Summary
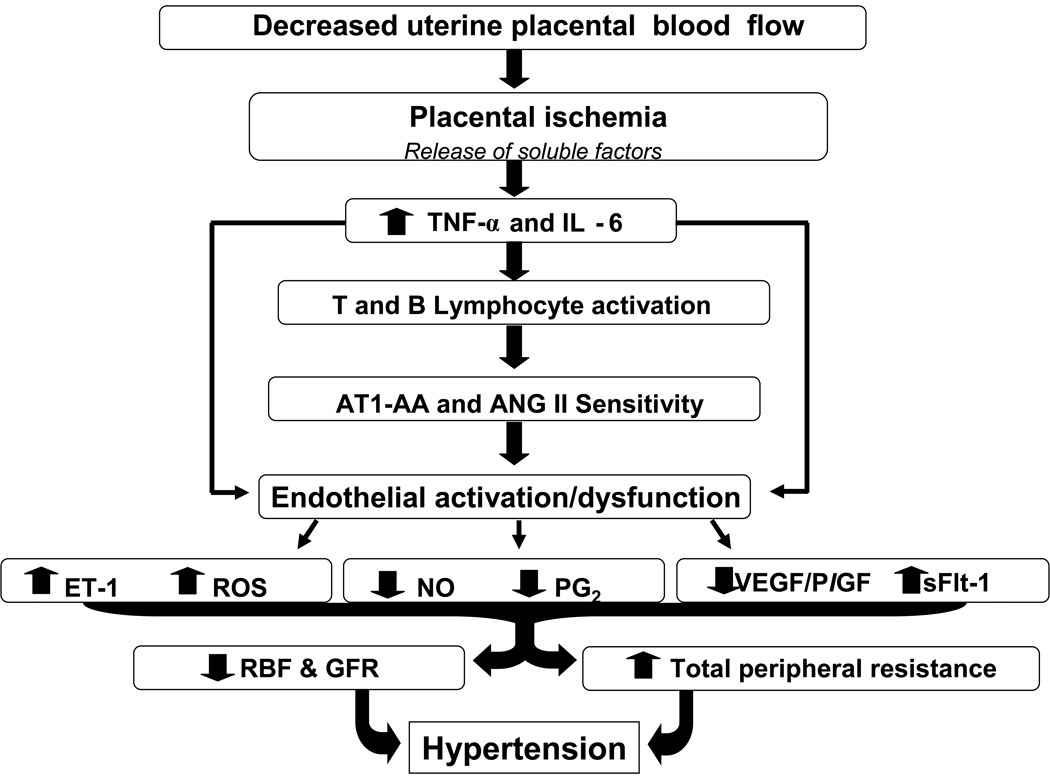
Alterations in vascular function contributes to hypertension as well as multi-organ dysfunction in women with preeclampsia (1,4, 11–14). Preterm preeclampsia remains a leading cause of maternal death and perinatal morbidity and most recently it has been recognized that women whom endure preeclampsia are at a greater risk for cardiovascular disease later in life. The pathophysiologic processes that underlie preeclampsia has been proposed to occur in two stages: stage 1, reduced placental perfusion, and stage 2, the maternal clinical syndrome (1,4). Placental ischemia/hypoxia is believed to result in the release of a variety of placental factors that have profound effects on blood flow and arterial pressure regulation (Figure 1) (1, 4, 10, 11). These factors include a host of molecules such as the soluble VEGF receptor-1 (sFlt-1), the angiotensin II type-1 receptor autoantibody (AT1-AA), and cytokines such as TNF-α and Interleukin 6 which in turn generate widespread dysfunction of the maternal vascular endothelium (1–11). This dysfunction results in formation of factors such as endothelin, reactive oxygen species (ROS), and augmented vascular sensitivity to angiotensin II (1–11). In addition, preeclampsia is also associated with decreased formation of vasodilators such as nitric oxide and prostacyclin (1–11). These alterations in vascular function not only lead to hypertension but multi-organ dysfunction, especially in women with early onset preeclampsia (1,4, 11–14). Therefore, identifying the connection between placental ischemia and maternal cardiovascular abnormalities is an important area of investigation (1,10,11,21). In addition, the quantitative importance of the various endothelial and humoral factors that mediate vascular dysfunction and hypertension during preeclampsia remains to be elucidated.
Immune activation and cytokine production in preeclampsia
Preeclampsia has long been considered an immunologically based disease (16–19). During normal pregnancy TNF alpha promotes expression of adhesion molecules in maternal endothelial cells and activates phagocytic cells that are important mediators of morphological changes in the uterine arteries. Under normal conditions the cytotrophoblasts undergo endovascular invasion allowing their replacement of the endothelial and muscular linings of the uterine arterioles. As a result of this invasion, the spiral arteriolar vessel diameter increases allowing enhanced perfusion to meet the metabolic needs of the uteroplacental unit. By the end of the second trimester the cytotrophoblasts line the decidua and endothelial cells are no longer visible. However, during preeclampsia, cytotrophoblast invasion of the uterus is shallow and the endovascular invasion is incomplete thus inhibiting essential morphological changes of the maternal uterine vasculature from occurring (16,17). As a result the mean arterial diameter of the first third of myometrial vessels is less than half of that of vessels isolated from normal pregnant placentas. Furthermore, the endothelial lining of the maternal vasculature remains, allowing interactions with activated immune cells and proinflammatory cytokines to persist leading to characteristics of a chronic inflammatory condition.
