed by 3-(4,5-dimthylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Left). Expression of Opa1 and Opa1-ΔS1 proteins in cells not harboring Higd-1a siRNA was verified by Western blotting (Right). Results shown are representative of three independent experiments. Data are mean ± SE; ***P < 0.001.
Finally, we examined whether the expression of L Opa1 alleviated the growth retardation of Higd-1a–silenced cells. HEK293T cells were transfected with Higd-1a siRNA in the presence and absence of Opa1 or Opa1-ΔS1, a deletion mutant lacking a cleavage site S1 (9). As shown in Fig. 5E, the total cell proliferation of the Higd-1a–depleted cells was only 36% of that of the control cells. Although overexpression of wild-type Opa1 only increased a few additional cells (40.3 ± 9.7%), and its minimal effect probably resulted from cleavage of wild-type Opa1 in the Higd-1a–depleted cells, the presence of Opa1-ΔS1, which generates a single L isoform, significantly increased the number of viable cells (75.5 ± 2.2%). This experiment demonstrates that the roles of Higd-1a in mitochondria are closely connected with maintenance of the L isoforms of Opa1 and the preservation of mitochondrial morphology.
Discussion
Opa1 is essential for mitochondrial fusion, cristae organization, and cell apoptosis (9, 16, 17). Balanced expression of the L and S isoforms is a prerequisite for their optimal functioning, and further processing of the L isoform completely destroys Opa1 activity (9). In this study, we characterized the role of Higd-1a in mitochondria and showed that it regulates mitochondrial fusion by inhibiting cleavage of Opa1. Our finding is based on several observations. First, depletion of Higd-1a led to cleavage of L Opa1 into its S isoforms (Fig. 2 A and B). Second, the role of Opa1 in mitochondrial fusion and cristae organization required both the L and S isoforms and, thus, the cellular changes in Higd-1a-depleted cells, such as mitochondrial fragmentation, irregular and hollow-shaped cristae, severe loss of mtDNA, and poor cell growth, mimicked those seen in Opa1 knockdown cells (Figs. 1–3). Third, overexpression of Higd-1a inhibited the processing of Opa1 induced by hypoxia and CCCP (Fig. 5 A and B). Concurrently, mitochondrial fragmentation induced by CCCP treatment could be inhibited by wild-type Higd-1a, but not by Higd-1a–ΔNT, a mutant form of Higd-1a with 26 N-terminal amino acids deleted (Fig. 5C). Fourth, expression of L Opa1 opposed the inhibition of proliferation of Higd-1a–silenced cells (Fig. 5E).
Inducible Opa1 cleavage occurs under various conditions, mainly associated with severely decreased mitochondrial membrane potential, apoptosis, and decreased ATP levels (10, 12, 13). It is likely that under our experimental conditions, Higd-1a directly inhibited the processing of Opa1: We observed no evidence of apoptosis, dissipation of membrane potential, or reduced cellular ATP (Fig. 3 F and G and Fig. S1), and the idea that Higd-1a directly regulates Opa1 processing is also based on the coprecipitation of Opa1 with Higd-1a (Fig. 4). The N-terminal domain of Opa1 appears essential for its interaction with Higd-1a: Our experiments showed that the soluble S forms of Opa1 did not interact with Higd-1a at all. Although Mfn1 can also be immunoprecipitated by anti-Higd-1a, that interaction seems to be indirect, because Opa1 knockdown or cleavage eliminated not only the interaction of Opa1 with Higd-1a but also that of Mfn1 with Higd-1a. Moreover, we showed that deletion of the 26-aa N-terminal region of Higd-1a completely eliminated its interaction with Opa1 and activity in mitochondrial fusion (Figs. 4F and 5C). This region includes some highly conserved basic amino acids and is present in the mitochondrial intermembrane space proximal to the transmembrane domain (18). Thus, its position provides the opportunity for Higd-1a to approach the N-terminal domain of Opa1. Our results indicate that the interaction between Higd-1a and Opa1 is specific and probably related to Opa1 cleavage.
