Abstract
Primates explore the visual world through the use of saccadic eye movements. Neuronal activity in the hippocampus, a structure known to be essential for memory, is modulated by this saccadic activity, but the relationship between visual exploration through saccades and memory formation is not well understood. Here, we identify a link between theta-band (3–12 Hz) oscillatory activity in the hippocampus and saccadic activity in monkeys performing a recognition memory task. As monkeys freely explored novel images, saccades produced a theta-band phase reset, and the reliability of this phase reset was predictive of subsequent recognition. In addition, enhanced theta-band power before stimulus onset predicted stronger stimulus encoding. Together, these data suggest that hippocampal theta-band oscillations act in concert with active exploration in the primate and possibly serve to establish the optimal conditions for stimulus encoding.
The use of saccadic eye movements to acquire information about the surrounding environment is perhaps the most conspicuous example of exploratory behavior in the primate. This behavior provides a mechanism for parsing incoming information into discrete, stable segments (i.e., snapshots of individual elements comprising a complex visual scene, allowing time for sufficient processing to occur before moving to the next fixation target). This mechanism of actively sampling sensory information from the environment may be similar to the behaviors engaged in by rodents exploring their environment through such activities as sniffing and whisking. Specifically, the fixation period following each saccade may be homologous to the period of incoming sensory information accompanying each sniff cycle in the rodent (1). Recently, it has been suggested that motor behaviors associated with information gathering are integral to the “active sensing” process in natural behavior (2). It is plausible that there may exist certain common neuronal elements across species that are associated with active sensing processes, such that the neuronal mechanisms underlying the encoding of information are intimately connected with the motor activities involved in acquiring that information.
In many mammalian species, voluntary, exploratory behaviors are often associated with theta-band activity, a prominent 3- to 12-Hz oscillatory activity in the hippocampus and other limbic structures. This activity has been studied extensively in the rodent hippocampus (3–5), but it has also been described in bats (6), cats (7), and, more recently, humans (8–11). In rodents, theta appears to show close temporal relationships with running (3, 12) and sniffing (13), suggesting an association between theta and the rate of sensory input. Although hippocampal theta has been identified in anesthetized monkeys (14), the lack of a clear demonstration of hippocampal theta in awake monkeys has been attributed to the fact that the recording methods typically require immobile, head-affixed monkeys, in contrast to rodent studies using freely moving animals (15). However, it is possible that exploration through saccadic eye movements may approximate the sensory acquisition that rodents engage in as they explore their physical environment. Accordingly, we examined hippocampal activity related to visual exploration and memory formation in monkeys performing a free-viewing visual recognition memory task.
Results
Behavioral Results.
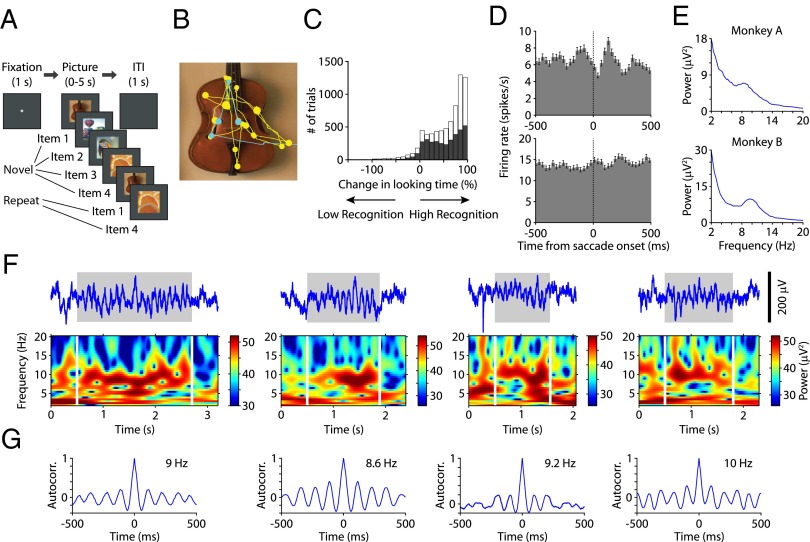
We recorded hippocampal activity in two rhesus monkeys performing the visual preferential looking task (VPLT) (16–18) (Fig. 1A). In each recording session, monkeys were presented with 200 novel complex stimuli (11° × 11° in size) on a computer screen. Each stimulus was presented twice during a given session, with up to 8 intervening stimuli between successive presentations. The eye movements of the monkeys were measured, and each stimulus remained on the screen until the monkey’s gaze moved off the stimulus or for a maximum of 5 s. Fig. 1B depicts an example of a monkey’s eye movements during the first (“novel,” yellow trace) and second (“repeat,” blue trace) presentations of a stimulus. Monkeys demonstrated recognition memory by spending less time exploring the stimulus when it was repeated compared with when it was novel. Across 45 sessions, the monkeys demonstrated robust recognition memory performance. There was a significant decrease in looking time for the repeated presentation (average looking times for novel and repeat trials were 2.7 s and 0.8 s, respectively; paired t test, P < 0.001; Fig. 1C). To control for varying interest in individual stimuli, recognition memory performance was calculated as the difference in looking time between presentations expressed as a percentage of the amount of time the monkey spent looking at the first presentation of each stimulus. The median reduction in looking time was 70.7% (67.3% in monkey A and 72.8% in monkey B; Fig. 1D). Similar tasks examining recognition memory through preference for novelty have been shown to depend on the integrity of the hippocampus in rodents, monkeys, and humans (19–22). Because the task involves minimal training and the monkeys are not explicitly rewarded for viewing the pictures, the behavior thus measured may approximate natural exploratory activity.
