Abstract
Objective
Restricted and repetitive behaviors, and a pronounced preference for behavioral and environmental consistency, are distinctive characteristics of autism spectrum disorders (ASD). Whether these clinical features of ASD are related to fundamental neuropsychological impairments in behavioral flexibility remains to be clarified.
Method
Forty-one individuals with ASD and 37 matched controls performed a probabilistic reversal learning task to assess behavioral flexibility. Participants learned to choose the correct stimulus location from a pair of locations to win points (acquisition). After making the correct choice over multiple trials, the rewarded stimulus location changed without warning (reversal). Feedback was provided on an 80:20 probabilistic schedule, with 80% of correct choices and 20% of incorrect choices randomly reinforced.
Results
ASD and control participants performed comparably during acquisition. At reversal, ASD participants initially chose the new correct location as quickly as controls, but then more frequently reverted back to the previously preferred response. The ASD group also more frequently shifted back to the previous response choice immediately following intermittent non-reinforcement of the new correct response. The number of regressive errors was positively correlated with independently ascertained clinical ratings of restricted and repetitive behaviors, but not other core features of ASD.
Conclusions
Restricted and repetitive behaviors in ASD are associated with neurocognitive deficits in flexible choice behavior. Preclinical research has established that frontostriatal circuitry supports flexibility on reversal learning tasks. Thus, alterations in this circuitry may contribute to behavioral rigidity in ASD and represent a target for therapeutic intervention.
Keywords: autism, behavioral flexibility, reversal learning, restricted and repetitive behaviors
Reduced Behavioral Flexibility in Autism Spectrum Disorders Autism spectrum disorders (ASD) are characterized by pervasive disturbances in social interactions and communication, and by circumscribed interests and restricted and repetitive behaviors (Diagnostic and Statistical Manual of Mental Disorders; 4th ed., text rev; DSM-IV-TR; American Psychiatric Association, 2000). Understanding of the latter symptom domain remains limited, despite it contributing significantly to clinical distress and behavioral problems (Bishop, Richler, Cain, & Lord, 2007; South, Ozonoff, & McMahon, 2005). Clarifying the cognitive bases of behavioral rigidity in ASD has the potential to provide clues as to its pathophysiology, improve its clinical assessment, and guide development of new treatments that can alleviate this core feature of ASD.
One possibility is that a specific impairment in the ability to transition away from preferred behaviors to new, more adaptive ones contributes to the occurrence of restrictive and repetitive behaviors. Some prior studies suggest that these behaviors are related to broad deficits in executive function and cognitive control in ASD (Lopez, Lincoln, Ozonoff, & Lai, 2005; Mosconi et al., 2009). However, results are inconsistent, and the specific cognitive impairments that may contribute to clinical manifestations of rigid behavior remain to be clarified. Studies have documented deficits in cognitive flexibility in ASD using the Wisconsin Card Sort Test and the CANTAB ID/ED set shifting task, showing that individuals with autism are impaired when learning to shift set to a new perceptual sorting category (Corbett, Constantine, Hendren, Rocke, & Ozonoff, 2009; Goldstein, Johnson, & Minshew, 2001; Hughes, Russell, & Robbins, 1994). It is of note that these tests place demands not only on behavioral flexibility but also on multiple higher-order cognitive processes that are known to be impaired in ASD, such as perceptual reasoning skills. Thus, it remains uncertain as to what degree previous findings reflect deficits in flexible behavioral control versus impaired cognition in other domains. Further, prior studies have not parsed apart different aspects of behavioral flexibility that are known to be supported by different cognitive and brain systems. For example, a behavioral flexibility deficit could result from the inability to initially inhibit a previously preferred choice pattern, or a deficit in maintaining a new choice pattern over time, which would point to impairments in frontal cortical and striatal functioning respectively (Dias, Robbins, & Roberts, 1996; Ragozzino, 2007; Robbins, 2007).
Reversal learning tasks provide a direct approach to examining flexible choice behavior. This methodology is widely used across species, and thus is useful for testing mechanistic biological models, and for translational studies that can facilitate drug development (Brown, Amodeo, Sweeney, & Ragozzino, 2012; Ghahremani, Monterosso, Jentsch, Bilder, & Poldrack, 2010; Glascher, Hampton, & O’Doherty, 2009; Ragozzino, Mohler, Prior, Palencia, & Rozman, 2009). In contrast to extradimensional shifting which is more dependent on prefrontal cortical functions, reversal learning is primarily dependent upon striatal circuitry (Robbins, 2007). Reversal learning tasks assess simple intradimensional shifts in behavior, e.g. shifting from choosing one spatial location to another, rather than shifting across dimensions, such as from the color to the shape of stimuli. This is accomplished by requiring subjects to acquire a behavioral response strategy using performance feedback, and then to reverse that response to an alternative option when the previously correct choice is no longer reinforced. Importantly, reversal learning tasks are designed to distinguish between deficits in disengaging from preferred behaviors versus maintaining new choice patterns.
