Abstract
Objectives
To determine the frequency of hypoxic-ischemic encephalopathy (HIE) in preterm infants of 33 to 35 weeks’ gestational age on the basis of physiological screening for perinatal acidosis and neurological assessment of encephalopathy and to correlate neurodevelopmental outcomes with brain magnetic resonance imaging findings.
Study design
This retrospective cohort study included all inborn infants of 33 to 35 weeks’ gestation admitted to the neonatal intensive care unit at Parkland Memorial Hospital with perinatal acidosis from October 2005 to September 2008. Their medical records were reviewed, and pertinent data were recorded.
Results
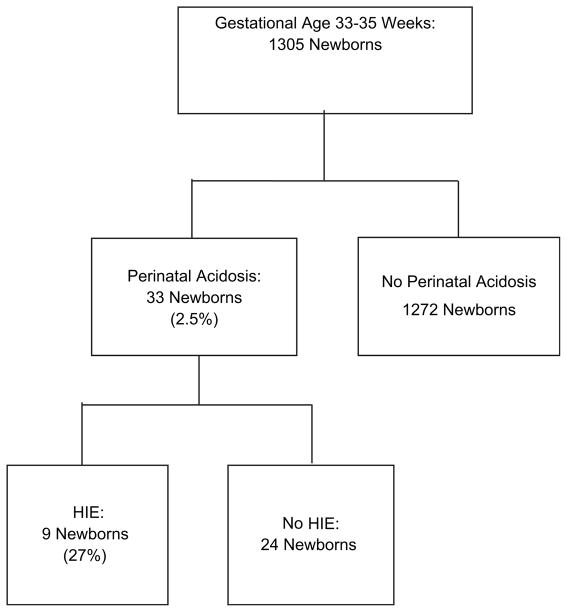
Of 1305 newborns, 2.5% (n = 33) had perinatal acidosis, and 27% (n = 9) of these had HIE (2, mild; 4, moderate; 3, severe). Persistence of metabolic acidosis on the first arterial blood gas obtained in the first hour of age was significantly associated with HIE (P < .005). Magnetic resonance imaging results were abnormal in 3 of 4 infants with moderate HIE and in both survivors with severe HIE. Death or disability occurred in no infants with mild or moderate HIE, but in all infants with severe HIE.
Conclusion
Screening criteria for HIE that use biochemical and neurological assessments as performed in term newborns can be applied to preterm infants of 33 to 35 weeks’ gestation.
Perinatal hypoxic-ischemic encephalopathy (HIE) remains a major cause of neurodevelopmental impairment. The diagnosis of HIE in full-term infants is made with well-defined and widely accepted criteria involving a step-wise combination of biochemical screening for perinatal acidosis and a standardized neurological examination for moderate-to-severe encephalopathy.1,2 Newborns who are ≥36 weeks’ gestation and have a diagnosis of moderate-to-severe HIE within the first 6 hours of age are selected for hypothermia therapy, which has improved neurodevelopmental outcomes and lessened the severity and extent of cerebral injury on magnetic resonance imaging (MRI).1,3 In preterm infants, however, the diagnosis of HIE remains problematic, and these newborns have been excluded from hypothermia therapy because of insufficient evidence for safety or efficacy. Moreover, it is not known whether the screening criteria used to identify term asphyxiated newborns for cooling are appropriate for preterm infants.
Brain MRI is considered to be the best surrogate marker of cerebral injury and predictor of long-term neurodevelopmental outcome in term infants with HIE.4 In term infants, the two major MRI patterns of brain injury are the “basal nuclei” predominant pattern following acute profound asphyxia and the “watershed” predominant pattern following partial prolonged asphyxia.5–7 It remains unclear how these or other MRI findings apply to the asphyxiated preterm infant.
Therefore, the objectives of this study are to: (1) determine the frequency of HIE on the basis of physiological screening for perinatal acidosis and neurological assessment of encephalopathy in preterm infants of 33 to 35 weeks’ gestational age; and (2) characterize the brain MRI findings in these preterm infants with clinical and laboratory evidence of HIE and correlate them with neurological outcomes after discharge from the hospital.