Fig. 1.
Task, behavioral results, and theta-band oscillations in the hippocampus. (A) VPLT design. (B) Representative example of one monkey’s scan path showing that the monkey spent more time looking at the image when it was novel (yellow) compared with when it was repeated (blue). Circles represent points of fixation between saccades, with the size of each circle proportional to the duration of the fixation period. (C) Percentage of change in looking time for all stimuli (gray, monkey A; white, monkey B). (D) Perievent histograms for two example units showing the cumulative firing activity across fixation periods during stimulus exploration. Error bars represent SEM. (E) Power spectra for two example LFP channels across all VPLT blocks showing peaks around 8–11 Hz. (F) Examples of theta bouts during VPLT performance. For each example, the theta bout in the raw LFP is marked by a gray square and the power spectrogram of the LFP signal is presented below. (G) Autocorrelograms (Autocorr.) of each example LFP shown in F during the theta bout.
Saccadic Modulation of Hippocampal Firing Rates.
We recorded single-unit activity and local field potentials (LFPs) from a total of 114 locations in the hippocampal formation of two rhesus monkeys (58 from monkey A and 56 from monkey B, primarily in the anterior hippocampal formation; recording locations are provided in ref. 18). Many units exhibited clear modulation of firing activity surrounding each saccade (Fig. 1E). Because this response was variable in time across units and was often biphasic in nature, we tested this modulation statistically using a template matching procedure (23), which allowed us to examine the consistency of the firing response pattern following each saccade. Based on this analysis, the spike patterns of 58 (44%) of 131 single units were predictable across individual saccades to a degree that was significantly greater than chance (test of proportions, P < 0.01). This proportion is in agreement with previously reported saccadic modulation in the monkey medial temporal lobe (MTL) (23, 24). In addition, we compared the incidence of saccade-modulated neurons with that of neurons that were significantly responsive to the onset of visual stimuli (16), and we found no correlation between the two distributions (Spearman’s rank correlation coefficient, ρ = 0.09; P > 0.1), indicating that the tendency of neurons to show saccade modulation was not simply a reflection of visual sensitivity.
Theta-Band Activity in Hippocampal LFPs.
Power spectral analysis of hippocampal LFPs during VPLT blocks revealed the presence of peaks throughout the theta frequency range (3–12 Hz), with the most prominent of these peaks occurring between 8 and 11 Hz (Fig. 1F and Fig. S1). The median saccade rate (5.1 Hz; Fig. S2) was outside the range of the peak hippocampal theta power observed in these data, yet it was within the 3- to 12-Hz theta band. Visual inspection of LFP signals revealed that unlike the long, continuous trains of oscillations seen in the rodent hippocampus during “active states,” oscillatory activity occurred in intermittent bouts separated by desynchronized, nontheta activity, similar to the activity previously described in the human hippocampus (8). Previous studies have evaluated the incidence of oscillatory components in continuous LFP data using the Pepisode measure (8, 25–27), which detects episodes of oscillatory activity exceeding amplitude and duration thresholds at each frequency of interest while ignoring the transient voltage fluctuations that may accompany artifacts or evoked potentials. We used this method to detect bouts of oscillatory activity in the theta frequency range that exceeded three cycles in duration and determined the incidence of these bouts across blocks of the VPLT. Four example bouts are depicted in Fig. 1F (Upper). The regular rhythmicity of these example oscillatory episodes was confirmed by calculating autocorrelograms of the LFP contained within each epoch, which revealed prominent oscillations in the 8- to 11-Hz range (Fig. 1G). Bouts of theta oscillations occurred with a median bout duration of 508 ms and a median interbout interval of 1,194 ms (Fig. S3). The average theta Pepisode across LFPs, taken across all components of the VPLT, was 0.24 ± 0.01. Similar measures, albeit with a lower incidence of bouts, were obtained from data recorded during a calibration task that monkeys performed during alternate blocks with the VPLT (SI Methods). As an additional measure to assess the prevalence of detected oscillations in particular frequency bands, the mean Pepisode of each LFP at each frequency was evaluated against the background value of 0.05, or 5%, using a two-tailed t test [P < 0.0014, after applying the Bonferroni correction (25)]. The resulting distribution reveals a majority of significantly high Pepisode values across LFPs falling within the range of 8–11 Hz (Fig. S4), indicating that bouts of oscillatory activity are most prevalent within this frequency band across hippocampal LFPs.
Saccade-Triggered Phase Reset of Hippocampal Theta.
