Abstract
Project RAP (Risk Avoidance Partnership) trained 112 active drug users to become peer health advocates (PHAs). Six months after baseline survey (Nbl = 522), 91.6% of PHAs and 56.6% of community drug users adopted the RAP innovation of giving peer intervention, and 59.5% of all participants (N6m = 367) were exposed to RAP innovation. Sociometric network analysis shows that adoption of and exposure to RAP innovation was associated with proximity to a PHA or a highly active interventionist (HAI), being directly linked to multiple PHAs/HAIs, and being located in a network sector where multiple PHAs/HAIs were clustered. RAP innovation has diffused into the Hartford drug-using community.
Keywords: diffusion of innovation, social networks, substance abuse, HIV/AIDS, peer interventions
INTRODUCTION
Peer intervention models have been theorized from a social ecological perspective as affecting the structural and environmental influences on individual behavior and social interactions (Latkin & Knowlton, 2005). These models are assumed to be effective because of the likelihood that peers have the greatest access to and can therefore reach those at highest risk within their social networks and communities at the times most critical for prevention messages and materials to be useful (Dickson-Gomez, Weeks, Martinez, & Convey, 2006). It is also assumed that building the capacity of members of the high-risk or target group to play a leadership role in delivering prevention intervention and promoting health to their peers and community contacts will indeed result in their doing so (Dickson-Gomez, et al., 2006). Further, it is expected that the messages that indigenous, trained peer interventionists deliver are more effective because of the perceived similarity between the messenger and the recipient, resulting in high levels of trust, believability, and cultural, contextual, and socio-linguistic appropriateness (Rogers, 1995). The act of social engagement itself is theorized as generating long-term change in both the providers of peer intervention and those to whom providers repeatedly deliver the peer intervention (Friedman, et al., 2004; Ramirez-Valles, 2002). For these reasons, peer intervention models have been promoted for decades worldwide in community settings to combat infectious diseases that can be prevented by changing social behaviors.
Evaluation of peer intervention diffusion and efficacy presents many challenges to scientists. Randomized control trial (RCT) test designs, the gold standard for efficacy studies and particularly for clinical trials, has limited value in evaluating efficacy of peer delivered interventions. First, most peer intervention models are implemented in community settings with the expectation that the interventions will diffuse through the community creating changes in community norms and practices. Therefore the unit of analysis is the community rather than the individual. It is generally too costly to have a large sample of communities for randomization to assess the intervention. Second, even if large scale resources can be invested in large samples of communities and can prove or disprove community level effects, alternative methods are still needed to demonstrate the diffusion process among inter-related rather than independent individuals, from individuals to different kinds of social networks, and from network pockets to changes in community.
Diffusion of Innovation Theory (Rogers, 1995) is one of the most important theories upon which peer intervention models are based. Diffusion theory assumes that the diffusion of innovation process starts with a few early innovators, and then diffuses to some early adopters in their social network, primarily as a result of peer modeling of the innovation and positive iterative feedback. The innovation is further adopted by more and more individuals in the community, which finally results in a change in general practice, or behavior norm change, in the population. Because the process of diffusion is beyond the individual level, evaluating the efficacy of peer intervention models requires not only demonstrating outcome changes at all levels, but also proving cross-level interactive dynamic processes consistent with diffusion theory. Using a social network approach is one way to demonstrate the diffusion and change process of a network based peer delivered intervention model.
We conducted a study called the Risk Avoidance Partnership (RAP) in which we tracked network relationships and dynamics in the course of implementing an innovative peer intervention to measure efficacy of this program to change group behavior. This paper uses ego and sociometric network analysis to test the RAP intervention diffusion process and effect based on diffusion theory, in order to illustrate the key processes of social change driven by drug users as community change agents within the networks of their peers.
THE RISK AVOIDANCE PARTNERSHIP (RAP) PROJECT
Theoretical Framework of the RAP Intervention
The RAP peer intervention model is based on multiple theories that guide understanding of the mechanisms effecting two types of behavior change (adoption of innovative peer intervention delivery and reduction of risk behaviors) among two types of participants (peer interventionists and their network members) at multiple levels (individual, network and community level), and the interaction among different levels. For example, social learning theory (Bandura, 1977) was used to shape intervention content as HIV prevention information and skills were modeled by trusted others who are behaviorally and culturally similar to the recipients. This theory applies to both peer interventionists and their peers at the individual level regarding both risk behavior reduction and adoption of peer intervention delivery. Health promotion and empowerment theory (Brown, 1991; Minkler, 1989) informed the peer intervention content and process, which included training drug users as peer interventionists, locally known as Peer Health Advocates (PHAs), to promote health activities at the individual level and group action around harm reduction at the network and community levels (Brown, 1991; Minkler, 1989). Diffusion theory (Granovetter, 1973; Rogers, 1995) provided a framework for understand the process by which “innovations” like peer intervention delivery and harm reduction practices are accepted, rejected, or transformed by drug users at the social network and community levels. The process of diffusion of the RAP intervention is the focus of this paper.
Selection of Drug Users to Become Peer Health Advocates (PHAs)
Numerous peer education intervention models for HIV prevention have been implemented worldwide with various at-risk populations (Medley, Kennedy, O’Reilly, & Sweat, 2009). While some peer intervention models emphasize careful selection of peer opinion leaders (Kelly, et al., 1997; Kelly, et al., 1991; Kelly, et al., 1992), others leave open the opportunity to become a peer interventionist to any member of the target population who is willing and has the potential to take on the new role (Broadhead, Heckathorn, & Weakhern, 1998; Broadhead, et al., 2006; Latkin, 1998; Latkin, et al., 2009; Latkin, Forman, Knowlton, & Sherman, 2003; Weeks, Convey, et al., 2009; Weeks, et al., 2006; Weeks, Li, et al., 2009). In the first few months of RAP, we sought PHA candidates with apparent potential to be peer leaders because of their network ties or evident influence on other drug users (e.g., as a gatekeeper of a drug-use site); however, throughout the training period we also opened it to any drug users who indicated interest and willingness to try this activity. We provided an intensive 5–10 session training program that built the capacity of all trainees to take on the health advocacy role regardless of their prior peer leadership.
Thus, to seek the pool of peer interventionist trainees, we recruited 176 PHA candidates based on: a) candidates’ self reported ability to referral 2–3 eligible network members (contact referrals—CRs) into the study (for baseline and follow-up surveys only) and the candidates’ willingness to take on the PHA role; b) outreach workers’ judgment, based on their familiarity with participants, about the candidates’ links in the drug-using community and their likelihood to complete at least 5 training sessions; and c) other eligibility criteria, including being 18 years or older and having used either heroin or cocaine/crack in the prior 30 days. Recruitment and training occurred in 28 cycles continuously over a two and a half-year period (December 2001 to May 2004). About 3–6 candidates were trained in each cycle. No participant was allowed to enter in the study twice, even in a different role. We compared the candidates at baseline from the early recruitment cycles with those from later cycles and found no statistical differences in gender, ethnicity, drug network size, retention rate, and self reported number of peers to whom they gave prevention information, materials or demonstration during the 6 months before their training started. The only difference we found was that early cycle PHA candidates were more likely to have been giving prevention information to others prior to initiation of their training according to peer reports. Out of the 176 PHA candidates, 131 (73.9%) initiated the training, and of those, 112 (86.2%) completed at least 5 sessions to become official trained PHAs.
PHA Training and Peer-Intervention Delivery
The RAP PHA Curriculum was a 10-session, theoretically driven interactive training program modeled after a similar one tested in Baltimore, Maryland (Latkin, 1998; Latkin, et al., 2003), adding a significant staff-PHA partnered community component based on community empowerment theory to emphasize advocacy action. Content of the training and intervention was modified on the basis of local ethnography (Weeks, et al., 2001) and PHA input during the pilot (Weeks, et al., 2006). The first 5 training sessions were conducted in-office for two hours each on consecutive days, using both didactic and interactive methods to provide information, model peer intervention activities, and role play delivery of the RAP Peer Intervention to other drug users in the community. The RAP Peer intervention was a harm reduction approach to reducing risky drug use and sexual practices and promoting general prevention and health enhancement. Up to 5 additional staff-accompanied field sessions were conducted in the community over the next 10 weeks at the convenience of the PHA and his or her staff partner in a variety of community locations chosen by the PHA, including in some of the PHAs’ drug-use sites. Field sessions allowed PHAs to practice effective communication and demonstration of prevention strategies in community situations where they could be expected to continue to apply them in the absence of project staff. Ongoing support for PHAs included monthly Community Advocacy Group (CAG) meetings to plan, organize, and implement activities to advocate for and promote drug users’ health and well-being at the community level, and to reinforce their new role as interventionists for peer and community change. PHAs received monetary compensation of $20 for participation in each of the 2-hour training sessions, and also received $10 for each CAG meeting they attended. However, they received no monetary incentives to deliver intervention to their peers.
