Abstract
Skin homeostasis is critical to preserve animal integrity. Although the skin of most vertebrates is known to contain a skin-associated lymphoid tissue (SALT), very little is known about skin B-cell responses as well as their evolutionary origins. Teleost fish represent the most ancient bony vertebrates containing a SALT. Due to its lack of keratinization, teleost skin possesses living epithelial cells in direct contact with the water medium. Interestingly, teleost SALT structurally resembles that of the gut-associated lymphoid tissue, and it possesses a diverse microbiota. Thus, we hypothesized that, because teleost SALT and gut-associated lymphoid tissue have probably been subjected to similar evolutionary selective forces, their B-cell responses would be analogous. Confirming this hypothesis, we show that IgT, a teleost immunoglobulin specialized in gut immunity, plays the prevailing role in skin mucosal immunity. We found that IgT+ B cells represent the major B-cell subset in the skin epidermis and that IgT is mainly present in polymeric form in the skin mucus. Critically, we found that the majority of the skin microbiota are coated with IgT. Moreover, IgT responses against a skin parasite were mainly limited to the skin whereas IgM responses were almost exclusively detected in the serum. Strikingly, we found that the teleost skin mucosa showed key features of mammalian mucosal surfaces exhibiting a mucosa-associated lymphoid tissue. Thus, from an evolutionary viewpoint, our findings suggest that, regardless of their phylogenetic origin and tissue localization, the chief immunoglobulins of all mucosa-associated lymphoid tissue operate under the guidance of primordially conserved principles.
Keywords: evolution, mucosal immunoglobulin, Ichthyophthirius multifiliis, cutaneous
The skin is the outermost organ of the body and the first line of defense from external aggressions. In all vertebrates, the skin presents a similar structure made up of two main layers, the epidermis and the dermis. It should be noted that the skin of all vertebrates has the same embryonic origin (1), with the epidermis originating from the embryonic ectoderm whereas the underlying layers have a mesodermic origin (2). Therefore, it would appear that the histological differences seen in the skin of vertebrates (i.e., scales, feathers, or hair, and secretion of mucus or sweat) are mainly the result of their adaptation into water-containing or dry-land environments. Interestingly, early vertebrates like fish originated in aquatic environments, and therefore their skin behaves as a mucosal surface that harbors abundant mucus-producing cells, lacks keratinization, and possesses living epithelial cells in direct contact with the water medium (3). When vertebrates colonized the land, their skin underwent important evolutionary changes, including loss of mucus production, acquisition of dead keratinized cell layers, and other adaptations such as hair follicles and sweat glands in the case of mammals (1). Thus, in contrast to the skin of mammals, that of fish can be considered a mucosal tissue.
Teleost fish represent the oldest living bony vertebrates exhibiting a skin-associated adaptive immune system (4), and containing immunoglobulins (Igs) (5). Only three Ig classes: IgM, IgD, and IgT/Z have been identified thus far in teleosts (3). Teleost IgM is by far the most prevalent Ig in plasma and is the principal Ig involved in systemic immunity although it has also been shown to be involved in mucosal responses (3). In that regard, antigen-specific IgM, albeit low, has been demonstrated in teleost gut and skin mucus (3). Although secreted IgD has been identified in the plasma of teleost species (6), its function remains unknown. Additionally, teleosts contain IgT (also called IgZ in some species), an Ig class first identified in 2005 (7, 8). In 2010, we demonstrated that, analogous to mammalian IgA, teleost IgT plays a specialized role in gut mucosal immune responses whereas IgM contributes predominantly to systemic immunity (9). This finding overturned the paradigm that specialized mucosal immunoglobulins arose during tetrapod evolution. Thus far, IgT represents the most ancient Ig specialized in mucosal immunity.
As previously stated, in contrast to the skin of mammals, that of fish can be considered a mucosal tissue. Thus, whether the adaptive immune response of teleost skin bears a resemblance to that from higher vertebrates or that of mucosal surfaces is an enigmatic question that we wanted to address. Although poorly understood, teleost fish have been shown to contain a skin-associated lymphoid tissue (SALT) (3). It is worth noting that the term SALT was first coined in mammals and that, in these species, the skin is not considered a mucosa-associated lymphoid tissue (MALT) (10, 11). The main cellular constituents of this tissue include epidermal keratinocytes, intraepidermal Langerhans cells (LCs) as antigen-presenting cells, the skin-homing T cells, dendritic epidermal T cells (DETCs), and endothelial cells. In addition, the mammalian SALT includes also the skin-draining lymph nodes where induction of immunity occurs by antigens that have been processed and transported by LCs. Thus far, very little is known about the components of the teleost SALT although it has been reported to contain secretory cells that produce mucus (i.e., goblet cells), lymphocytes, granulocytes, macrophages, and Langerhans-like cells (3, 12, 13). Because the skin of fish lacks keratinization and is coated by a mucosal layer and because fish don’t contain organized lymphoid structures, the teleost SALT cannot be considered a counterpart of that of mammals. Interestingly, the structure of the teleost SALT resembles that of the gut-associated lymphoid tissue (GALT), and, similar to the fish gut, the skin mucosa harbors a large and diverse microbiota (14). Thus, we hypothesized that because teleost SALT and GALT have probably been subjected to similar evolutionary selective forces, their B-cell responses would be comparable.