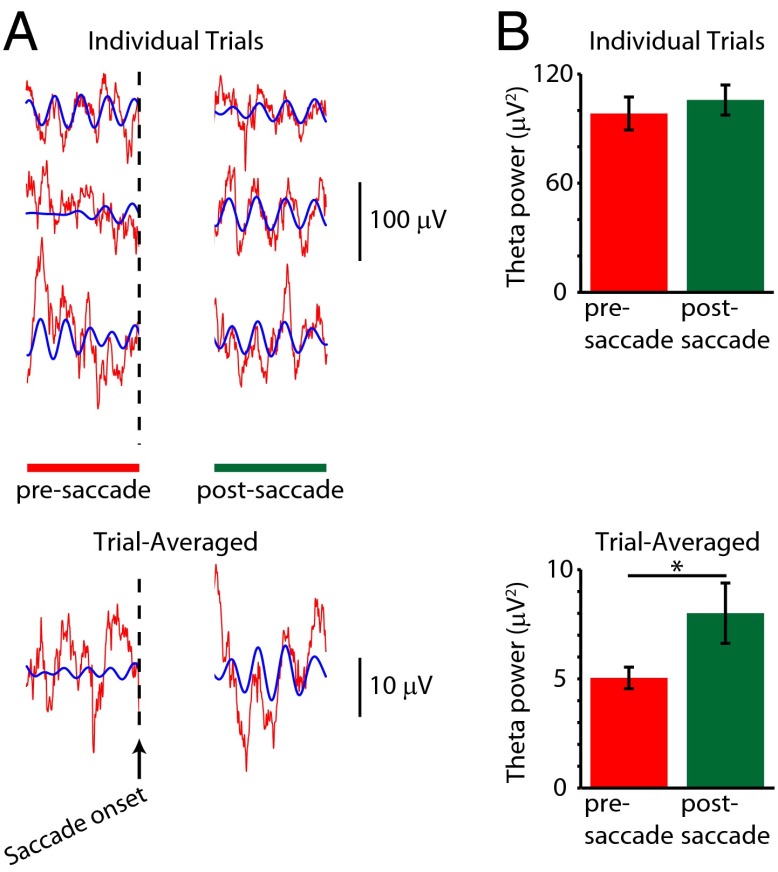
Previous work in rodents and humans has shown that ongoing theta oscillations undergo a phase reset during salient events (e.g., stimulus onset) in certain memory tasks (28–30), suggesting that phase-locking of hippocampal theta to incoming sensory information is an important element of stimulus processing during encoding. Only neural events for which there is an increase in phase concentration without a concurrent change in power at the dominant frequency meet the criteria for a “true” phase reset (31–33). The former requirement may be assessed through multiple means, including the observation of oscillatory activity in the postevent signal after averaging across multiple events (i.e., ref. 28), or through the calculation of phase concentration in the form of the Rayleigh statistic, after deriving the phase at the frequency of interest using the Hilbert transform (i.e., ref. 34). To assess the relationship between theta oscillations and saccades, we first examined hippocampal LFPs around the saccade to the center fixation cross that was presented at the start of each VPLT trial. Only trials in which no saccades occurred within 600 ms before, or 1,000 ms after, the saccade to the center cross were included (SI Methods). These trials were divided into “presaccade” and “postsaccade” periods, with the former consisting of the 600-ms period leading up to saccade onset and the latter consisting of 600 ms following a 400-ms “buffer” period immediately after saccade onset. The buffer period was included to account for any visually evoked voltage response driven by the appearance of the center cross. To assess the degree of pre- and postsaccade phase consistency across trials, we examined LFP signals in two ways: at the single-trial level (Fig. 2A, Upper) and after calculating the trial-averaged LFP (Fig. 2A, Lower). We observed an increased tendency of trial-averaged LFPs to display oscillatory activity in the postsaccade period compared with the presaccade period, similar to the increased poststimulus oscillatory activity seen in trial-averaged LFPs in the rodent hippocampus (28). Power spectra were calculated for the pre- and poststimulus periods for single-trial LFP signals and for the trial-averaged LFPs. Theta power in individual trials was not significantly different postsaccade compared with presaccade (Fig. 2B, Upper), indicating that each individual saccade did not evoke a change in theta power in the LFP. However, theta power was significantly higher in the postsaccade period than in the presaccade period in the trial-averaged signal (Fig. 2B, Lower; P < 0.01). This postsaccade increase in theta-band activity in the averaged LFP, but not the LFP taken from individual trials, suggests that ongoing theta oscillations in the primate hippocampus undergo a reset to a consistent phase following saccadic eye movements, producing event-locked oscillatory patterns in the average signal postsaccade. The prolonged time course of this effect (which outlasts a typical evoked response that may be present in the trial-averaged LFP signal) further argues in favor of a phase reset of ongoing oscillatory activity rather than an additive evoked response. Further evidence supporting a phase reset after saccade onset comes from the calculation of the Rayleigh statistic (35), which compares the distribution of phases across fixation periods against a uniform (random) distribution at each time point relative to saccade onset. Average Rayleigh statistic values for theta-filtered (6–12 Hz) LFPs were higher during the postsaccade period than during the presaccade period (Fig. S5; P < 0.01), indicating a higher degree of phase concentration during the fixation period following saccade onset. A similar analysis performed on LFP data taken from the calibration task did not show similar saccade-related changes in power and phase (Fig. S6 and SI Methods), indicating that this effect was specific to saccades made in anticipation of an exploratory state.
Fig. 2.
Saccade-triggered phase reset of hippocampal theta. (A) Example LFP traces (Upper) and trial-averaged LFP (Lower) aligned to saccade onset during the prestimulus fixation period. Theta oscillations show phase alignment across trials during the last 600 ms of the fixation period (represented by the green scale bar), which translates to visible theta oscillations in the trial-averaged LFP during the same time period. (Scale bar = 600 ms.) (B) Theta (6.7–11.6 Hz) power calculated across all single trials (Upper) and for each trial-averaged signal (Lower) for presaccade (red) and postsaccade (green) periods. Theta power was significantly higher for the postsaccade period than for the presaccade period for the trial-averaged signals (*P < 0.05) but not for the individual trial signals (P > 0.1).