Survey Sample and Outcome Evaluation Design
The survey sample includes two primary participant groups related to peer intervention diffusion. The first was 112 PHAs who completed 5 or more of the 10-session training curriculum. The second participant group was 411 Contacts, comprising primarily contact referrals the trained PHAs brought into the study for the baseline and 6-month surveys, plus PHA candidates who did not finish the training and their network referrals. We have reported elsewhere more extensively on the characteristics of the subgroups within the Contact group (Weeks, Li, et al., 2009); for the purposes of this network analysis we will treat them all as non-PHA potential recipients of the PHA-delivered RAP peer intervention.
We conducted a 1½-hour long risk behavioral and social network survey at baseline with PHAs and their contact referrals in each training cycle prior to the PHAs’ entry into the training program. All PHA candidates were recruited via street outreach. Each of the PHA candidates was asked to refer 2–3 drug using peers (injection or non-injection heroin or cocaine/crack users) for the baseline survey prior to their training cycle. All participants who completed a baseline were sought for 6-month follow-up surveys regardless of participation in or exposure to RAP interventions. Since the 28 PHA training cycles were conducted over two and a half years, there were two years of time in which our staff were conducting parallel baseline and 6-month surveys. Therefore, it is possible that Contacts recruited for later cycles might have already been exposed to RAP peer intervention at the time of their baseline survey (discussed more fully below). PHAs and Contacts received $25 for completion of the survey at each time point; PHAs additionally received $10 for each contact referred into the baseline survey or if they assisted with follow-up to relocate their contacts at 6 months. All procedures for recruitment, follow-up, survey content, and peer intervention content and delivery were reviewed and approved by an Institutional Review Board.
Prior Findings and Remaining Study Questions
Analyses of changes in risk behaviors among study participants in relation to the project interventions demonstrated the success of the RAP PHA training program and its effectiveness in mobilizing PHAs, their direct network members and other drug users in the community to adopt prevention practices and reduce risk behaviors (Dickson-Gomez, et al., 2006; Weeks, Convey, et al., 2009; Weeks, et al., 2006). Ethnographic process and outcome evaluation additionally documented the PHAs’ use of prevention materials and project tools (e.g., the RAP “Flipbook,” a PHA field manual containing the prevention intervention components) to teach, promote, and support adoption of health promotion attitudes and practices among their peers. Ethnography also documented the feedback process of change whereby PHA successes in positively influencing their peers reinforced PHAs’ continued health promotion efforts for their own and their peers’ benefits (Convey, Dickson-Gomez, Weeks, & Li, 2010);(Convey et al., 2010); Dickson-Gomez, et al., 2006; Weeks, et al., 2006).
Analyses comparing baseline risk behaviors and attitudes with 6-month follow-up reports among trained PHAs, their contact referrals, and the other study participants (i.e., PHA candidates who did not start or did not finish the training and their network referrals) showed significant risk reduction for all sub-groups from baseline to the 6-month survey (Weeks, Li, et al., 2009). The most significant injection risk reduction outcomes included cessation of drug injection or reduced number of injections per month (74.1%), and reduced sharing of syringes (95.9%), other injection works (91.7%), and drug solutions (93.1%). The most significant sexual risk reduction outcomes included reduced number of sex partners (41.7%), stopping or reducing all unprotected sex or continuing no unprotected sex (76.3%), and stopping or reducing unprotected sex with non-primary partners (96.5%) and in exchange for money/drugs (95.0%). This evidence clearly demonstrated behavior norm change in the Hartford drug using community. Within this broad sample of illicit drug users in the city, the predominance of risky practices had been changed into a predominance of harm reduction practices.
However, despite greater intensity of staff-delivered intervention with PHAs, we found no significant differences in risk reduction between PHAs, their direct contacts and other study participants (Weeks, Li, et al., 2009). This lack of differentiation between trained PHAs and other drug users called for a closer look at the mechanisms at work that might account for these findings. Was it the RAP intervention or something else that caused the risk behavior norm change? To answer this question, methods other than over time risk assessment comparisons are needed in order to reveal the RAP intervention diffusion process, and to determine the relationship between RAP intervention diffusion and its possible effects on risk behavior change. This paper uses sociometric network methods to explore this process further. Specifically, we examine the location of PHAs and others in the network of drug users in the study, the social network structure of all study participants, the intervention diffusion processes, non-linear change dynamics, the effect of social action on the individual and network levels, and social influence patterns within the sociometric of drug users in the study.
ANALYTICAL FRAMEWORK AND HYPOTHESES
The “innovation” introduced by the RAP project is engagement in peer intervention. Adoption of the RAP innovation was measured by both self reported “giving” and peer reported “receiving” of specific intervention via the social network survey. To demonstrate RAP intervention diffusion, we needed to find evidence of the following:
A high percentage of PHAs were actively delivering RAP intervention to their peers (the innovation), and reduced their own risk behaviors. This supports the proposition that active drug users can be transformed into peer/public health advocates and become “change agents” in the community.
A high percentage of other drug users (Contacts) adopted the RAP innovation and also reduced their risk behaviors. This supports the proposition that other drug users can adopt the innovation and can also become “change agents” in the drug using community, and that community behavioral norms can be changed from a predominance of risky practices into a predominance of support (peer intervention) and harm reduction practices through this process.
- The patterns of adoption of the RAP innovation are associated with several sociometric structural factors. These include the following:
- Additive effect: The likelihood of adoption increases with the number of people in one’s personal network who have already adopted the RAP innovation. Therefore, we hypothesize that the number of PHAs in a person’s personal network will be correlated with the number of to and from whom the person gives and receives intervention.
- Proximity effect: Adoption of the RAP innovation is associated with proximity to early innovators (PHAs). We hypothesize that minimum distance from (i.e., number of ties it takes to reach) any active PHA within the sociometric network will be correlated with the number of peers to and from whom a person receives intervention.
- Clustering effect: Within sectors of the sociometric network where the most active PHAs are clustered, the density of RAP innovation adoption is hypothesized to be high and to include more early adopters (Contacts delivering intervention to peers), compared to sectors where active PHAs are lacking or are isolated.
Evidence supporting these hypotheses can further demonstrate that the pattern of the RAP innovation diffusion process is consistent with diffusion theory, and the new community behavior norm of peer intervention is the result of the RAP intervention.
A challenge for our analyses was to find evidence that supported an association between the RAP innovation (peer intervention adoption/exposure) and risk behavior change. Innovation diffusion creates a paradox when the desired effect of the innovation (RAP peer intervention) is to create a sea change from risky to harm reduction practices in the population. If behavior norms change so much that the vast majority of the population has adopted harm reduction practices, it would be impossible, and counter to expectations of diffusion theory, to find evidence of an association between adoption/exposure to the RAP innovation and the reduced risk behaviors at the individual level. That is, diffusion of the RAP peer intervention and its effects would create the potential for a Type 2 error, masking evidence of intervention effects and making it difficult to distinguish effects on different subgroups within the population.
High on the health promotion priority list of RAP’s peer intervention content were messages to stop/reduce syringe and other injection equipment sharing, to bleach used needles prior to injection, and to use condoms during sex, especially with casual or paying partners and those who use drugs. Because 91.7% – 96.5% of all study participants adopted drug injection related harm reduction practices and unprotected sex with casual and paying partners by 6 months (Weeks, Li, et al., 2009), these variables were not appropriate for analysis to identify associations between the RAP innovation and behavioral outcomes. We had to choose behaviors not so high on the priority list of harm reduction messages and not yet adopted by the vast majority of the sample. Therefore, we selected “entered any drug treatment in the last 6 months” (47.7%). For injectors specifically, we selected “total number of injections in the last 30 days” (74.1% ceased or reduced injection and 92 remained injectors at 6-month follow-up) and “entered a methadone program in the last 6 months” (45.2%). For crack users, we selected “number of days used crack in the last 30 days” (68.3% ceased or reduced crack use and 139 remained crack users at 6 month follow-up). Additionally, regarding sexual behavior, we select “number of partners had unprotected sex with in the last 30 days” (76.3% ceased or reduced unprotected sex and 211 remained sexually active at 6-month follow-up).
In order to test the association between the RAP intervention and behavior change on the individual and network levels, we used two primary measures of exposure to the RAP peer intervention in addition to reports on the network survey of giving and receiving prevention intervention to network members described below. The first was a question asked at the 6-month follow-up about recognition of the RAP Flipbook, a field manual PHAs used to facilitate delivery of the peer intervention components consistently with different recipients and over time. This unique laminated manual was project specific and only available to trained PHAs. The second measure was a set of project specific slogans PHAs were trained to use during peer intervention delivery that encourage harm reduction practices (e.g., “15 seconds to safety,” “Be aware, don’t share, carry a spare,” etc.). Slogans passed on by PHAs could also have been adopted by Contacts. We asked all participants about recognition of each of six slogans at the 6-month survey.
SOCIAL NETWORK ANALYSIS OF THE DIFFUSION PROCESS
Network Construction and Measures
The baseline and follow-up surveys included an inventory of participants’ personal network members. These network data were used to analyze ego-centric (personal) network characteristics and potential influences, as well as to create and analyze the sociometric network of named ties (types of relationships among connected individuals) in the total sample of drug users in the study. This sociometric network of ties also provided the pathways through which RAP intervention was hypothesized to be delivered and diffused.