Methods
This retrospective cohort study included all inborn infants of 33 to 35 weeks’ gestation who were admitted to the neonatal intensive care unit (NICU) at Parkland Memorial Hospital (PMH) with perinatal acidosis from October 2005 to September 2008. These infants were identified by review of a prospective neonatal database and resuscitation registry8 and included all admissions to the PMH NICU with perinatal acidosis. Umbilical cord arterial blood was routinely sampled on all deliveries at PMH from double-clamped sections of umbilical cord.9 The infants’ medical records were reviewed, and pertinent demographic, clinical, laboratory, and neuroimaging data were recorded. Gestational age assessment was based on the best obstetrical estimate with the date of the last menstrual period and ultrasonography, when performed, and then confirmed with a Ballard examination performed on admission to the NICU. Newborns who were not resuscitated or had lethal anomalies or other causes of encephalopathy not related to HIE were excluded. The study was approved by the institutional review board of the University of Texas Southwestern Medical Center.
Biochemical Screening for Perinatal Acidosis
Newborns with perinatal acidosis were identified with the same criteria used to screen for HIE in the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network study of whole body hypothermia.1 Specifically, these criteria consisted of: (1) A pH ≤7.0 or a base deficit ≥16 mEq/L on umbilical cord blood or any postnatal blood sample within 1 hour of age; or (2) history of an acute perinatal event and either no blood gas available, or a pH from 7.01 to 7.15 or a base deficit from 10 to 15.9 mEq/L, with a 10-minute Apgar score ≤5, or assisted ventilation initiated at birth and continued for at least 10 minutes.
Neurologic Assessment
The medical records of newborns with perinatal acidosis were reviewed for details of the neurological examination for HIE that was performed by the attending neonatologist within 24 hours of birth. Specifically, the results of the physical examination were reviewed for: (1) level of consciousness; (2) spontaneous activity; (3) posture; (4) tone; (5) primitive reflexes; and (6) autonomic nervous system signs, as described in the NICHD Neonatal Research Network study of whole body hypothermia.1 Infants were categorized as having mild (stage I), moderate (stage II), or severe (stage III) HIE with the modified Sarnat staging for HIE.1
Magnetic Resonance Imaging
Imaging was done at >1 week of age by using T1 and T2 images, T2 gradient echo, and diffusion-weighted imaging. Two experienced pediatric neuroradiologists who were blinded to the infant’s gestational age and clinical status reviewed the MRI studies. Images were assessed for presence and severity of pathologic T1 shortening and T2 prolongation, restricted diffusion, intracranial/intraventricular hemorrhage and periventricular leukomalacia. MRI findings were further graded according to published criteria in term newborns: grade 0, normal; grade 1a, minimal cerebral abnormality without involvement of the basal ganglia or of the posterior limb of the internal capsule (PLIC); grade 1b, more extensive cerebral involvement, but no involvement of the basal ganglia or PLIC; grade 2a, basal ganglia or PLIC abnormalities, but no cerebral abnormalities; grade 2b, both basal ganglia and PLIC involvement; and grade 3, hemispheric devastation.5,6
Neurodevelopmental Assessment
Infants in whom HIE was diagnosed were seen at the Follow-up Clinic at Children’s Medical Center Dallas as per standard practice. When available, information on psychometric testing performed by a certified psychometrician with the Bayley Scales of Infant Development III (BSID; mean ± SD score, 100 ± 15) was obtained. Severe disability was defined by a BSID III score >2 SD less than the mean (ie, <70). Moderate disability was defined as a BSID III score between 1 and 2 SD less than the mean (ie, 85–70).
Statistical Analysis
Data analysis was performed with Sigma Stat software version 11.0 (SPSS, Chicago, Illinois), with results reported as the mean ± SD or as the number and percentage. Non-parametric analyses were used when indicated to compare clinical and laboratory variables in infants with and without HIE. A paired t test was used to compare umbilical cord blood gas and the first arterial blood gas parameters for each patient. Two-tailed tests were considered significant when the P value was ≤.05.