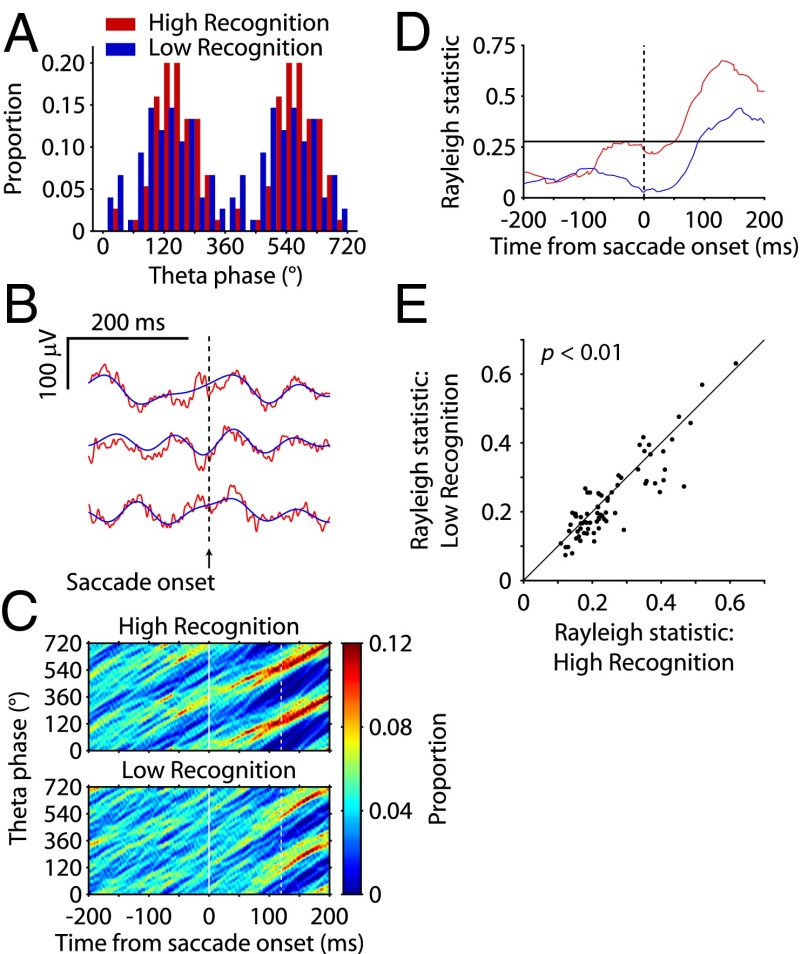
Modulation of the phase of theta may have important implications for cognition, as demonstrated in some studies of hippocampal long-term potentiation (36–38) and sensory processing (34, 39). For example, neural activity in the hippocampus may be influenced by theta phase in such a way that resetting to an “ideal” phase on saccade initiation may set up the optimal conditions for plasticity, and thus memory formation. Accordingly, we next considered whether there was any relationship between theta phase reset after saccades and memory formation as measured in the VPLT. Due to the relatively short duration of fixation periods during naturalistic viewing compared with cued fixation, we focused on the 200-ms period immediately following each saccade the monkeys made as they explored the visual stimuli during novel trials. We limited our analysis to novel trials in which the monkey made at least three saccades during exploration of the image, with all resultant postsaccade fixation periods lasting at least 200 ms. LFPs were filtered in the 3- to 12-Hz theta band, and the time-resolved phase of the filtered signal was calculated using the Hilbert transform. This phase distribution was averaged within each LFP channel for each of two memory conditions: the 30 stimuli for which the monkey showed the best subsequent recognition memory [“high recognition” (i.e., largest percentage of reduction in looking time for repeated stimuli)] and the 30 stimuli for which the monkey showed the worst subsequent recognition memory (“low recognition”). These trials represented, on average, the upper and lower 30.8% of all trials within a recording session that met the inclusion criteria. While both conditions showed similar latencies to the first saccade and intersaccade intervals, high recognition trials were significantly longer, and thus contained more saccades, than low recognition trials (Table S1); therefore, only the first three post-saccade fixation periods were analyzed for each trial. The phase distribution for both conditions showed a high degree of organization during the postsaccade period; in other words, at any given time point, the phase was highly predictable across fixation periods (Fig. 3B). This change in oscillatory phase was not accompanied by a concomitant increase in power (Fig. S7), supporting the hypothesis that this shift in phase at saccade onset represents a phase reset (31–33), similar to that seen with prestimulus saccades. However, the reliability of theta phase following saccade that occurred during image viewing was variable across memory conditions, with high recognition trials showing greater postsaccade phase specificity than low recognition trials (Fig. 3 A and C). Rayleigh statistic values for each LFP tended to be higher during the postsaccade fixation period in the high recognition condition than in the low recognition condition (Fig. 3D). For each LFP, we calculated the difference between memory conditions of the average Rayleigh statistic value in the 40- to 200-ms time window after saccade onset; the distribution of differences across LFPs was significantly greater than zero (sign test, P < 0.01; Fig. 3E). This time window of significant modulation by memory condition represents times at which the LFP theta phase was significantly more consistent, or predictable, during the encoding of subsequently well-remembered stimuli than for poorly remembered stimuli. These data suggest that hippocampal theta phase resetting by saccades may contribute to memory formation.
Fig. 3.
