Abstract
In a treatment re-infection study of 206 Papua New Guinean school children, we examined risk of reinfection and symptomatic malaria caused by different Plasmodium species. Although children acquired a similar number of polymerase chain reaction–detectable Plasmodium falciparum and P. vivax infections in six months of active follow-up (P. falciparum = 5.00, P. vivax = 5.28), they were 21 times more likely to develop symptomatic P. falciparum malaria (1.17/year) than P. vivax malaria (0.06/year). Children greater than nine years of age had a reduced risk of acquiring P. vivax infections of low-to-moderate (>150/μL) density (adjusted hazard rate [AHR] = 0.65 and 0.42), whereas similar reductions in risk with age of P. falciparum infection was only seen for parasitemias > 5,000/μL (AHR = 0.49) and symptomatic episodes (AHR = 0.51). Infection and symptomatic episodes with P. malariae and P. ovale were rare. By nine years of age, children have thus acquired almost complete clinical immunity to P. vivax characterized by a very tight control of parasite density, whereas the acquisition of immunity to symptomatic P. falciparum malaria remained incomplete. These observations suggest that different mechanisms of immunity may be important for protection from these malaria species.
INTRODUCTION
The epidemiology of Plasmodium falciparum malaria suggests children first acquire immunity against severe disease after relatively few infections.1,2 However, uncomplicated P. falciparum malaria remains common throughout most of childhood, and a significant decrease in risk of infection is only seen in adolescence and early adulthood.1 Similar patterns have also been described in area of areas of Papua New Guinea highly endemic for malaria.3,4 It has therefore been argued that the mechanisms responsible for protection against severe disease may be distinct from those that protect against infections per se and mild episodes of disease,1,5 and that immunity might be acquired in stages. Although many potential targets and mechanisms of protective immunity have been identified,1,5–8 we still know little about mechanisms involved in the acquisition of protective immunity against P. falciparum.
Even less is known about the acquisition of immunity to non-P. falciparum malarias. In highly endemic areas such as Papua New Guinea where the different species co-occur, prevalence of infection with P. vivax peaks at younger ages3,9,10 and contributes proportionally less to the burden of febrile illness11 than P. falciparum. Conversely, P. malariae reaches maximum prevalence only in adolescents.9,10 These data indicate that immunity to P. vivax may be acquired more quickly than immunity to P. falciparum despite lower transmission rates.12 Although a number of potential targets and mechanisms for immunity have been identified for P. vivax,13–15 little is known about acquisition of immunity to P. malariae and P. ovale.
A better understanding of the incidence of infection and disease caused by different Plasmodium species in areas co-endemic for all species is needed to properly assess differences in the acquisition of clinical immunity to different species. Because mixed infections are common in malaria-endemic areas, but often remain undetected by light microscopy,10,16 polymerase chain reaction (PCR)–based diagnostic methods are needed for quantifying risk of infection and morbidity reliably.
To determine epidemiologic patterns of infections and disease with P. falciparum and P. vivax and investigate possible mechanisms of immune protection, we conducted a longitudinal treatment re-infection study of 206 Papua New Guinean elementary school children that combines repeated blood sampling and molecular detection of parasitemia with a large array of classic and functional immune assays. We describe the general study design and report patterns of incidence of infection and disease with all four human malaria parasite species. Detailed investigations of immunity to P. falciparum and P. vivax malaria will be the topic of future reports.
MATERIALS AND METHODS
Field study
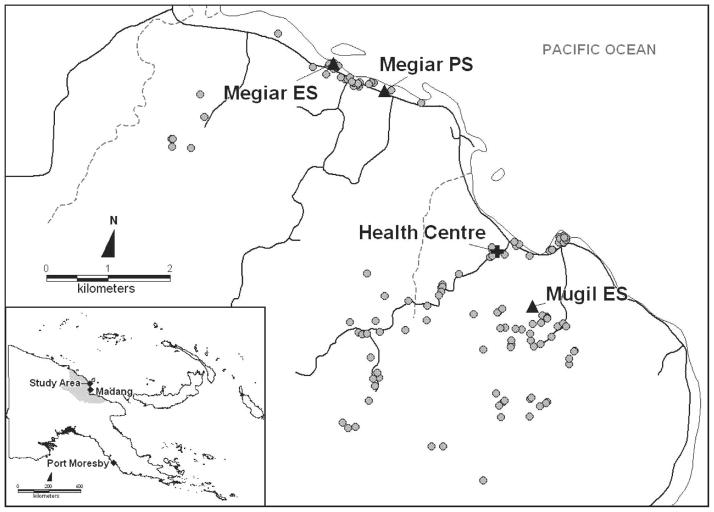
This study was conducted between June and December 2004 at the Mugil and Megiar elementary schools situated on the northern coast of Papua New Guinea, 50 km north of Madang. The catchment area of both schools is serviced by a single health center at Mugil (Figure 1) run by the Catholic Health Services. Although the Mugil school is within easy walking distance of the health center, the Megiar schools are 4 km away along a sealed road but with frequent transport available. Bed net use in the study area is limited, with retreatment of bed nets virtually absent. This study was reviewed and approved by institutional review boards of the Papua New Guinea Medical Research Advisory Council, the Walter and Eliza Hall Institute, and the Veteran’s Affairs Medical Center (Cleveland, OH).
Figure 1.
Location of study site, schools, and participants’ houses in Papua New Guinea.
After obtaining community support and written parental consent, children from all three grades in Mugil and grades 1 and 2 in Megiar were enrolled. Demographic information was collected from all participating children; the location of each child’s home was recorded using a hand-held global positioning system (GPS) receiver (GPS 315; Magellan, Santa Clara, CA).
Before starting treatment, each child was clinically examined: axillary temperature was measured using digital thermometers, the spleen was palpated, and a standard questionnaire of common signs and symptoms of malarial illness was administered. Hemoglobin (Hb) levels were measured using a portable device (HemoCue, Ångholm, Sweden). A 10-mL venous blood sample was collected using EDTA-Vacutainer® tubes (Becton Dickinson, Franklin Lakes, NJ) and two blood slides (thick and thin films) were made for determination of malarial infection. All children were subsequently treated with a seven-day course of artesunate monotherapy according to Papua New Guinea National treatment guidelines (i.e., 4 mg/kg on day 1 and 2 mg on days 2–7). All children received all seven doses with at least five of them directly observed.
After treatment, children were actively followed-up at the schools every two weeks for new infections and febrile illness with the first visit taking place two weeks after receiving the first artesunate treatment. A total of 12 follow-up visits were conducted. Because of national holidays that prevented field work, the eighth follow-up period had to be extended to three weeks, which resulted in a total length of active follow-up of 25 week. Follow-up visits were conducted on a class-by-class basis with one class checked every day. Children that did not attend school on the day of scheduled follow-up were checked the next day or at their homes at the earliest possible time within the next week. During school holidays (follow-up time points weeks 4 and 14), special clinics that included the screening of children’s videos were set up at the schools or in the villages that regrouped most students. Children who did not come to these clinics were individually followed-up at their homes, when possible.
At each active follow-up, all children were clinically examined, their axillary temperatures were taken, their health books were checked for recent antimalarial treatments, and they were questioned for recent bed net use. Hemoglobin levels were measured every four weeks and spleens were palpated every eight weeks. At the same time, two malarial blood films were made, a rapid diagnostic test (ICT Diagnostics, Brookvale, New South Wales, Australia) was conducted and a 250-μL blood sample was collected from each child into an K+-EDTA Microtainer® tube (Becton Dickinson) by finger prick using a retractable lancet (Genie® Lancet; Becton Dickinson). Children with symptoms of clinical malaria were transported to the Mugil health center after a third blood slide was made for parasitologic assessment, and treatment was given at the health center according to blood slide results. The scheduled active follow-ups, together with collection of additional venous blood samples, resulted in the study team visiting the Mugil school five times and the Megiar schools three times over each two-week period.