Results
In 1305 newborns admitted to the PMH NICU at 33 to 35 weeks’ gestation, biochemical screening was performed on umbilical cord blood (n = 1275) or a blood sample obtained within 1 hour of birth (n = 30). Thirty-three newborns (2.5%) were identified with perinatal acidosis that was detected in 32 of the infants with umbilical cord blood sampling. In one newborn, acidosis was detected on the first arterial blood gas within 1 hour of age (Figure; Table I). Overall, 22 of those 33 newborns (67%) met the first biochemical screening criterion, and 11 met the second one. Most mothers of newborns with perinatal acidosis were of Latino/Hispanic ethnicity (n = 26; 79%), 4 (12%) were black, and 3 (9%) were Caucasian. These percentages were similar to the ethnicity and race of the delivery population at PMH. Mode of delivery was cesarean delivery in 25 newborns (78%) and vaginal delivery in 8 newborns (22%). Pregnancy was complicated by hypertension in 12 newborns (36%), diabetes mellitus in 6 newborns (18%), and placental abruption in 3 newborns (9%). Blood cultures from all 33 newborns on admission to the NICU were sterile, and no infant had a lumbar puncture.
Figure.
Flow chart of infants born at PMH from October 2005 to September 2008 with perinatal acidosis and HIE.
Table I.
Characteristics of newborns with perinatal acidosis
| HIE
|
No HIE
|
Total
|
|
|---|---|---|---|
| n = 9 | n = 24 | n = 33 | |
| Birth weight, g | 2184 ± 741 | 2083 ± 516 | 2162 ± 561 |
| Gestational age, weeks | 34 ± 1 | 34 ± 1 | 34 ± 1 |
| 35 (33–35) | 34 (34–35) | 34 (34–35) | |
| Sex, male | 5 (55%) | 15 (60%) | 19 (57%) |
| Apgar 1 minute | 3 (1–3) | 3 (2–4) | 3 (2–4) |
| Apgar 5 minutes | 4 (3–7) | 6 (4–8) | 6 (4–7) |
| Apgar 10 minutes | 4 (2–5) | 6 (4–8) | 5 (3–8) |
| Intubated in delivery room | 7 (78%) | 5 (20%) | 11 (33%) |
| CPR in delivery room | 2 (22%) | 0 | 2 (6%) |
| Umbilical cord gas | |||
| pH | 6.9 ± 0.14 | 7.01 ± 0.07 | 7.00 ± 0.10 |
| Base deficit | 21 ± 6 | 16 ± 4 | 17 ± 4 |
| First arterial blood gas | |||
| pH* | 7.11 ± 0.11 | 7.23 ± 0.13† | 7.26 ± 0.10 |
| Base deficit* | 18 ± 9 | 9 ± 5† | 10 ± 6 |
CPR, cardiopulmonary resuscitation.
Data presented as mean ± SD or median (IQR).
P < .001 between groups.
P < .005 within group.
Neurologic Assessment
Clinical signs of encephalopathy were documented in 9 of the 33 newborns (28%) with perinatal acidosis (Figure). These newborns did not differ significantly from newborns without HIE in birth weight, gestational age, sex, Apgar scores, need for resuscitation at birth, and umbilical cord blood pH and base deficit (Table I). However, the first arterial blood gas obtained within the first hour of age showed a significantly lower blood pH and a higher base deficit in infants with HIE than infants without HIE (7.11 ± 0.11, base deficit 18 ± 9 versus 7.23 ± 0.13, base deficit 9 ± 5, respectively; P < .001). Paired analyses within each group between umbilical cord blood pH and first arterial blood gas showed significant resolution of metabolic acidosis only in newborns without HIE (P < .005; Table I).
The modified Sarnat criteria was used to describe the severity of HIE (Table II). Two infants had mild (stage I) encephalopathy, 4 infants had moderate (stage II) encephalopathy, and 3 infants had severe (stage III) HIE. Three of the infants with moderate-to-severe encephalopathy had clinical seizures and received anticonvulsant therapy. Overall, the rate of moderate-to-severe HIE was 5 per 1000 live births of 33 to 35 weeks’ gestation.