Postsaccade phase reliability during novel image exploration predicts subsequent recognition. (A) Phase distribution of an example theta-filtered LFP signal at 120 ms after saccade onset for the first three saccades in all high recognition (red) and Low recognition (blue) conditions, in which 0° was defined as the trough of a theta cycle. (B) Raw (red) and theta (3–12 Hz)-filtered (blue) segments from an example LFP showing reset to a consistent phase following saccade onset. (C) Phase concentration for the 400-ms period centered on saccade onset for high recognition and low recognition conditions for the example LFP in A. White dashed lines mark the time point of the phase distribution shown in A. (D) Rayleigh statistic as a function of time relative to saccade onset for high recognition and low recognition conditions for the example LFP in A. The black solid line represents the threshold for significant (P < 0.01) deviation from a uniform phase distribution. (E) Rayleigh statistic values for the high recognition (x axis) and low recognition (y axis) trials for all LFPs (n = 74). Each point represents the average Rayleigh statistic value in the 40- to 200-ms time window after saccade onset across all fixation periods analyzed (n = 90 in each condition). Rayleigh statistic values were significantly higher for high recognition than low recognition trials across LFPs (paired t test, P < 0.01).
Prestimulus Theta-Band Power and Memory.
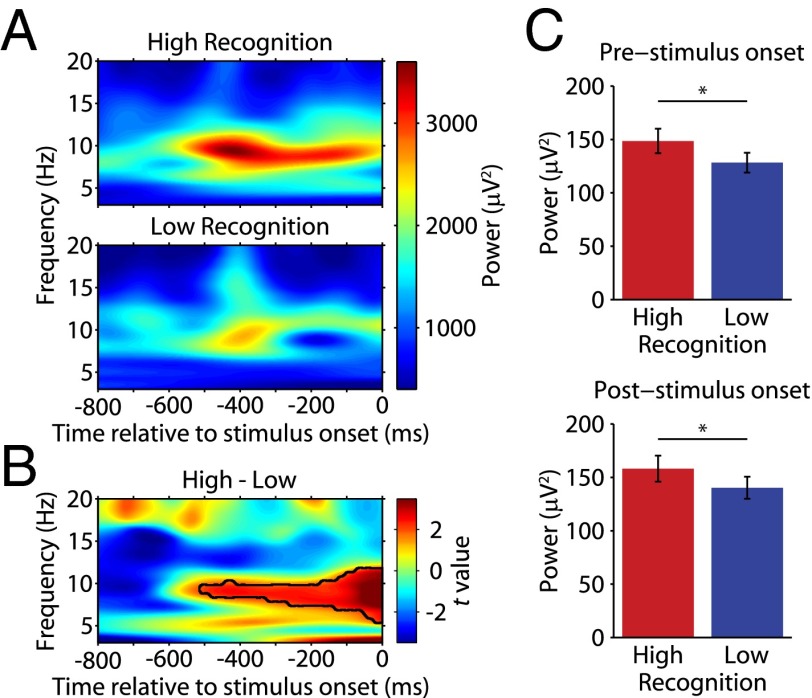
We next considered whether there was any relationship between modulations in theta-band power and recognition memory performance on the VPLT. In view of previous studies showing positive correlations between prestimulus theta in the human MTL and memory (40–42), we examined the possibility that the strength of theta-band activity during the baseline period leading up to stimulus onset might predict the strength of memory encoding. We calculated the power in the 3- to 20-Hz range for each LFP during the 800 ms before novel presentations of the 30 stimuli for which the monkey showed the best subsequent recognition memory (high recognition) and before novel presentations of the 30 stimuli for which the monkey showed the worst subsequent recognition memory (low recognition). These trials represented the upper and lower 22.2% of trials in terms of memory performance. In the representative example LFP power spectra shown in Fig. 4A and across the population (Fig. 4B), there was an enhancement in prestimulus theta power for trials with the best subsequent memory performance compared with trials with the worst subsequent memory performance. A nonparametric permutation test (43) revealed a cluster of significant memory modulation starting ∼500 ms before stimulus onset, initially restricted within an 80- to 10-Hz band but gradually encompassing a larger frequency range (6–12 Hz) approaching stimulus onset. Due to the temporal smoothing inherent in this method, a poststimulus onset influence cannot be ruled out in the later part of this effect; therefore, the time-averaged power at 9 Hz was calculated for 444-ms windows immediately preceding and immediately following stimulus onset. In both windows, power was significantly higher for the high recognition condition than for the low recognition condition (P < 0.01; Fig. 4C). This result indicates that not only is there a true correlation between theta power immediately preceding stimulus onset and memory performance but that this prestimulus effect persists into at least the initial period of the stimulus presentation.
Fig. 4.
Theta-band power preceding stimulus onset for novel stimuli is predictive of subsequent recognition. (A) Power spectrograms from one example LFP during the 800-ms time period immediately preceding stimulus presentation. (B) Modulation of theta power for high recognition and low recognition trials across 114 LFPs. The area of significant power modulation across conditions is outlined in black. (C) Power in the 9-Hz band for the 444-ms window preceding (Left) and following (Right) stimulus onset, for high recognition (red) and low recognition (blue) trials. Power was significantly higher for high recognition than for low recognition in both time windows (paired t test, *P < 0.01).
