Abstract
Within the TGF-β superfamily, there are approximately forty ligands divided into two major branches: the TGF-β/Activin/Nodal ligands and the BMP/GDF ligands. We studied the ligand GDF3 and found that it inhibits signaling by its co-family members, the BMPs; however, GDF3 has been described by others to have Nodal-like activity. Here, we show that GDF3 can activate Nodal signaling, but only at very high doses and only upon mRNA over-expression. In contrast, GDF3 inhibits BMP signaling upon over-expression of GDF3 mRNA, as recombinant protein, and regardless of its dose. We therefore further characterized the mechanism through which GDF3 protein acts as a specific BMP inhibitor and found that the BMP inhibitory activity of GDF3 resides redundantly in the unprocessed, predominant form and in the mature form of the protein. These results confirm and extend the activity that we described for GDF3 and illuminate the experimental basis for the different observations of others. We suggest that GDF3 is either a bi-functional TGF-β ligand, or, more likely, that it is a BMP inhibitor that can artificially activate Nodal signaling under non-physiological conditions.
Keywords: GDF3, BMP, TGF-β, Embryo
Introduction
Many of the key processes of embryonic development and adult pathophysiology are regulated by the TGF-β superfamily of ligands. These factors mediate communication between cells to control signal transduction and transcription in the target cell. In the mammalian genome, there are approximately forty TGF-β ligands, divided into two main branches based on homology and function (Lander et al., 2001).
These two branches are roughly distinguished by homology, and functionally by the major signal transducer that is activated by the ligands, such that typical TGF-β/Activin/Nodal ligands activate Smad2 and Smad3, while typical BMP/GDF ligands activate Smad1, Smad5, and Smad8 (reviewed (Shi and Massague, 2003). The high number of TGF-β ligands could reflect significant redundancy. However, it is also likely that there are many levels of subtle variation amongst the activities of this family of ligands.
There are several atypical TGF-β ligands that have been placed into one of these two main branches by homology but have different activities. For instance, GDF1 activates Smad2/3 signaling rather than Smad1/5/8 (Rankin et al., 2000; Wall et al., 2000), and BMP3 (Daluiski et al., 2001) and Lefty (Cheng et al., 2004; Meno et al., 1999) act as ligand inhibitors, blocking classic BMP and Nodal signaling, respectively.
We previously found that GDF3, classified as a BMP/GDF ligand by homology, also inhibits BMP signaling through Smad1/5/8, thereby adding GDF3 to this group of unusual TGF-β ligands (Levine and Brivanlou, 2006). This conclusion was strengthened by the recent observation that reduction of endogenous GDF3 levels in human embryonic stem cells results in enhanced levels of BMP pathway activation. However, other groups have found that GDF3 is instead a Nodal-like ligand (Andersson et al., 2007; Chen et al., 2006). Interestingly, we and others used several common assays, most prominently over-expression of the mammalian-specific GDF3 mRNA in the frog embryo.
To understand the basis for these conflicting observations, we performed studies on GDF3 function using several different sources of GDF3 throughout a range of doses. We find that GDF3 has the unique feature of acting as a BMP inhibitor throughout its dose range and as a Nodal-like ligand at high doses. Further, we observed that both the unprocessed and mature forms of the GDF3 protein inhibit BMP signaling, but did not observe Nodal-like activity of the GDF3 protein. This suggests that GDF3 is either a bi-functional ligand, or, more likely, that GDF3 is a BMP inhibitor with artificial Nodal activity at high doses.
GDF3 is now considered to be a classic embryonic stem cell gene and a required regulator of mammalian embryogenesis. Our findings here confirm and extend our previous conclusion that the mechanism of GDF3 action is through BMP inhibition. We further suggest that previously observed Nodal-like activity may be an artifact of over-expression. This discrepancy now requires a reevaluation of the critical roles of GDF3 in developmental contexts.
Results
GDF3 dose determines its function upon over-expression in frog embryos
We first confirmed the findings that our construct for GDF3 acts as a BMP inhibitor while the constructs for GDF3 used by Chen et al. act like a Nodal-like ligand. We performed this test in the animal cap assay of frog embryos in which endogenous BMP signaling induces epidermal fates, inhibition of this BMP activity promotes neural fates (Wilson and Hemmati-Brivanlou, 1995), and activation of the Nodal pathway induces mesoderm (Smith, 1987; Thomsen et al., 1990) (data not shown).