To gain evidence for the aforementioned hypothesis and to obtain further insight into the evolution of skin-associated B-cell responses, here we performed an in-depth study of cutaneous B-cell and Ig responses in an ancient vertebrate species. Confirming our hypothesis here, we show that B-cell and Ig responses in the skin of rainbow trout (Oncorhynchus mykiss), a teleost fish, are strikingly similar to those of their gut, and that IgT plays a prevailing role in skin mucosal immune responses.
Results
Protein Characterization of Skin Mucus IgT and Identification of IgT+ B Cells in Skin of Trout.
In 2010, we reported for the first time the protein characterization of gut and serum IgT of rainbow trout (9). Here, we used antibodies raised against this Ig to detect by immunoblot a protein in skin mucus consistent with the reported molecular mass of IgT. To gain insights into the identity of this immunoreactive band, putative skin mucus IgT was isolated by affinity purification with an anti-IgT affinity column, and the immunoreactive IgT band was subjected to liquid chromatography–tandem mass spectrometry (LC-MS/MS). This technique confirmed that the immunoreactive purified protein corresponded to IgT (Fig. S1). As a first step in the protein characterization of skin IgT, we collected and processed skin mucus and loaded it into a gel filtration column. As reported for gut mucus IgT, most of the IgT present in the skin mucus was found in polymeric form, as it eluted at a fraction similar to that of trout IgM, a tetrameric Ig (Fig. 1A). However, by SDS/PAGE under nonreducing conditions, skin polymeric IgT migrated in the same position as monomeric IgT (Fig. 1B, red arrowhead), similar to what has previously been observed for gut mucosal or serum IgT (9). This finding indicates that the monomeric subunits of skin polymeric IgT are associated by noncovalent interactions. In contrast, under the same SDS/PAGE conditions, skin IgM migrated as polymeric (Fig. 1B, Left). Next, we analyzed the protein concentrations of IgT and IgM in skin mucus and serum, and we found that the concentration of IgT in both fluids was ∼15- and ∼465-fold lower than that of IgM, respectively (Fig. 1 C and D). Although the concentration of both immunoglobulins in the skin mucus was much lower than that in serum (Fig. 1 C and D), the ratio of IgT to IgM was 38-fold higher in the skin mucus compared with that of serum (Fig. 1E), thus suggesting that IgT is an important Ig in the skin mucosa. To study the specific abundance of IgT+ B cells in the skin, we adapted to rainbow trout a reported protocol for the isolation of leukocyte from catfish skin (15). Thereafter, we stained skin leukocytes with monoclonal antibodies against trout IgT and IgM and analyzed them by flow cytometry (Fig. 1F). We found that, although both IgT+ and IgM+ B cells were present at low percentages in the skin of trout (Fig. 1F), IgT+ B cells represented the main skin B-cell subset in this tissue (60.9% of all B cells) (Fig. 1G). Similar to what we previously found in other lymphoid tissues (9), we did not detect the presence of double positive IgT+IgM+ B cells (Fig. 1F). The preponderant presence of IgT+ B cells in skin suggested an important role of IgT in cutaneous mucosal immunity.
Fig. 1.
Structural characterization of IgT and IgT+ B cells in trout skin. (A) Fractionation of skin mucus by gel filtration (Upper) followed by immunoblot analysis of the fractions with anti-IgM and anti-IgT specific mAbs (Lower). A280, absorbance at 280 nm. (B) 4–15% SDS/PAGE of gel-filtration fractions corresponding to elution volumes of 8.5 mL and 11.5 mL under nonreducing conditions followed by immunoblot analysis with mAbs to trout IgM or IgT. Red arrowhead indicates IgT monomers. (C and D) Immunoblot and densitometric analysis of the concentration of IgT and IgM in skin mucus (C) and serum (D) (n = 12 fish). Data in C and D are representative of at least three independent experiments. (E) Serum and skin mucus IgT/IgM ratios calculated from the values shown in C and D. (F) Representative flow cytometry dot plot showing the percentage of IgT+ and IgM+ B cells from skin leukocytes. (G) Percentage of IgM+ and IgT+ B cells from the total B cells in skin. Results in G are expressed as mean ± SEM obtained from 15 individual fish.
Large Fraction of Trout Skin Bacteria Are Coated with IgT.