Sociometric network data regarding peer intervention has advantages over ego network data in three respects. First, sociometric network data provide a fuller picture of the whole network’s dynamics and allow deeper assessment of specific participants and others linked with them through specific ties (e.g. giving or receiving HIV prevention information, or sharing drugs). Second, we are able to assess both self-reported and peer-reported ties regarding giving and/or receiving the intervention. Third, we are able to combine self-reported and peer-reported intervention ties to obtain a more complete measure of intervention ties, and thus address measuring errors to some degree. However, as described below, sociometric data are limited to study participants whose links were confirmed by our study team. Intervention ties with community drug users not in our study were excluded in the sociometric data. Ego-network reports may also include ties among some study participants who were identified by first name or nickname only and therefore could not be reliably linked with study participants. These unverified links were also not included in the sociometric network data. Therefore, it is necessary to retain ego network measures to assist with tracking RAP intervention diffusion effect.
Constructing the Ego-centric (Ego) Network
Collecting network data during the baseline survey began by generating each participant’s personal network name list. The interviewer asked each participant to list all the people with whom he or she: did any kind of drugs; injected drugs; had sex; and was close to or received some kind of support from (participant’s subjective judgment) in the prior 6 months. Following this, participants were asked to list any additional names of people to whom they had given HIV prevention or other health information, materials or demonstration of prevention techniques in the prior 6 months, and names of any people from whom the participant had received these prevention information, materials or demonstrations. After generating the participant’s personal network name list, we asked a series of additional questions about each network member, including the person’s sex, ethnicity, age, relationship to the participant, how much the participant trusts him/her, frequency of recent contact, shared HIV risk, and social and economic support (tell problems to, lend money or provide a place to stay). This full process was repeated at the 6-month survey.
Ego network data were used to create aggregate measures of the participant’s personal network size (total number of names listed) and types and degree of specific relationship ties. The latter included the number of network members to whom the participant gave specific intervention components, the number of people from whom the participant received specific intervention components, and the number of each type of shared risk behavior tie. These data were incorporated into the SPSS database on individual participant characteristics that included the baseline and follow-up risk and attitude surveys for individual-level data analyses. They were also used as attribute data for sociometric network analyses in UCINET (Borgatti, Everett, & Freeman, 2002).
Constructing the Sociometric Network
Ego network data were used to construct a sociometric network of all participants in the study who were linked to each other, as indicated on their network name lists. To construct the sociometric network, project outreach interviewers, ethnographers, and other staff reviewed the names indicated on each participant’s list and sought to determine if they matched any of the names with those of other study participants, thereby generating a link between the participants. A link was only indicated if staff could confirm it on the basis of street observation or familiarity with the participants and their frequent hang-out partners or other frequent contacts. Directionality of the named tie (who named whom) was also indicated in this process. We confirmed that 39.3% of all baseline reported ties (n=1628) and 44.7% of all 6-month ties (n=1549) were among RAP participants. The rest, including ties with non-RAP participants (33.8% at baseline and 26.6% at 6-month) and unknown ties (26% at baseline and 28.7% at 6-month) were not included in the sociometric network database for analysis, though they were retained in ego network analyses.
We completed construction of the sociometric network first with the baseline data, and then added links if others were indicated on the 6-month surveys. Thus, the sociometric network represents the maximum number of ties we could confirm at any time during the study. Though we recognize that participants’ networks change over time with regard to close and frequent interaction (e.g., as a result of mobility, incarceration, ending of some relationships, and the beginning of others), we assumed that a network tie at any point in time meant a potential for significant interaction, even if only recently, intermittently or periodically.
Computed Sociometric Network Measures of the RAP Innovation
Construction of the sociometric network allowed us to compute new network measures for participants in the study sample. We computed a series of measures in UCINET (Borgatti, Everett, & Freeman, 2002.) based on the original network survey data. Many of these measures were added as personal (node) attributes for network analyses in UCINET or network mapping in Netdraw (Borgatti, 2002). Some were also added to the SPSS database for individual level analysis.
The sociometric network data comprise three components: nodes (participants), node attributes (individual characteristics, collected in the original survey or computed later), and ties (types of relationships between participants). Peer intervention ties are important ones for tracking the intervention diffusion process. On the network survey, we asked both giving and receiving specific types of interventions with peers, stated as, “Have you given (or received from) [named peer] prevention information in the last 6 months?” and “Have you given (or received from) [named peer] prevention materials or harm reduction demonstration in the last 6 months?” Each type of intervention given or received at different time points (i.e., at the baseline or 6-month survey) was counted as a separate tie. We separated information sharing from delivery of materials and their demonstration because the latter are a deeper level of peer prevention intervention (i.e., peer modeling and provision of prevention resources are more effective for behavior change than information sharing alone).
Having identified the total number and various types of ties among study participants, we then calculated in UCINET the following measures of the RAP innovation (peer intervention delivery) for each participant: a) the number of self-reported intervention ties to peers; b) the number of peer-reported intervention ties to that participant; and c) the total number of intervention ties from each participant, including both self-reported and peer-reported ties. So for example, “giving prevention information” for each participant included three measures: a) number of peers to whom the participant self-reported giving information; b) number of peers who reported that the participant gave them information; and c) the total number of people to whom the participant gave information, whether reported by the participant or by his/her peers. We calculated these three measures for giving prevention information, giving prevention materials/demonstrations, receiving prevention information, and receiving prevention materials/demonstrations for each participant in the sociometric network.
In addition, we created several other key characteristics using the sociometric data and peer intervention activity measures. These included the following:
Active PHA includes any trained PHA who gave information or materials/demonstrations to at least one person in the prior 6 months (using the 6-month follow-up survey data).
Highly Active Interventionists (HAIs) are PHAs or early adopter Contacts who are among the top 5% of all study participants with respect to the total number of peers to whom they gave prevention information or materials/demonstrations in the prior 6 months (using the 6-month follow-up survey data, including self- and peer reports).
Number of active PHAs or HAIs directly tied to ego (used to measure the Additive effect) is calculated as the number of active PHAs/HAIs the participant named or who named the participant on their personal (ego) network name lists. This measure also captures whether a participant’s position in the sociometric network is located in a cluster of early innovators (PHAs) or early adopter Contacts (used to assess the Cluster effect).
Proximity to an active PHA/HAI refers to the minimum number of links from each participant (whether PHA or Contact) to any active PHA or HAI in the network.
FINDINGS
Comparison of Ego-Centric Network and Sociometric Network Measures of Peer Intervention Ties
To understand the validity of different types of giving/receiving RAP innovation measures we used to track the diffusion process, and to understand intervention tie reporting behaviors, we compared three factors: 1) sociometric network measures of self-reported and 2) peer-reported intervention ties (among study participants only), and 3) ego-centric network measures of intervention ties (self-reported only, including ties beyond study participants), against the reference: sociometric total measure of giving/receiving intervention ties (combining self- and peer-reported ties in the sociometric network). Tables 1 and 2 demonstrate that all the measures show moderate to very good correlation with sociometric measures of total giving/receiving peer intervention, while variations were observed over time, across different types of participants, and for different kinds of intervention.
Table 1.
Comparison of Different Measures of Giving Peer Intervention Ties at Baseline and 6-month Surveys [Mean (Standard Deviation)/Correlationa with Total Number of Giving Peer Intervention Tiesb]
| Baseline (n=523)c | 6-Month (n=367) d | |||
|---|---|---|---|---|
| Gave prevention information | Gave harm reduction materials or demonstration | Gave prevention information | Gave harm reduction materials or demonstration | |
| Sociometric Network Ties | ||||
| Self-reported giving intervention ties: | ||||
| PHAs | .33 (.73)/857** | .22 (.60)/.848** | 1.17 (1.07)/.239* | 1.72 (1.57)/.569** |
| Contacts | .16 (.43)/.865** | .07 (.30)/.754** | .20 (.50)/.617** | .22 (.57)/.805** |
| Total | .20 (.51)/.863** | .10 (.39)/.804** | .46 (.82)/.526** | .62 (1.16)/.736** |
| Peer-reported giving intervention ties: | ||||
| PHAs | .27 (.54)/.636** | .16 (.42)/.655** | 2.11 (3.20)/.910** | 2.01 (3.02)/.898** |
| Contacts | .08 (.30)/.587** | .07 (.27)/.680** | .24 (.58)/.780** | .20 (.53)/.749** |
| Total | .12 (.37)/.626** | .09 (.31)/.674** | .74 (1.91)/.905** | .68 (1.81)/.900** |
| Total giving intervention tiesb: | ||||
| PHAs | .54 (.87) | .37 (.71) | 3.63 (3.61) | 3.41 (3.44) |
| Contacts | .22 (.50) | .14 (.41) | .53 (.98) | .39 (.81) |
| Total | .29 (.61) | .19 (.50) | 1.36 (2.46) | 1.20 (2.32) |
|
| ||||
| Ego-Network Giving Intervention Ties e | ||||
| PHAs | .65 (1.33)/.660** | .33 (.81)/.649** | 4.34 (3.51)/.427** | 4.21 (3.96)/.460** |
| Contacts | .51 (1.13)/.484** | .21 (.71)/.473** | 1.04 (1.37)/.518** | .73 (1.24)/.573** |
| Total | .54 (1.18)/.536** | .24 (.73)/.528** | 1.97 (2.65)/.616** | 1.72 (2.82)/.643** |
We report Pearson correlation coefficients.