To understand the different observations of GDF3 activity, we first analyzed the constructs that each group used to produce GDF3 mRNA. We had previously studied GDF3 over-expression with a construct containing only open reading frame (ORF) of GDF3. In contrast, Chen et al. (2006) and Andersson et al. (2007) used either constructs containing both the ORF and vector-derived polyA or a chimeric GDF3 mRNA encoding the prepro domain of BMP2 with the mature domain of GDF3 (Michael Shen, personal communication, (Andersson et al., 2007), a strategy that has been used frequently in enhancing processing of TGF-β ligands (Thomsen and Melton, 1993).
We tested the hypothesis that the presence of the polyA signal or the enhanced processing of GDF3 could promote higher levels of GDF3 mature protein and thus account for the different activities as a dose threshold effect. To study the effects of different doses of GDF3, we injected 1 pg, 10 pg, 100 pg, and 1000 pg of GDF3 ORF mRNA with and without endogenous GDF3 UTRs and polyA and analyzed the amount of GDF3 protein produced. We found that the presence of the UTRs and polyA allowed production of ten to one hundred times more GDF3 protein for each dose of mRNA injected (Fig. 1A). Therefore, injection of the ORF or the ORF plus UTRs/polyA of GDF3 gives rise to very different ‘doses’ of GDF3 protein.
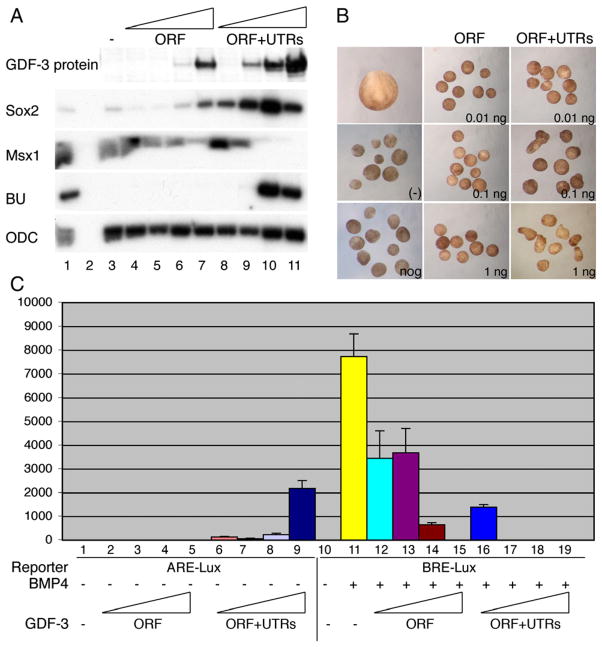
Fig. 1.
Dose effects of GDF3 ORF and ORF plus untranslated regions (UTRs). Embryos were injected with increasing doses (1 pg, 10 pg, 100 pg, 1000 pg) of GDF3 RNA for the open reading frame (ORF) or the coding region plus UTRs (ORF+UTRs). (A) Top panel: western blot with goat anti-GDF3 of embryos injected with increasing doses of GDF3 constructs. Other panels: RT-PCR showing the effect of GDF3 constructs on cell fate. In lanes 1 and 2, whole embryo mRNA is shown with (1) and without (2) reverse-transcription is shown as controls for each marker gene. Lane 3 shows uninjected animal caps that express Msx1 (epidermis). Increasing doses of GDF3 induce Sox2 (neural tissue) and high doses of GDF3 ORF+UTRs induce BU (mesoderm). ODC is shown as a loading control. (B) Whole embryo (stage 17) or animal caps isolated at stage 9 and cultured until sibling stage 17. Caps were uninjected (−), injected with noggin RNA (200 pg) or injected with increasing doses of GDF3 ORF or ORF+UTRs. Morphology of caps injected with 10, 100, 1000 pg of each GDF3 construct are shown. (C) Luciferase assay using an Activin-responsive element (ARE-Lux) or a BMP-responsive element (BRE-Lux) driving luciferase reporters. For the BRE-Lux experiments, embryos were injected with BRE alone or with BRE and 100 pg of BMP4 RNA to activate the reporter. Increasing doses (1 pg, 10 pg, 100 pg, 1000 pg) of GDF3 ORF or ORF+UTRs were injected with each reporter.