Discussion
In a naturalistic task of recognition memory through free viewing, we found that single neurons and LFPs in the primate hippocampus were modulated by saccades during visual exploration. Further, saccades produced a phase resetting of theta activity in the hippocampal LFP. Importantly, we observed significant effects on memory of prestimulus theta power and postsaccade theta phase variability during visual exploration. Together, these data suggest that modulations in hippocampal theta during exploratory behaviors may be important for memory storage in the primate hippocampus. Because the timing of neuronal spikes in the hippocampus is influenced by theta oscillations (9, 44–46), theta phase resetting could represent a mechanism by which stimulus-related activity in the hippocampus, in the form of neuronal firing, may be coordinated with ongoing behavior to optimize memory formation.
Previous studies of saccadic modulation in the primate hippocampus have drawn parallels between this modulation and theta activity in rodents (24, 47). Saccadic eye movements partition sensory input into discrete elements in much the same way that whisking and sniffing, behaviors often associated with hippocampal theta in rodents, break up somatosensory and olfactory information, respectively (1). Furthermore, the modulation of hippocampal activity by saccadic eye movements is reminiscent of previous findings showing that theta in rodents becomes phase-synchronized with whisking and sniffing during exploration (13, 48). Although this relationship may exist only during certain behavioral states or only during the presence of sensory input (49), it suggests that one function of hippocampal theta across species may be as a mechanism to promote neural processing of information gathered during the performance of “sensing” behaviors.
Our finding that hippocampal theta undergoes a phase reset with saccade onset is similar to the phase resetting observed in both rodent (28, 29) and human (30, 50, 51) hippocampus with stimulus events during performance of memory tasks. This phase resetting has several important implications for memory formation. For example, the induction of long-term potentiation in the hippocampus is preferential for particular phases of theta (36–38, 52, 53). Thus, phase resetting of the hippocampal theta rhythm may represent a mechanism to ensure that the hippocampus is in an “ideal phase” to integrate incoming sensory information into memory via synaptic plasticity. Additionally, hippocampal theta may be important for organizing interactions between disparate brain regions. Neurons in multiple brain regions, including prefrontal cortex, are phase-locked to the hippocampal theta rhythm (54–56), and the amplitude of locally generated gamma-band (30–140 Hz) oscillations is also modulated by hippocampal theta phase (55, 57, 58). This latter phenomenon may be particularly important for memory, because gamma synchrony within and between MTL regions is enhanced during successful memory task performance (18, 59, 60). The degree to which interactions between brain regions are modulated by hippocampal theta phase may thus represent a mechanism by which theta organizes the routing of information within and between functional networks during memory formation and retrieval (57, 61, 62). It has also been suggested that theta serves as a “frame-setting” mechanism, where the theta cycle provides a reference in which multiple items are represented at different phases of the cycle (63, 64). In view of these functional models of hippocampal theta, phase resetting may represent a mechanism to align mnemonic processing temporally with potentially significant behavioral events (e.g., saccade/stimulus onset).
We also observed an effect of prestimulus theta-band power on memory, such that higher theta power preceding novel stimulus presentation, and persisting through at least the initial period of stimulus presentation, predicted better subsequent recognition. This result is consistent with studies in human patients with epilepsy showing positive correlations between prestimulus theta in the MTL and memory (40–42), and it suggests that theta oscillations are involved in “preparatory” mechanisms that bring the hippocampus into an “online” state (5). Because the monkeys in the current study were not explicitly trained to form a memory of the stimuli, it is unlikely that this preparatory state reflects an overt intention, or motivation, to remember the stimulus. An alternative explanation is that this activity is a component of an attentional process related to the anticipation of a novel stimulus, and that this anticipation is conducive to processes involved in memory storage (65). It was recently demonstrated that target detection performance fluctuates rhythmically within the theta-band frequency range (66), providing evidence that covert attentional processes, like some exploratory behaviors, are rhythmic in nature and possibly synchronize with oscillatory neural patterns.
In summary, these results provide a glimpse into a possible mechanism for memory encoding in the hippocampus during active exploration. This mechanism may involve linking saccadic behavior and hippocampal activity in such a way that the former establishes the optimal conditions for synaptic plasticity. The present results suggest that encoding of sensory information in the hippocampus is closely linked to the sampling routines used in active sensing, and that the precise timing of hippocampal activity relative to behavioral events affects the strength of memory formation.
Methods
Behavioral Testing and Data Acquisition.
Procedures used for behavioral testing and electrophysiological recording are described in detail by Jutras et al. (18) and in SI Methods. Neuronal recordings were carried out in two adult male rhesus monkeys (Macaca mulatta).
Monkeys were tested on the VPLT while their eye movements were recorded using a noninvasive IR eye-tracking system (ISCAN). The monkey initiated each trial by fixating a white cross (1°) at the center of a computer screen for 1 s. Following this, the cross disappeared and a picture stimulus (11°) was presented and remained on the screen as long as the monkey continued to look at it, up to a maximum looking time of 5 s. The VPLT was given in 51 daily blocks of 6, 8, or 10 trials each, chosen pseudorandomly.
LFP and single-unit recordings were obtained from tungsten microelectrodes placed in the anterior part of the left hippocampus, in the CA3 field, dentate gyrus, and subiculum (recording locations are provided in ref. 18). Data amplification, filtering, and acquisition were performed with a multichannel acquisition processor system from Plexon, Inc. The neural signal was split to extract the spike and the LFP components separately. For spike recordings, the signals were filtered from 250 Hz to 8 kHz, further amplified, and digitized at 40 kHz. For LFP recordings, the signals were filtered with a passband of 0.7–170 Hz, further amplified, and digitized at 1 kHz. Any additional filtering was performed in MATLAB (The Mathworks, Inc.). Eye movement data were digitized and stored with a 240-Hz resolution.