Total giving peer intervention ties is the total number of either self-reported or peer-reported ties to whom each participant is indicated to have given intervention.
Baseline PHAs n = 112; baseline Contacts n = 411
Six-month PHAs n = 98; 6-month Contacts n = 269.
Number of named network members to whom ego/participant self reported having given intervention. This includes to network members who are not study participants.
p < .05 (2-tailed)
p < .01 (2-tailed)
Table 2.
Comparison of Different Measures of Receiving Peer Intervention Ties at Baseline and 6-month Surveys [Mean (Standard Deviation)/Correlationa with Total Number of Receiving Peer Intervention Tiesb]
| Baseline (n=523) c | 6-Month (n=367) d | |||
|---|---|---|---|---|
| Received prevention information | Received harm reduction materials or demonstration | Received prevention information | Received harm reduction materials or demonstration | |
| Sociometric Network Ties | ||||
| Self-reported receiving intervention ties: | ||||
| PHAs | .23 (.66)/.794** | .19 (.51)/.871** | .77 (1.07)/.801** | .71 (1.21)/.885** |
| Contacts | .09 (.31)/.593** | .07 (.27)/.573** | .83 (1.19)/.831** | .77 (1.08)/.858** |
| Total | .12 (.41)/.688** | .09 (.34)/.675** | .81 (1.16)/.822** | .76 (1.12)/.864** |
| Peer-reported receiving intervention ties: | ||||
| PHAs | .32 (.54)/.693** | .10 (.30)/.548** | .36 (.65)/.547** | .47 (.68)/.433** |
| Contacts | .17 (.44)/.857** | .10 (.37)/.809** | .36 (.59)/.408** | .49 (.80)/.632** |
| Total | .20 (.47)/.800** | .10 (.36)/.741** | .36 (.61)/.446** | .49 (.76)/.586** |
| Total receiving intervention tiesb: | ||||
| PHAs | .50 (.79) | .27 (.55) | 1.34 (1.38) | 1.13 (1.30) |
| Contacts | .23 (.51) | .17 (.46) | 1.26 (1.41) | 1.14 (1.33) |
| Total | .29 (.59) | .19 (.48) | 1.28 (1.40) | 1.14 (1.32) |
|
| ||||
| Ego-Network Receiving Intervention Ties e | ||||
| PHAs | .33 (.91)/.751** | .24 (.59)/.768** | 1.25 (1.52)/.638** | 1.02 (1.47)/.780** |
| Contacts | .21 (.54)/.372** | .14 (.48)/.369** | 1.74 (2.06)/.664** | 1.54 (1.87)/.699** |
| Total | .23 (.64)/.535** | .16 (.51)/.489** | 1.60 (1.93)/.650** | 1.39 (1.78)/.712** |
We report Pearson correlation coefficients.
Total receiving peer intervention ties is the total number of either self-reported or peer-reported ties from whom each participant is indicated to have received intervention (maximized degree of receiving intervention).
Baseline PHAs n = 112, Contacts n = 411
Six-month PHAs n = 98, Contact n = 269
Number of named network members from whom ego/participant self-reported having received intervention. This includes network members who are not study participants.
p < .05 (2-tailed)
p < .01 (2-tailed)
Table 1 shows the following patterns of reporting giving intervention, using sociometric total giving intervention ties as a reference. At baseline giving intervention ties were more likely to be self-reported than peer-reported for both PHAs and Contacts regardless of intervention type. However, at 6 months this pattern reversed, especially for PHAs reporting giving prevention information. The ratio of PHAs’ self-reported giving information ties over the total number of giving information ties decreased from 61% at baseline to 32% at 6 months, while the ratio of peer-reported receiving information ties from PHAs over the reference total number of giving information ties slightly increased from 50% at baseline to 58% at 6 months. As a result of PHAs’ significant under-reporting of giving prevention intervention at 6 months, this measure’s correlation with the total number of giving information ties significantly decreased (r=.857 vs .239 for baseline and 6-month, respectively). Finally, all ego-network measures of giving intervention have a stable range of correlation coefficients (r = .45 – .65 for most measures) with the correspondent reference sociometric total giving intervention ties. Similarly, the correlation coefficients were higher for PHAs than Contacts at baseline, but again the pattern reversed at 6 months. We also ran correlations comparing each ego network measure of giving intervention with its correspondent sociometric network measure of self-reported giving intervention. The results, not shown in Table 1, show good correlations (Pearson r ranged from .526 to .798) for all measures for both PHAs and Contacts, except for the number of peers to whom PHAs reported giving prevention information at 6 months (r =. 292). These ego-network measure findings suggest, again, that PHAs tended to under report intervention ties at the 6-month survey, especially giving information ties within study participants. It is also possible that PHA-reported 6-month intervention ties had a higher proportion of non-study-participants, who are not included in the sociometric database.
Table 2 shows the following reporting patterns regarding receiving intervention, using the sociometric total receiving intervention ties as a reference. First, baseline receiving intervention was more likely to be self-reported than peer-reported for PHAs regardless of intervention type, but the opposite pattern was found among Contacts. Second, at 6 months receiving intervention was more likely to be self-reported than peer-reported for both PHAs and Contacts, and the differences between these two types of participants diminished. Similarly, the lower part of Table 2 shows generally good correlations between ego-network measures of receiving intervention and the correspondent sociometric total receiving interventions ties. Reported behavior was similar for PHAs and Contacts at baseline regardless of intervention type, yet Contacts’ baseline reporting of intervention behaviors was less correlated with the corresponding sociometric total receiving intervention measures. Not shown in tables, the correlation between each ego network measure with its correspondent sociometric self-reported receiving intervention ties was also generally good (Pearson r range from .681 to .921). Ego-network measures of receiving intervention ties were generally more reliable than giving intervention ties and more consistent across intervention type and participant type.
Increased Level of Adoption and Exposure to RAP Innovation from Baseline to 6-Month Surveys
All sociometric and ego-centric measures in Table 1 consistently show remarkable increase over time in the number of giving intervention ties for all participants, thought the increase among PHAs was greater than among Contacts. With the total giving intervention ties (Table 1), PHAs’ mean number of giving information ties increased 6.7 times, and giving harm reduction materials/demonstration ties increased 9.2 times from baseline to the time of the 6-month survey. Contacts’ mean number of total giving information ties increased 2.4 times, and giving materials/demonstration ties increased 2.8 times from baseline to the 6-month survey. Table 1 also shows more giving information ties (self-reported, peer-reported, and total reported) than giving materials/demonstration ties for all participant types at baseline. However, by 6 months, the difference between giving information ties and giving materials/demonstration ties appears to have diminished.
All ego-centric and sociometric measures in Table 2 demonstrated significant baseline to 6-month increase in the number of receiving intervention ties, both for PHAs and Contacts. At baseline, PHAs started off receiving more intervention than Contacts, but this difference diminished by the time of the 6-month survey. At baseline, both groups received more prevention information than harm reduction materials or demonstration. But by 6 months, the differences in the level of receiving information and receiving materials or demonstration also diminished for both groups.
Table 3 shows the percentage of our study participants who gave peer intervention to or received it from at least one person at the time of the baseline and 6-month surveys. At baseline, both ego network data and sociometric network data showed that about one-third of study participants were giving and receiving any type of peer intervention. The high proportion of active peer interventionists and intervention receivers at baseline may be evident in part because baseline survey recruitment occurred over two and a half years while cycles of PHA training were ongoing; participants who joined the study at a later time might already have been exposed to peer intervention or become peer interventionists prior to their baseline surveys. This possibility is supported by the positive correlation between recruitment cycle (larger numbers indicate later cycles) and baseline peer-reported receiving of materials and demonstration (r=.119, p<.05) as well as baseline ego-network reported receiving of HIV prevention at the participants’ primary drug use site (r=.309, p<.01). Only sociometric network data showed that a higher proportion of PHAs were giving and receiving peer intervention than Contacts at the time of the baseline survey. By the 6-month survey, almost all trained PHAs were actively giving peer intervention. Among the 10 inactive PHAs, six were in a methadone drug treatment program and three had been in jail during at least part of the six months prior to their 6-month survey. The proportion of active peer interventionists among Contacts also increased from baseline to the 6-month survey although to a lesser degree. The same proportion of PHAs and Contacts reported having received peer intervention. Overall, over half of our study participants were giving peer intervention and over 60% of them reported receiving peer intervention at the 6-month survey.
Table 3.