We next analyzed whether these different constructs possess different cell fate inductive activities using the animal cap assay in the frog embryo. We found that throughout the dose range of the ORF alone construct (up to 3 ng/embryo), GDF3 acted as a BMP inhibitor, inducing neural tissue directly (Fig. 1A). However, we found that at low doses (1, 10 pg), the ORF plus UTRs/polyA construct acted as a direct neural inducer, the hallmark of BMP inhibition, while at higher doses (100 pg,1 ng), it acted as a mesoderm inducer, a classic response to Nodal signaling (Fig. 1A).
In addition to our analysis of cell fate in animal caps, we tested the ability of GDF3 constructs to induce the typical elongated morphology of dorsal mesoderm in animal caps. We injected RNA for increasing doses of GDF3 ORF alone and GDF3 ORF plus UTRs/polyA. We found that the ORF alone did not induce elongated dorsal mesoderm and caps maintained a spherical shape, similar to uninjected caps or caps injected with noggin, another BMP inhibitor. In contrast, the ORF plus UTRs/polyA induced dorsal mesoderm elongation at 100 pg and 1 ng of RNA injection (Fig. 1B).
We confirmed the effects of a dose range of the ORF plus UTRs/polyA using a luciferase assay. We tested the effects of this construct on activation of a luciferase gene regulated by a BMP-responsive element (BRE) and an Activin/nodal responsive element (ARE) (Hata et al., 2000; Huang et al., 1995). These experiments showed that even 1 pg of the GDF3 coding region plus UTRs/polyA is sufficient for BMP inhibition, while 100 pg–1 ng is required to activate Nodal-like signaling (Fig. 1C). To determine whether this type of activity switching dose response is a common feature of nodal-like ligands, we tested the luciferase response to a similar dose curve using a known nodal-like ligand, Xnr1. Xnr1 was not able to inhibit BMP signaling at low doses (Supplemental Fig. 1).
TGF-β ligands typically exert their activities upon over-expression of mRNA in the picogram range and can lead to non-specific activation of low affinity receptors at high concentrations. Therefore, our results suggest that function of GDF3 within its physiological dose range is solely to inhibit BMP signaling.
High doses of GDF3 preserve its BMP inhibitory function and yield Nodal-like activity
We next sought to determine whether GDF3 switches activity at a certain dose threshold or acquires Nodal-like activity while retaining the ability to inhibit BMP signaling. To do this, we analyzed the effects of over-expressing 1 ng (high dose) of GDF3 ORF plus UTRs/polyA with or without α-amanitin, an inhibitor of transcription that prohibits secondary transcriptional responses. This approach allowed us to observe the primary effects of GDF3 protein, but not secondary effects mediated by new transcription. This is important because activation of Nodal signaling induces dorsal mesoderm which then expresses new transcripts of BMP inhibitors such as noggin and chordin and thus includes primary mesoderm induction and secondary BMP inhibition. We found that a high dose of GDF3 caused inhibition of Smad1/5/8 and direct activation of Smad2/3 in either the absence or presence of α-amanitin (Fig. 2). Therefore, we demonstrated that GDF3 is a Smad1/5/8 inhibitor throughout its dose range and that, at very high doses, this activity is coincident with Smad2/3 activation.
Fig. 2.
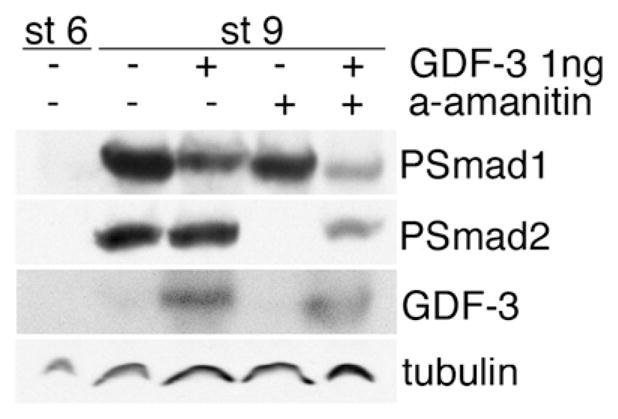
Direct effects of GDF3 over-expression in frog embryos. GDF3 was injected with and without α-amanitin, which blocks protein translation. Embryos were harvested at stage 9 and analyzed for activation of BMP and Nodal signaling (Psmad1 and Psmad2, respectively). Stage 6 embryos are shown as a negative control for TGF-β activation, tubulin is shown as a loading control.