Our observations suggest that the role of Higd-1a in Opa1 cleavage can be explained in two different conditions of normal and stressed one. In normal cells, Higd-1a depletion directly disturbs the maintenance of the L Opa1 isoform and has several consequences that contribute to mitochondrial disorganization and decreased cell proliferation. The decreased proliferation is clearly a consequence of L Opa1 cleavage, as demonstrated in our reconstitution experiment with the noncleavable L Opa1 isoform (Opa1-ΔS1), which restored the cells’ proliferative capacity (Fig. 5E). Meanwhile, under conditions of cellular stress, Higd-1a overexpression is necessary, although not sufficient to completely inhibit Opa1 cleavage (Fig. 5 A and B). In other words, constitutive expression of Higd-1a is sufficient to maintain Opa1 integrity in normal conditions, and its overexpression alleviates cellular stress by delaying Opa1 cleavage, although it does not completely prevent it. The processing and preservation of Opa1 is regulated via a series of complex steps. Multiple proteases such as PARL, m-AAA, and OMA1 metalloprotease are known to be involved in the inducible cleavage (14, 15, 32). OMA1, in particular, appears to play a key role in Opa1 cleavage in the absence of m-AAA proteases, and its activity is thought to be controlled by protease-dependent digestion. It has also been postulated that Opa1 cleavage is regulated by spatial sequestration in lipid microdomains of the inner membrane with the help of prohibitin complexes that bind to m-AAA proteases (33–35). Finally, Opa1 cleavage may depend on the type of stress and tissue, both of which affect the expression of proteases. To sum up, cleavage of Opa1 appears to be tightly regulated by different sets of proteases and some inner membrane proteins in response to external stimuli. Based on our observations, Higd-1a expression alone is unlikely to completely protect Opa1 against cleavages induced by the different proteases and stress stimuli. However, although this protection is imperfect, it plays a crucial role in maintaining mitochondrial morphology and promoting cell survival. Specifically, overexpression of Higd-1a in our study clearly inhibited CCCP-induced mitochondrial fragmentation (Fig. 5C). In addition, when cells were treated with 10–20 µM CCCP and counted after CCCP washout, there were two- to threefold more total viable Higd-1a–transfected cells than viable mock-transfected cells (Fig. 5D). These findings are consistent with previous reports showing that Higd-1a promotes cell survival and opposes apoptosis caused by hypoxia and low glucose levels. Wang and coworkers showed that such apoptosis was inhibited by ectopic expression of Higd-1a in pancreatic β cells (18, 36). We have shown that hypoxia-induced apoptosis can be prevented by overexpression of Higd-1a in RAW264.7 macrophages (22).
Although Higd-1a is ubiquitous, it is induced by hypoxia in a HIF-1–dependent manner (22). The human and mouse forms of Higd-1a are highly expressed in the brain, heart, and kidney (18, 22). By regulating mitochondrial homeostasis, Higd-1a may permit these major organs to resist external stimuli such as hypoxia. Recently it was suggested that Higd-1a has another function in mitochondria, namely to bind and inhibit the mitochondrial γ-secretase, which digests a broad spectrum of substrates belonging to the family of type 1 transmembrane proteins including Notch and amyloid precursor protein (37). This functional role of Higd-1a was suggested by the observation that hypoxia-induced mitochondrial dysfunction was associated with amyloid β accumulation. To improve our understanding of the role Higd-1a plays in cellular responses to hypoxia and in the maintenance of mitochondrial integrity, and to develop strategies to combat hypoxia-related diseases, more detailed studies are warranted.
Materials and Methods
For further detail, please see SI Materials and Methods.
Antibodies, Reagents, Transfection Constructs, and siRNA Oligonucleotides.
Rabbit anti-Higd-1a antibody was raised against a synthetic peptide corresponding to residues 9–21 of human Higd-1a and further purified by affinity chromatography before use.
Mitochondrial Isolation, Immunoprecipitation, and Digitonin/Proteinase K Assays.
Mitochondrial samples each derived from 2 × 107 cells were prepared by using a mitochondrial isolation kit (Pierce), following option A of the manufacturer’s protocol.
Assessment of Cell Growth, Analysis of ATP Content, and Quantification of mtDNA.
ATP levels in total cell lysates, cytoplasm, and mitochondrial fractions were measured by using an ATP Lite Kit (Perkin-Elmer Life Sciences).
Fluorescence Microscopy and Transmission Electron Microscopy.
For the experiments in Fig. 1, HeLa cells were stained with 125 nM MitoTracker red CMXRos and fixed with formaldehyde.
BN-PAGE and MALDI-TOF.
For BN-PAGE, mitochondria were resuspended in 40 µL of buffer (0.75 M aminocaproic acid, 50 mM Bis-Tris at pH 7.0, and protease inhibitors) followed by the addition of 7.5 µL of 10% (wt/vol) n-dodecyl-d-maltopyranoside.
Supplementary Material
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by Ministry of Education Grant NRF-2010-359-C00025.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307170110/-/DCSupplemental.