Giving and Receiving Any Type of Peer Intervention at Baseline and 6-months (Percents of sample or subsample) a
| Baseline | 6-month | |||||
|---|---|---|---|---|---|---|
| PHAs | Contacts | Totalb | PHAs | Contacts | Totalb | |
| N: | 112 | 411 | 523 | 98 | 269 | 367 |
| Gave intervention (ego-network) | 36.6 | 29.6 | 31.1 | 91.6 | 56.6 | 66.1** |
| Gave intervention (sociometric network) | 42.9 | 22.9 | 27.2** | 90.2 | 42.2 | 54.9** |
| Received intervention (ego-network) | 25.9 | 18.0 | 19.8 | 58.9 | 59.8 | 59.5 |
| Received intervention (sociometric network) | 41.1 | 24.1 | 27.7** | 69.9 | 68.0 | 68.4 |
P values of all baseline and 6-month comparisons over time within each group and the total sample are all less than .01.
Significance is reported for comparisons between PHAs and Contacts at each time point.
p < .01 (2-tailed)
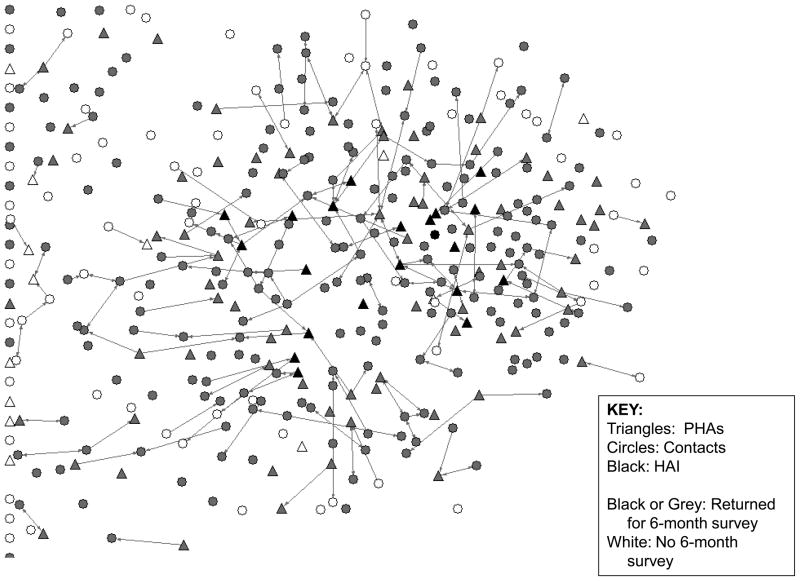
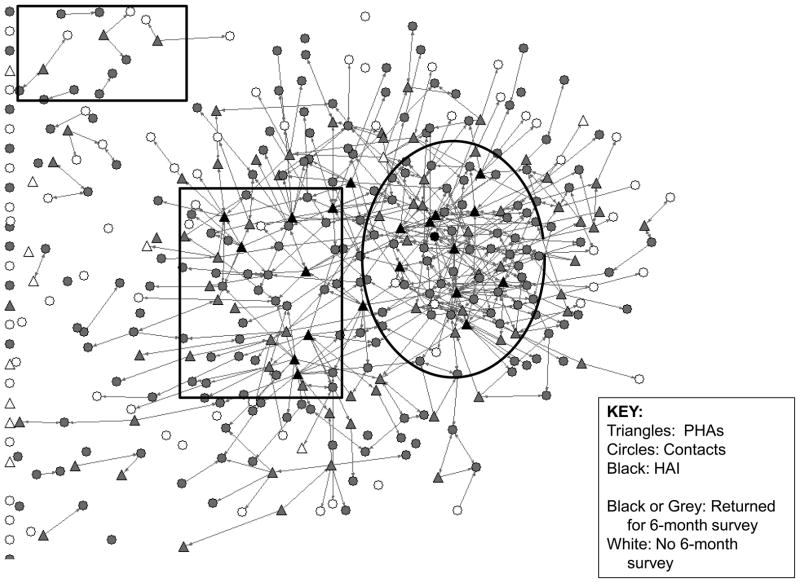
Figures 1 and 2 provide network graphs of giving/receiving either type of intervention at baseline and 6-months. We can clearly see an overall diffusion of the RAP peer intervention innovation between these two time points, as illustrated by the increased number of intervention ties reported by all study participants between the two assessment points.
Figure 1.
Total Giving Intervention Ties at Baseline survey
Figure 2.
Total Giving Intervention Ties at 6-month survey
Patterns of RAP Innovation Diffusion in the Drug User Social Network
Additive Effect
Table 4 shows that the number of ties to active PHAs or Highly Active Interventionists (HAIs) (whether self-reported, peer-reported, or total reported) is generally associated with giving intervention, and that the association patterns for PHAs and Contacts are different. For Contacts, the level of adopting RAP innovation (giving peer intervention) is generally positively correlated with the number of PHAs or HAIs directly linked with them, regardless whether contacts named PHAs/HAIs or PHAs/HAIs named contacts in the ego networks (Pearson r range from .124 to .211, except total ties with active PHAs, either naming direction). For PHAs, the number of named ties to other PHAs did not affect their level of giving peer intervention except that being named by other PHAs is positively correlated with the total reported ties of giving information. However, number of ties with HAIs, especially being named by HAIs, was highly correlated with PHAs’ level of giving peer intervention. Being named by other PHAs or HAIs was also strongly correlated with the level of receiving peer intervention for PHAs, but PHAs naming other PHAs or HAIs did not have this effect. For Contacts, however, the number of ties with active PHAs or HAIs, no matter which naming direction, all correlated with the level of exposure to peer intervention.
Table 4.
Association Between Individual Characteristics and RAP Peer Intervention Delivery at 6-months (Correlationa with Total Number of Giving/Receiving Peer Intervention Tiesb) (N = 367)c
| Individual Characteristics | Total Number of Giving Peer Intervention Ties | Total Number of Receiving Peer Intervention Ties | |||
|---|---|---|---|---|---|
|
| |||||
| Information | Materials or Demonstration | Information | Materials or Demonstration | ||
| Sociometric Network Structural Characteristics | |||||
|
| |||||
| Number of active PHAsd who named ego | PHAs | .253* | .198 | .455** | .367** |
| Contacts | .171** | .124* | .290** | .293** | |
| Total | .051 | .013 | .319** | .306** | |
|
| |||||
| Number of active PHAs who ego named | PHAs | .080 | .045 | .042 | .070 |
| Contacts | .198** | .213** | .176** | .181** | |
| Total | .012 | −.006 | .141** | .155** | |
|
| |||||
| Total # of ties with active PHAs, either naming direction | PHAs | .156 | .112 | .251* | .210** |
| Contacts | .201** | .211** | .298** | .299** | |
| Total | .038 | .015 | .280** | .275** | |
|
| |||||
| Number of HAIse who named ego | PHAs | .511** | .442** | .341** | .271** |
| Contacts | .103 | .096 | .161** | .179** | |
| Total | .150** | .124* | .194** | .209** | |
|
| |||||
| Number of HAIs who ego named | PHAs | .200* | .148 | .128 | .159 |
| Contacts | .166** | .213** | .167** | .182** | |
| Total | .070 | .058 | .155** | .176** | |
|
| |||||
| Total # of HAIs directly tied to ego, either naming direction | PHAs | .367** | .319** | .181 | .155 |
| Contacts | .146** | .179** | .177** | .199** | |
| Total | .115* | .103* | .174** | .188** | |
|
| |||||
| Minimum distance to an active PHA | PHAs | −.139 | −.150 | −.053 | −.012 |
| Contacts | −.206** | −.210** | −.260** | −.227** | |
| Total | −.078 | −.076 | −.202** | −.163* | |
|
| |||||
| Minimum distance to a HAI | PHAs | −.297** | −.291** | −.045 | −.037 |
| Contacts | −.127* | −.154* | −.169** | −.164** | |
| Total | −.141** | −.141** | −.136** | −.131** | |
|
| |||||
| RAP Recognition/Exposure | |||||
| Has seen RAP Flipbookf | Contacts | .128* | .135* | .199** | .194* |
|
| |||||
| Number of RAP slogans heard g | Contacts | .161** | .162** | .323** | .301** |
We report Pearson correlation coefficients.
Total giving/receiving peer intervention ties is the total number of either self-reported or peer-reported ties to whom each participant is indicated to have given/received intervention.
PHAs N = 98; Contacts N = 269.
Active PHAs reported giving intervention (information or materials/demonstration) to at least one person at the 6-month survey.
Highly Active Interventionists are participants whose total number of giving peer intervention ties was among the top 5% of the total sample, regardless of whether a PHA or Contact.
The RAP Flipbook is an intervention field manual provided to all PHAs to facilitate their delivery of the RAP peer intervention.
PHAs were trained to incorporate the unique RAP project slogans into their intervention messages, such as “15 seconds to safety,” “Be aware, don’t share, carry a spare,” etc.
p < .05 (2-tailed)
p < .01 (2-tailed)
p < .001 (2-tailed)
Proximity to Active PHAs or HAIs
Also shown in Table 4, for PHAs, being close to another PHA, that is, the minimum number of links in the network to connect to another PHA, had no effect on the level of peer intervention they gave or received; however, being close to an HAI was correlated with increased level of giving peer intervention. For Contacts, being close to any PHA or HAI was correlated with increased level of giving and receiving peer intervention, and the distance to a PHA was more meaningful than the distance to a HAI.