Few studies have examined reversal learning in ASD. Most have used small samples of young children who showed alterations in the ability to learn an initial response pattern in addition to reversal deficits (Coldren & Halloran, 2003; Lionello-Denolf, McIlvane, Canovas, de Souza, & Barros, 2008). If initial acquisition of a response is impaired, that can confound the interpretation of problems in switching to a new response, because this could result from a generalized learning deficit rather than a specific impairment in response shifting. Reports from larger and primarily adolescent samples using intradimensional subtests of the CANTAB ID/ED task do not show deficits in reversal learning in ASD (Edgin & Pennington, 2005; Goldberg et al., 2005; Ozonoff et al., 2004; Ozonoff, South, & Miller, 2000). However, a number of important issues remain to be resolved. First, reversal learning studies to date have not clarified whether there are deficits in the specific processes of selecting or maintaining new responses; whether one or the other is selectively affected could indicate alterations in distinct cognitive and brain systems. Second, studies have not systematically examined whether reversal learning performance is related to clinical manifestations of behavioral rigidity. Third, because delayed maturation of behavioral flexibility in ASD may result in deficits that are more pronounced at younger ages, studies with older adolescents and young adults may have missed deficits evident in younger individuals.
Finally, critical to a comprehensive understanding of behavioral flexibility in ASD is an understanding of how dynamically changing consequences for choice behaviors support or disturb flexible behavioral control. Probabilistic reversal learning paradigms, in which accurate feedback or reinforcement for response choices is provided on only a proportion of trials, allow for an examination of the effect of inconsistent reinforcement on behavioral flexibility. The intermittent non-reinforcement used in probabilistic tasks increases the difficulty associated with establishing, maintaining, and reversing a behavioral set. For this reason, such tasks may be more sensitive to behavioral flexibility deficits, as misleading feedback might slow learning of new responses after reversal, or increase the likelihood of reverting back to a previously reinforced and preferred response choice. A psychometric advantage is that probabilistic paradigms may be less susceptible to ceiling effects in test performance that could contribute to the failure to identify deficits in prior studies in ASD, in which all correct responses were accurately reinforced. The unpredictable and inconsistent nature of reinforcement for choice behaviors used in probabilistic tasks also corresponds more closely to the behavioral flexibility demands of typical day-to-day life.
In the present study, individuals with ASD and matched controls performed a probabilistic reversal learning task. Performance at acquisition and at reversal was examined. The primary measures of interest were the number of trials required to learn a behavioral response and to shift to a new response when reinforcement contingencies changed, the number of errors made after reversal when sustaining a new response over a previously preferred choice, and the number of errors made following intermittent non-reinforcement. We evaluated test performance in relation to independently ascertained clinical measures of restricted and repetitive behaviors, and other clinical features of ASD. Given reports of altered cognitive development across the lifespan in ASD (Luna, Doll, Hegedus, Minshew, & Sweeney, 2007; Solomon, Ozonoff, Cummings, & Carter, 2008), in secondary analyses we examined performance across a broad age range to identify preliminary indications of an altered trajectory in the development of behavioral flexibility.
Method
Forty-one individuals with an ASD (34 males) and 37 typically developing controls (30 males) participated in the study (Table 1). Eleven additional individuals were excluded who did not meet performance criteria during the training tasks described below. Individuals with an ASD were recruited from outpatient clinics at the University of Illinois Medical Center and via flyers posted in the community. Participants in the ASD group met cut-off points for an ASD on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). Thirty seven of 41 individuals meeting this criterion that had a parent available to provide historical information also met cut-off points for an ASD on the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994). In addition, all participants in the ASD group received a consensus clinical diagnosis of DSM-IV-TR Autistic Disorder (n=22), Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS; n=12), or Asperger’s Disorder (n=7). There were no performance differences amongst the three diagnostic groups, and thus ASD participants were pooled for statistical analyses as planned. Control participants were recruited from the community and were required to have a Social Communication Questionnaire score of eight or lower (SCQ; Berument, Rutter, Lord, Pickles, & Bailey, 1999), no personal history of psychiatric or neurologic disorders, and no first- or second-degree relative with a suspected ASD or other familial neuropsychiatric illness. The ASD and control groups were matched on age, sex and IQ.
Table 1.
Demographics and clinical characteristics of autism spectrum disorder (ASD) and typically developing control participants.
| Variable | ASD group (n=41, 8 females) | Controls (n=37, 6 females) | Statistical value |
|---|---|---|---|
|
| |||
| Mean ± SD | Mean ± SD | ||
| Age (years) | 15.34 ± 7.75 | 18.24 ± 8.12 | .12 |
| Full-scale IQ | 103.90 ± 16.48 | 108.70 ± 11.97 | .15 |
| Verbal IQ | 102.00 ± 18.70 | 109.00 ± 12.59 | .06 |
| Performance IQ | 104.73 ±15.50 | 107.59 ± 12.55 | .38 |
| Repetitive behavior (ADI-R algorithm) | 6.05 ± 2.46) | -- | -- |
| RBS-R total score | 35.85 ± 24.49 | -- | -- |
Note. ADI-R; Autism Diagnostic Interview – Revised (Lord, Rutter & LeCouter, 1994); RBS-R; Repetitive Behavior Scales – Revised (Bodfish, Symons, Parker, & Lewis, 2000);
n.s., not significant (p>.05).