References
- 1.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 2.Santel A, Fuller MT. Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 2001;114(Pt 5):867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 3.Rojo M, Legros F, Chateau D, Lombès A. Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci. 2002;115(Pt 8):1663–1674. doi: 10.1242/jcs.115.8.1663. [DOI] [PubMed] [Google Scholar]
- 4.Koshiba T, et al. Structural basis of mitochondrial tethering by mitofusin complexes. Science. 2004;305(5685):858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 5.Olichon A, et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523(1-3):171–176. doi: 10.1016/s0014-5793(02)02985-x. [DOI] [PubMed] [Google Scholar]
- 6.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA. 2004;101(45):15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12(8):2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delettre C, et al. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109(6):584–591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25(13):2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178(5):757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvezin-Caubet S, et al. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J Biol Chem. 2006;281(49):37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 13.Baricault L, et al. OPA1 cleavage depends on decreased mitochondrial ATP level and bivalent metals. Exp Cell Res. 2007;313(17):3800–3808. doi: 10.1016/j.yexcr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187(7):959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehses S, et al. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187(7):1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olichon A, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278(10):7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 17.Frezza C, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126(1):177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, et al. Pancreatic beta cells lack a low glucose and O2-inducible mitochondrial protein that augments cell survival. Proc Natl Acad Sci USA. 2006;103(28):10636–10641. doi: 10.1073/pnas.0604194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denko N, et al. Epigenetic regulation of gene expression in cervical cancer cells by the tumor microenvironment. Clin Cancer Res. 2000;6(2):480–487. [PubMed] [Google Scholar]
- 20.Jin K, et al. cDNA microarray analysis of changes in gene expression induced by neuronal hypoxia in vitro. Neurochem Res. 2002;27(10):1105–1112. doi: 10.1023/a:1020913123054. [DOI] [PubMed] [Google Scholar]
- 21.Bedo G, Vargas M, Ferreiro MJ, Chalar C, Agrati D. Characterization of hypoxia induced gene 1: Expression during rat central nervous system maturation and evidence of antisense RNA expression. Int J Dev Biol. 2005;49(4):431–436. doi: 10.1387/ijdb.041901gb. [DOI] [PubMed] [Google Scholar]
- 22.An HJ, et al. The survival effect of mitochondrial Higd-1a is associated with suppression of cytochrome C release and prevention of caspase activation. Biochim Biophys Acta. 2011;1813(12):2088–2098. doi: 10.1016/j.bbamcr.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem. 2003;134(3):333–344. doi: 10.1093/jb/mvg150. [DOI] [PubMed] [Google Scholar]
- 24.Möpert K, et al. Loss of Drp1 function alters OPA1 processing and changes mitochondrial membrane organization. Exp Cell Res. 2009;315(13):2165–2180. doi: 10.1016/j.yexcr.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Hudson G, et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: A novel disorder of mtDNA maintenance. Brain. 2008;131(Pt 2):329–337. doi: 10.1093/brain/awm272. [DOI] [PubMed] [Google Scholar]
- 26.Amati-Bonneau P, et al. OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain. 2008;131(Pt 2):338–351. doi: 10.1093/brain/awm298. [DOI] [PubMed] [Google Scholar]
- 27.Parone PA, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE. 2008;3(9):e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141(2):280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landes T, et al. The BH3-only Bnip3 binds to the dynamin Opa1 to promote mitochondrial fragmentation and apoptosis by distinct mechanisms. EMBO Rep. 2010;11(6):459–465. doi: 10.1038/embor.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillery O, et al. Metalloprotease-mediated OPA1 processing is modulated by the mitochondrial membrane potential. Biol Cell. 2008;100(5):315–325. doi: 10.1042/BC20070110. [DOI] [PubMed] [Google Scholar]
- 31.Wada J, Kanwar YS. Characterization of mammalian translocase of inner mitochondrial membrane (Tim44) isolated from diabetic newborn mouse kidney. Proc Natl Acad Sci USA. 1998;95(1):144–149. doi: 10.1073/pnas.95.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cipolat S, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126(1):163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Steglich G, Neupert W, Langer T. Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol Cell Biol. 1999;19(5):3435–3442. doi: 10.1128/mcb.19.5.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osman C, Merkwirth C, Langer T. Prohibitins and the functional compartmentalization of mitochondrial membranes. J Cell Sci. 2009;122(Pt 21):3823–3830. doi: 10.1242/jcs.037655. [DOI] [PubMed] [Google Scholar]
- 35.Merkwirth C, et al. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22(4):476–488. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, et al. β-Cells with relative low HIMP1 overexpression levels in a transgenic mouse line enhance basal insulin production and hypoxia/hypoglycemia tolerance. PLoS ONE. 2012;7(3):e34126. doi: 10.1371/journal.pone.0034126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi H, et al. HIG1, a novel regulator of mitochondrial γ-secretase, maintains normal mitochondrial function. FASEB J. 2012;26(6):2306–2317. doi: 10.1096/fj.11-196063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.