Clustering Effect
As shown in Figure 2, in the sector indicated by the large oval in which multiple PHAs were connected to each other, overall density of giving/receiving peer intervention appears to be the highest. In this sector, some Contacts were also more active in giving intervention. One of them even became a HAI. In sectors where HAIs were located but were less connected to other HAIs or PHAs, the intervention tie density was still relatively high (the large square). However, among sectors where PHAs are less active, and neither they nor their intervention recipients are connected to other PHAs (the small square), the density of intervention ties was very low. This suggests the potential of a dynamic influence that is greater than the sum of its parts when measured using the additive effect. This may indicate a continuous feedback loop or evidence of exponential change as a result of the network sector having reached a “tipping point.” However, while mapping the sociometric network provides an illustration of these ties that suggests such an effect, a deeper examination of these network dynamics and interactive factors affecting different individuals and subgroups in the “cluster” is needed to verify this effect, which is beyond the scope of this paper.
Diffusion of RAP Innovation Adoption and RAP Exposure
As indicated in Table 4, exposure to RAP intervention, measured by the participant reporting having seen the RAP Flipbook and the number of RAP slogans reportedly heard, was positively correlated with both giving and receiving peer intervention among Contacts. (All PHAs were trained in the use of the Flipbook and slogans and therefore were not reported in Table 4 for these measures.)
The Association Between RAP Innovation and Risk Behavior Reduction
The goal of the RAP intervention study was to reduce impoverished urban drug users’ HIV risk. Diffused RAP innovation (peer intervention delivery) would have no value without resulting in risk behavior change. We ran a series of exploratory correlation analyses to assess the association between RAP innovation adoption/exposure (all sociometric and ego-centric measures of giving and receiving each type of peer intervention) reported at 6 months (that is, receiving or giving intervention that occurred between the baseline and 6-month surveys) and selected outcome behavior measures. The following summarizes only the statistically significant correlation coefficients.
For the whole sample, having entered any drug treatment between baseline and 6-month surveys was found to be positively correlated with the sociometric total giving information (r=.137, p<.01) and giving materials/demonstration (r=.129, p<.05), total receiving materials or demonstration (r=.126, p<.01), and self-reported receiving information (r=.142, p<.01) and self-reported receiving materials or demonstration (r=.126, p<.05). Within the Contact sub-group, no measures of giving or receiving peer intervention were found to be correlated with entering any drug treatment between baseline and 6-month. But for PHAs, having entered drug treatment was found to be positively correlated with total receiving materials/demonstration ties (r=.199, p<.05) and self-reported receiving information ties (r=.206, p<.05).
For all baseline or 6-month injectors, the frequency of injection at the 6-month survey was found to be negatively correlated with sociometric total giving information ties (r= −.176, p<.05) and giving materials/demonstration ties (r= −.164, p<.05), self-reported giving materials/demonstrations (r= −.182, p<.05), and ego-network reported giving information (r= −.188, p<.05) during the 6 months after their baseline surveys. Within the Contact injector sub-group, no giving or receiving peer intervention measures were correlated with drug injection frequency. However, for PHA injectors, injection frequency was found to be negatively correlated with ego-network reported giving prevention information only (r= −.321, p<.05). ANOVA analysis showed that PHA injectors injected less frequently than Contacts at the 6-month survey (prior 30-day Mean = 18.56 vs. 38.65 times, p<.05), although no statistical difference was found at the baseline survey. For all injectors, having entered methadone maintenance between the time of the baseline and 6-month surveys was found to be positively correlated with the sociometric total number of giving information ties (r=.176, p<.05) and peer-reported giving information ties (r=.180, p<.05). For Contact injectors, having entered methadone maintenance was not correlated with any giving or receiving peer intervention ties. But for PHA injectors, peer-reported number of receiving information ties was correlated with having entered methadone maintenance (r=.385, p<.05).
Before presenting findings for crack users, it is worth pointing out that a higher percentage of crack users were among PHAs than Contacts at baseline (67.9% vs. 57.6%, p<.05), but the percentage decreased for both groups at 6 months and the group difference was no longer statistically significant (39.8% and 46.8% among PHAs and Contacts, respectively, p>.05 for both groups). In addition, PHA crack users at baseline were more likely to be frequent users of crack (Mean of number of days used = 18.8 vs. 15.6 for PHAs and Contacts, respectively, p<.05) and tended to be clustered together. That is, among PHAs, frequent crack use at baseline was positively correlated with the number of other PHAs directly connected, but this was no longer the case at the 6-month survey. It was also not true for Contacts or the total sample at either baseline or 6 months. Once again, this finding supports the proposition that PHAs’ positive social role change was a very powerful motivator for changing their crack use behavior. The lack of association between giving intervention and crack use at 6-month, indicates that giving intervention had become a prevailing norm. Yet the association between crack use and a few measures of receiving intervention suggests that the peer intervention was continuously targeted to heavy crack users. Findings from the social network analysis demonstrate that PHAs started at a high level of crack use related risk before intervention and benefited more from the RAP intervention than Contacts in terms of risk reduction.
Among all baseline or 6-month crack users, more frequent crack use at baseline was associated with a greater probability of giving peer intervention later (between the time of baseline and 6 month surveys). The number of days used crack in the prior month reported at baseline was correlated with all measures of later giving intervention for all crack users (r ranged from .141 to .185, p<.05). A similar pattern was also observed among Contact crack users in that their baseline number of days used crack was correlated with sociometric total reported (r=.171, p<.05) and ego-network reported giving materials or demonstration at 6 months (r=.223, p<.01). For PHA crack users, however, the results were very different. PHAs who used crack less frequently at baseline were more likely to give intervention between baseline and follow-up. The baseline number of days used crack was negatively correlated with 6-month sociometric total reported giving materials/demonstration ties (r= −.268, p<.01) and self-reported giving information ties (r= −.264, p<.05). At the time of the 6-month survey, crack use frequency was no longer correlated with any measures of giving intervention among crack users, while heavier users continued to receive more peer intervention. The latter was evident in that the number of days the participant used crack was correlated with both the total and self-reported number of ties from whom they received materials/demonstrations (r= .131 and 152, respectively, p<.05) among all crack users.
In terms of sexual risk for the whole sample, the 6-month number of sex partners with whom the participant had unprotected sex was negatively correlated with several measures of giving intervention, such as sociometric self-reported giving information (r= −.125, p<.05), and ego-network reported giving information (r= −.132, p<.05) and giving materials/demonstrations (r= −.150). For Contacts, only ego-network reported giving materials/demonstration was negatively correlated with the number of sex partners with whom they had unprotected sex (r= −.161, p<.05), while no similar pattern was found among PHAs. However, significant sexual risk reduction among PHAs was noted to be associated with their position in the sociometric network. At baseline we found that among PHAs being directly connected to multiple other PHAs in the sociometric network was strongly correlated with a high level of unprotected sex with casual partners (r=.293, p<.01), unprotected sex in exchange for money or drugs (r=.313, p<.01) and number of sex partners with whom the participant had unprotected sex (r=.256, p<.01). (Again, note that PHAs at baseline included more heavy crack users than Contacts did.) However, by the 6-month survey, this same additive effect among PHAs was strongly correlated with a reduced level of unprotected sex (r=.417, .423 and .365, respectively, for each of the risk variables reported above, p<.01). This finding suggests very significantly greater sexual risk reduction among PHAs than Contacts, a finding that was not identifiable via previous individual-level analyses (Weeks, Li, et al., 2009).
DISSCUSSION
Strong Evidence of RAP Innovation Diffusion
Results of these analyses support all our hypotheses and demonstrate that the RAP innovation of peer prevention intervention delivery and modeling had clearly diffused from PHAs to their network members and to the broader drug using community. Within only 6 months of their training, over 90% of trained PHAs had become active in doing their “job” as peer interventionists, and over two-thirds of all study participants had adopted the peer intervention delivery and modeling innovation. Literature suggests that when 10–15% or more of a population adopts an innovation that is consistent with a favorable peer norm, innovation diffusion is likely to reach a “critical mass” and will more efficiently be adopted by the rest of the population (Kelly, et al., 1991; Kelly, et al., 1992 (Valente, 1995). With the high adoption rate of RAP innovation by the time of the 6-month survey, we believe this innovation could quickly diffuse to the rest of study participants, and might likely make a significant impact among the Hartford drug using community, though the size of this population is unknown. Contacts’ adoption of RAP innovation also supports social learning theory in that they adopted peer intervention by exposure to and mimicking of well recognized and respected peers (i.e., PHAs) who were similar to themselves.
Transformation of PHAs
The RAP intervention made a remarkable impact on transforming active high-risk drug users into peer health advocates and community change agents. Social network analyses support that PHAs reduced their own HIV risks at a greater level than other drug users, a result not found from prior individual level analyses (Weeks, Li, et al., 2009). Besides their own health benefits, PHAs also made a significant impact on their fellow drug users. Sociometric network analysis revealed that Contacts’ exposure to RAP innovation (i.e., receiving peer intervention) was clearly associated with proximity to any PHA/HAI, being directly link to multiple PHAs/HAIs, and being located in a network sector where multiple PHAs/HAIs were clustered. The fact that PHAs had a stronger impact on Contacts than HAIs supports our prior ethnographic findings that PHAs had established a positive admirable identity and image in the community (Convey et al., 2010; Dickson-Gomez, Weeks, Li, & Convey, 2011, Dickson-Gomez, et al., 2006). The role model PHAs presented motivated Contacts to adopt the innovation of peer intervention delivery and modeling, and the adoption of RAP innovation among Contacts further diffused and resulted in behavior norm change among the community of drug users. Indeed, some Contacts surrounded by multiple highly active PHAs became HAIs and role models for other Contacts.