For six months after treatment, a passive case-detection system was maintained at the Mugil health center. In addition, children, their parents, and teachers were encouraged to report any illness to the study team at any time the team was visiting the school. All study children attending the outpatient clinic at Mugil health center with any illness, as well as those diagnosed by the study teams during their school visit, were referred to specific study staff based at the Mugil health center for clinical and parasitologic assessment. After a detailed clinical assessment, a 250-μL finger prick sample was collected, 3 blood slides were made, and Hb levels were measured. One of the slides was stained using rapid Giemsa staining (10% Giemsa for 10 minutes) and read immediately for diagnostic purposes. The other two slides were stained for research readings (5% Giemsa for 30 minutes). If children had signs of a febrile illness and a P. falciparum parasitemia > 500/μL or a P. vivax parasitemia > 250/μL, an additional 5-mL venous blood sample was collected within 24 hours. All children with clinical signs of malaria and a positive blood slide (irrespective of parasite density) were treated by the study team according to Papua New Guinea national treatment guidelines with chloroquine (three days) and sulfadoxine-pyrimethamine (single dose). A concurrent in vivo efficacy study at Mugil health center showed an overall 28-day failure rate (corrected by polymerase chain reaction [PCR]) of 12% for the treatment of P. falciparum infections (4% late clinical failures and 8% parasitologic failures) (Marfurt J, Mueller I, Genton B, unpublished data) Any other medical conditions were referred to the health center for appropriate treatment.
During the study period, monthly mosquito sampling was conducted in two villages using the all-night landing catch method17 and specimens were identified morphologically as described by Belkin.18 A subset of morphologically identified mosquitoes was processed by PCR to assess species identity.19 Mosquitoes were tested by enzyme-linked immunosorbent assay (ELISA)20 to detect circumsporozoite antigens using monoclonal antibodies specific for P. falciparum, P. vivax (PK210), and its variant (PK240).
Laboratory methods
All venous blood samples were separated into plasma, peripheral mononuclear cells, and remaining blood cells, centrifuged, and aliquoted accordingly. Finger prick blood samples were separated into plasma and cell pellets. DNA was extracted using the QIAamp 96 DNA Blood kit (Qiagen, Valencia, CA) from the cell pellet fraction of all samples.
All research blood films were read by two expert microscopists independently. Slides with discrepant results were reread by a third microscopist. Thick blood films were examined by light microscopy (LM) for 100 thick-film fields (under a 100× oil-immersion lens) before being declared infection negative. Parasite species in positive films were identified and densities were recorded as the number of parasites per 200 white blood cells (WBCs). Densities were converted to the number of parasites per microliter of blood assuming 8,000 WBCs/μL (population average WBC count3). Slides were scored as LM positive for an individual Plasmodium species if the species was detected independently by at least two microscopists and subsequent PCR-based analysis confirmed the presence of the species. Densities were calculated as the geometric mean densities of all positive results.
Infection by each of the four human malaria species was assessed in all blood samples collected using a semi-quantitative post-PCR, ligase detection reaction–fluorescent microsphere assay (LDR-FMA).21 This assay combines PCR amplification of the small subunit (SSU) ribosomal RNA gene (491–500-basepair fragments) using genus specific primers, followed by a multiplex species-specific LDR. The LDR products are hybridized to FlexMAP™ classification bead sets (5′) (Luminex, Austin, TX) and receive reporter labeling after incubation with streptavidin-R-phycoerythrin that binds to biotin (3′). Double-labeled species-specific LDR complexes are detected using a Bio-Plex array reader (Bio-Rad Laboratories, Hercules, CA). Species-specific fluorescence data were collected with Bio-Plex Manager version 3.0 software (Bio-Rad Laboratories). To ensure maximum sensitivity for the detection of Plasmodium infections, the PCR cycle number was set at 35. Differentiation of negative from positive fluorescent signals was performed by comparing median fluorescent intensity from study participants with values obtained from uninfected North American controls. Cut-off values for positivity were set at the 99% quantile of signals in controls as reported by Kasehagen and others.10 The design and sensitivity of this assay has been previously described.10,21,22 In these studies, the assay demonstrated high sensitivity compared with LM and real-time PCR. Nevertheless, we are not able to exclude the possibility that sequence polymorphisms in the Plasmodium species SSU ribosomal RNA gene sequences may contribute to false-negative results. However, to date, we have observed no direct evidence identifying specific variants of the P. falciparum, P. vivax, P. malariae, or P. ovale target sequences in our malaria prevalence studies in Papua New Guinea (Zimmerman PA, unpublished data).
To differentiate between treatment failures and newly established blood stage infections, all infections with P. falciparum or P. vivax (by PCR or LM) that occurred between the pre-treatment time point and six weeks post-treatment were genotyped to identify individual P. falciparum merozoite surface protein 2 alleles (P. falciparum infections23,24) or the highly polymorphic P. vivax Duffy binding protein alleles (P. vivax infections25).
Statistical analysis
Children were monitored for acquiring new infections until they withdrew from the study, did not provide two consecutive bi-weekly blood samples, or were re-treated with antimalarial drugs. Time-to-first infection with each species was calculated as the time between the date of first treatment and an infection-positive result by PCR or LM by either active or passive case detection. In addition, we calculated time-to-first infections with > 500 and > 5,000 parasites/μL for P. falciparum and > 150 parasites/μL for P. vivax. Children that remained negative for a particular species and/ or density cut-off until the end of active follow-up were censored after the last active follow-up time point (after 169–181 days of follow-up). Prevalence of infection during follow-up was calculated for only those children who had not been retreated.
The risk of symptomatic malarial illness was assessed as time-to-first clinical episode and incidence of clinical episodes during follow-up. Clinical malaria was defined as a measured fever (axillary temperature ≥ 37.5°C) or history of febrile illness during the 48 hours preceding examination in conjunction with any malaria infection. The cut-off value for clinical disease was set at 5,000/μL for P. falciparum26 and 1,000/μL for all other species (Mueller I, unpublished data). Children were monitored for clinical disease until they either withdrew from the study or were re-treated with antimalarial drugs. Children without a clinical episode were excluded after the last bi-weekly follow-up time point.
For the calculation of incidence rates, all disease episodes observed during active follow-up and passive morbidity surveillance were considered. In contrast to analyses for risk of infections, a child was considered at risk until he or she withdrew or reached the end of the study with the exception of the four weeks after further antimalarial treatments for the analyses of difference in incidence rates. If a child was recorded as ill with the same species twice within a two-week period, this was considered a single episode.
Time-to-event (infection or clinical episode) data were analyzed using standard survival analysis techniques. A log-rank test was used to evaluate the difference in non-parametric survival curves. Because hazards for the different explanatory variables were generally proportional over the follow-up study, Cox regression was used to test for univariate and multivariate risk factors. Poisson regression was used for univariate and multivariate analyses of factors associated with the incidence of clinical P. falciparum disease. In all multivariate analyses, backwards selection and likelihood ratio tests were used to identify the best fitting models. All statistical analyses were performed using STATA 8 statistical analysis software (Stata Corporation, College Station, TX).
RESULTS
Enrollment and baseline characteristics
After obtaining informed consent and baseline health assessment, 206 children 5–14 years of age (inter-quartile range = 8.1–9.3 years) were enrolled into the study. Of these, 152 attended Mugil Elementary School, 44 attended Megiar Elementary School, and 10 attended Megiar Primary School. Of these children, 51.5% were girls and 35.9% reported having slept under a bet net the previous night.
At the time of enrollment, 92 children (44.7%) were positive for Plasmodium trophozoites by LM with P. falciparum the most common infection followed by P. vivax and P. malariae (Table 1). An additional 12 children were positive for P. falciparum gametocytes only. Geometric mean parasite densities for all species were generally low (Table 1). Densities of infections with P. vivax were significantly lower than those with P. falciparum (P < 0.001) or mixed infections (P < 0.001). The prevalence of infection by all species increased significantly when infections were diagnosed by LDR-FMA. Overall, 166 children (80.6%) were positive for any malarial infection with 54 (26.2%) having mixed species infections (Table 1). There was no significant association with prevalence between any species (by LDR-FMA) and bed net use.
Table 1.