Table II.
Characteristics of the 9 newborns with HIE
| Sarnat stage | Perinatal history | Resuscitation at birth | Seizure | Organ dysfunction | EEG | MRI | Outcome |
|---|---|---|---|---|---|---|---|
| 3 | 33 weeks stat C/S (placental abruption) | Intubation, CPR, epinephrine | Yes | Troponin 2.5, ALT 1366, creatinine 3.7, PPHTN | Status, low voltage, burst suppression | No MRI (cranial ultrasound scanning with diffuse cerebral edema, PVWM echogenicity, grade 2 IVH) | Death, DOL 2 |
| 3 | 35 weeks NVD, Class C diabetic mother | Intubation | No | Troponin 0.3, ALT 202, creatinine 1.2 | Cerebral coma, burst suppression | DOL 9, grade 3 Severe diffuse white matter, deep gray and cortical infarction, elevated lactate with MRS | DNR, Gastrostomy-tube, death (1 year) |
| 3 | 35 weeks C/S (TEF, growth restriction) | Intubation | Yes | ALT 60, creatinine 1.3 | Long tracé discontinue, low amplitude | DOL 57, grade 3 Chronic bilateral parieto-occipital infarctions |
Discharge (day 50) developmental delay |
| 2 | 33 weeks stat C/S (decelerations) | CPAP | Yes | Not done | Right temporal lobe seizure | DOL 10, grade 1b PVWM abnormal, cerebellar hemorrhage, grade 1 IVH |
Discharge (day 22) Normal exam |
| 2 | 35 weeks stat C/S, PIH (75% placental abruption) | Intubation, hypothermia therapy (72 hours) | No | Not done | None | DOL 16, grade 0 | Discharge (day 47) Normal exam |
| 2 | 33 weeks stat C/S, PIH (75% placental abruption) | Intubation | No | Troponin 1.7, ALT 181, creatinine 2.3, hypotension | None | DOL 11, grade 0 White matter edema without restricted diffusion; MRI DOL 80, grade 0 |
Discharge (day 22), normal examination results |
| 2 | 35 weeks stat C/S (decelerations, meconium) | Intubation | No | ALT 469, creatinine 1.5, PPHTN | None | DOL 17, grade 1b Focal intraparenchymal hematoma of occipital lobe; basal ganglia normal |
Discharge (day 25), normal examination results |
| 1 | 35 weeks stat C/S (low fetal heart rate) | Intubation | No | ALT 176, creatinine 0.9, hypotension | None | DOL 17, grade 1b Focal right IVH with multiple foci of cystic hemorrhagic PVL |
Discharge (day 30), normal examination results |
| 1 | 35 weeks NVD, meconium, histologic chorioamnionitis | CPAP, then intubation | No | AST 212, Creatinine 1.7, PPHTN | None | DOL 20, grade 0 White matter edema, abnormal T2 signal of cerebellar white matter |
Discharge (day 24), normal examination results |
EEG, electroencephalogram; C/S, cesarean delivery; ALT, alanine aminotransferase (Units/L); PPHTN, persistent pulmonary hypertension; IVH, intraventricular hemorrhage; DOL, day of life; NVD, normal vaginal delivery; MRS, magnetic resonance spectroscopy; DNR, do not resuscitate; TEF, tracheoesophageal fistula; CPAP, continuous positive airway pressure; PIH, pregnancy-induced hypertension; PVL, periventricular leukomalacia; AST, aspartate aminotransferase (units/L).
Mild HIE
These two newborns required ventilator support briefly and had mild elevation in creatinine and liver enzyme levels that resolved within 24 hours. The results of the neurologic examination were normal within 24 to 48 hours, and they were discharged to home by 1 month of age on full nipple feeds.