Data Analysis.
All analyses were performed using custom programming in MATLAB and using FieldTrip (fieldtrip.fcdonders.nl).
LFP Spectral Analysis, Bout Detection, and Phase Concentration.
We calculated power spectra from LFP data after first limiting data segments to those recorded during blocks of VPLT performance, including intertrial intervals, prestimulus fixation periods, and stimulus presentation/exploration periods. Initial spectral analysis was performed using the multitaper method (67, 68) on nonoverlapping LFP segments of 5 s each. Bouts of theta activity in LFP data were quantified using an oscillatory episode detection algorithm that estimates the background power spectrum of the LFP to determine power and duration criteria; this method is described in detail in refs. 8, 25–27 and in SI Methods. Pepisode measures were calculated from LFP segments taken from blocks of VPLT performance, with each segment consisting of a block in its entirety.
To calculate theta power for pre- and postsaccade periods for individual trial power measures, we multiplied the raw LFP traces for each pre- and postsaccade period with one orthogonal taper function before Fourier transformation, providing spectral smoothing of ±1.67 Hz (for 600-ms segments taken from the prestimulus period or the calibration task) or ±4 Hz (for 200-ms segments taken from the stimulus viewing period). To calculate theta power for pre- and postsaccade periods for trial-averaged power measures, the same method was used, with the exception that before taper multiplication and Fourier transformation, we calculated the average LFP signal (i.e., evoked signal) across trials. Taper multiplication and Fourier transformation were then applied to each trial-averaged LFP segment. We then calculated the average power in the theta frequency band (6.7–11.6 Hz for prestimulus and calibration task data and 4–12 Hz for data taken from the stimulus viewing period) to obtain a theta power value for each LFP for presaccade and postsaccade periods.
Supplementary Material
Acknowledgments
We thank Megan Jutras for technical assistance. This study was supported by Emory Alzheimer’s Disease Research Center Grant AG025688 (to E.A.B.); National Institute of General Medical Sciences and National Institute of Neurological Disorders and Stroke grants (to M.J.J.); National Institute of Mental Health Grants MH080007 (to E.A.B.), MH093807 (to E.A.B.), and MH082559 (M.J.J.); and National Center for Research Resources Grant P51RR165 (currently the Office of Research Infrastructure Programs/OD P51OD11132).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302351110/-/DCSupplemental.
References
- 1.Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses. 2006;31(2):167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder CE, Wilson DA, Radman T, Scharfman H, Lakatos P. Dynamics of Active Sensing and perceptual selection. Curr Opin Neurobiol. 2010;20(2):172–176. doi: 10.1016/j.conb.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(4):407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 4.Winson J. Patterns of hippocampal theta rhythm in the freely moving rat. Electroencephalogr Clin Neurophysiol. 1974;36(3):291–301. doi: 10.1016/0013-4694(74)90171-0. [DOI] [PubMed] [Google Scholar]
- 5.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 6.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10(2):224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 7.Grastyán E, Lissák K, Madarász I, Donhoffer H. Hippocampal electrical activity during the development of conditioned reflexes. Electroencephalogr Clin Neurophysiol. 1959;11(3):409–430. doi: 10.1016/0013-4694(59)90040-9. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrom AD, et al. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15(7):881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- 9.Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464(7290):903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- 10.Lega BC, Jacobs J, Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22(4):748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- 11.Cantero JL, et al. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23(34):10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland WL, Teitelbaum H, Hedges EK. Relationship between hippocampal theta activity and running speed in the rat. J Comp Physiol Psychol. 1975;88(1):324–328. doi: 10.1037/h0076177. [DOI] [PubMed] [Google Scholar]
- 13.Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. J Neurosci. 1982;2(12):1705–1717. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart M, Fox SE. Hippocampal theta activity in monkeys. Brain Res. 1991;538(1):59–63. doi: 10.1016/0006-8993(91)90376-7. [DOI] [PubMed] [Google Scholar]
- 15.Skaggs WE, et al. EEG sharp waves and sparse ensemble unit activity in the macaque hippocampus. J Neurophysiol. 2007;98(2):898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- 16.Jutras MJ, Buffalo EA. Recognition memory signals in the macaque hippocampus. Proc Natl Acad Sci USA. 2010;107(1):401–406. doi: 10.1073/pnas.0908378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killian NJ, Jutras MJ, Buffalo EA. A map of visual space in the primate entorhinal cortex. Nature. 2012;491(7426):761–764. doi: 10.1038/nature11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jutras MJ, Fries P, Buffalo EA. Gamma-band synchronization in the macaque hippocampus and memory formation. J Neurosci. 2009;29(40):12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zola SM, et al. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20(1):451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/parahippocampal regions and recognition memory: Insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci. 2004;24(8):2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKee RD, Squire LR. On the development of declarative memory. J Exp Psychol Learn Mem Cogn. 1993;19(2):397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- 22.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20(23):8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobotka S, Nowicka A, Ringo JL. Activity linked to externally cued saccades in single units recorded from hippocampal, parahippocampal, and inferotemporal areas of macaques. J Neurophysiol. 1997;78(4):2156–2163. doi: 10.1152/jn.1997.78.4.2156. [DOI] [PubMed] [Google Scholar]
- 24.Ringo JL, Sobotka S, Diltz MD, Bunce CM. Eye movements modulate activity in hippocampal, parahippocampal, and inferotemporal neurons. J Neurophysiol. 1994;71(3):1285–1288. doi: 10.1152/jn.1994.71.3.1285. [DOI] [PubMed] [Google Scholar]