GDF3 unprocessed and mature protein forms both inhibit BMP signaling
The activities observed upon over-expression of mRNA may not reflect true functions of a TGF-β ligand, and protein assays may be more informative. Therefore, we analyzed the activity of recombinant human GDF3 protein (rhGDF3) on cell fate in the frog embryo animal cap. It has previously been shown that this manipulation removes endogenous BMP signals and allows the animal cap cells to adopt a default neural fate, rather than the epidermal fate that BMPs induce (Grunz and Tacke, 1989; Wilson and Hemmati-Brivanlou, 1995). We then challenged these cells with exogenous BMP protein and tested the ability of rhGDF3 to block the effects of this exogenous BMP signaling.
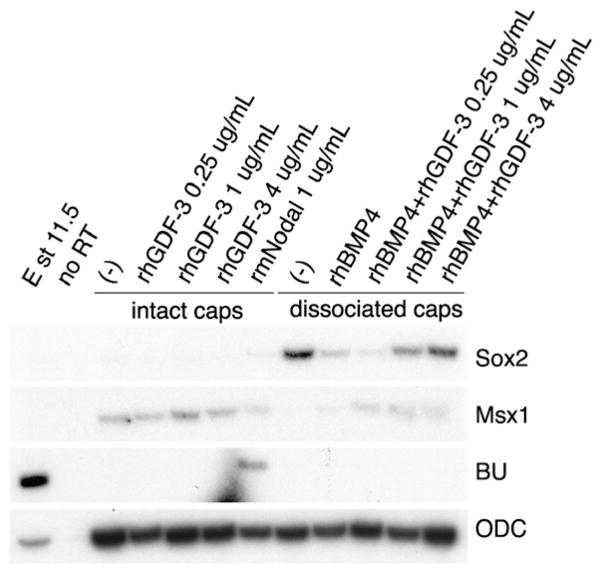
We found that, at the doses that we tested, rhGDF3 protein did not induce mesoderm, although mesoderm was induced by the same dose of recombinant mouse Nodal protein (rmNodal), produced from a similar source (Fig. 3). While untreated disassociated cells became neural, rhBMP4 reverted these cells to epidermis. Increasing doses (0.5, 1 and 4 ng/mL) of rhGDF3 blocked this activity of rhBMP4 (Fig. 3 and data not shown). We also tested 15 ng/mL of rhGDF3 and found that it had the same effect (data not shown). Therefore, at these protein doses, GDF3 is a BMP inhibitor and does not act like a Nodal ligand. We also tested the effects of rhGDF3 protein alone on disassociated cells and found a synergism in neural induction, with no mesoderm induction (data not shown).
Fig. 3.
Recombinant human GDF3 protein (rhGDF3) inhibits BMP activity in animal caps. Animal caps were isolated from stage 9 embryos and were cultured intact or were disassociated. The intact caps were treated with increasing doses of rhGDF3 or with recombinant mouse Nodal (rmNodal) to test for BU (mesoderm) induction. Disassociated caps express Sox2 (neural tissue) but treatment with BMP4 alone induces Msx1 (epidermis). Increasing doses of rhGDF3 protein antagonize BMP4 protein to revert the cells to a neural state. ODC is shown as a loading control. No RT is a control for genomic DNA.
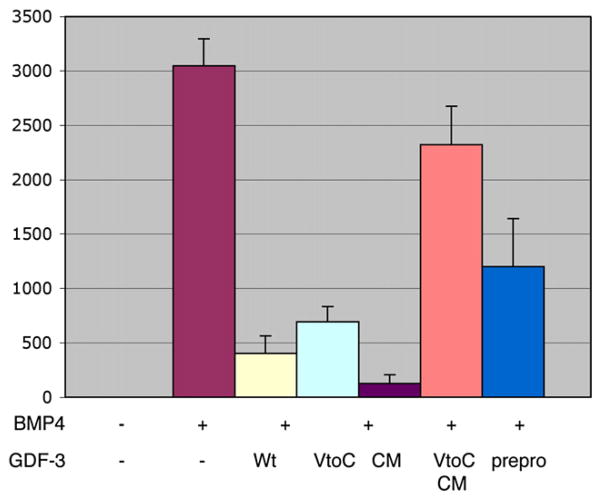
To determine whether the unusual ‘missing cysteine’ of GDF3 mediated its inhibitory function, we mutated the valine in GDF3 to a cysteine and tested its function upon BMP induction of the BRE-luciferase reporter. We found that this ‘V to C’ mutation alone did not change the ability of GDF3 to block BMP signaling (Fig. 4).