Moderate HIE
The 4 infants had evidence of multiple organ injury with elevations in the levels of liver enzymes, creatinine, and troponin (Table II) that resolved within 72 hours of age. Signs of encephalopathy also resolved by 72 hours, and all 4 infants had normal neurological examination results at discharge from 20 to 47 days of age. One infant had clinical seizures, but was not discharged to home on antiepileptic therapy.
Severe HIE
One of the 3 infants with severe HIE died after intensive care was discontinued on the third day of age for liver failure (alanine aminotransferase, 1766 units/L; aspartate aminotransferase, 308 units/L), renal failure (creatinine, 3.7 mg/dL), cardiac failure (peak troponin, 4 μg/L), disseminated intravascular coagulopathy, refractory hypotension, persistent pulmonary hypertension, and status epilepticus. Electroencephalogram showed low voltage burst suppression and head ultrasound scanning findings were consistent with diffuse cerebral edema. Brain MRI was not done. The other two newborns also had multiple organ injury, prolonged respiratory support, and persistent hypotonia at hospital discharge. One infant had a tracheoesophageal fistula with chylothorax and seizures, and the other was discharged to home with gastrostomy tube feeds and on hospice care.
All surviving infants with moderate and severe HIE had brain MRI performed (Table II).
Moderate HIE
Of the 4 infants, one had normal study results. This was an infant of 35 weeks’ gestational age who was treated with systemic hypothermia on a compassionate basis according to the Neonatal Research Network’s published trial1 without complications. The remaining 3 infants had grade 1b MRI results. Two of these infants had multifocal periventricular white matter (PVWM) injury, which resolved in one infant on follow-up MRI performed 12 weeks later. These two infants with preterm patterns of white matter brain injury were both at the lower gestational age of 33 weeks.
Severe HIE and MRI
Both surviving infants showed extensive grade 3 abnormalities on MRI. One infant had global cerebral infarction involving cortical and deep gray matter nuclei and white matter, and the other infant had large focal areas of symmetric infarction in the parietal and occipital lobes. The MRI on the latter infant was performed at 55 days of age because of prolonged respiratory support and surgical repair of a tracheoesophageal fistula. The newborn who died on day 2 without MRI studies was born at 33 weeks, and neuroimaging was limited to head ultrasound examination that revealed PVWM injury, intra-ventricular hemorrhage, and diffuse cerebral edema.
Follow-up
The two infants with mild HIE had a normal neurological evaluation at 12 months of age. The 4 infants with moderate HIE had a normal examination results and appropriate developmental milestones at 12 months of age.
All 3 infants with severe HIE had an abnormal outcome, with either death or severe disability. One infant died on the second day of age, and another infant died at 12 months of age while receiving hospice care. Before death, the latter patient had severe neurodevelopmental delay with inability to nipple feed that required placement of a gastrostomy tube, inability to sit, nystagmus, hypertonia with clonus, and contractures. The third patient had severe disability and impairment and was functioning developmentally at a chronological age of 3 to 5 months at the 12- and 24-month assessments, respectively. His composite BSID III cognitive score was 55, language score was 62, and motor score was 46, all <2 SDs less than the mean at 12 months. Follow-up BSID III examination at 24 months of age showed a language score of 59 and a motor score of 46.