The gut mucosa in mammals is populated by a large and diverse community of bacterial microbiota. One important feature of secreted IgA (sIgA) is its capacity to prevent these bacteria from intruding into the gut epithelium. To this end, sIgA is known to coat a large percentage of these bacteria, thus playing a key role in immune exclusion in gut (16, 17). Similarly, we have previously shown that trout IgT is found coating a large fraction of the fish intestinal bacteria (9). Here, we tested the hypothesis that, similar to gut bacterial microbiota, bacteria found in the skin mucosa of fish would also be prevalently coated with IgT. To this end, we adapted to the skin mucosa the methodology we had used to assess Ig coating of trout gut luminal bacteria (9). Flow cytometry analysis showed that a large fraction of skin bacteria stained for IgT (∼38%) whereas a smaller proportion (∼12%) was coated with IgM (Fig. 2 A–C). Immunofluorescence microscopy substantiated the results obtained by flow cytometry, showing a predominance of bacteria stained with IgT and very few bacteria stained with IgM (Fig. 2 D–H; isotype-matched control antibodies, Fig. S2). Immunoblot analysis corroborated the presence of IgT or IgM on skin bacteria (Fig. 2I). Interestingly, we found that more than 50% of the IgT present in the skin mucus was coating bacteria whereas only 8% of skin mucosal IgM was being used for bacterial coating (Fig. 2J). All combined, these data suggested a key role of IgT in the control of skin bacterial microbiota.
Fig. 2.
A majority of trout skin bacteria are predominantly coated with IgT. (A and B) Histograms with representative IgT (A) and IgM (B) staining of 30,000 BacLight–positive skin bacteria. Bacteria were stained with anti-IgT or anti-IgM Abs (solid line) or their respective isotype controls (shaded histograms). (C) Percentage of bacteria coated with IgT or IgM from 17 individual fish. The median percentage is shown by a red line. Statistical differences between the percentage of bacteria coated with IgT or IgM were evaluated by the nonparametric Mann–Whitney test. (D–H) Differential interference contrast images of skin bacteria stained with a DAPI-Hoeschst solution (blue, D), BacLight (magenta, E), anti-IgM (red, F), anti-IgT (green, G), or merged IgT and IgM staining (H). (Isotype-matched control antibody staining in Fig. S2). (Scale bars, 5 μm.) (I) Immunoblot analysis of IgT and IgM on skin bacteria. Lane 1, 0.2 μg of purified IgT or IgM; lanes 2–8, skin bacteria (n = 7 fish). (J) Percentage of total skin mucus IgT and IgM coating skin bacteria. The median percentage is shown by a red line. Statistical differences were evaluated by the nonparametric Mann–Whitney test. Data are representative of at least three independent experiments.
IgT Is the Dominant Cutaneous Ig Responding to a Skin Parasite in Trout.
The prevalence of IgT+ B cells in the skin of trout, as well as the predominant coating of skin bacteria with IgT, led us to hypothesize an important role of IgT in skin mucosal immunity. To assess this hypothesis, we evaluated skin and serum immune responses in trout infected with the ciliated parasite Ichthyophtirius multifiliis (Ich). Ich is a parasite with a strong tropism for the skin of fish and is the causal agent of the white spot disease, a very widespread condition that affects farmed, wild, and aquarium fish in which the trophont feeding stage of the parasite is visible in the skin as small white spots (18).
Using immunofluorescence microscopy, we observed that the skin of noninfected control fish presented very few IgT+ B cells and even fewer IgM+ B cells (Fig. 3A), a finding that is in agreement with the results presented in Fig. 1F. The fish from the infected group (21 d postinfection) presented a significant number of visible trophonts in the skin. Although these fish showed a moderate increase in the number of IgT+ B cells in the skin epidermis, it was noticeable that the IgT+ B cells had a brighter IgT-associated fluorescence signal compared with those of control fish (Fig. 3B). Fish from the survivor group (3 mo postinfection) did not contain parasites or signs of infection on their skin. In this group, a notable accumulation of IgT+ B cells was observed in their skin (Fig. 3C), with several of these IgT+ B cells apparently secreting IgT (Fig. 3D). To assess whether there was an increase in putative IgT-secreting plasma cells as suggested by Fig. 3D, we performed flow cytometric intracellular staining using the anti-IgT antibody on skin leukocytes from control and survivor fish. As observed in Fig. S3, the percentage of large IgT+ B cells (putative plasma cells) increased by 4.1-fold in the skin of survivor fish compared with that of control.
Fig. 3.
Accumulation of IgT+ B cells in the skin epidermis of trout infected with Ich. Differential interference contrast (DIC) images of immunofluorescence staining on trout skin cryosections from uninfected control fish (A, n = 6), fish infected with Ich after 21 d (B, n = 9), and fish that survived Ich infection (C and D, n = 9), stained for IgT (green) and IgM (red); nuclei are stained with DAPI (blue) (isotype-matched control antibody staining, Fig. S5A). (D) Enlarged images of the areas outlined in C, without DIC, showing some IgT+ B cells possibly secreting IgT (white arrowhead). Skin structure is always displayed with the outside part of the epidermis (Ep) down, and the dermis (De) up. Scales (Sc) and melanophores (Me) are also indicated with white letters. (Scale bars, 20 μm.) Data are representative of at least three different independent experiments.