No participants were receiving medications known to affect cognitive abilities, including antipsychotics, psychostimulants, antidepressants, and anticonvulsants. All participants were either medication naïve, or had been free from medications for 5.5 half-lives of the drug they had been taking prior to testing. Participants were at least eight years of age (up to 44 years of age), and had Full-Scale, Verbal and Performance IQs ≥ 70. For individuals with an ASD diagnosis, a family member completed the Repetitive Behavior Scales-Revised (RBS-R; Bodfish, Symons, Parker, & Lewis, 2000), a questionnaire used to assess repetitive, ritualistic, and obsessive-compulsive behaviors in ASD. All participants completed informed consent or assent, and study procedures were approved by the Institutional Review Board at the University of Illinois at Chicago.
Probabilistic Reversal Learning Task
Participants were presented with two identical stimuli (animal pictures) and were required to select the picture that was in the correct location in order to win points (Figure 1). Prior to starting the task, participants were given the following instructions; “Choose the animal that is usually in the correct location. After a while the correct location may change”. Participants made their stimulus choice on a touchscreen monitor using their dominant hand (Hewlett Packard TouchSmart 600). During the acquisition phase of the task, the criterion for demonstrating that the response had been learned was a correct response on eight out of ten consecutive trials. Participants were presented with up to 50 trials to achieve this criterion. Participants who did not achieve this criterion were not included in the analyses (one ASD and one control participant). This was followed immediately, and without warning to the participant, by the reversal phase of the task, in which the correct stimulus was changed to the picture in the other location. Again, participants received up to 50 trials to achieve the criterion of eight out of ten consecutive correct responses.
Figure 1.

Schematic of probabilistic reversal learning task. Participants were instructed to select the correct stimulus location from a pair and won points for making the correct choice (acquisition). After correct response choices, “+10” was displayed between the two stimuli, and a coin appeared that moved into the bag displayed on the lower right of the screen. After incorrect choices, a red “X” appeared in the center of the screen. Once participants had achieved the performance criterion of eight out of ten consecutive correct choices, the correct stimulus location was changed without warning (reversal). Reinforcement was provided on an 80:20 probabilistic schedule during both acquisition and reversal, such that inaccurate feedback was presented on 20% of randomly determined trials throughout the task.
On all trials, immediate feedback was provided after participants made their response choice. Responses were reinforced accurately on 80% of trials. On such trials, after correct choices, a coin appeared and then moved into a money bag on the lower right corner of the screen; the message “+10” was presented, and the participant’s total winnings for the game increased by 10 points. On trials when the incorrect choice was made, a large red “X” appeared in the center of the screen. On 20% of randomly determined trials, participants received inaccurate feedback, i.e. choice of the correct location was not reinforced and instead choice of the incorrect location yielded a coin win. If participants did not respond after four seconds, the word “CHOOSE” was presented in the center of the screen. Studies of probabilistic learning, without subsequent reversals to assess behavioral flexibility, have reported that individuals with ASD can acquire response preferences as quickly as healthy individuals when 80% of correct responses are reinforced (Solomon, Smith, Frank, Ly, & Carter, 2011), which was the reinforcement probability used for correct responses in the present study.
Prior to performing this reversal learning task, participants completed two training tasks. In the first task, they learned to select the correct animal picture from one of two locations on nine out of ten consecutive trials, and then to reverse that response to choose the other location, whilst receiving 100% accurate feedback throughout. This was done to ensure that participants understood task directions, and to familiarize them with basic task demands. In the second training task, participants learned to select the correct location on ten consecutive trials, but received probabilistic feedback on an 80:20 schedule, as in the task itself. This allowed participants to experience a task condition in which they needed to keep choosing a stimulus location that was correct most, but not all of the time. There was no reversal after this second training condition. Animal pictures during each training task, and their locations on-screen, were different from those used in the task itself. Participants were presented with a maximum of 50 trials in each training task to meet performance criteria. Individuals who did not reach learning criteria for both training conditions did not proceed with further testing and are not included in the analyses reported here (seven ASD participants, and two control participants). Excluding these individuals permitted testing of reversal learning in ASD and healthy control groups with an established ability to both switch behavioral set and to acquire an initial response preference that was probabilistically reinforced. The task was administered by research assistants who were unaware of participants’ clinical ratings.