Discussion
This retrospective study examined how the biochemical screening criteria and neurological assessment for HIE that are used in full-term infants to assess their eligibility for hypothermia therapy apply to the preterm infant of 33 to 35 weeks’ gestation.1 Overall, 2.5% of these preterm newborns met physiological screening criteria for perinatal acidosis, and 27% of them had neurological signs of moderate to severe HIE on the first day of age. This finding is similar to that seen in term newborns, in which one-third of infants screened for biochemical fetal acidosis have evidence of moderate to severe HIE.1 The basis of physiological screening for fetal acidosis to capture infants at risk for HIE is based on the previously reported association in term newborns of pH <7.0 and low 1- and 5-minute Apgar scores with neonatal seizures, permanent neurological injury, and death.9,10 A pH <7.0 reflects the degree of acidosis below which end organ dysfunction is likely to be detected in term infants.10,11 Earlier work from our institution showed that approximately 0.3% of term deliveries are complicated by severe fetal acidosis, and approximately one-third of the newborns required admission to the NICU, and 15% had moderate to severe HIE.12
This study establishes baseline data on which to base future trials of neuroprotective strategies such as hypothermia in this preterm population.13 The literature describing the incidence of HIE in preterm infants is limited. In a 10-year retrospective study at our institution that identified 62 neonates born at 31 to 36 weeks’ gestation who had an umbilical cord arterial blood gas with a pH <7.0, 7 received a diagnosis of moderate or severe HIE with Sarnat staging criteria for an incidence of 1.3 per 1000 live births of this gestation.14 Most of these newborns with HIE, however, were 36 weeks’ gestation, and only 2 of the newborns were 33 to 35 weeks’ gestation. We found that the incidence of moderate to severe HIE in this latter gestational age cohort was 5 of 1000 live births of gestational ages from 33 to 35 weeks, a rate greater than the reported one to two of 1000 in term newborns, but lower than 9 of 1000 live births of 32 to 36 weeks’ gestation reported by Schmidt and Walsh.15 This latter study screened infants of 32 to 36 weeks’ gestation for HIE on the basis of a 5-minute Apgar score <6. In addition, these criteria were required for classifying an infant as having perinatal HIE: umbilical cord or initial blood pH <7.0 or base deficit >15 mmol/L, evidence of encephalopathy at or shortly after birth, history of a sentinel event at the time of delivery, and no other cause of encephalopathy (eg, sepsis, hypermagnesemia, hemorrhage, hyponatremia, drug induced).15
Overall, the higher incidence of HIE in preterm infants could be attributed to additional maturational and trophic disturbances in the developing brain because more than one-third of brain growth occurs from 33 to 40 weeks of gestation. In addition, in our cohort of preterm infants, those with HIE were more likely to have persistent metabolic acidosis with a lower pH and two-fold higher base deficit at 1 hour of age, and resolution of the metabolic acidosis was associated with absence of HIE. The association of persistent metabolic acidosis with HIE merits further study as a potential marker of brain injury.
This study also uses the neurological examination to establish the diagnosis of HIE. There are inherent difficulties in the diagnosis of HIE in preterm infants because signs may be lacking or mistaken as abnormal because of their developmental immaturity. Normal preterm infants typically have systematic developmental differences in primitive reflexes, tone, and posture.16 However, sufficient signs of HIE were noted in our cohort of preterm infants that allowed classification of the stage of HIE. The finding that all infants with clinical signs of severe encephalopathy had an abnormal outcome (ie, predictive value 100%) suggests that the neurological examination can be used in future neuroprotective trials for this cohort of infants. In addition, the neurological assessment of autonomic signs of HIE includes the use of mechanical ventilation, which occurred in 77% of our infants who had HIE. Similarly, Perlman and Risser17 found that in term newborns with severe fetal acidemia, mechanical ventilation was a marker for seizures. In preterm infants, our data and those of Salhab and Perlman14 suggest that HIE is unlikely to develop in those with perinatal acidemia who do not require ventilatory support. Thus, the lack of mechanical ventilation may identify a subgroup of preterm infants with perinatal acidemia who may not benefit from neuroprotective therapies.18
Ultimately, the optimal method of diagnosing HIE may be neuroimaging, with MRI being the best modality currently available. Logitharajah et al19 reviewed the MRI findings of 55 preterm infants of 26 to 36 weeks’ gestation with HIE and found that the main sites of injury were basal ganglia (75%), white matter (89%), brainstem (44%), and cortex (58%). MRI abnormalities were highly predictive of 2-year outcomes, with death in 32% of infants, cerebral palsy in 26%, mild impairment in 10%, and normal results in only 32%. Overall, the outcomes were worse than that seen in term infants with HIE.20 Similarly, in our cohort of preterm infants, severe HIE was associated with extensive grade 3 MRI abnormalities that included basal ganglia injury and either death or severe disability in the first 2 years of age. However, the MRI findings in mild and moderate HIE were more variable and included PVWM changes, intracranial hemorrhage, and cerebellar edema. These additional findings usually are not seen in term neonates and are likely caused by regional differences in white matter vulnerability to hypoxic ischemic injury, with preterm infants showing selective vulnerability of the preoligodentrocyte.20–22 Unfortunately, MRI testing is impractical for enrollment into a trial of neuroprotection that requires diagnosis within 6 hours of birth.