Parasitologic and clinical assessment prior to treatment*
| Infection species† | PCR-LDR-FMA
|
Light microscopy
|
|||
|---|---|---|---|---|---|
| No. pos | % | No. pos | % | Geometric mean density‡ | |
| Pf | 85 | 41.3 | 68 | 33.0 | 361 (240, 544) |
| Pv | 25 | 12.1 | 17 | 8.3 | 102 (74, 142) |
| Pm | 2 | 1.0 | 4 | 1.9 | 421 (38, 4,646) |
| Pf and Pv | 40 | 19.4 | 3 | 1.5 | 315 (183, 540) |
| Pf and Pm | 8 | 3.9 | 0 | 0.0 | – |
| Pf and Po | 1 | 0.5 | 0 | 0.0 | – |
| Pf, Pv, and Pm | 4 | 1.9 | 0 | 0.0 | – |
| Pf, Pv, and Po | 1 | 0.5 | 0 | 0.0 | – |
| Pf gametocyte only | – | 12 | 5.8 | 68 (51, 90) | |
| all positive | 166 | 80.6% | 104 | 50.5% | |
PCR = polymerase chain reaction; LDR = ligation detection reaction; FMA = fluorescent microsphere assay; Pf = Plasmodium falciparum; Pv = P. vivax; Pm = P. malariae; Po = P. ovale.
Only species combinations that were observed are shown.
Mean number of parasites per microliter (95% confidence interval).
Ninety-eight children (47.6%) had an enlarged spleen that was associated with a concurrent malarial infection by either LM (odds ratio [OR] = 2.49, P = 0.001) or LDR-FMA (OR = 2.51, P = 0.01). Six children (2.9%) had moderate-to-severe anemia (Hb level = 5–8 g/dL) and 74 (35.9%) had mild anemia (Hb level = 8–11 g/dL) with no significant difference between boys and girls. All five children who had an axillary temperature ≥37.5°C were positive for P. falciparum by both LM and LDR-FMA.
Initial antimalarial treatment
Of the 139 children with an LDR-FMA-positive P. falciparum infection at baseline, only 12 (8.6%) showed evidence of infection with the same merozoite surface protein 2 genotype in the six weeks after the seven-day treatment with artesunate. In one child, the genotype of the first re-infection could not be ascertained and the infection was thus considered as a suspected treatment failure. Genotyping for P. vivax showed that only one PCR-positive infection observed within the first six weeks after treatment had the same DBP genotype as the infection at baseline. Among P. malariae infections, there was one infection that remained PCR-positive at day 14. Because of the lack of a genotyping assay for P. malariae, this case was therefore also treated as a suspected treatment failure. No treatment failure was observed for P. ovale. Overall, the artesunate treatment had an efficacy of 91.4% for treating infections with P. falciparum, 92.9% for infections with P. malariae, and 98.6% for infections with P. vivax. All treatment failure children were excluded from further analysis of re-infection with the homologous Plasmodium species. However, they were included in analyses for heterologous Plasmodium infections.
Risk of re-infections during active follow-up
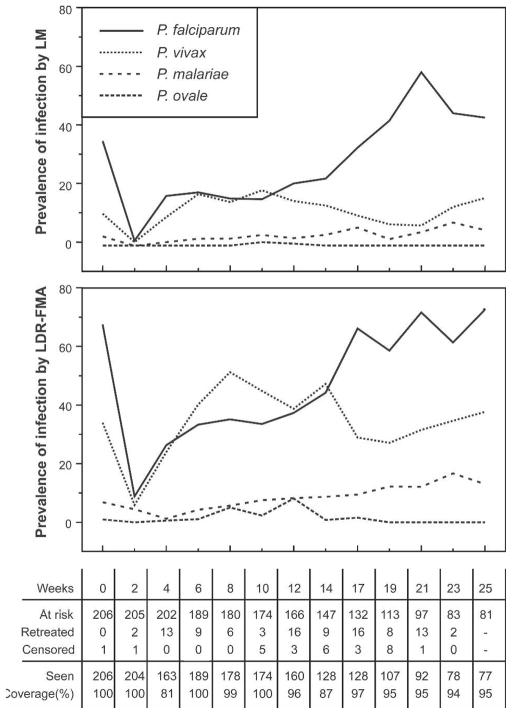
When treatment failure cases were excluded, only one child was positive for P. falciparum and none for any other species by LM two weeks after treatment. However, when we used LDR-FMA, 17 (8.9%) of 192 children were positive for P. falciparum, 12 (5.9%) of 204 were positive for P. vivax, and 9 (4.4%) of 203 were positive for P. malariae two weeks after treatment. Prevalence of infection increased steadily over the active follow-up period (Figure 2), with prevalence rates for P. vivax and P. malariae reaching pre-treatment levels 6 and 10 weeks after treatment, respectively. The prevalence of P. falciparum reached pre-treatment levels after 16 weeks. The comparatively slower increase in P. falciparum prevalence may be partly due to the large number of P. falciparum infections that became symptomatic (see below), which needed to be treated and were thus excluded from the analyses of prevalence rates after re-treatment time points (Figure 2).
Figure 2.
Prevalence of malarial infections at baseline and at active bi-weekly follow-up obtaining of blood samples. Top, Diagnosis by light microscopy (LM). Bottom, Diagnosis using post–polymerase chain reaction and ligation detection reaction–fluorescent microsphere assay (LDR-FMA). The number of children at risk, treated, and withdrawn during each two-week period and number of children seen during each follow-up are shown below the bottom panel.
The risk of acquiring blood stage Plasmodium infection was thus better estimated by the time to re-infection (Table 2 and Figure 3). When diagnosed by LDR-FMA, re-infection rates for P. falciparum and P. vivax were identical (incidence of 5.00 new infections with P. falciparum and 5.28 with P. vivax/ child/year; P = 0.29, by log-rank test). However, the relative incidence of infections with either species changed over the course of follow-up. A significantly higher incidence of new P. vivax infections compared with P. falciparum was observed during the first 90 days of follow-up (Figure 3) (0–90 days: 4.33 P. falciparum and 5.28 P. vivax infections/child/year; P = 0.04), whereas the incidence of P. falciparum was significantly higher in the second 90 days (90–180 days: 9.04 P. falciparum and 5.23 P. vivax infections/child/year; P = 0.03). Infections with P. malariae (0.98/child/year) and P. ovale (0.42/child/year) were significantly less frequent than those with the two other species (P < 0.001).
Table 2.
Median time to first infections, incidence of new infections, and multivariate risk factors for acquiring new infections with different Plasmodium species diagnosed by LM and LDR-FMA*
|
P. falciparum
|
P. vivax
|
P. malariae
|
P. ovale
|
|||||
|---|---|---|---|---|---|---|---|---|
| PCR | LM | PCR | LM | PCR | LM | PCR | LM | |
| % Re-infected† | 95.3 | 87.6 | 82.0 | 49.5 | 29.3 | 8.3 | 13.6 | 1.5 |
| Median time to re-infections (days) | 55 | 99 | 54 | 118 | –‡ | –‡ | –‡ | –‡ |
| Incidence rate (years) | 5.00 (4.30, 5.80) | 3.23 (2.76, 3.78) | 5.28 (4.54, 6.14) | 2.03 (1.68, 2.46) | 0.98 (0.76, 1.26) | 0.24 (0.15, 0.39) | 0.42 (0.29, 0.61) | 0.04 (0.01, 0.13) |
| Multivariate predictors§ | AHR | AHR | AHR | AHR | AHR | AHR | AHR | –¶ |
| Age > 9 years | 0.67 (0.44, 0.96) | 0.41 (0.17, 0.95) | ||||||
| Distance from Mugil HC# | 1.14 (1.02, 1.27) | 1.44 (1.03, 2.01) | ||||||
| Distance from school# | 0.76 (0.64, 0.91) | 1.68 (1.16, 2.45) | 1.54 (1.08, 2.19) | |||||
| Enrolled at Megiar Elementary School | 5.68 (2.45, 13.2) | |||||||
| Pf LDR-FMA + at baseline | 1.67 (1.18, 2.35) | |||||||
| Pf LM + at baseline | 3.79 (1.75, 8.18) | |||||||
| Pv LM + at baseline | 1.70 (1.03, 2.83) | |||||||
LM = light microscopy; AHR = adjusted hazard ratio. For definitions other abbreviations, see Table 1. Values in parentheses are 95% confidence intervals.
Proporation of children with at least one positive sample while at risk. Total children at risk: Pf: n = 194, Pv: n = 206; Pm: n = 205; Po: n = 206.
Not estimated because less than 50% of children re-infected.
Only significant factors (p < 0.05) from multivariate Cox regression analyses are given. Other factors included sex, distance from seacoast, presence of enlarged spleen and/or mild anemia (hemoglobin < 11 g/dL), reported bed net use (slept > 50% of nights under bed net), and infection status with any infection by PCR and LM at baseline.