Data Analyses
In both the acquisition and reversal phases of the task, the number of incorrect trials and the total number of trials to successfully reach the learning criterion were recorded for each participant. Following classification of errors on reversal learning tasks used in rodent studies, errors in the reversal phase were considered as either perseverative or regressive (Ragozzino, Jih, & Tzavos, 2002). Perseverative errors occurred when participants chose the previously reinforced response before choosing the new correct response. Regressive errors occurred when participants chose the previously reinforced response after having already selected the new correct choice at least once. Thus, this distinction allowed the number of perseverative errors to provide an index of how quickly a participant shifted their response after reversal, whilst the number of regressive errors provided a measure of how well the new correct choice pattern was maintained.
A particular advantage of probabilistic reversal learning paradigms is the ability to examine individuals’ behavior following unexpected non-reinforcement of their response choices. When a preferred response is not reinforced, individuals may incorrectly shift back to a previous response choice because of a difficulty maintaining behavioral set or a heightened response to non-reinforcement. These shifts are referred to as lose:shift errors, and were calculated for both the acquisition and reversal phases of the task. All tests for group differences used analysis of covariance (ANCOVA) with age as a covariate.
For participants in the ASD group, the relationship of reversal learning performance to clinical manifestations of behavioral rigidity was examined using ratings of repetitive and stereotyped behaviors from the RBS-R (total score) and the ADI-R (using the restricted-repetitive behavior algorithm). To determine the specificity of any association between behavioral flexibility impairments and restrictive and repetitive behaviors, correlational analyses also were conducted to assess the relationship of reversal learning task performance with social and communication deficits using the respective ADI-R algorithms. All ADI-R algorithm scores were from the “4–5 years/ever” age items. RBS-R items are endorsed based on behaviors observed within the last month.
Results
Probabilistic Reversal Learning Task Performance
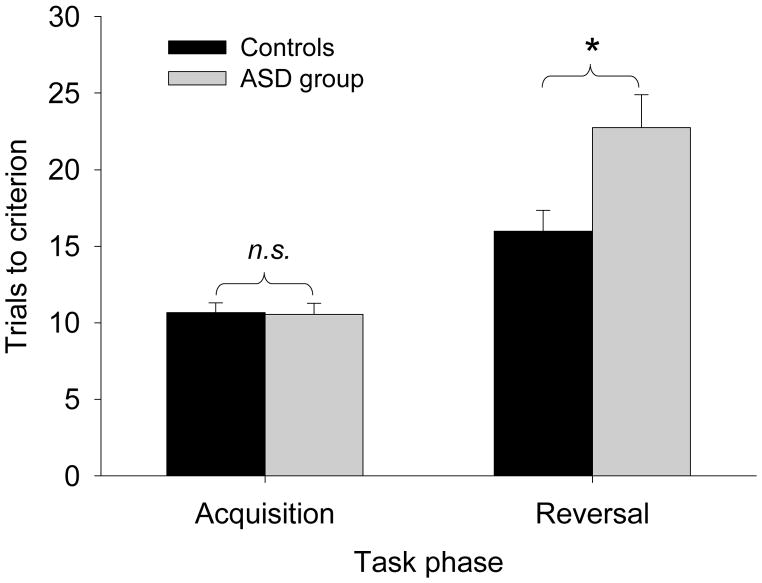
During the acquisition phase, there was no difference between the control and ASD groups in the number of incorrect responses (MCON=3.01, SDCON=2.96; MASD=3.71, SDASD=4.93; F(1, 76)=0.21, p=.65, η2=.003), nor in the number of trials required to reach the learning criterion of eight out of ten consecutive correct responses (MCON=10.68, SDCON=3.82; MASD=10.56, SDASD=4.60; F(1, 76)=.11, p=.74, η2=.001). During acquisition, there was also no difference between groups in the number of lose: shift errors (MCON=3.00, SDCON=1.78; MASD=2.93, SDASD=1.82; F(1, 76)=0.79, p=.07, η2=.001).