Fig. 4.
The prepro form and domain of GDF3 can inhibit BMP signaling. Luciferase assay using a BMP-responsive element (BRE) driving expression of a luciferase reporter (BRE-Lux). Embryos were injected with BMP4 RNA (100 pg/embryo) alone or with 1 ng of GDF3 constructs for wild-type GDF3 (Wt), a cleavage mutant of GDF3 (CM), or the prepro domain of GDF3 (prepro). Reporter alone is shown as a baseline control.
The results above showed that GDF3 mature protein could inhibit BMP signaling but we also examined whether the GDF3 unprocessed, ‘prepro’ form had this function because GDF3 mainly exists in the unprocessed form in vivo. We created a cleavage mutant of GDF3 by mutating the cleavage recognition sequence from amino acids RKRR to GNVG. This construct produces no mature protein when over-expressed in frog embryos. Using the luciferase and animal cap cell fate assays, we found that cleavage mutated GDF3 (CM GDF3) was as effective as wild-type GDF3 in inhibiting BMP signaling (Fig. 4). Further, we found that the prepro domain of GDF3 alone is sufficient for mild BMP inhibition (Fig. 4).
To determine whether GDF3 has redundant mechanisms for inhibiting BMP signaling, we created a doubly mutated form, having both the V to C mutation in the mature domain and the cleavage mutation blocking processing. As shown in Fig. 4, this double mutant failed to block BMP induction of the luciferase reporter, demonstrating that redundant regions are responsible for BMP inhibition in the GDF3 protein.
Our results demonstrate that both the unprocessed form of the GDF3 – the major endogenous form – and the mature form of the protein – the active form of typical TGF-β ligands, are endowed with BMP inhibitory function. This redundancy may highlight the importance of this function. Further, the identification of the ‘missing cysteine’ as a key residue in this activity suggests that other TGF-β with this feature may also act as inhibitors.
Discussion
Many TGF-β ligands act as morphogens, inducing different cell fates at different doses (Dosch et al., 1997; Ferguson and Anderson, 1992; Green et al., 1992; Green and Smith, 1990; Wilson et al., 1997). Importantly, these activities generally rely on graded activation of a single pathway, and do not involve changing signal transduction activity. We show here that upon exogenous GDF3 expression in the frog embryo, GDF3 is a BMP inhibitor, blocking signaling through Smads1/5/8, but that at high doses, it can also act as a Nodal-like ligand, stimulating activation of Smads2/3. However, it is unlikely that this reflects a true bi-functionality because GDF3 protein acts solely as a BMP inhibitor.
Both Levine and Brivanlou (2006) and Chen et al. (2006) performed principal mechanistic experiments on GDF3 function in frog embryos, despite the fact that no frog homolog of the mammalian GDF3 has been described (Chen et al., 2006; Levine and Brivanlou, 2006). While GDF3 is very similar by overall homology to xVg1, and is considered by Chen and colleagues to be a Vg1 homolog, several critical characteristics demonstrate that GDF3 is not the mammalian homolog of Vg1. First, GDF3 has a defining structural peculiarity, not shared by Vg1, in that it is missing the fourth of seven canonical cysteines that form the defining cysteine knot of the TGF-β superfamily. Second, xVg1 is syntenic with a different GDF ligand, GDF-1. Xenopus tropicalis Vg1 (CAJ82217, peptide Tegg005K01.1) is next to the genes COPE, Ddx49, then Homer3, the precise arrangement of genes neighboring human GDF-1 on chromosome 19. Vg1 also shares a high overall homology with GDF-1 (Lee, 1990), the same complement of canonical cysteines (Lee, 1990), and a functional similarity (Joseph and Melton, 1998; Thomsen and Melton, 1993; Wall et al., 2000). We therefore do not consider GDF3 to be related to Vg1. In agreement with Andersson et al. (2007), we suggest that GDF-1 is the mammalian homolog of Vg1.
Despite the lack of an amphibian homolog of GDF3, there are several advantages of performing GDF3 over-expression experiments in frog embryos, including the ability to study in vivo the outcome of this factor on multiple well defined pathways and the absence of endogenous GDF3 which allows ectopic expression of a full range of GDF3 doses with a ‘zero’ baseline. However, a significant disadvantage of this technique is the risk of artifactual results due to exogenous factor overdose and the observation of non-physiological functions. Indeed, DNA or RNA mediated over-expression of exogenous have previously yielded artifactual functional readouts of other TGF-β ligands in the frog embryo.