Not estimated due to very low re-infection rate (n = 3).
Distances calculated as straight line distance (km) from child’s residence to Mugil Health Center (HC) or school.
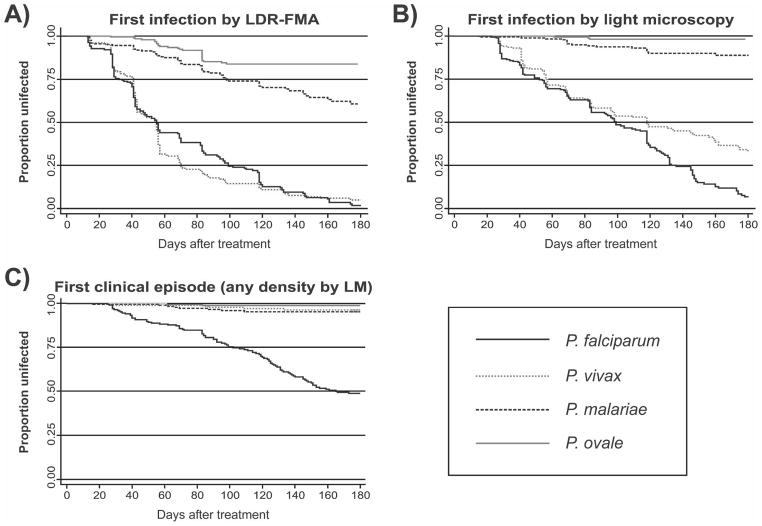
Figure 3.
Risk of acquiring new infections after treatment of blood-stage infections. Kaplan-Meier curves for A, Asymptomatic infections diagnosed by post–polymerase chain reaction and ligation detection reaction–fluorescent microsphere assay (LDR-FMA), B, Asymptomatic infections diagnosed by light microscopy (LM), and C, Episodes of febrile illness with concurrent malarial infection of any density (by LM).
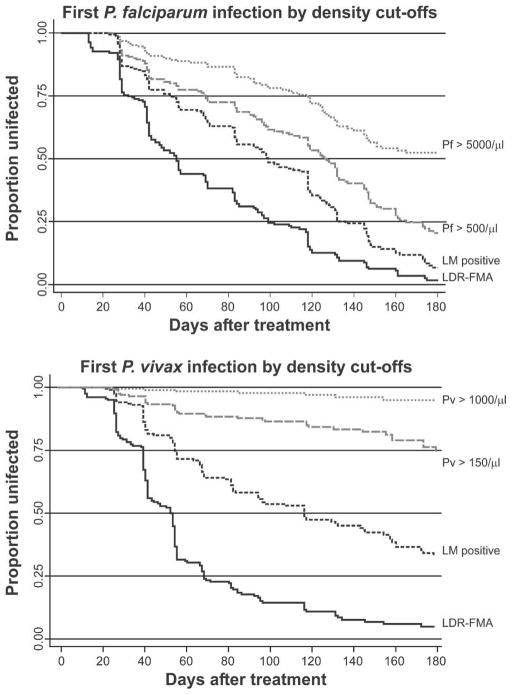
Re-infection rates decreased significantly in all Plasmodium species when infections were diagnosed by LM (P < 0.001), with children acquiring 3.25 LM-detectable P. falciparum infections, 2.03 P. vivax infections, 0.24 P. malariae infections, and 0.04 P. ovale infections/child/year. Although children had significantly less new P. vivax infections than P. falciparum infections (P < 0.001), this effect was almost exclusively observed in the second half of follow-up (Figure 3: 0–90 days: 2.23 P. falciparum and 1.98 P. vivax infections/ child/year; P = 0.46; after 90 days, 6.63 P. falciparum and 2.19 P. vivax infections/child/year; P < 0.001). The difference in incidence between these two species increased when only new moderate (P. falciparum > 500/μL and P. vivax > 150/μL) and high-density (P. falciparum > 5,000/μL and P. vivax > 1,000/ μL) infections were considered (Figure 4). Although 78.9% and 47.8% of the children were re-infected with P. falciparum infections > 500/μL and > 5,000/μL, respectively, only 25.1% and 5.6% had P. vivax infections > 150/μL and > 1000/μL, respectively (P < 0.001, by log-rank test).
Figure 4.
Kaplan-Meier curves of time to first Plasmodium falciparum (Pf) and P. vivax (Pv) infections exceeding increasing density thresholds. LM = positive by light microscopy; LDR-FMA = positive by post–ligation polymerase chain reaction ligation detection reaction–fluorescent microsphere assay. Shown are species-specific density cut-offs: higher cut-offs are set at species-specific pyrogenic thresholds and lower cut-offs are set at approximately 1.5 times the geometric mean density observed at baseline.
For all Plasmodium species except P. vivax, some geographic variation in infection rates was observed, with children in more remote areas of the study area (those further away from health center and schools) having a significantly higher risk of acquiring new infections (Table 2). No significant associations were found between frequency of bed net use (average use = 35.9%) and risk of re-infections with any Plasmodium species. However, effects were small and not consistent between LDR-FMA- and LM-positive infections. Although some significant associations were observed between infection risk during the active follow-up period and infection status prior to artesunate treatment, no consistent relationships were detected. Time-to-first LM-positive P. falciparum infection was decreased in children who were PCR positive for P. falciparum at baseline but not in those who were only LM positive (Table 2). Infection status at baseline was also not significantly associated with time-to-first PCR-positive infections. Similarly, no clear association of infection status at baseline with risk of infection during follow-up was found for P. vivax. Although the risk of acquiring a new PCR-positive P. vivax infection was associated with an increased risk of having a P. vivax-positive blood slide at baseline, this association was not found with PCR positivity at baseline or with a risk of acquiring new LM-positive infections (Table 2). Although having a PCR-positive P. malariae infection was associated with a decreased risk for later infections with P. ovale, no association in infection status was observed for risk of infection with P. malariae.
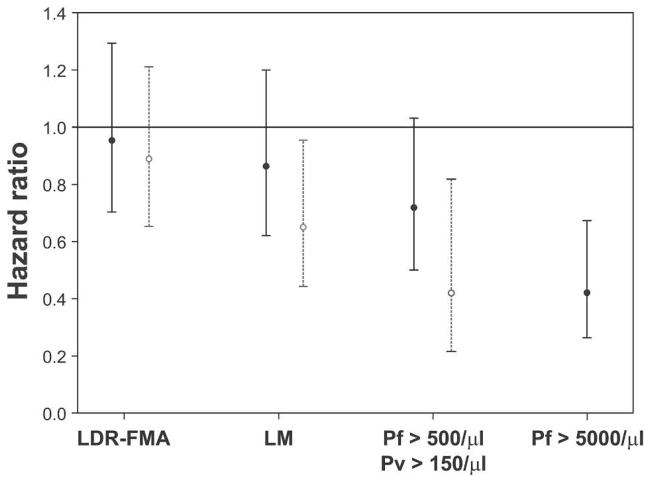
A decrease in risk of acquiring infections with increasing age was only observed in LM-detectable P. vivax (adjusted hazard ratio [AHR] = 0.65, P = 0.028) and PCR-diagnosed P. ovale (AHR = 0.41, P = 0.038) infections. However, progressively larger decreases in risk with age were observed not only for increasing density cut-offs of P. vivax infections (> 150/μL, AHR = 0.42, P = 0.01) but also for P. falciparum infections (Figure 5). However, this effect only reached statistical significance for moderate and high-density infections (i.e. P. falciparum > 500/μL, AHR = 0.71, P = 0.053 and P. falciparum > 5,000/μL, AHR = 0.48, P = 0.002).
Figure 5.
Risk of acquiring new infections in relations to age and increasing density cut-offs. Values are adjusted hazard ratios and 95% confidence intervals. ● = Plasmodium falciparum (Pf); ○ = P. vivax (Pv). Because of low number of infections, no age effect for Pv parasitemias > 1,000/mL was estimated. LDR-FMA = positive by post–polymerase chain reaction ligation detection reaction–fluorescent microsphere assay; LM = positive by light microscopy. Shown are species-specific density cut-offs: higher cut-offs for Pf are set at species-specific pyrogenic thresholds and lower cut-offs set at approximately 1.5 times the geometric mean density observed at baseline.