Interestingly the vast majority of IgT+ B cells were observed in the skin epidermis whereas a few of them localized in the dermis area between the scales, and almost none in the basal dermis layer (Fig. S4; isotype-matched control antibodies, Fig. S5A). In contrast to the increase of IgT+ B-cell number observed in infected and survivor fish, there was no change in the number of IgM+ B cells in these groups compared with the controls (Fig. 3 A–C).
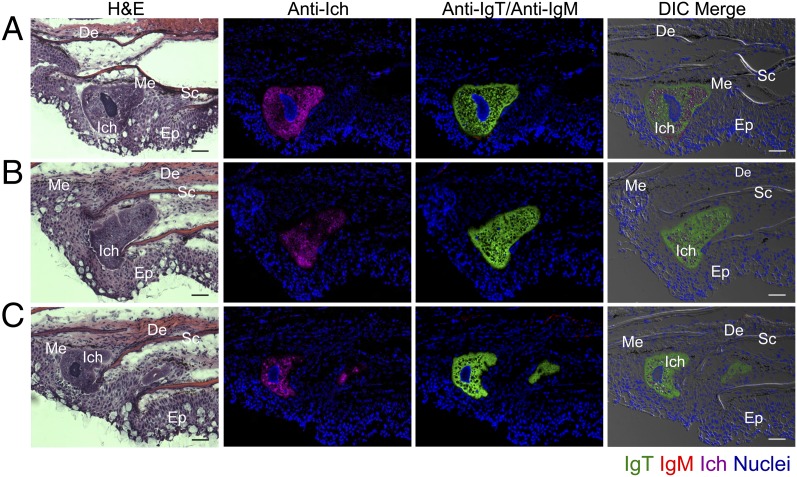
In the fish of the infected group, Ich trophonts (up to 200 μm in size) were localized within the skin epidermis and were easily identified by hematoxylin/eosin staining or anti-Ich antibody (Fig. 4 A–C). Importantly, all of these parasites were intensely stained with anti-IgT, but not with anti-IgM antibodies (Fig. 4 A–C; isotype-matched control antibodies, Fig. S5B). Cell count in the immunosections described above showed that the IgT+ B cells almost doubled in number in the skin epidermis of the infected fish whereas they increased a staggering ∼ninefold in survivors compared with control fish (Fig. 5A). In contrast, the number of IgM+ B cells did not change significantly in the same sections (Fig. 5A). In agreement with the greater abundance of IgT+ B cells in skin observed in infected and survivor fish, the IgT protein concentration in the skin mucus of the same animal groups also increased by ∼8- and ∼10-fold, respectively, whereas the levels of IgM remained unchanged (Fig. 5B). Interestingly, the serum concentration of IgT increased by ∼sixfold with respect to control fish, only in survivor fish, whereas that of IgM remained unchanged (Fig. 5C).
Fig. 4.
IgT coats Ich parasites located in the skin epidermis of 21 d infected fish. Three different microscope images (A–C) of consecutive slides of hematoxylin/eosin (H&E) staining and immunofluorescence staining of Ich parasites in skin cryosections from trout infected with Ich after 21 d (n = 5). From left to right: H&E staining; Ich (magenta) and nuclei stained with DAPI (blue); IgT (green), IgM (red) and nuclei stained with DAPI (blue); differential interference contrast (DIC) images showing merged staining (isotype-matched control antibody staining, Fig. S5B). Skin structure is always displayed with the outside part of the epidermis (Ep) down and the dermis (De) up. Scales (Sc), melanophores (Me), and Ich are also indicated with white letters. (Scale bars, 50 μm.) Data are representative of at least three different independent experiments.
Fig. 5.
Immune responses in the skin of trout infected with Ich are mediated by the IgT system. (A) Number of IgT+ and IgM+ B cells in skin cryosections of uninfected control fish, 21 d infected fish, and fish that survived infection with Ich, counted in average 10 fields. (Original magnification, 200×.) (B and C) Concentration of IgT and IgM in skin mucus (B) and serum (C) of control, infected, and survivor fish (n = 12–15 per group). (D) Immunoblot analysis of IgT and IgM specific binding to Ich in skin mucus (dilution 1:2) from infected and survivor fish. (E and F) IgT and IgM specific binding to Ich in dilutions of skin mucus from infected (E) and survivor (F) fish, measured by densitometric analysis of immunoblots and presented relative to values of control fish (n = 7 per group). (G) Immunoblot analysis of IgT and IgM specific binding to Ich in serum (dilution 1:10) from infected and survivor fish. (H and I) IgT and IgM specific binding to Ich in dilutions of serum from infected (H) and survivor (I) fish, measured by densitometric analysis of immunoblots and presented relative to values in control fish (n = 7 per group). *P < 0.05, **P < 0.01, and ***P < 0.001 (unpaired Student t test). Data are representative of at least three independent experiments (mean and SEM).