To more directly assess the physiological role of GDF3, we studied the function of the recombinant protein. Our results from experiments with GDF3 protein show that it is a BMP inhibitor but we did not find any evidence of Nodal-like activity. It is possible that this finding reflects several technical factors.
First, exogenous recombinant GDF3 protein could be missing partners with which the protein must be co-synthesized to function. For instance, in cell culture, GDF3 has been shown to robustly activate a Nodal-responsive luciferase reporter if it is co-expressed with Nodal (Andersson et al., 2007).
Second, this could be due to the source of recombinant GDF3 protein, which was bacterial. It is possible that mammalian proteins produced in E. coli are not properly folded and it is likely that active GDF3 protein is only a fraction of the total bacterially produced recombinant protein used in the experiments. For this reason, we also used bacterially produced recombinant mammalian Nodal protein, as a control, although the two proteins were not prepared in parallel and could have different fractions of active protein. Recombinant mouse Nodal induced mesoderm at 1 μg/mL, comparable to the range that we tested for GDF3 (0.25–15 μg/mL).
Third, it could be that we did not test high enough doses of GDF3 protein to reach a threshold of Nodal-like activity. This could not be done because we could not test one hundred times the active BMP inhibitory dose of the GDF3 protein, which is a minimum of 0.5 μg/mL. However, within the range of 0.25–15 μg/mL, already a very significant dose of protein, we found no Nodal-like activity. The company that produced the GDF3 protein that we used, Peprotech, reports that 0.1– 0.15 μg/mL of protein represents the ED50 for inhibiting alkaline phosphatase induction in ATDC5 cells, while classic BMP proteins such as BMP2 and BMP6 induce alkaline phosphatase (data sheet for product 120–22).
For these reasons, a role for very high levels of GDF3 in promoting Nodal-like signaling cannot be ruled out, and it is possible that GDF3 is a bi-functional ligand. Interestingly, Nodal itself has been reported to inhibit BMP signaling, providing a precedent for this type of dual role (Yeo and Whitman, 2001). However, it is unclear whether endogenous Nodal functions through a role in blocking BMP signaling, in addition to its typical activating role.
The required activities of GDF3 must be analyzed through loss-of-function, both for examining their effect on BMP and Nodal signaling, and for incorporating these results into a more comprehensive understanding of the functions of GDF3 and TGF-β pathways in their endogenous contexts.
In human embryonic stem cells, Peerani and colleagues showed that siRNA mediated GDF3 knockdown results in enhanced levels of Smad1/5/8 activation — the hallmark of BMP pathway signaling (Peerani et al., 2007). These authors do not demonstrate whether this regulation is direct, nor whether Nodal signaling is perturbed. It is not known whether GDF3 also supports stemness through activation of Nodal-like signaling that has been shown to be required for and to promote pluripotency in human embryonic stem cells. In human embryonic stem cells, GDF3 acts to maintain an inner cell mass/epiblast pluripotent cell type, while inhibiting differentiation to classic extra-embryonic tissues, a fate promoted by classic BMPs (Levine and Brivanlou, 2006; Xu et al., 2002). Together, these results strengthen the argument that the primary role of endogenous GDF3 is to block BMP signaling.
GDF3 has also been studied in the mouse embryo through a knockout allele and through a genetrap allele. Both reports describe defects in anterior visceral endoderm induction or anterior migration, which likely represents a requirement for GDF3 in the epiblast during early post-implantation stages. Nodal signaling has a well-documented role in anterior visceral endoderm formation (Brennan et al., 2001), and both papers considered GDF3 activity in this context. However, neither paper directly assesses the status of Nodal or BMP pathway signaling.
BMP signaling has also been shown to regulate anterior visceral endoderm establishment, migration, and maintenance. It has been suggested that BMP4 in the extra-embryonic ectoderm accounts for the ability of this tissue to inhibit anterior visceral endoderm formation (Rodriguez et al., 2005). Interestingly, in BMP4-dsRNA treated mouse embryos, the anterior visceral endoderm either fails to form, remains at the distal tip of the embryo, or is distal and expanded with ectopic patches, although this reflects reduced levels of BMP4, rather than enhanced levels of signaling as would be predicted in the GDF3 mutants (Soares et al., 2005). In the chordin/noggin double mutant that also would have enhanced BMP signaling, the anterior visceral endoderm is formed, but not maintained (Bachiller et al., 2000).