Incidence and risk factors for symptomatic malarial infections
Overall, 162 febrile episodes with concurrent parastemia were observed in 109 children (52.9%, Table 3). Of these, 111 (65.7%) exceeded species-specific pyrogenic thresholds of 5,000/μL for P. falciparum and > 1,000/μL for non-P. falciparum. Most of these malaria-attributable illness episodes (92.8%) were caused by P. falciparum, with only five episodes caused by P. vivax (4.5%), two each by P. malariae and P. ovale (1.8%), and one by a P. falciparum/P. vivax mixed infection (0.9%). The distribution of febrile illness episodes with any concurrent parasitemia and malaria-attributable episodes was consistent with a Poisson distribution both overall and for P. falciparum episodes (P > 0.5, by Kolmogorow-Smirnov test). Although 20 children (9.7%) had more than one P. falciparum-attributable malaria episode, none had more than one non-P. falciparum malaria episode. With an incidence rate of 1.17 episodes/child/year, P. falciparum-attributable illness episodes were 21 times more frequent than those caused by P. vivax (0.06/child/year) and 52 times more frequent than P. malariae or P. ovale (0.02/child/year) (P < 0.001).
Table 3.
Incidence of symptomatic malaria episodes by species and case definition*
| Any episode
|
P. falciparum
|
P. vivax
|
P. malariae
|
P. ovale
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any | Threshold | Any | > 5,000/μL | Any | < 1,000/μL | Any | > 1,000/μL | Any | > 1,000/μL | |
| No. episodes | 162 | 111 | 148 | 103 | 10 | 5 | 8 | 2 | 3 | 2 |
| No. mixed | 7 | 1 | 5 | 1 | 4 | 1 | 5 | 0 | 0 | 0 |
| No. of children with | ||||||||||
| 0 episodes | 97 | 123 | 103 | 126 | 196 | 201 | 198 | 204 | 203 | 204 |
| 1 episode | 62 | 57 | 64 | 60 | 10 | 5 | 8 | 2 | 3 | 2 |
| 2 episodes | 36 | 23 | 33 | 17 | ||||||
| 3 episodes | 9 | 3 | 6 | 3 | ||||||
| 4 episodes | 2 | 0 | 0 | 0 | ||||||
| % children with ≥ 1 episode | 52.9 | 40.3 | 50.0 | 38.8 | 4.9 | 2.4 | 3.9 | 1.0 | 1.5 | 1.0 |
| Incidence rate† (/child/year) | 1.92 (1.65, 2.23) | 1.27 (1.06, 1.53) | 1.68 (1.43, 1.98) | 1.17 (0.97, 1.42) | 0.11 (0.06, 0.21) | 0.06 (0.02, 0.14) | 0.09 (0.05, 0.18) | 0.02 (0.01, 0.09) | 0.03 (0.01, 0.11) | 0.02 (0.01, 0.09) |
Values in parentheses are 95% confidence intervals.
Estimated from Poisson regression model. Total time at risk = 32,125 person days.
The incidence of febrile illness with any P. falciparum density was significantly lower in children greater than nine years of age (adjusted incidence rate ratio [IRR] = 0.67, 95% CI = 0.48–0.93, P = 0.019) and in children with an LM-positive P. falciparum infection at baseline (IRR = 0.66, 95% CI = 0.45, 0.97, P = 0.032). In addition, children living within one kilometer of the coastline more frequently had a fever and concurrent parasitemia (IRR = 1.57, 95% CI = 1.11–2.23, P = 0.011).
The decrease in incidence of P. falciparum illness with increasing age was more pronounced when only febrile episodes with a P. falciparum parasitemia greater than 5,000/μL were considered (IRR = 0.55, 95% CI = 0.37–0.83, P = 0.005). However, the incidence of high P. falciparum density episodes was no longer significantly associated with infection status at baseline (P > 0.1). As for fevers with any P. falciparum density, spatial differences in incidence of high P. falciparum density episodes were observed with those children living near the coastline (IRR = 1.61, 95% CI = 1.05, 2.46, P = 0.030) and/or with those attending Mugil Elementary School (IRR = 2.02, 95% CI = 1.20, 3.40, P = 0.008) who had an increased risk. A tendency for lower incidence of high-density clinical P. falciparum infections was observed in mildly anemic children (Hb level < 11 g/dL, IRR = 0.69, 955 CI = 0.45–1.05, P = 0.084).
Of the 103 children with ≥ 1 febrile illness episodes and concurrent P. falciparum parasitemia of any density, five (4.9%) were re-treated with antimalarials prior to the episode and were thus excluded from time-to-event analyses. Because all febrile episodes with any concurrent parastaemia were treated, 12 (15.0%) of 80 children who had ≥ 1 episode with a P. falciparum parasitemia > 5,000/μL were excluded because of earlier re-treatment. Similar to our preceding analysis, the hazard of having a P. falciparum episode, both overall and for high-density infections, was higher in children attending the Mugil school and/or coastal hamlets, but lower in children greater than nine years of age (Table 4 and Figure 6). In addition, female children tended to present more quickly with P. falciparum illness than males. Children with mild anemia (Hb level < 11 g/dL) had a significantly a lower risk of having a febrile illness (all episodes: AHR = 0.59, P = 0.020 and P. falciparum > 5,000/μL: AHR = 0.52, P = 0.016).
Table 4.
Multivariate predictors for time to first Plasmodium falciparum (Pf) episode: infections with any density versus infections > 5,000/μL*
| Any Pf density
|
Pf > 5,000/μL
|
|||
|---|---|---|---|---|
| AHR | (95% CI) | AHR | (95% CI) | |
| Multivariate predictors† | ||||
| Enrolled at Mugil Elementary School | 1.62 | (0.98, 2.68) | 3.35 | (1.58, 7.10) |
| Coastal residence‡ | 2.08 | (1.34, 3.21) | 1.98 | (1.19, 3.30) |
| Female | 1.41 | (0.94, 2.12) | 1.52 | (0.93, 2.40) |
| Age > 9 years | 0.56 | (0.37, 0.85) | 0.51 | (0.31, 0.84) |
| Hb < 11 g/dL | 0.59 | (0.38, 0.92) | 0.52 | (0.30, 0.88) |
AHR = adjusted hazard ratio; CI = confidence interval; Hb = hemoglobin.
Only factors with a P value < 0.05 and 0.05–0.10 (bold) from multivariate Cox regression analyses are given. Other factors included distance from school, distance from health center, presence of enlarged spleen, reported bed net use (slept > 50% of nights under bed net) and infection status with any Plasmodium species diagnosed by PCR and LM at baseline.
Residence of child situated less than one kilometer from coastline.
Figure 6.
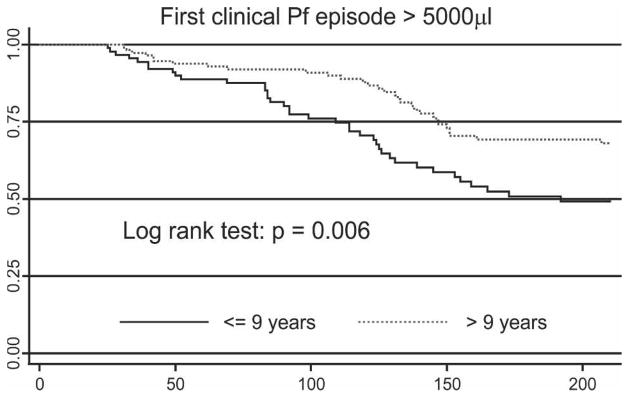
Kaplan-Meier curves of risk of experiencing an episode of symptomatic Plasmodium falciparum (Pf) malaria with a parasite density greater than 5,000 parasites/μL in relation to age.
Mosquito transmission dynamics
The biting rates of Anopheles sp. in the coastal and inland (> 2.5 km) villages were 1.2 bites/person/night and 5.81 bites/person/night, respectively. A sampling effort of 188 person-nights over a 12-month period, identified 659 mosquitoes belonging to the An. punctulatus group. These included An. punctulatus (34.1%), An. koliensis (51.6%), and An. farauti (14.3%). Anopheles farauti (41.6%) and An. koliensis (41.6%) were equally abundant in coastal villages where An. punctulatus accounted for only 16% of mosquitoes caught. In inland villages, An. punctulatus (37.3%) and An. koliensis (51.9%) were notably more prevalent than An. farauti (8.6%).