The one infant with moderate HIE in this study who had a normal brain MRI result received systemic hypothermia therapy. Rutherford et al4 described a significant reduction of moderate and severe basal ganglia lesions after hypothermia in a small number of term neonates with HIE. It is not known whether the same occurs in preterm infants, but will need to be studied in future neuroprotective trials in this population. In term infants, moderate to severe HIE correlates well with neurodevelopmental impairment in infancy and childhood. Moderate HIE is associated with death or childhood physical disabilities in 6% and 30% of these infants, respectively, and with severe HIE, mortality is high and 100% of survivors have physical or mental disabilities.21–24 Our findings in preterm infants of 33 to 35 weeks’ gestation are consistent with that seen in full-term infants, because death or disability occurred in all infants with severe HIE. With moderate encephalopathy, these outcomes were more variable and consisted of only mild grade 1 MRI abnormalities and no death or disability.
Limitations of this study include the small sample size, the retrospective design, lack of uniform neurologic assessment and timing of MRI studies, and lack of data on infants with fetal acidosis but no clinical signs of HIE at birth. Therefore, our findings may represent only the most severely affected newborns. Strengths, however, include a large inborn cohort and a detailed prospective registry that allowed the application of the same sequence of biochemical screening for fetal acidosis and neurological assessment for encephalopathy that was used in the whole body hypothermia trial conducted by the NICHD Neonatal Research Network.1
Screening criteria for HIE that use biochemical and neurological assessments as performed in at-risk term newborns can be applied to preterm infants of 33 to 35 weeks’ gestation. This study lays the groundwork for the selection of preterm infants of 33 to 35 weeks’ gestation in future neuroprotective trials and provides some estimate of the number of infants needed for such trials. Finally, this descriptive study adds to our understanding of HIE in preterm infants by describing neurological assessments and neuroimaging findings.
Acknowledgments
L.C. is supported by the National Center for Research Resources (grants KL2RR024983 and UL1 RR024982).
The authors thank Lucy Christie, RN, and P. Jeannette Burchfield, RN, for their assistance in the database management and data entry and Karen Kirby for secretarial assistance.
Glossary
- BSID
Bayley scales of infant development
- HIE
Hypoxic-ischemic encephalopathy
- MRI
Magnetic resonance imaging
- NICHD
National Institute of Child Health and Human Development
- NICU
Neonatal intensive care unit
- PLIC
Posterior limb of internal capsule
- PMH
Parkland Memorial Hospital
- PVWM
Periventricular white matter
Footnotes
The authors declare no conflicts of interest.
Presented in part at the Pediatric Academic Societies’ Meeting, May 5 to 8, 2007, Toronto, Canada (abstract 751111).