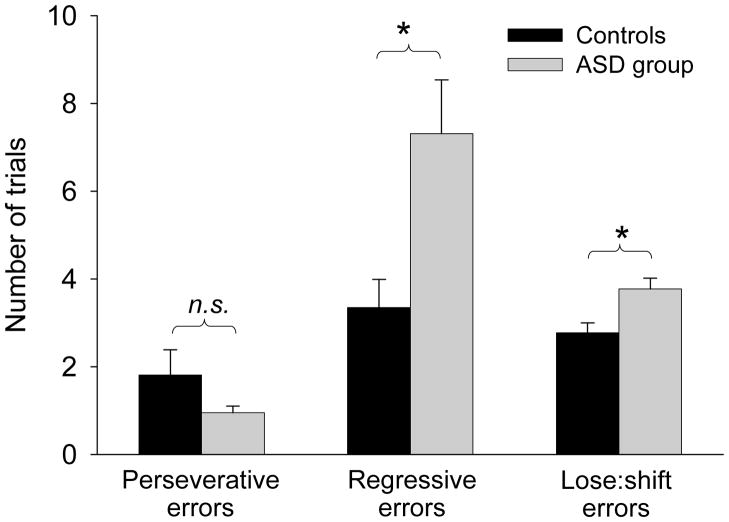
After reversal, individuals in the ASD group required more trials to achieve the criterion for learning the new response choice than did controls (MCON=15.97, SDCON=8.44; MASD=22.76, SDASD=13.67; F(1, 76)=4.45, p=.04, η2=.056; Figure 2). The number of perseverative errors did not differ between the ASD and control groups (MCON=1.81, SDCON=3.49; MASD=0.95, SDASD=1.00; F(1, 76)=3.18, p=.08, η2=.041; Figure 3). However, the ASD group made significantly more regressive errors (MCON=3.35, SDCON=3.91; MASD=7.32, SDASD=7.81; F(1, 76)=5.37, p=.02, η2=.067; Figure 3) such that after initially choosing the new correct response, they regressed back to choosing the previously rewarded response more often than did controls. This was due, in part, to their greater number of lose:shift errors at reversal, when they incorrectly shifted their choice back to the previously preferred response after intermittent non-reinforcement (MCON=2.78, SDCON=1.34; MASD=3.78, SDASD=1.56; F(1, 76)=6.94, p=.01, η2=.085; Figure 3). In control and ASD participants, lose:shift errors accounted for 90% and 40% of regressive errors respectively.
Figure 2.
Trials to achieve criterion of 8 of 10 correct choices in the acquisition and reversal phases of the probabilistic reversal learning task for individuals with an autism spectrum disorder (ASD) and typically developing control participants (* significant at p<.05).
Figure 3.
Types of errors made by typically developing controls and individuals with an autism spectrum disorder (ASD) in the reversal phase of the probabilistic reversal learning task. Perseverative errors occurred when participants chose the previously reinforced response before choosing the new correct response. Regressive errors occurred when participants chose the previously reinforced response after having already selected the new correct choice at least once. Lose:shift errors occurred when participants incorrectly shifted away from a correct response choice following inaccurate feedback that their choice was incorrect. (* significant at p<.05).
Age-Related Changes in Reversal Learning Performance
Visual inspection revealed a non-linear relationship of age and reversal learning performance, consistent with many studies showing slower rates of cognitive development through late childhood and adolescence (Luna, Garver, Urban, Lazar, & Sweeney, 2004). Consequently, the log natural transform (ln) of each participant’s age was computed, and then related to their performance. Multiple regression analysis revealed a significant interaction of ln(age) with group for regressive errors, indicating slower age-related improvement in task performance in the ASD group (βgroup*ln(age)=−.49, p=.03). Performance deficits in the ASD group were most pronounced at younger ages, with sequential testing of group differences over increasing age showing that group differences evident at younger ages were no longer significant beyond 14 years of age (β=3.91, p<.01). There was no significant interaction of age and group with either the number of trials to learn the new response choice after reversal, or with lose:shift errors. Exploratory regression analyses did not indicate a significant influence of IQ on group differences in performance.
Relationship of Reversal Learning Performance to Restricted and Repetitive Behaviors
For ASD participants, there was a positive correlation between the number of regressive errors and total score on the RBS-R (r=.34, p=.02; Table 2). The number of regressive errors was also positively correlated with ADI-R ratings of stereotyped and restricted behaviors, need for routine, and difficulty with behavioral transitions (ADI-R C-subscale; r=.37, p=.04). In addition, there was also a significant correlation between regressive errors and stereotyped, repetitive, or idiosyncratic speech (ADI-R B3-subscale; r=.38, p=.02). Correlations of reversal learning errors with ADI-R subscales that assess communication and social skill impairments were not significant.
Table 2.
Correlational analyses of probabilistic reversal learning errors and clinical characteristics for autism spectrum disorder participants
| Clinical Scores | Pearson’s r value |
|---|---|
| ADI-R Scores | |
| A: Reciprocal social interaction | .28 |
| B: Communication | .25 |
| B3: Stereotyped, repetitive, or idiosyncratic speech | .38* |
| C: Restricted, repetitive, and stereotyped patterns of behavior | .37* |
| RBS-R total score | .34* |
Note. ADI-R; Autism Diagnostic Interview – Revised (Lord, Rutter & LeCouter, 1994); RBS-R; Repetitive Behavior Scales – Revised (Bodfish, Symons, Parker, & Lewis, 2000);
signifies significant at p<.05.