Due to the early defects of the GDF3 knockout and genetrap allele, later roles for GDF3 in the mammalian embryo have not been analyzed. Based on the localization of GDF3 in the node, anterior axial mesoderm, and ventral neural tube, it may play a role in neural induction and patterning. It is possible that GDF3 could inhibit BMP2 and BMP4 to compensate for the loss of noggin and chordin in the double knockout and allow posterior neural induction. Another possibility is that GDF3, as a ligand type of inhibitor, has a distinct role from noggin and chordin, although they are expressed in similar domains. We have recently analyzed a genetrap allele of GDF3 and found mostly normal cell fates throughout the embryo, but a significant defect in morphogenesis. These abnormalities are preceded by expanded activation of BMP signaling, as analyzed by the distribution of activated Smad1/5/8 (A.J.L. and A.H.B. manuscript submitted).
The biochemical mechanism through which GDF3 inhibits BMPs is still unknown. We tested whether the ‘missing’ fourth cysteine in the GDF3 mature domain endows GDF3 with its unusual activity because other TGF-β superfamily ligands with this same structural characteristic are also inhibitors, such as LeftyA and LeftyB. However, we found that this motif is only required for GDF3 function if the cleavage of the prepro domain is also mutated, preventing maturation. Therefore, there seem to be multiple, redundant mechanisms involving both the prepro domain of GDF3 and the mature domain, which is sufficient to block BMP signaling.
These data confirm and extend our previous finding that GDF3 is a BMP inhibitor and explain the different findings of Chen, Andersson and colleagues, who observed that GDF3 can also act like Nodal (Andersson et al., 2007; Chen et al., 2006).
Materials and methods
Animal cap assays
For intact animal caps, caps of stage 9 embryos were isolated in 0.1X MMR, washed once and transferred immediately to 0.5X MMR with gentamycin for culture. For animal cap disassociation, caps were isolated in CMFM media (88 mM NaCl,1 mM KCl, 2.4 mM NaHCO3, 5 mM HEPES). Cut caps were transferred to fresh CMFM with 0.5% BSA in an agarose coated dish for several minutes with the sensorial layer down, to allow the epithelial layer to peel away; this step was aided with a hair knife. Disassociated cap cells were then transferred to 100 μL/well of CMFM/BSA in pre-coated 96-well plates for protein co-culture for 4 h. During culture, cells were mixed every 30 min by pipetting up and down. Cells were then transferred to a pre-BSA-coated Eppendorf tube containing 0.75X MMR and 10 mM Ca2+/Mg2+ and spun at 800 rpm for 2 min to reaggregate the cells. The cell pellet was then cultured until sibling embryos reached stage 11.5 and harvested for mRNA.
Luciferase assays
All luciferase assays were done in three separate experiments, each in triplicate; representative individual triplicate experiments are shown in the results section. In Xenopus embryos, 20 pg of luciferase DNA construct (BRE-Lux (Hata et al., 2000) or ARE-Lux (Huang et al., 1995) was injected into the animal region of two cell embryos together with the indicated RNAs. Pools of four embryos were harvested at stage 11 in 50 μL of lysis buffer. The error bars indicate standard deviation.
Constructs and reagents
The open reading frame (ORF) of GDF3 and the ORF plus UTRs/polyA were generously provided by S.J. Lee and R.M. Harland, respectively. They were both subcloned into the EcoR1/Not1 sites of pCS2++. For sense RNA, GDF3 was linearized with Not1 and transcribed with SP6. BMP-4 is in pSP64T and RNA was produced with EcoR1/SP6. Xnr1 is in pCS2++ and RNA was produced with Not1/SP6. The cleavage mutant of GDF3 (coding region alone) in pCS2++ was produced by site-directed mutagenesis with the Stratagene QuickChange kit using the following primers: CATCCTTCTTCCG-GAAACGTGGGGGCGGCCATCTCTGTCCCC (sense) and GGGGACAGA-GATGGCCGCCCCCACGTTTCCGGAAGAAGGATG (anti-sense). The amino acid coding was converted from RKRR to GNVG. Prepro GDF3 was cloned by PCR into the EcoR1/Not1 sites of pCS2++. The primers for prepro cloning were: ATGATGAATTCCACCATGCAGCCT-TATCAACG (sense) and ATTATGCGGCCGCCCTCCTTTTGCG (anti-sense). Recombinant human GDF3 protein was purchased from Peprotech. Recombinant mouse Nodal and human BMP4 proteins were purchased from R&D Systems. Antibodies for western blots were goat anti-GDF3 (R&D systems), rabbit anti-Phospho Smads (1,5,8 or 2,3) (Cell Signaling), and mouse anti-tubulin (Santa Cruz).