References
- 1.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 2.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 3.Rutherford M, Ramenghi LA, Edwards AD, Brocklehurst P, Halliday H, Levene M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9:39–45. doi: 10.1016/S1474-4422(09)70295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutherford M, Pennock J, Schwieso J, Cowan F, Dubowitz L. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome. Arch Dis Child Fetal Neonatal Ed. 1996;75:F145–51. doi: 10.1136/fn.75.3.f145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol. 1998;19:143–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999;20:1399–405. [PMC free article] [PubMed] [Google Scholar]
- 7.Rutherford M, Srinivasan L, Dyet L, Ward P, Allsop J, Counsell S, et al. Magnetic resonance imaging in perinatal brain injury: clinical presentation, lesions and outcome. Pediatr Radiol. 2006;36:582–92. doi: 10.1007/s00247-006-0164-8. [DOI] [PubMed] [Google Scholar]
- 8.Perlman JM, Risser R. Cardiopulmonary resuscitation in the delivery room. Associated clinical events. Arch Pediatr Adolesc Med. 1995;149:20–5. doi: 10.1001/archpedi.1995.02170130022005. [DOI] [PubMed] [Google Scholar]
- 9.Goldaber KG, Gilstrap LC, 3rd, Leveno KJ, Dax JS, McIntire DD. Pathologic fetal acidemia. Obstet Gynecol. 1991;78:1103–7. [PubMed] [Google Scholar]
- 10.Winkler CL, Hauth JC, Tucker JM, Owen J, Brumfield CG. Neonatal complications at term as related to the degree of umbilical artery acidemia. Am J Obstet Gynecol. 1991;164:637–41. doi: 10.1016/s0002-9378(11)80038-4. [DOI] [PubMed] [Google Scholar]
- 11.Belai Y, Goodwin TM, Durand M, Greenspoon JS, Paul RH, Walther FJ. Umbilical arteriovenous PO2 and PCO2 differences and neonatal morbidity in term infants with severe acidosis. Am J Obstet Gynecol. 1998;178(1 Pt 1):13–9. doi: 10.1016/s0002-9378(98)70619-2. [DOI] [PubMed] [Google Scholar]
- 12.King TA, Jackson GL, Josey AS, Vedro DA, Hawkins H, Burton KM, et al. The effect of profound umbilical artery acidemia in term neonates admitted to a newborn nursery. J Pediatr. 1998;132:624–9. doi: 10.1016/s0022-3476(98)70350-6. [DOI] [PubMed] [Google Scholar]
- 13.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–14. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 14.Salhab WA, Perlman JM. Severe fetal acidemia and subsequent neonatal encephalopathy in the larger premature infant. Pediatr Neurol. 2005;32:25–9. doi: 10.1016/j.pediatrneurol.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt JW, Walsh WF. Hypoxic-ischemic encephalopathy in preterm infants. J Neonatal-Perinatal Med. 2010;3:277–84. [Google Scholar]
- 16.Volpe JJ. Neurologic outcome of prematurity. Arch Neurol. 1998;55:297–300. doi: 10.1001/archneur.55.3.297. [DOI] [PubMed] [Google Scholar]
- 17.Perlman JM, Risser R. Can asphyxiated infants at risk for neonatal seizures be rapidly identified by current high-risk markers? Pediatrics. 1996;97:456–62. [PubMed] [Google Scholar]
- 18.Walsh WF, Azzopardi D, Hobson A, Clark R. Survival of infants between 32–35 weeks gestation treated with hypothermia for HIE. Pediatric Academic Societies’ Annual Meeting E-PAS201136754; Denver, CO. May 2011. [Google Scholar]
- 19.Logitharajah P, Rutherford MA, Cowan FM. Hypoxic-ischemic encephalopathy in preterm infants: antecedent factors, brain imaging, and outcome. Pediatr Res. 2009;66:222–9. doi: 10.1203/PDR.0b013e3181a9ef34. [DOI] [PubMed] [Google Scholar]
- 20.Volpe JJ. The encephalopathy of prematurity—brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16:167–78. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low JA, Galbraith RS, Muir DW, Killen HL, Pater EA, Karchmar EJ. The relationship between perinatal hypoxia and newborn encephalopathy. Am J Obstet Gynecol. 1985;152:256–60. doi: 10.1016/s0002-9378(85)80205-2. [DOI] [PubMed] [Google Scholar]
- 22.Robertson CM, Finer NN. Long-term follow-up of term neonates with perinatal asphyxia. Clin Perinatol. 1993;20:483–500. [PubMed] [Google Scholar]
- 23.Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long term outcomes: systematic review and meta-analysis. BMJ. 2010;340:c1471. doi: 10.1136/bmj.c1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankaran S, Woldt E, Koepke T, Bedard MP, Nandyal R. Acute neonatal morbidity and long-term central nervous system sequelae of perinatal asphyxia in term infants. Early Hum Dev. 1991;25:135–48. doi: 10.1016/0378-3782(91)90191-5. [DOI] [PubMed] [Google Scholar]