Discussion
The present study used a probabilistic reversal learning task to demonstrate behavioral flexibility deficits in ASD. The impairment in reversal learning test performance was related significantly and specifically to clinical ratings of restricted interests, repetitive and stereotyped behavior and speech, and ritualistic behavior in ASD; associations with communication and social deficits in ASD were not significant. These findings indicate that a neuropsychological impairment in establishing new behavioral routines over previously preferred alternatives is related to clinical manifestations of behavioral rigidity in ASD. Further, although the study design was cross-sectional, the observation that deficits were particularly pronounced in younger individuals suggests a delayed maturation of flexible behavioral control in ASD. By clarifying the nature of behavioral flexibility deficits present in ASD and their relationship to clinical manifestations of the disorder, the present findings advance understanding of the neurocognitive underpinnings of an important and understudied clinical characteristic of ASD.
At the start of the reversal phase of our task, individuals with an ASD selected the new correct response as quickly as did controls. Thus, impaired reversal learning was not due to perseveration on the previously reinforced response immediately after reversal. Rather, the ASD group demonstrated a persistent preference for the previously learned response over the new correct choice, reflected in their significantly higher rate of regressive errors after reversal. Notably, this difficulty in changing response preference was observed in individuals who were unimpaired during the initial acquisition phase of the reversal learning test, when they were able to learn a response preference even when only 80% of correct choices were reinforced. It is possible that both groups performed sufficiently close to ceiling during the acquisition phase of the task such that some level of learning deficit might not have been detected. However in our reversal condition, the higher rate of regressive errors suggest a specific deficit in switching response preferences that is not likely the result of a generalized deficit in learning new choice behaviors.
In the present study, the reversal learning paradigm was only administered to individuals whom training tasks established were able to learn a response set under probabilistic reinforcement conditions. Training also established that all individuals were able to reverse behavioral choices when given 100% reinforcement for correct choices. The ability of participants to achieve this latter criterion is consistent with findings from prior reversal learning studies in ASD using the CANTAB’s intradimensional reversal subtest (Corbett et al., 2009; Goldstein et al., 2001; Hughes et al., 1994). Thus, the reversal deficit we observed in ASD was specific to conditions in which reinforcement was probabilistic, suggesting that new behavioral preferences are unstable relative to previously learned choices, and have an increased susceptibility to interference from occasional non-reinforcement.
The ability to shift behavioral preference in reversal learning requires a number of interacting cognitive processes. First, the ability to inhibit a previously learned choice pattern is important so that new choice behaviors can be implemented. Past studies have demonstrated deficits in behavioral inhibition and withholding of prepotent response tendencies in ASD, which in turn have been linked to clinical measures of restricted and repetitive behaviors (Agam, Joseph, Barton, & Manoach, 2010; Mosconi et al., 2009). Second, individuals must learn and consistently implement a new choice pattern. A third relevant cognitive process is reinforcement learning, as individuals must learn whether to maintain or change choice patterns based on performance feedback cues. Determining the relative importance of alterations in these three different cognitive processes to behavioral inflexibility in ASD remains a target for future research.
Rodent and human neuroimaging studies have made significant progress in defining the functional neuroanatomy of behavioral flexibility. In particular, frontostriatal pathways including dorsal and ventral striatum, anterior cingulate cortex, and dorsolateral and orbital prefrontal cortex are known to support reversal learning (Cools, Clark, Owen, & Robbins, 2002; D’Cruz, Ragozzino, Mosconi, Pavuluri, & Sweeney, 2011; Ghahremani et al., 2010; O’Doherty, Critchley, Deichmann, & Dolan, 2003). In disorders with an overlapping behavioral phenotype of rigid behavior and adherence to routine, such as obsessive-compulsive disorder, frontostriatal dysfunction is believed to represent a substrate for deficits in behavioral and cognitive flexibility (Britton et al., 2010; Jacob, Landeros-Weisenberger, & Leckman, 2009). A similar relationship between the structural and functional integrity of the striatum with restricted and repetitive behaviors has been suggested in ASD (Hollander et al., 2005; Rojas et al., 2006).
The persistent bias towards previously preferred response tendencies (regressive errors) in our intradimensional reversal learning paradigm in the absence of an increased rate of perseverative errors suggests a prominent striatal contribution to reduced behavioral flexibility in ASD. However, some previous data also implicates prefrontal systems. For example, extradimensional shifting performance deficits have been demonstrated and correlated with restricted and repetitive behaviors in ASD (Lopez et al., 2005; Yerys et al., 2009). Shifts across perceptual dimensions, i.e. extradimensional shifts, require higher cognitive processes that are more dependent on prefrontal cortical functions than striatal circuitry (Robbins, 2007). Thus, whilst previous evidence implicates prefrontal system dysfunction in rigid and restricted behaviors in ASD, the present findings suggest that striatal impairments may also contribute to this clinical problem.
Distinct neural circuitry within the striatum has been associated with the ability to switch to a new response versus the ability to later maintain the new response pattern (O’Doherty et al., 2004; Ragozzino, 2007). This distinction is particularly relevant given the dissociation in perseverative and regressive errors we observed. Inactivation of ventral striatum in rodents results in increased perseverative responding, whereas inactivating dorsomedial striatum selectively impairs reversal learning by causing an increase in regressive errors without increasing perseveration (Ragozzino & Choi, 2004; Ragozzino et al., 2002), a pattern similar to that observed in ASD participants in the present study.