Supplementary Material
Acknowledgments
We appreciate the comments of Drs. Julie Baker and Richard Harland. AJL was supported by NIH MSTP grant GM07739 and NIH grant 5 F30 NS051965.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2008.09.006.
References
- Andersson O, et al. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol. 2007;311:500–511. doi: 10.1016/j.ydbio.2007.08.060. [DOI] [PubMed] [Google Scholar]
- Bachiller D, et al. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Brennan J, et al. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Chen C, et al. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development. 2006;133:319–329. doi: 10.1242/dev.02210. [DOI] [PubMed] [Google Scholar]
- Cheng SK, et al. Lefty blocks a subset of TGFbeta signals by antagonizing EGF-CFC coreceptors. PLoS Biol. 2004;2:E30. doi: 10.1371/journal.pbio.0020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daluiski A, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- Dosch R, et al. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997;124:2325–2334. doi: 10.1242/dev.124.12.2325. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. Decapentaplegic acts as a morphogen to organize dorsal–ventral pattern in the Drosophila embryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Green JB, et al. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Grunz H, Tacke L. Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ Dev. 1989;28:211–217. doi: 10.1016/0922-3371(89)90006-3. [DOI] [PubMed] [Google Scholar]
- Hata A, et al. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell. 2000;100:229–240. doi: 10.1016/s0092-8674(00)81561-5. [DOI] [PubMed] [Google Scholar]
- Huang HC, et al. Identification of a potential regulator of early transcriptional responses to mesoderm inducers in the frog embryo. EMBO J. 1995;14:5965–5973. doi: 10.1002/j.1460-2075.1995.tb00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EM, Melton DA. Mutant Vg1 ligands disrupt endoderm and mesoderm formation in Xenopus embryos. Development. 1998;125:2677–2685. doi: 10.1242/dev.125.14.2677. [DOI] [PubMed] [Google Scholar]
- Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Identification of a novel member (GDF-1) of the transforming growth factor-beta superfamily. Mol Endocrinol. 1990;4:1034–1040. doi: 10.1210/mend-4-7-1034. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. GDF3, a BMP inhibitor, regulates cell fate in stem cells and early embryos. Development. 2006;133:209–216. doi: 10.1242/dev.02192. [DOI] [PubMed] [Google Scholar]
- Meno C, et al. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–298. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- Peerani R, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CT, et al. Regulation of left–right patterning in mice by growth/differentiation factor-1. Nat Genet. 2000;24:262–265. doi: 10.1038/73472. [DOI] [PubMed] [Google Scholar]
- Rodriguez TA, et al. Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm. Development. 2005;132:2513–2520. doi: 10.1242/dev.01847. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Smith JC. A mesoderm-inducing factor is produced by Xenopus cell line. Development. 1987;99:3–14. doi: 10.1242/dev.99.1.3. [DOI] [PubMed] [Google Scholar]
- Soares ML, et al. Functional studies of signaling pathways in peri-implantation development of the mouse embryo by RNAi. BMC Dev Biol. 2005;5:28. doi: 10.1186/1471-213X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen G, et al. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Thomsen GH, Melton DA. Processed Vg1 protein is an axial mesoderm inducer in Xenopus. Cell. 1993;74:433–441. doi: 10.1016/0092-8674(93)80045-g. [DOI] [PubMed] [Google Scholar]
- Wall NA, et al. Mesendoderm induction and reversal of left–right pattern by mouse Gdf1, a Vg1-related gene. Dev Biol. 2000;227:495–509. doi: 10.1006/dbio.2000.9926. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Wilson PA, et al. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–3184. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- Xu RH, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Yeo C, Whitman M. Nodal signals to Smads through Cripto-dependent and Cripto-independent mechanisms. Mol Cell. 2001;7:949–957. doi: 10.1016/s1097-2765(01)00249-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.