- 25.Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J Neurophysiol. 2001;86(1):368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- 26.Hughes AM, Whitten TA, Caplan JB, Dickson CT. BOSC: A better oscillation detection method, extracts both sustained and transient rhythms from rat hippocampal recordings. Hippocampus. 2012;22(6):1417–1428. doi: 10.1002/hipo.20979. [DOI] [PubMed] [Google Scholar]
- 27.van Vugt MK, Sederberg PB, Kahana MJ. Comparison of spectral analysis methods for characterizing brain oscillations. J Neurosci Methods. 2007;162(1-2):49–63. doi: 10.1016/j.jneumeth.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams JM, Givens B. Stimulation-induced reset of hippocampal theta in the freely performing rat. Hippocampus. 2003;13(1):109–116. doi: 10.1002/hipo.10082. [DOI] [PubMed] [Google Scholar]
- 29.Givens B. Stimulus-evoked resetting of the dentate theta rhythm: Relation to working memory. Neuroreport. 1996;8(1):159–163. doi: 10.1097/00001756-199612200-00032. [DOI] [PubMed] [Google Scholar]
- 30.Mormann F, et al. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15(7):890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- 31.Shah AS, et al. Neural dynamics and the fundamental mechanisms of event-related brain potentials. Cereb Cortex. 2004;14(5):476–483. doi: 10.1093/cercor/bhh009. [DOI] [PubMed] [Google Scholar]
- 32.Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev. 2007;31(7):1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Sauseng P, et al. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146(4):1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Rajkai C, et al. Transient cortical excitation at the onset of visual fixation. Cereb Cortex. 2008;18(1):200–209. doi: 10.1093/cercor/bhm046. [DOI] [PubMed] [Google Scholar]
- 35.Fisher NI. Statistical Analysis of Circular Data. Cambridge, UK: Cambridge Univ Press; 1993. [Google Scholar]
- 36.Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15(5):1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 37.Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364(6439):723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- 38.Hölscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997;17(16):6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakatos P, Chen C-M, O’Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53(2):279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proc Natl Acad Sci USA. 2011;108(26):10702–10707. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guderian S, Schott BH, Richardson-Klavehn A, Düzel E. Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci USA. 2009;106(13):5365–5370. doi: 10.1073/pnas.0900289106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fell J, et al. Medial temporal theta/alpha power enhancement precedes successful memory encoding: Evidence based on intracranial EEG. J Neurosci. 2011;31(14):5392–5397. doi: 10.1523/JNEUROSCI.3668-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. J Neurosci. 2007;27(14):3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6(2):149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Fox SE, Wolfson S, Ranck JB., Jr Hippocampal theta rhythm and the firing of neurons in walking and urethane anesthetized rats. Exp Brain Res. 1986;62(3):495–508. doi: 10.1007/BF00236028. [DOI] [PubMed] [Google Scholar]
- 47.Sobotka S, Ringo JL. Saccadic eye movements, even in darkness, generate event-related potentials recorded in medial sputum and medial temporal cortex. Brain Res. 1997;756(1-2):168–173. doi: 10.1016/s0006-8993(97)00145-5. [DOI] [PubMed] [Google Scholar]
- 48.Komisaruk BR. Synchrony between limbic system theta activity and rhythmical behavior in rats. J Comp Physiol Psychol. 1970;70(3):482–492. doi: 10.1037/h0028709. [DOI] [PubMed] [Google Scholar]
- 49.Berg RW, Whitmer D, Kleinfeld D. Exploratory whisking by rat is not phase locked to the hippocampal theta rhythm. J Neurosci. 2006;26(24):6518–6522. doi: 10.1523/JNEUROSCI.0190-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rizzuto DS, et al. Reset of human neocortical oscillations during a working memory task. Proc Natl Acad Sci USA. 2003;100(13):7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesche CD, Karhu J. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci USA. 2000;97(2):919–924. doi: 10.1073/pnas.97.2.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCartney H, Johnson AD, Weil ZM, Givens B. Theta reset produces optimal conditions for long-term potentiation. Hippocampus. 2004;14(6):684–687. doi: 10.1002/hipo.20019. [DOI] [PubMed] [Google Scholar]
- 53.Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23(37):11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46(1):141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 55.Sirota A, et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60(4):683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15(6):739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- 57.Colgin LL, et al. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462(7271):353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 58.Bragin A, et al. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15(1 Pt 1):47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fell J, et al. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nat Neurosci. 2001;4(12):1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 60.Montgomery SM, Buzsáki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104(36):14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fries P. The model- and the data-gamma. Neuron. 2009;64(5):601–602. doi: 10.1016/j.neuron.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 62.Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14(4):793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- 63.Lisman J. The theta/gamma discrete phase code occurring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15(7):913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- 64.Lisman JE, Idiart MA. Storage of 7 +/- 2 short-term memories in oscillatory subcycles. Science. 1995;267(5203):1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 65.Wittmann BC, Bunzeck N, Dolan RJ, Düzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. Neuroimage. 2007;38(1):194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landau AN, Fries P. Attention samples stimuli rhythmically. Curr Biol. 2012;22(11):1000–1004. doi: 10.1016/j.cub.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 67.Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13(4):717–749. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
- 68.Mitra PP, Pesaran B. Analysis of dynamic brain imaging data. Biophys J. 1999;76(2):691–708. doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.