While the observation of increased regressive but not perseverative errors suggests dorsal striatal involvement, increased lose:shift errors also suggest a potential role for the ventral striatum. The ventral striatum is important for reversal learning because of its role in processing reinforcement cues about the consequences of shifting or maintaining a response strategy (O’Doherty et al., 2004). Reduced responsivity of ventral striatum is believed to increase perseverative errors because reinforcement cues fail to signal a need to change future choice behaviors. Rather than a reduced response to non-reinforcement, the increase of lose:shift errors at reversal in the ASD group suggests a heightened response to non-reinforcement. Together, these observations suggest a specific reinforcement learning deficit in ASD, in which heightened response to non-reinforcement impairs maintenance of stable new behavior patterns and increases regression to previous response preferences. This is interesting in light of recent functional neuroimaging studies showing an altered response to reward in ventral striatum in ASD (Dichter, Richey, Rittenberg, Sabatino, & Bodfish, 2012; Kohls et al., 2012; Scott-Van Zeeland, Dapretto, Ghahremani, Poldrack, & Bookheimer, 2010). Further research is needed to develop a broader understanding of what may be multiple cognitive and affective processes contributing to behavioral inflexibility, and to develop mechanistic cognitive and neurobiological models of how these alterations translate to the clinical problem of rigid thinking and behavior in ASD (Geurts, Corbett, & Solomon, 2009).
A secondary aim of the present study was to investigate the developmental trajectory of reversal learning performance in ASD. The slower rate of improvement in task performance with age in the ASD group relative to controls suggests that there could be a delayed development of behavioral flexibility, and potentially also of the frontostriatal circuitry that supports flexible behavioral transitions. Further work is needed to test this idea, as it is possible that the psychometric characteristics of our task might make it more sensitive to deficits early in development. Whilst ours is a cross-sectional study not designed to formally test developmental models, these preliminary findings highlight the importance of considering developmental trends in reversal learning ability and potentially the broader neurocognitive profile of ASD. In light of prior studies that have reported delayed and altered development of other cognitive functions in ASD (Luna et al., 2007; Ozonoff & McEvoy, 1994), our pattern of age-related findings may explain why several prior studies of behavioral flexibility using samples of older individuals with an ASD failed to detect significant performance deficits. By studying behavioral flexibility over broad range of ages and more heterogeneous samples, and with longitudinal follow-up studies, future work can further address issues of developmental alteration and comorbid psychopathology in relation to behavioral rigidity in ASD.
In conclusion, individuals with ASD demonstrated a deficit in behavioral flexibility, as evidenced by a preference for a previously learned behavior on a probabilistic reversal learning task. This deficit was related specifically to clinical manifestations of rigid and repetitive behaviors, but not to social and communication deficits in ASD. Individuals with ASD also made more frequent lose:shift errors, suggesting an increased sensitivity to non-reinforcement that may have exacerbated their difficulty maintaining a new choice pattern. Reversal learning impairments suggest that alterations in frontostriatal systems may contribute to clinical features of rigid behavior and cognition in ASD, a hypothesis that can be tested in future functional brain imaging studies. Studies of reversal learning can also be readily conducted in rodent models, and thus represent a useful translational strategy for testing mechanistic hypotheses about the neurobiology of behavioral rigidity and its pharmacologic treatment. As such, our findings inform understanding into a clinical dimension of ASD for which effective treatments are not yet available, and suggest important new approaches and targets for clinical assessment and intervention.
Acknowledgments
This work was supported by funding from the National Institutes of Health Autism Center of Excellence P50HD055751, MH092696, and a Dennis Weatherstone Predoctoral Fellowship from Autism Speaks.
Contributor Information
Anna-Maria D’Cruz, Department of Psychology, University of Illinois at Chicago. Institute for Juvenile Research, University of Illinois at Chicago.
Michael E. Ragozzino, Department of Psychology, University of Illinois at Chicago
Matthew W. Mosconi, Departments of Psychiatry and Pediatrics, University of Texas Southwestern Medical Center
Sunil Shrestha, Departments of Psychiatry and Pediatrics, University of Texas Southwestern Medical Center.