To assess whether infected and survivor fish produced parasite-specific immunoglobulins, we measured the capacity of serum and skin mucus IgT and IgM to bind to the parasite using a pull-down assay. Importantly, we detected parasite-specific binding of IgT, but not IgM, in the skin mucus of infected and survivor fish. Thus, we measured significant increases in parasite-specific IgT binding in up to 1/10 diluted skin mucus of infected and survivor fish, in which we found a ∼2.3- and ∼4.5-fold binding increase, respectively, compared with that of control skin mucus (Fig. 5 D–F). Conversely, in serum, IgM was the main parasite-specific Ig induced in infected and survivor fish. Accordingly, significant increases in parasite-specific IgM binding were observed in up to 1/100 (∼fourfold) and 1/1,000 (∼10-fold) serum dilutions from infected and survivors fish, respectively (Fig. 5 G–I). In contrast, IgT-specific binding was detected only at the 1/10 serum dilution of the survivor group, in which binding to the parasite was only ∼threefold higher than that of control serum (Fig. 5I). All these data support the notion that IgT is the main Ig responding against a parasite infection in the skin mucosa.
Polymeric Ig Receptor in the Skin of Rainbow Trout.
In mammals, immunoglobulins are transported to mucosal surfaces by making use of the polymeric Ig receptor (pIgR) (19). We have previously cloned a rainbow trout pIgR and generated antibodies to detect it, showing that its secretory component (SC) is found associated to gut mucosal IgT and IgM (9). In the present study, we investigated the association of pIgR with the skin mucus IgT and IgM, and its localization in the skin of rainbow trout. We detected by immunoblotting a trout secretory component-like molecule (tSC) in the skin mucus, but not in the serum (Fig. S6A). In addition, we found that both the anti-IgT and anti-IgM antibodies were able to coimmunoprecipitate tSC from the skin mucus in association with the immunoprecipitated IgT or IgM (Fig. S6B). Conversely, we were also able to coimmunoprecipitate IgT and IgM in the skin mucus using the anti-pIgR antibody (Fig. S6C). To identify trout pIgR-containing cells in the skin, we conducted immunofluorescence analysis, observing that a large portion of the skin epithelial cells localized at the interface between the epidermis and the mucus layer stained for pIgR, with some of them presenting also positive staining for IgT (Fig. S6 D and E) or at a lesser degree for IgM (Fig. S6 F and G; isotype-matched control antibodies, Fig. S5C).
Discussion
Here, we show that B-cell and Ig responses in the skin of rainbow trout, a teleost fish, are strikingly similar to those of their gut. The structural characteristics of IgT in skin mucus resemble those previously described by us for gut mucus IgT (9). Thus, IgT was present in skin mucus mainly as a polymer. In addition, the IgT/IgM ratio in skin is much higher than that found in serum. However, skin mucus IgT levels were significantly lower than those previously found in gut mucus. Thus, similar to the case of mammalian sIgA (20–22), differences exist in IgT levels depending on the mucosal surface examined. However, it is interesting to note that, whereas in most mammalian mucosal surfaces IgA is the most abundant Ig, under homeostatic conditions, trout IgM is more abundant than IgT in the gut (9) and the skin mucus (as shown in this study). In mammals, it has been reported that the production and abundance of IgA in the gut is dependent on both the presence and amount of microbiota respectively. In that regard, it has been shown that, in mice depleted of gut microbiota, the IgA levels are very low and that reconstitution of these mice with microbiota leads to a high production of IgA in the gut (23). Thus, we speculate that, in mammals, the relative amount of bacterial microbiota in mucosal surfaces is far superior than that of fish, which may in turn induce a proportionally more elevated production of IgA, compared with that of IgT. To substantiate this hypothesis, future work will have to address the relative abundance of bacterial commensals in mucosal surfaces of fish and mammals.
Comparable to what we described for the gut mucus, here we found that the putative trout secretory component (tSC) of TpIgR was associated with both skin mucus IgT and IgM. Moreover, we also found that TpIgR was expressed in the epithelial layer of the skin. These findings support the idea that skin mucosal IgT and IgM require the association to TpIgR for their transport into the skin mucosal surface. Our results concur with those reported on pIgR from fugu fish in which the putative fugu SC was found associated to skin mucus IgM and fugu pIgR was also found expressed on the epithelial layer of the skin (24). Nevertheless, association of fugu pIgR with IgT was not addressed in the previous study.
Mammalian sIgA plays a key role in immune exclusion by coating a portion of the bacterial microbiota of the gut (17),skin (25), mouth (26), nose (27), and vagina (28). Except for the gut, it is unclear whether sIgA is the main Ig isotype participating in the bacterial coating of the microbiota at the other mucosal surfaces. As in the gut, the rainbow trout skin mucus contains high densities of bacterial microbiota (14). Here, we show that IgT was the main Ig coating bacteria from the skin microbiota. This finding represents a unique example of a nonmammalian mucosal Ig playing a dominant role in the recognition and coating of the bacterial microbiota outside of the gut luminal area. Importantly, this finding may point to a conserved role of mucosal immunoglobulins in the control of the bacterial microbiota at all mucosal surfaces.