Only 113 Anopheles mosquitoes were caught in the coastal village and none of the 53 processed by ELISA was positive for malaria parasites. In inland villages, the P. falciparum and P. vivax sporozoite rates for An. punctulatus s.l were 1.89% and 1.26%, respectively. The corresponding entomologic inoculation rates were 36.56 and 24.38 infective bites/person/ year for P. falciparum and P. vivax, respectively.
DISCUSSION
The present study shows that Papua New Guinean children 5–13 years of age have acquired significantly different levels of immunity against P. falciparum and P. vivax. Despite comparable number of PCR-detectable new blood infections, children were 21 times more likely to have illness from P. falciparum than from P. vivax infections. By the time they reach elementary school age, children in the study appeared to have acquired almost complete clinical immunity to P. vivax, whereas the acquisition of clinical immunity against P falciparum was ongoing, with older children showing reduced risk.
After treatment with a very short half-life drug, we saw rapid re-infection of children. In particular, prevalence of P. vivax increased quickly and parasite prevalence (by LDR-FMA and LM) exceeded those observed prior to treatment. Although by the end of follow-up virtually all children had been infected at least once with both parasites, it took significantly longer for P. falciparum prevalence rates to reach the levels seen at baseline. The fact that we did not use a drug with activity against liver stages may have influenced the rapid recurrence of P. vivax infections. However, in an area with year-round, albeit moderately seasonal, transmission such as Madang,27 the rates of acquiring new liver stage infections through mosquito bites and establishing new blood stage infections from liver stages are likely to be comparable. The clearance of liver stages by drug treatment may lead to an underestimation of the incidence of P. vivax blood stage infections, particularly early in the follow-up period when new liver stage infections are acquired, but no new blood stage infections are established from existing liver stages. The high number of blood stage infections observed is thus likely to be reflective of the true burden of infection with P. vivax in the study community.
The varying delay between time of initial infection by mosquito bite and appearance of blood stages in P. vivax may mean that seasonality of blood stage infections is partially uncoupled from seasonality of transmission. Some evidence for this is seen in this study cohort. The study was started and treatment was given in June, at the end of the high transmission season with the first half of the follow-up coinciding with the period of lowest transmission (July–September).11,27 Although the incidence of new P. falciparum infections (by LDR-FMA and LM) was lower during the first half of follow-up, the incidence of P. vivax was relatively constant over time. Relapses from long-lasting liver stages may be an important contributor to the more limited seasonality of P. vivax that was also observed in earlier population surveys in the Madang area.11,27
It was evident that the children in the cohort were significantly better at controlling moderate and high-density P. vivax infections than P. falciparum infections (Figure 4). A considerable number of LDR-FMA–detectable P. vivax infections never became detectable by LM. Of those that did, few reached the pyrogenic threshold of 1,000 parasite/μL and only five children had a symptomatic P. vivax episode. This low incidence of clinical P. vivax disease in children 5–13 years of age is consistent with data from a passive morbidity surveillance system in another community in Papua New Guinea,4 which showed that the incidence of P. vivax illness peaks in children 1–2 years of age and becomes rare in children greater than five years of age (Mueller I, Genton B, unpublished data). Conversely, almost half of all children had at least one P. falciparum infection > 5,000/μL and 80 (39%) children were diagnosed with 1–3 episodes of P. falciparum illness during the six months of follow-up. In a similar treatment re-infection study conducted in northern Ghana,28 the incidence of P. falciparum disease after radical treatment for blood stage infections was higher than that usually observed for the same age group. On the basis of evidence that in older children asymptomatic infections of high multiplicities offered protection against subsequent morbidity,29 Smith and others reported that the initial treatment of asymptomatic infections increased the children’s risk of clinical illness. Although the incidence of P. falciparum illness in our study was high, it was similar to that observed in an earlier study in a neighboring population that measured malarial associated fever rates in children 2–15 years of age using a weekly morbidity surveillance program.11 Because this earlier study did not treat children prior to follow-up and overall P. falciparum prevalence in asymptomatic individuals (39%) was comparable to what we observed at baseline, we believe it is unlikely that the initial treatment led to an increase in incidence of P. falciparum disease.
The relatively limited heterogeneity in the cohort in reinfection risk in relation to sex and place of residence indicates that age is likely to be closely correlated with lifetime exposure and thus is a good proxy for overall immune status. Comparing differences in risk of infections with different parasite densities not only shows that high-density P. vivax infections are rare, but that despite a similar number of new blood stage infections (as detected by LDR-FMA), the risk of acquiring even moderate and low-density P. vivax infections is significantly lower in older children (i.e. > 9 years of age). This indicates that by nine years of age children have acquired an ability to control P. vivax densities at levels well below pyrogenic thresholds with many infections not becoming patent by LM. In contrast, control of P. falciparum densities is less developed. Most blood stage P. falciparum infections do eventually become LM positive, and only at moderate-to-high densities is a reduction in risk apparent in older children. Nevertheless, some older children still have frequent high-density P. falciparum infections and clinical illness. These differences in ability to control parasite densities may explain why in most studies in areas with moderate-to-high endemicity for malaria, P. vivax prevalence3,10,11,27 and incidence rates30 peak in younger age groups than P. falciparum prevalence and incidence rates.
The observed difference in rates of immune acquisition for P. falciparum and P. vivax is remarkable, given that entomologic inoculation rates, as well as re-infection rates (by PCR), for both species are comparable. By the time children reach elementary school age, i.e. 6–7 years, they are likely to have been exposed to 150–200 infected bites and had 30 successful blood stage infections with each species. Why a similar amount of exposure can lead to these apparent differences in immunity to the two species is not clear. However, similar differences in rates of immune acquisition have been described in Vanuatu30 and other areas of the world where the species co-existed.31,32 These results are consistent with data from malaria therapy patients in whom immunity to P. vivax was more rapidly acquired than that to P. falciparum.33 Whereas a single infection with P. vivax resulted in a strongly reduced incidence of a febrile episode upon homologous and to a lesser extent heterologous re-infection,34 most secondary P. falciparum infections were associated with fever, and in some cases high-density parasitemia, even if re-infected with the same strain.35 Similar patterns were observed under natural conditions in Sri Lankan patients.36
Contrary to the relative homogeneity in re-infection risk in relation to sex and place of residence, there were significant, spatially structured differences in risk of P. falciparum disease between children attending the Mugil Elementary School and those living near the coast who more often had a febrile episode of P. falciparum parasitemia; girls also tended to be at higher risk of illness. However, these differences are more likely to be related to differences in accessibility and health-seeking behavior rather than reflect true difference in risk of disease. The higher frequency of study team visits to the Mugil school indicated that children who were sick while attending school were more likely to be reported to the study team on non-surveillance days than at the other schools. Similarly, the vicinity to the schools and the sealed road leading to the health center indicated that it was substantially simpler for children living in costal hamlets to seek treatment from either study team or health center. Such a decrease in attendance at health centers with increasing distance has been previously observed in Papua New Guinea.37
The observed association of mild anemia (Hb level < 11 g/dL) with protection against clinical P. falciparum disease is intriguing because such a mild degree of anemia is by itself unlikely to be an impediment to parasite growth. Because malaria is an important contributor to anemia,38–40 Hb levels at baseline may be a marker of recent malarial exposure rather than directly affecting risk of P. falciparum illness.
Although almost 40% of children did have at least one LDR-FMA–detectable P. malariae infection, most of these infections were not apparent by LM, and as in areas of Africa highly endemic for malaria,41,42 episodes of P. malariae illness were rare. The low number of episodes precludes drawing firm conclusion with regards to acquisition on immunity to P. malariae in this population. However, because seven of eight clinical episodes occurred in children greater than nine years of age, older children are still at least as susceptible as younger children to P. malariae and the acquisition of immunity to this species may consequently be rather slow. Similar slow acquisition of immunity to P. malariae is also supported by observations by Smith and others9 and Kasehagen and others10 that in another population in Papua New Guinea the prevalence of P. malariae infections peaked later than those for P. vivax and P. falciparum. As observed in earlier cross-sectional surveys10,16 infections and illness with P. ovale were rare and, as observed in Africa,43 most P. ovale infections were of very short duration. In contrast to infections with other species, P. ovale infections were clustered in time and space with significantly higher risk of infection between weeks 6 and 12 of follow-up and in children attending Megiar Elementary School.