Edwin H. Cook, Institute for Juvenile Research, University of Illinois at Chicago
John A. Sweeney, Departments of Psychiatry and Pediatrics, University of Texas Southwestern Medical Center
References
- Agam Y, Joseph RM, Barton JJS, Manoach DS. Reduced cognitive control of response inhibition by the anterior cingulate cortex in autism spectrum disorders. NeuroImage. 2010;52(1):336–347. doi: 10.1016/j.neuroimage.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. The British Journal of Psychiatry: The Journal of Mental Science. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Richler J, Cain AC, Lord C. Predictors of perceived negative impact in mothers of children with autism spectrum disorder. American Journal of Mental Retardation: AJMR. 2007;112(6):450–461. doi: 10.1352/08958017(2007)112[450:POPNII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. Journal of Autism and Developmental Disorders. 2000;30(3):237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Britton JC, Rauch SL, Rosso IM, Killgore WDS, Price LM, Ragan J, et al. Cognitive inflexibility and frontal-cortical activation in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(9):944–953. doi: 10.1016/j.jaac.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HD, Amodeo DA, Sweeney JA, Ragozzino M. The selective serotonin reuptake inhibitor, escitalopram, enhances inhibition of prepotent responding and spatial reversal learning. Journal of Psychopharmacology (Oxford, England) 2012 doi: 10.1177/0269881111430749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldren JT, Halloran C. Spatial reversal as a measure of executive functioning in children with autism. The Journal of Genetic Psychology. 2003;164(1):29–41. doi: 10.1080/00221320309597501. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22(11):4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. 20026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Research. 2009;166(2–3):210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz A, Ragozzino ME, Mosconi MW, Pavuluri MN, Sweeney JA. Human reversal learning under conditions of certain versus uncertain outcomes. NeuroImage. 2011;56(1):315–322. doi: 10.1016/j.neuroimage.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral Neuroscience. 1996;110(5):872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2012;42(2):147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin JO, Pennington BF. Spatial cognition in autism spectrum disorders: Superior, impaired, or just intact? Journal of Autism and Developmental Disorders. 2005;35(6):729–745. doi: 10.1007/s10803-005-0020-y. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Monterosso J, Jentsch JD, Bilder RM, Poldrack RA. Neural components underlying behavioral flexibility in human reversal learning. Cerebral Cortex. 2010;20(8):1843–1852. doi: 10.1093/cercor/bhp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Hampton AN, O’Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex. 2009;19(2):483–495. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, et al. Subtle executive impairment in children with autism and children with ADHD. Journal of Autism and Developmental Disorders. 2005;35(3):279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Johnson CR, Minshew NJ. Attentional processes in autism. Journal of Autism and Developmental Disorders. 2001;31(4):433–440. doi: 10.1023/a:1010620820786. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry. 2005;58(3):226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Neuropsychologia. 1994;32(4):477–492. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Jacob S, Landeros-Weisenberger A, Leckman JF. Autism spectrum and obsessive-compulsive disorders: OC behaviors, phenotypes and genetics. Autism Research: Official Journal of the International Society for Autism Research. 2009;2(6):293–311. doi: 10.1002/aur.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, et al. Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-Denolf KM, McIlvane WJ, Canovas DS, de Souza DG, Barros RS. Reversal learning set and functional equivalence in children with and without autism. The Psychological Record. 2008;58(1):15–36. doi: 10.1007/bf03395600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35(4):445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61(4):474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Kay M, D’Cruz AM, Seidenfeld A, Guter S, Stanford LD, et al. Impaired inhibitory control is associated with higher-order repetitive behaviors in autism spectrum disorders. Psychological Medicine. 2009:1–8. doi: 10.1017/S0033291708004984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. Journal of Neuroscience. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, et al. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: Evidence from the collaborative programs of excellence in autism network. Journal of Autism and Developmental Disorders. 2004;34(2):139–150. doi: 10.1023/B:JADD.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, McEvoy RE. A longitudinal study of executive function and theory of mind development in autism. Development and Psychopathology. 1994;6(03):415. doi: 10.1017/S0954579400006027. [DOI] [Google Scholar]
- Ozonoff S, South M, Miller JN. DSM-IV-defined asperger syndrome: Cognitive, behavioral and early history differentiation from high-functioning autism. Autism. 2000;4(1):29–46. doi: 10.1177/1362361300041003. [DOI] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Annals of the New York Academy of Sciences. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learning & Memory. 2004;11(1):70–77. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: Role of muscarinic cholinergic receptors. Brain Research. 2002;953(1–2):205–214. doi: 10.1016/S0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiology of Learning and Memory. 2009;91(1):13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Shifting and stopping: Fronto-striatal substrates, neurochemical modulation and clinical implications. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6(56) doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research: Official Journal of the International Society for Autism Research. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Smith AC, Frank MJ, Ly S, Carter CS. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Research: Official Journal of the International Society for Autism Research. 2011;4(2):109–120. doi: 10.1002/aur.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. International Journal of Developmental Neuroscience. 2008;26(2):239–247. doi: 10.1016/j.ijdevneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35(2):145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy LE. Set-shifting in children with autism spectrum disorders: Reversal shifting deficits on the Intradimensional/Extradimensional shift test correlate with repetitive behaviors. Autism: The International Journal of Research and Practice. 2009;13(5):523–538. doi: 10.1177/1362361309335716. [DOI] [PMC free article] [PubMed] [Google Scholar]