Here, we show that large accumulation of IgT+ but not IgM+ B cells occurred in the skin epidermis of infected and surviving fish exposed to Ich. These data correlated with large increases in the concentration of IgT but not IgM in the skin mucus of the same animals. Interestingly, these results parallel those obtained in our previous study showing that fish surviving infection with Ceratomyxa shasta, a gut parasite, also exhibited large accumulations of IgT and IgT+ B cells in the gut (9). In addition, infection with Ich induced pathogen-specific IgT titers mainly in the skin mucus and to a much lesser degree in the serum. Strikingly, pathogen-specific IgM titers were detected only in the serum. Similar to what we had previously observed in the gut (9), these results indicate a compartmentalization of IgT- and IgM-specific responses in skin mucosa and systemic areas, respectively. These findings strongly indicate that IgT responses are specifically confined to all mucosal areas of the fish whereas IgM responses are typically of systemic nature.
The reported skin compartmentalized responses suggest that the inductive sites for IgT and IgM responses are localized in different lymphoid sites. It would appear that the inductive site for the observed IgT responses is the skin because IgT-specific titers against Ich in infected animals can be observed only in the skin mucus and not in the serum. In addition, these specific titers in the skin mucus correlate with a significant increase in the number of skin IgT+ B cells. Taking into account that this parasite stays in the skin, it is unlikely that the inductive site is another lymphoid tissue. As for the inductive site of IgM responses, IgM-specific titers against Ich in infected animals can be observed only in serum. These data suggest that the inductive site for IgM is not the skin, but a systemic organ (i.e., spleen or head-kidney). In that regard, it will be of interest to elucidate how the antigen reaches these organs to induce the IgM response. Interestingly, because this skin infection induces the generation of both a systemic IgM and local IgT response against Ich, it would seem as if the trout SALT is somehow a combination of mammalian SALT (which generates systemic immunity) and MALT (which generates local immunity).
Overall, the reported data strongly suggest that, in the skin, IgT would play a major role in the neutralization of both the bacterial microbiota and pathogens whereas the role of IgM would be limited to the neutralization of a small fraction of the microbiota. As shown here, the Ich infection induced only skin IgT but not IgM responses. Given the fact that IgT+ and IgM+ B-cell numbers are not that dissimilar under homeostatic conditions, these differential IgM and IgT responses against Ich are difficult to explain at this point. However, it is tempting to speculate that this disparity in the response is caused by potential differences in chemokine receptors expressed by IgM+ and IgT+ B cells. Accordingly, the skin of infected fish would produce a chemokine that would initially attract only the B-cell subset (IgT+ B cells) expressing its receptor. This hypothesis is based on the very significant accumulation of IgT+ but not IgM+ B cells on the skin of infected and survivor fish. However, whether this accumulation of IgT+ B cells is solely the result of infiltration of cells into the infected area or also the product of subsequent local B-cell proliferation is presently unknown. Whether IgT+ B cells generated in the skin can later be found dispersed to all mucosal sites is a question that remains to be investigated. We speculate that this “common mucosal immune response” will greatly depend on either the shared or the exclusive expression of adhesion and chemokine receptor pairs in all mucosal sites.
With regard to skin antibody and B-cell responses against Ich, those have been reported for catfish only in which infection of the skin by the parasite induces specific IgM responses in the skin mucus (15). Interestingly, catfish appear to lack IgT whereas all other studied teleosts contain it (3, 29); therefore, it is possible that this species use IgM for both mucosal and systemic responses. From a more general perspective, skin Ig responses have been poorly studied in teleosts, and the involvement of IgT has never been addressed. The limited research in that area appears to indicate that natural skin infections in teleosts rarely induce pathogen-specific IgM titers in skin mucus (3). In that regard, our results support this restricted IgM response in teleosts. Moreover, it has been reported that fish vaccination by bath or immersion rarely induces IgM-specific skin responses and that, when present, such responses are poor (3). Thus, this lack or restriction of skin IgM responses upon natural infection or immersion vaccination may indicate a deficiency of teleost fish in stimulating local IgM immunity in the skin. In contrast, skin IgT immunity can be effectively mounted in response to a local skin infection, as evidenced here by our results, thus supporting a specialized role of IgT in local mucosal responses. Whether similar IgT responses can be induced by bath or i.p. vaccination remains to be investigated.