Since the suggestion by Williams and others that an increased incidence of P. vivax infection in early life may protect children with α-thalassemia from later severe P. falciparum malaria,44 a number of studies have investigated potential cross-species protection. Although clinical studies from Thailand,45,46 as well as an epidemiologic study from Papua New Guinea,47 provide some support to the idea of cross-species protection by P. vivax against P. falciparum morbidity, it has been remarkably difficult to find consistent evidence that infection with one species protects against (concurrent or subsequent) heterologous infections.16,47–50 With the exception of a possible positive interaction between prior infection with P. falciparum and subsequent risk of infection with P. ovale, the present study did not find any evidence that the presence of a heterologous infection at baseline provided protection against subsequent re-infections or P. falciparum illness. However, persistent, concurrent parasitemia may be required for cross-species protection. Thus, it is possible that the lack of associations between heterologous species is caused by clearance of blood stage infections by the initial treatment rather than truly indicating a lack of cross-species protection. The existence of cross-species protection is thus better studied in conventional cohort rather than a treatment re-infection design.
The current study clearly demonstrates differences in the acquisition of clinical immunity to P. falciparum and P. vivax among Papua New Guinean children. Despite similar incidence of infections, P. vivax is no longer a consistent source of morbidity by the time children reach school age. Although it continues to decrease with age, the burden of P. falciparum illness remains high. In the age group studied (i.e., 5–14 years), clinical immunity seems to be linked closely to the ability to control parasite densities, with children able to control P. vivax at much lower densities than P. falciparum. Potential reasons for the differential rates in the acquisition of immunity may include key biologic differences between the two species, particularly in relation to red blood cell invasion, adhesion or sequestration in vascular beds, and the nature of variant surface antigens. Understanding these differences may provide key insights into immunity and pathogenesis with P. falciparum and P. vivax and is the focus of on-going studies. Comparative studies on immune acquisition to both P. falciparum and P. vivax species may thus provide valuable insight into the important different immune targets and mechanisms underlying acquisition of clinical protection.
Acknowledgments
We thank the children for participating in the study; the guardians, teachers, and support staff at Mugil and Megiar schools for their support; Livingstone Tavul, Mary Goroti, and Mugil health centre staff for assistance with the field work; and Kay Baea, Lina Lorri, and Moses Lagog for reading the blood films and David T. McNamara for technical assistance with LDR-FMA.
Financial support: This study was supported by the National Health and Medical Research Council of Australia (NHMRC; grants 215201 and 406601), the National Institutes of Health (grant AI063135), and the United States Veterans Association. Peter A. Zimmerman was supported by the National Institutes of Health (grants AI-46919 and AI-52312). Jennifer L. Cole-Tobian was supported by grant AI07024. Louis Schofield is an International Research Scholar of the Howard Hughes Medical Institute. James G. Beeson was supported by an NHMRC Career Development Award and a Miller Fellowship of the Walter and Eliza Hall Institute.
References
- 1.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 3.Genton B, Al Yaman F, Beck HP, Hii J, Mellor S, Narara A, Gibson N, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. I. Malariometric indices and immunity. Ann Trop Med Parasitol. 1995;89:359–376. doi: 10.1080/00034983.1995.11812965. [DOI] [PubMed] [Google Scholar]
- 4.Genton B, Al Yaman F, Beck HP, Hii J, Mellor S, Rare L, Ginny M, Smith T, Alpers MP. The epidemiology of malaria in the Wosera area, East Sepik Province, Papua New Guinea, in preparation for vaccine trials. II. Mortality and morbidity. Ann Trop Med Parasitol. 1995;89:377–390. doi: 10.1080/00034983.1995.11812966. [DOI] [PubMed] [Google Scholar]
- 5.Schofield L, Mueller I. Clinical immunity to malaria. Curr Mol Med. 2006;6:205–221. doi: 10.2174/156652406776055221. [DOI] [PubMed] [Google Scholar]
- 6.Yazdani SS, Mukherjee P, Chauhan VS, Chitnis CE. Immune responses to asexual blood-stages of malaria parasites. Curr Mol Med. 2006;6:187–203. doi: 10.2174/156652406776055212. [DOI] [PubMed] [Google Scholar]
- 7.Wipasa J, Elliott S, Xu H, Good MF. Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol. 2002;80:401–414. doi: 10.1046/j.1440-1711.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 8.Bull PC, Marsh K. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 2002;10:55–58. doi: 10.1016/s0966-842x(01)02278-8. [DOI] [PubMed] [Google Scholar]
- 9.Smith T, Hii JL, Genton B, Muller I, Booth M, Gibson N, Narara A, Alpers MP. Associations of peak shifts in age–prevalence for human malarias with bednet coverage. Trans R Soc Trop Med Hyg. 2001;95:1–6. doi: 10.1016/s0035-9203(01)90314-1. [DOI] [PubMed] [Google Scholar]
- 10.Kasehagen LJ, Mueller I, McNamara DT, Bockarie MJ, Kiniboro B, Rare L, Lorry K, Kastens W, Reeder JC, Kazura JW, Zimmerman PA. Changing patterns of Plasmodium blood-stage infections in the Wosera region of Papua New Guinea monitored by light microscopy and high throughput PCR diagnosis. Am J Trop Med Hyg. 2006;75:588–596. [PMC free article] [PubMed] [Google Scholar]
- 11.Cox MJ, Kum DE, Tavul L, Narara A, Raiko A, Baisor M, Alpers MP, Medley GF, Day KP. Dynamics of malaria parasitaemia associated with febrile illness in children from a rural area of Madang, Papua New Guinea. Trans R Soc Trop Med Hyg. 1994;88:191–197. doi: 10.1016/0035-9203(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 12.Hii JL, Smith T, Mai A, Ibam E, Alpers MP. Comparison between anopheline mosquitoes (Diptera: Culicidae) caught using different methods in a malaria endemic area of Papua New Guinea. Bull Entomol Res. 2000;90:211–219. doi: 10.1017/s000748530000033x. [DOI] [PubMed] [Google Scholar]
- 13.Arevalo-Herrera M, Herrera S. Plasmodium vivax malaria vaccine development. Mol Immunol. 2001;2001:443–455. doi: 10.1016/s0161-5890(01)00080-3. [DOI] [PubMed] [Google Scholar]
- 14.Cole-Tobian JL, Cortes A, Baisor M, Kastens W, Xainli J, Bockarie M, Adams JH, King CL. Age-acquired immunity to a Plasmodium vivax invasion ligand, the duffy binding protein. J Infect Dis. 2002;186:531–539. doi: 10.1086/341776. [DOI] [PubMed] [Google Scholar]
- 15.Michon P, Fraser T, Adams JH. Naturally acquired and vaccine-elicited antibodies block erythrocyte cytoadherence of the Plasmodium vivax Duffy binding protein. Infect Immun. 2000;68:3164–3171. doi: 10.1128/iai.68.6.3164-3171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehlotra RK, Kasehagen LJ, Baisor M, Lorry K, Kazura JW, Bockarie MJ, Zimmerman PA. Malaria infections are randomly distributed in diverse holoendemic areas of Papua New Guinea. Am J Trop Med Hyg. 2002;67:555–562. doi: 10.4269/ajtmh.2002.67.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkot TR, Graves PM, Paru R, Wirtz RA, Heywood PF. Human malaria transmission studies in the Anopheles punctulatus complex in Papua New Guinea: sporozoite rates, inoculation rates, and sporozoite densities. Am J Trop Med Hyg. 1988;39:135–144. doi: 10.4269/ajtmh.1988.39.135. [DOI] [PubMed] [Google Scholar]