Differently from Contacts, PHAs’ exposure to RAP innovation (i.e., peer intervention they received from others) was not associated with proximity to any other PHA/HAI, nor the number of PHAs/HAIs they named. On one hand, this is not surprising because PHAs were all well connected with each other through the project’s monthly Community Advocacy Group (CAG) meetings, open to all trained PHAs to re-stock their prevention supplies, give support to each other, and receive ongoing training and support from staff. On the other hand, this non-association suggests that the impact of transforming from “street drug users” to “peer health advocates” itself was more powerful. We also found that PHAs’ level of conducting peer intervention and modeling was more likely to be affected by the additive and clustering effect of HAIs than the additive and clustering effects of PHAs, and more affected by proximity to a HAI than proximity to another PHA. This finding suggests that the actual positive action of peers (i.e., peer intervention delivery and modeling) was more important than who, by identity, was surrounding them in motivating and sustaining PHAs to conduct peer intervention. Thus, the number of PHAs/HAIs who named the participant was more likely than number of PHAs/HAIs who the participant named to be associated with giving intervention among PHAs. This suggests that PHAs were not consciously aware that other highly active peer interventionists had a strong influence on them. We conclude that PHAs’ adoption of RAP peer intervention delivery and modeling, i.e., their transformation from “street drug users” into peer interventionists and change agents in the community, was primarily the results of project training, which is consistent with health promotion empowerment theory and social engagement theory. However, sustained peer intervention delivery and modeling among PHAs was not only motivated and supported by monthly project CAG meetings, but also by observing other very active peer interventionists’ actions, though PHAs might not have been fully aware of the latter influence. Qualitative analyses also suggested that PHAs’ behavior change mechanisms could be the function of their altruism (Convey, et al., 2010), the development of their pro-social roles, positive social reinforcement from drug users and other community members, and cognitive dissonance associated with continued risk behavior while engaging in health advocacy (Dickson-Gomez et al., 2011).
Our findings show that heavy drug use, especially heavy crack use, is a barrier to PHAs’ performance of the role of peer heath advocate. For example, among the 10 inactive trained PHAs, two of them injected 13 times or more per day and one smoke crack more than 10 times a day at baseline. Among PHA crack users, post-training delivery of peer intervention was more likely to be found among those who were less heavy crack users at baseline. Despite the challenge from drug addiction, PHA crack users managed to control their drug use and continued doing PHA work. By 6 months, crack use frequency was no longer associated with the level of peer intervention delivery and modeling. The two extreme heavy injectors at baseline were in a methadone program and reduced their drug injection rate by the 6-month survey. The negative association between 6-month injection rate and ego-network reported giving prevention information also confirmed the addiction related challenge PHA injectors faced.
The Link Between RAP Innovation and Risk Behavior Reduction
Clear evidence supports the hypothesis that the RAP innovation (peer intervention delivery and demonstration) had diffused through the Hartford drug using community. We are confident that the diffused RAP peer intervention resulted in reducing this high risk group’s level of drug use, injection risk behaviors, and sexual risk behaviors. First, the most promoted harm reduction practices in RAP peer intervention, which were most relevant to HIV risk (eliminating drug and injection equipment sharing risk, using condoms) became prevailing practices among our sample. Second, the less often promoted harm reduction practices, such as reducing drug use, getting into drug treatment, and reducing the number of unprotected sex partners, also showed promising change, and some changes were demonstrated to be associated with adoption of and/or exposure to RAP peer intervention delivery and modeling. The different correlation patterns between PHAs, Contacts, and the whole sample suggest different mechanisms of risk behavior change in relation to adoption of or exposure to RAP peer intervention. For PHAs, their positive role change in the community was the main mechanism for entering treatment and injection behavior change, while receiving intervention from peers and positive support from other PHAs appeared to reinforce these changes. For Contacts, while mimicking PHAs appeared to encourage entering drug treatment, no consistent association was found between the level of exposure to peer intervention and drug treatment entry, nor drug use reduction.
One possible explanation is that drug users’ reduction of risk behavior is a function of peer behavior norm change. To test this hypothesis, we ran a correlation between participants’ harm reduction behavior change and the number and percentage of their direct network members’ harm reduction behavior change. We found that entering into any drug treatment was correlated with the number (r = .130, p<.05) and percentage (r=.117, p<.05) of network members who also entered drug treatment. Reduction in total number of unprotected sex partners was also correlated with the number and percentage of network members who also reduced their total number of unprotected sex partners. Exposure to other (non-RAP) interventions in the community was not likely to explain this significant change in our study sample. Our intensive ethnography and survey interviewing did not identify any other significant intervention programs in the community that could have caused the dramatic changes we observed during the three year period of the RAP intervention training and 6-month follow-up assessments.
LIMITATIONS
Network analysis for this study was limited by the three-year long pre- and post-intervention design with only two time-point measures for each participant, meaning that baseline and follow-up assessments were running currently for more than two years while PHA training was going on. With this design, we were not fully able to understand different stages of the RAP intervention diffusion process, such as whether there was a point in time when the RAP innovation delivery reached a critical mass. Also, network ties used in these analyses reflected relations at two time points for each participant (his/her own baseline and 6-month surveys); some of these ties may not have been continuous over the three-year period of data collection. The study also relies on self-reported data on sensitive risky practices and network ties. Another limitation of the study is the lack of a control group. The city of Hartford is not big enough for us to recruit a control group without contaminating it with the desired intervention diffusion effect. Further, more research is needed to observe and analyze the longer term outcomes and sustainability of these changes among peer interventionists and the broader community of drug users.
However, these limitations do not undermine the validity of the RAP intervention study and the social network analyses. The overall risk reduction was very dramatic and consistent with planned intervention content. Strong evidence in the social network analysis also supports the hypothesis that RAP indeed had a significant impact on the drug using community of Hartford. This was confirmed through multiple analyses on the individual level as well. Social desirability responses in self-reports were unlikely to explain this dramatic change in the total sample because Contacts (the majority of the sample) had little relation to the project (only participating in baseline and 6-month surveys), and therefore had no incentive to over report their own or others’ peer intervention activities or to report risk behavior differently at the baseline vs. the 6-month time points. While PHAs may have felt compelled to over report their peer intervention work to project staff during the follow-up assessment, in fact, they consistently appeared to under report it as indicated by the larger number of contacts and other PHAs who reported receiving an intervention from PHAs.
IMPLICATIONS FOR FUTURE INTERVENTION AND EVALUATION
This network analysis of the RAP intervention diffusion process demonstrated that training active drug users as peer interventionist can be successful. The trained peer interventionists not only benefited the most by reducing their own risk, but were also empowered to become change agents among their at-risk peers and the broader community. Diffusion of peer intervention behavior is key to the cost-effectiveness of network based peer intervention, because more and more untrained drug users can mimic the peer interventionists’ behavior and become change agents themselves, thereby further impacting their network until behavior norms of the community change.
Future network based peer intervention for drug users should consider adopting several key components found to contribute to RAP intervention success, such as promoting PHAs’ pro-social values as health advocates for their peers, supporting the establishment of PHAs’ positive image as change agents in the community, maintaining monthly Community Advocacy Group meetings for PHAs to restock with intervention materials and receive continuous training and support, and offering them “safe” opportunities to conduct peer-intervention should they cease drug use in order to prevent relapse while retaining the benefits of their new role as peer/public health advocates. These efforts are needed in order to support their sustained peer-intervention efforts over time. To evaluate the efficacy and diffusion process of similar network-based peer intervention programs, especially diffusion of peer intervention behavior, individual-level analysis alone is of limited value and randomized controlled trial study designs are likely to miss key change processes resulting from network diffusion influences. This is because the intervention is not targeted to independent randomizable individuals, but rather, to dependent network members within various network structures composed of members with differing levels of social influence. Network data collection should be an essential part of peer intervention program evaluation. Ego-centric network data collection is much more feasible and less expensive than sociometric network data collection. However, our comparison of self-reported, peer-reported, and total reported giving/receiving intervention suggests that sociometric network measures have superior value in overcoming some important shortcomings of ego network self-reported measures. Most importantly, because many PHAs became highly active in giving prevention intervention by 6 months, it may have been difficult for them to remember all to whom they had given intervention, particularly prevention information. For Contacts, receiving prevention from PHAs had important meaning to them; therefore, it was easier for them to report what PHAs had forgotten. Receiving intervention from other PHAs or HAIs had significant meaning to PHAs, yet they were not always conscious of it. The issue of forgetting network members related to highly central individuals is consistent with the literature of other types of network studies (Bell, Belli-McQueen, & Haider, 2007; Brewer, Garrett, & Kulasingam, 1999; Brewer & Webster, 2000). However, peer report alone is also limited because peers may not always be aware of all their network members’ intervention efforts.