Here, we have shown that trout SALT contains many common features with MALT-containing mammalian mucosal surfaces (Table S1) such as those of the gut, lung, uterus, and nose (30). In addition, immune responses in the aforementioned mammalian mucosal surfaces present also commonalities with those observed here in the fish skin. Thus, it has also been shown that microbial infections in these mammalian mucosal surfaces generally induce a local mucosal response governed by a mucosal Ig (sIgA) and that, in some cases, the systemic response is dominated by IgG/IgM (31, 32). Moreover, similar to the accumulations of IgT+ B cells shown here in the skin epidermis of infected fish, significant accumulations of B cells have also been described upon infection in the MALT of nose (33), gut (16, 34), lung (35), and uterus (36). Interestingly the only tetrapod skin known to be coated by a mucus layer is that of amphibians. However, like that of mammals, amphibian skin is keratinized (1). One report has shown the presence of IgY, IgX, and IgM in skin mucus of frog (37). Whether IgX represents the prevalent skin mucosal Ig in amphibians remains to be determined.
In conclusion, here we report that, in contrast to the situation of mammals, secretory Ig and B-cell responses in rainbow trout SALT parallel those previously observed by us in its GALT (9). From an evolutionary viewpoint, our findings suggest that, regardless of their phylogenetic origin and tissue localization, mucosal immunoglobulins of MALT-containing surfaces operate under the guidance of primordially conserved principles. Moreover, our findings in this phylogenetically primitive vertebrate strongly suggest a universal requirement for vertebrate MALT-containing mucosal surfaces to use a dedicated Ig for maintenance of homeostasis. Dedicated mucosal immunoglobulins in these surfaces have thus far been detected in all studied bony vertebrates, from teleosts to mammals. Based on the aforementioned hypothesis, it is tempting to predict that phylogenetically older nonbony vertebrates, including lampreys and sharks, will also contain dedicated antigen receptors [i.e., immunoglobulins in sharks and variable lymphocyte receptors (VLRs) in lampreys] in analogous mucosal surfaces. Further studies on the fish SALT and the IgT system will contribute to revealing some of these principles common to fish and mammals. In that regard, recent comparative studies on the fish immune system have been instrumental in unraveling previously unrecognized aspects of mammalian immunity (38). Additionally, from a more practical perspective, it is well known that the fish skin is a major portal of pathogens for which vaccines are still unavailable, including that used in this study. Thus, the knowledge derived from our findings will be critical for the future evaluation and rational design of fish vaccines that effectively stimulate IgT skin responses, a parameter thus far never assessed in the last 30 y of fish vaccine development.
Materials and Methods
Fish maintenance and Ich isolation are described in SI Materials and Methods. Two types of challenges with Ich were performed. In the first one (infected group), fish were exposed to a single dose of 5,000 theronts per fish added into the aquarium, and fish samples were taken after 21 d. For the second type of challenge (survivor group), fish were monthly exposed during 3 mo to 10,000 theronts per fish and subsequently challenged with a dose of 10,000 theronts per fish; fish samples were taken 2 h after this last challenge. Both sets of infections were performed at least three times. Control fish were maintained in a similar tank without being exposed to parasites. Serum, skin tissue, and skin mucus from all fish groups were collected as described in SI Materials and Methods.
All animal procedures were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania and the Committee for Animal Experimentation, Ministry of Law, Copenhagen.
Isolation of trout skin bacteria and leukocytes, SDS/PAGE, Western blot, flow cytometry, gel filtration, immunofluorescence microscopy, intracellular staining, binding assay, coimmunoprecipitation studies, and statistical analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Molecular Pathology and Imaging Core (University of Pennsylvania), funded by the Center for Molecular Studies in Digestive and Liver Diseases (NIH P30 DK050306), and especially Adam Bedenbaugh for technical assistance and advice provided for the immunohistochemistry work. We especially thank Bryan Vorbach for his contribution in immunoblot analysis. This work was supported by the National Science Foundation Grant NSF-MCB-0719599 (to J.O.S.) and National Institutes of Health Grant R01GM085207 (to J.O.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304319110/-/DCSupplemental.