- 18.Belkin JN. The Mosquitoes of the South Pacific (Diptera: Culicidae) Vol. 1. Berkeley, CA: University of California Press; 1962. [Google Scholar]
- 19.Benet A, Mai A, Bockarie F, Lagog M, Zimmerman P, Alpers MP, Reeder JC, Bockarie MJ. Polymerase chain reaction diagnosis and the changing pattern of vector ecology and malaria transmission dynamics in Papua New Guinea. Am J Trop Med Hyg. 2004;71:277–284. [PubMed] [Google Scholar]
- 20.Wirtz RA, Burkot TR, Graves PM, Andre RG. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987;24:433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- 21.McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg. 2006;74:413–421. [PMC free article] [PubMed] [Google Scholar]
- 22.McNamara DT, Thomson JM, Kasehagen LJ, Zimmerman PA. Development of a multiplex PCR-ligase detection reaction assay for diagnosis of infection by four human malaria parasite species. J Clin Microbol. 2004;42:2403–2410. doi: 10.1128/JCM.42.6.2403-2410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felger I, Beck HP. Genotyping of Plasmodium falciparum: RFLP analysis. Methods Mol Med. 2002;72:117–129. doi: 10.1385/1-59259-271-6:117. [DOI] [PubMed] [Google Scholar]
- 24.Irion A, Felger I, Abdulla S, Smith T, Mull R, Tanner M, Hatz C, Beck HP. Distinction of recrudescences from new infections by PCR-RFLP analysis in a comparative trial of CGP 56 697 and chloroquine in Tanzanian children. Trop Med Int Health. 1998;3:490–497. doi: 10.1046/j.1365-3156.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- 25.Cole-Tobian J, Zimmerman PA, King CL. High throughput identification of the predominant malaria parasite clone in complex blood stage infections using a multi-SNP molecular haplotyping assay. Am J Trop Med Hyg. 76:12–19. [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T, Genton B, Baea K, Gibson N, Taime J, Narara A, Al Yaman F, Beck HP, Hii J, Alpers M. Relationships between Plasmodium falciparum infection and morbidity in a highly endemic area. Parasitology. 1994;109:539–549. doi: 10.1017/s0031182000076411. [DOI] [PubMed] [Google Scholar]
- 27.Cattani JA, Tulloch JL, Vrbova H, Jolley D, Gibson FD, Moir JS, Heywood PF, Alpers MP, Stevenson A, Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986;35:3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- 28.Owusu-Agyei S, Binka F, Koram K, Anto F, Adjuik M, Nkrumah F, Smith T. Does radical cure of asymptomatic Plasmodium falciparum place adults in endemic areas at increased risk of recurrent symptomatic malaria? Trop Med Int Health. 2002;7:599–603. doi: 10.1046/j.1365-3156.2002.00902.x. [DOI] [PubMed] [Google Scholar]
- 29.Smith T, Felger I, Tanner M, Beck HP. Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg. 1999;93(Suppl 1):59–64. doi: 10.1016/s0035-9203(99)90329-2. [DOI] [PubMed] [Google Scholar]
- 30.Maitland K, Williams AI, Bennett NM, Newbold C, Peto TE, Viji J, Timothy R, Clegg A, Weatherall DJ. The interaction of between Plasmodium falciparum and P. vivax in children on Espiritu Santo island, Vanuatu. Trans R Soc Trop Med Hyg. 1996;90:614–620. doi: 10.1016/s0035-9203(96)90406-x. [DOI] [PubMed] [Google Scholar]
- 31.Balfour MC. Malaria studies in Greece. Am J Trop Med Hyg. 1935;15:301–329. [Google Scholar]
- 32.Earle WC. Epidemiology of malaria in Puerto Rico. Puerto Rico J Pub Health Trop Med. 1939;15:3–27. [Google Scholar]
- 33.Ciuca M, Ballif L, Chelarescu-Vieru M. Immunity in Malaria. Trans R Soc Trop Med Hyg. 1934;6:619–622. [Google Scholar]
- 34.Collins WE, Jeffery GM, Roberts JM. A retrospective examination of reinfection of humans with Plasmodium vivax. Am J Trop Med Hyg. 2004;70:642–644. [PubMed] [Google Scholar]
- 35.Collins WE, Jeffery GM. A retrospective examination of secondary sporozoite- and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity following secondary infection. Am J Trop Med Hyg. 1999;61:20–35. doi: 10.4269/tropmed.1999.61-020. [DOI] [PubMed] [Google Scholar]
- 36.Gunewardena DM, Carter R, Mendis KN. Patterns of acquired anti-malarial immunity in Sri Lanka. Mem Inst Oswaldo Cruz. 1994;89:63–65. doi: 10.1590/s0074-02761994000600015. [DOI] [PubMed] [Google Scholar]
- 37.Muller I, Smith T, Mellor S, Rare L, Genton B. The effect of distance from home on attendance at a small rural health centre in Papua New Guinea. Int J Epidemiol. 1998;27:878–884. doi: 10.1093/ije/27.5.878. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheimer SJ, Macfarlane SB, Moody JB, Bunari O, Williams TE, Harrison C, Hendrickse RG. Iron and infection in infancy–report on field studies in Papua New Guinea: 1. Demographic description and pilot surveys. Ann Trop Paediatr. 1984;4:135–143. doi: 10.1080/02724936.1984.11748324. [DOI] [PubMed] [Google Scholar]
- 39.Wildig J, Michon P, Siba P, Mellombo M, Ura A, Mueller I, Cossart Y. Parvovirus B19 infection contributes to severe anemia in young children in Papua New Guinea. J Infect Dis. 2006;194:146–153. doi: 10.1086/505082. [DOI] [PubMed] [Google Scholar]
- 40.McElroy PD, ter Kuile FO, Lal AA, Bloland PB, Hawley WA, Oloo AJ, Monto AS, Meshnick SR, Nahlen BL. Effect of Plasmodium falciparum parasitemia density on hemoglobin concentrations among full-term, normal birth weight children in western Kenya, IV. The Asembo Bay Cohort Project. Am J Trop Med Hyg. 2000;62:504–512. doi: 10.4269/ajtmh.2000.62.504. [DOI] [PubMed] [Google Scholar]
- 41.Greenwood BM, Bradley AK, Greenwood AM, Byass P, Jammeh K, Marsh K, Tulloch S, Oldfield FS, Hayes R. Mortality and morbidity from malaria among children in a rural area of The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1987;81:478–486. doi: 10.1016/0035-9203(87)90170-2. [DOI] [PubMed] [Google Scholar]
- 42.Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, Legros F, Badji A, Ndiaye G, Ndiaye P. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–137. doi: 10.4269/ajtmh.1994.51.123. [DOI] [PubMed] [Google Scholar]
- 43.Faye FB, Konate L, Rogier C, Trape JF. Plasmodium ovale in a highly malaria endemic area of Senegal. Trans R Soc Trop Med Hyg. 1998;92:522–525. doi: 10.1016/s0035-9203(98)90900-2. [DOI] [PubMed] [Google Scholar]
- 44.Williams TN, Maitland K, Bennett S, Ganczakowski M, Peto TE, Newbold CI, Bowden DK, Weatherall DJ, Clegg JB. High incidence of malaria in alpha-thalassaemic children. Nature. 1996;383:522–525. doi: 10.1038/383522a0. [DOI] [PubMed] [Google Scholar]
- 45.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–240. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 46.Snounou G, White NJ. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 2004;20:333–339. doi: 10.1016/j.pt.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Smith T, Genton B, Baea K, Gibson N, Narara A, Alpers MP. Prospective risk of morbidity in relation to malaria infection in an area of high endemicity of multiple species of Plasmodium. Am J Trop Med Hyg. 2001;64:262–267. doi: 10.4269/ajtmh.2001.64.262. [DOI] [PubMed] [Google Scholar]
- 48.McKenzie FE, Bossert WH. Mixed-species Plasmodium infections of humans. J Parasitol. 1997;83:593–600. [PMC free article] [PubMed] [Google Scholar]
- 49.Bruce MC, Donnelly CA, Packer M, Lagog M, Gibson N, Narara A, Walliker D, Alpers MP, Day KP. Age- and species-specific duration of infection in asymptomatic malaria infections in Papua New Guinea. Parasitology. 2000;121:247–256. doi: 10.1017/s0031182099006344. [DOI] [PubMed] [Google Scholar]
- 50.Bruce MC, Galinski MR, Barnwell JW, Donnelly CA, Walmsley M, Alpers MP, Walliker D, Day KP. Genetic diversity and dynamics of Plasmodium falciparum and P. vivax populations in multiply infected children with asymptomatic malaria infections in Papua New Guinea. Parasitology. 2000;121:257–272. doi: 10.1017/s0031182099006356. [DOI] [PubMed] [Google Scholar]