Sociometric network data makes graphic social network analysis possible for the measurement of network structural factors beyond the intervention itself, needed in order to understand the broader context of risk, intervention, and prevention. It additionally offers a visual understanding of network structures and further hypothesis testing. However, constructing sociometric network data requires a significant amount of outreach work and direct community observation through ethnography or participant tracking to gain necessary familiarity with the study population needed to verify ties among named network members and study participants. Network data analysis also requires special training. Future network based peer intervention evaluation design should fully consider pros and cons of ego- versus sociometric network design and make careful choices.
Training average active drug users to deliver network based peer intervention has great potential to change the context of risk in drug using communities. RAP demonstrated the great capacity of trained PHAs to reduce their own risk, but also to reduce risky behavior among their contacts, and thereby to alter the environment of risk within the broader network of drug users in the city. Strong evidence of the breadth and depth of their impact, as demonstrated in our analysis of network changes associated with the RAP peer intervention program, suggest both the long-term value and potential cost effectiveness of these multi-level prevention intervention efforts.
Acknowledgments
The authors wish to acknowledge the following individuals for their contributions to the work that led to this paper. Oscar Woods, Eduardo Robles, and Maria Martinez went through the tedious work of verifying thousands of network member ties. Their work was critical to the construction of sociometric network databases. Jun-Jie Lispent considerable time preparing sociometric network measures and databases. Maria Martinez, Mark Convey, Oscar Woods, Eduardo Robles, Kim Radda, Michelle Garner, Julie Gonzalez, Chris Ortiz, and others contributed to different aspects of RAP project implementation. This study was funded by the National Institute on Drug Abuse, grant #R01 DA13356. RAP is an affiliated study of the Center for Interdisciplinary Research on AIDS (P30 MH62294).
Contributor Information
Jianghong Li, Institute for Community Research.
Margaret R. Weeks, Institute for Community Research
Stephen P. Borgatti, Gatton College of Business & Economics, University of Kentucky
Scott Clair, Iowa State University
Julia Dickson-Gomez, Medical College of Wisconsn.
References
- Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice Hall; 1977. [Google Scholar]
- Bell DC, Belli-McQueen B, Haider A. Partner naming and forgetting: Recall of network members. Social Networks. 2007;29(2):279–299. doi: 10.1016/j.socnet.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti SP. NetDraw: Graph Visualization Software. Harvard, MA: Analytic Technologies; 2002. [Google Scholar]
- Borgatti SP, Everett MG, Freeman LC. Ucinet for Windows: Software for Social Network Analysis. Harvard, MA: Analytic Technologies; 2002. [Google Scholar]
- Brewer DD, Garrett SB, Kulasingam S. Forgetting as a Cause of Incomplete Reporting of Sexual and Drug Injection Partners. Sexually Transmitted Diseases. 1999;26(3):166–176. doi: 10.1097/00007435-199903000-00008. [DOI] [PubMed] [Google Scholar]
- Brewer DD, Webster CM. Forgetting of friends and its effects on measuring friendship networks. Social Networks. 2000;21(4):361–373. [Google Scholar]
- Broadhead RS, Heckathorn DD, Weakhern D. Harnessing peer education networks as an instrument for AIDS prevention. Public Health Reports. 1998;113(Supplement 1):42–57. [PMC free article] [PubMed] [Google Scholar]
- Broadhead RS, Volkanevsky VL, Rydanova T, Ryabkova M, Borch C, van Hulst Y, et al. Peer-driven HIV interventions for drug injectors in Russia: First year impact results of a field experiment. International Journal of Drug Policy. 2006;17(5):379–392. [Google Scholar]
- Brown ER. Community action for health promotion: a strategy to empower individuals and communities. Int J Health Serv. 1991;21(3):441–456. doi: 10.2190/AKCP-L5A4-MXXQ-DW9K. [DOI] [PubMed] [Google Scholar]
- Convey M, Dickson-Gomez J, Weeks MR, Li J. Altruism and Peer-Led HIV Prevention Targeting Heroin and Cocaine Users. Qualitative Health Research. 2010;20(11):1546–1557. doi: 10.1177/1049732310375818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson-Gomez J, Weeks MR, Li J, Convey M. Social psychological dynamics of enhanced HIV risk reduction among peer interventionists. Journal of Community Psychology. 2011;39(4):369–389. doi: 10.1002/jcop.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson-Gomez J, Weeks MR, Martinez M, Convey M. Times and places: Process evaluation of a peer-led HIV prevention intervention. Substance Use & Misuse. 2006;41(5):669–690. doi: 10.1080/10826080500411403. [DOI] [PubMed] [Google Scholar]
- Friedman SR, Maslow C, Bolyard M, Sandoval M, Mateu-Gelabert P, Neaigus A. Urging others to be healthy: “intravention” by injection drug users as a community prevention goal. AIDS Education & Prevention. 2004;16(3):250–263. doi: 10.1521/aeap.16.3.250.35439. [DOI] [PubMed] [Google Scholar]
- Granovetter MS. The strength of weak ties. American Journal of Sociology. 1973;78(6):1360–1380. [Google Scholar]
- Kelly JA, Murphy DA, Sikkema KJ, McAuliffe TL, Roffman RA, Solomon LJ, et al. Randomised, controlled, community-level HIV-prevention intervention for sexual-risk behaviour among homosexual men in US cities. The Lancet. 1997;350(9090):1500–1505. doi: 10.1016/s0140-6736(97)07439-4. [DOI] [PubMed] [Google Scholar]
- Kelly JA, St Lawrence JS, Diaz YE, Stevenson YL, Hauth AC, Brasfield TL, et al. HIV risk behavior reduction following intervention with key opinion leaders of population: an experimental analysis. American Journal of Public Health. 1991;81(2):168–171. doi: 10.2105/ajph.81.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JA, St Lawrence JS, Stevenson LY, Hauth AC, Kalichman SG, Diaz YE, et al. Community AIDS/HIV Risk Reduction: The Effects of Endorsements by Popular People in Three Cities. [Article] American Journal of Public Health. 1992;82(11):1483–1489. doi: 10.2105/ajph.82.11.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin CA. Outreach in natural settings: the use of peer leaders for HIV prevention among injecting drug users’ networks. Public Health Reports. 1998;1:151–159. [PMC free article] [PubMed] [Google Scholar]
- Latkin CA, Donnell D, Metzger D, Sherman S, Aramrattna A, Davis-Vogel A, et al. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Social Science & Medicine. 2009;68(4):740–748. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin CA, Forman V, Knowlton AR, Sherman S. Norms, social networks, and HIV-related risk behaviors among urban disadvantaged drug users. Social Science & Medicine. 2003;56(3):465–476. doi: 10.1016/s0277-9536(02)00047-3. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Knowlton AR. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;17(Supplement 1):S102–S113. doi: 10.1080/09540120500121185. [DOI] [PubMed] [Google Scholar]
- Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV Prevention in developing countries: A sysmatic review and meta-analysis. [Article] AIDS Education & Prevention. 2009;21(3):181–206. doi: 10.1521/aeap.2009.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler M. Health Education, Health Promotion and the Open Society: An Historical Perspective. Health Education & Behavior. 1989;16(1):17–30. doi: 10.1177/109019818901600105. [DOI] [PubMed] [Google Scholar]
- Ramirez-Valles J. The protective effects of community involvement for HIV risk behavior: a conceptual framework. Health Education Research. 2002;17(4):389–403. doi: 10.1093/her/17.4.389. [DOI] [PubMed] [Google Scholar]
- Rogers EM. Diffusion of Innovations. 4. New York: The Free Press; 1995. [Google Scholar]
- Valente TW. Network models of the diffusion of innovations. Cresskill, NJ: Hampton Press; 1995. [Google Scholar]
- Weeks MR, Clair S, Singer M, Radda K, Schensul JJ, Wilson DS, et al. High risk drug use sites, meaning and practice: Implications for AIDS prevention. Journal of Drug Issues. 2001;31(1):781–808. [Google Scholar]
- Weeks MR, Convey M, Dickson-Gomez J, Li J, Radda K, Martinez M, et al. Changing Drug Users’ Risk Environments: Peer Health Advocates as Multi-level Community Change Agents. American Journal of Community Psychology. 2009;43(3 special issue):330. doi: 10.1007/s10464-009-9234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks MR, Dickson-Gomez J, Mosack KE, Convey M, Martinez M, Clair S. The Risk Avoidance Partnership: Training active drug users to be Peer Health Advocates. Journal of Drug Issues. 2006 (Summer):541–570. doi: 10.1177/002204260603600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks MR, Li J, Dickson-Gomez J, Convey M, Martinez M, Radda K, et al. Outcomes of a Peer HIV Prevention Program with Injection Drug and Crack Users: The Risk Avoidance Partnership. Substance Use & Misuse. 2009;44(2):253–281. doi: 10.1080/10826080802347677. [DOI] [PMC free article] [PubMed] [Google Scholar]