References
- 1.Schempp C, Emde M, Wölfle U. Dermatology in the Darwin anniversary. Part 1: Evolution of the integument. J Dtsch Dermatol Ges. 2009;7(9):750–757. doi: 10.1111/j.1610-0387.2009.07193.x. [DOI] [PubMed] [Google Scholar]
- 2. Kanitakis J (2002) Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol 12(4):390–399. [PubMed]
- 3.Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev Comp Immunol. 2011;35(12):1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wölfle U, Martin S, Emde M, Schempp C. Dermatology in the Darwin anniversary. Part 2: Evolution of the skin-associated immune system. J Dtsch Dermatol Ges. 2009;7(10):862–869. doi: 10.1111/j.1610-0387.2009.07202.x. [DOI] [PubMed] [Google Scholar]
- 5.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat Rev Genet. 2010;11(1):47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramirez-Gomez F, et al. Discovery and characterization of secretory IgD in rainbow trout: Secretory IgD is produced through a novel splicing mechanism. J Immunol. 2012;188(3):1341–1349. doi: 10.4049/jimmunol.1101938. [DOI] [PubMed] [Google Scholar]
- 7.Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nature Immunology. 2005;6(3):295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 8.Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci USA. 2005;102(19):6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YA, et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11(9):827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: Nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008;1(1):31–37. doi: 10.1038/mi.2007.9. [DOI] [PubMed] [Google Scholar]
- 11.Streilein JW. Skin-associated lymphoid tissues (SALT): Origins and functions. J Invest Dermatol. 1983;80(Suppl):12s–16s. doi: 10.1111/1523-1747.ep12536743. [DOI] [PubMed] [Google Scholar]
- 12.Lugo-Villarino G, et al. Identification of dendritic antigen-presenting cells in the zebrafish. Proc Natl Acad Sci USA. 2010;107(36):15850–15855. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovy J, Wright GM, Speare DJ. Comparative cellular morphology suggesting the existence of resident dendritic cells within immune organs of salmonids. Anat Rec (Hoboken) 2008;291(4):456–462. doi: 10.1002/ar.20674. [DOI] [PubMed] [Google Scholar]
- 14.Austin B. The bacterial microflora of fish, revised. ScientificWorldJournal. 2006;6:931–945. doi: 10.1100/tsw.2006.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X, Findly RC, Dickerson HW. Cutaneous antibody-secreting cells and B cells in a teleost fish. Dev Comp Immunol. 2008;32(5):500–508. doi: 10.1016/j.dci.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28(6):740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokes CR, Soothill JF, Turner MW. Immune exclusion is a function of IgA. Nature. 1975;255(5511):745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- 18.Dickerson H, Clark T. Ichthyophthirius multifiliis: A model of cutaneous infection and immunity in fishes. Immunol Rev. 1998;166:377–384. doi: 10.1111/j.1600-065x.1998.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 19.Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- 20.Aufricht C, et al. Salivary IgA concentration is influenced by the saliva collection method. Eur J Clin Chem Clin Biochem. 1992;30(2):81–83. [PubMed] [Google Scholar]
- 21.Powell KR, Shorr R, Cherry JD, Hendley JO. Improved method for collection of nasal mucus. J Infect Dis. 1977;136(1):109–111. doi: 10.1093/infdis/136.1.109. [DOI] [PubMed] [Google Scholar]
- 22.Okada T, Konishi H, Ito M, Nagura H, Asai J. Identification of secretory immunoglobulin A in human sweat and sweat glands. J Invest Dermatol. 1988;90(5):648–651. doi: 10.1111/1523-1747.ep12560807. [DOI] [PubMed] [Google Scholar]
- 23.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamuro K, Suetake H, Saha NR, Kikuchi K, Suzuki Y. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin. J Immunol. 2007;178(9):5682–5689. doi: 10.4049/jimmunol.178.9.5682. [DOI] [PubMed] [Google Scholar]
- 25.Metze D, Kersten A, Jurecka W, Gebhart W. Immunoglobulins coat microorganisms of skin surface: A comparative immunohistochemical and ultrastructural study of cutaneous and oral microbial symbionts. J Invest Dermatol. 1991;96(4):439–445. doi: 10.1111/1523-1747.ep12469908. [DOI] [PubMed] [Google Scholar]
- 26.Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62(1):71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirkeby L, Rasmussen TT, Reinholdt J, Kilian M. Immunoglobulins in nasal secretions of healthy humans: Structural integrity of secretory immunoglobulin A1 (IgA1) and occurrence of neutralizing antibodies to IgA1 proteases of nasal bacteria. Clin Diagn Lab Immunol. 2000;7(1):31–39. doi: 10.1128/cdli.7.1.31-39.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajan N, et al. Roles of glycoproteins and oligosaccharides found in human vaginal fluid in bacterial adherence. Infect Immun. 1999;67(10):5027–5032. doi: 10.1128/iai.67.10.5027-5032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bengtén E, et al. Structure of the catfish IGH locus: Analysis of the region including the single functional IGHM gene. Immunogenetics. 2006;58(10):831–844. doi: 10.1007/s00251-006-0139-9. [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 31.Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. 2006;208(2):270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 32.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 33.Tamura S, et al. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J Gen Virol. 1998;79(Pt 2):291–299. doi: 10.1099/0022-1317-79-2-291. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Ha SA, Tsuji M, Fagarasan S. Intestinal IgA synthesis: A primitive form of adaptive immunity that regulates microbial communities in the gut. Semin Immunol. 2007;19(2):127–135. doi: 10.1016/j.smim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Onodera T, et al. Memory B cells in the lung participate in protective humoral immune responses to pulmonary influenza virus reinfection. Proc Natl Acad Sci USA. 2012;109(7):2485–2490. doi: 10.1073/pnas.1115369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milligan GN, Bernstein DI. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology. 1995;206(1):234–241. doi: 10.1016/s0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect Immun. 2010;78(9):3981–3992. doi: 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunyer JO. Fishing for mammalian paradigms in the teleost immune system. Nat Immunol. 2013;14(4):320–326. doi: 10.1038/ni.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.