Many inflammatory cells are activated in the circulation and infiltrate into renal and placental tissues. Macrophages, neutrophils and T Lymphocytes of the Th1 subset are the predominant cell type mediating the inflammatory cascade in women with preeclampsia (16–20). Furthermore, the cytokine profile of women with preeclampsia is consistent with a cell mediated immune response that utilizes neutrophils, macrophages and CD4+Th1 cells as a defense mechanism against microbial infections. As a result, elevated inflammatory cytokines and the oxidative burst of phagocytic cells persist resulting in vascular oxidative stress during preeclampsia (16–20)
Inflammatory cytokines
While several groups have suggested a potential role for inflammatory cytokines in the etiology of preeclampsia much remains unknown concerning factors stimulating the increase of these cytokines (20–22). Freeman and colleagues recently examined changes in inflammatory markers prospectively during pregnancy and the current inflammatory status of women who had a pregnancy complicated by preeclampsia 20 years previously against matched controls and found that preeclampsia was associated with short- and long-term changes in inflammatory status (22). While Armanini and Calo (23) suggested that aldosterone could play an important role in the genesis of this increased susceptibility of inflammatory process in preeclampsia, other factors such as obesity, diabetes, and placental ischemia could also be involved (24–26).
Preeclampsia is characterized by compromised vascular remodeling which results in decreased placental perfusion and creates a hypoxic environment for placental and fetal tissues. Under hypoxic conditions, placental explants from preeclamptic women exhibit a twofold increase in TNF alpha compared to explants from normal pregnant women (20). Preeclamptic women have a twofold elevation in placental and plasma TNF alpha protein levels (21). Circulating levels of other pro-inflammatory cytokines such as IL-8 and IL-6 are also significantly elevated in preeclamptic women. Interestingly, circulating IL-10, an anti-inflammatory cytokine, is decreased in these same women (18).
While inflammatory cytokines such as IL-6 and TNF-α have been reported by some laboratories to be elevated in preeclamptic women, it has been uncertain whether moderate and long-term increases in cytokines during pregnancy could result in elevations in blood pressure. We previously reported that chronic reductions in uterine perfusion pressure (RUPP) in the pregnant rat increases arterial pressure and impairs endothelial function (24,25). We have previously reported that RUPP in pregnant rats results in a chronic inflammatory response, characterized by elevated circulating TNF alpha and immune infiltrates in the placenta (24,25).Furthermore our laboratory has shown increased TNF alpha transcript in renal cortices and medulla of RUPP rats compared to normal pregnant rats. Moreover, recent studies from our laboratories indicate that chronic overexpression of TNF-α or IL-6 during pregnancy, at concentrations mimicking that observed in preeclamptic women, increases arterial pressure and decreases renal plasma flow and glomerular filtration rate (24,25,27). In addition we have demonstrated activation of various immune cells infiltrated into tissues in response to placental ischemia. These immune cells are a constant source allowing the persistence of these proinflammatory cytokines
T lymphocytes
Macrophages and dendritic cells are antigen presenting cells (APC) for activation of T helper (Th1) lymphocytes (28). Activation of Th1 cells proceeds through co-stimulatory pathways involving B7 receptors on the APC binding with CD28 on the T cell (28–33). Recent evidence implicates an imbalance between regulatory (Treg) and effector T cells in preeclamptic women (31). There is considerable evidence both in humans and in mice for the importance of Interleukin 17 in the development and progression of chronic inflammatory and autoimmune diseases. IL17-producing CD4(+) T cells (Th17 cells) are the dominant pathogenic cellular component in autoimmune inflammatory diseases, including autoimmune arthritis, psoriasis and multiple sclerosis (32). In contrast, Tregs(foxp3+Tcells) have anti-inflammatory properties and can cause quiescence of autoimmune diseases (32). In a recent study the frequencies of circulating Tregs(foxp3+CD4+Tcells), IFN-gamma, IL-10, or IL-17 at the end of the third trimester of healthy and preeclamptic pregnancies were compared (31). Th17 cells and regulatory T (Treg) cells were detected by flow cytometry. Preeclamptic women showed abnormal ratios of Foxp3(+) Treg to IL-17-expressing CD4(+) T cells (31). The percentage of CD4(+)IL-17-producing T cells were decreased significantly in normal pregnant women compared with preeclamptic pregnancies. Although preeclampsia is associated with increased IL-17 and decreased Tregs it is unknown if placental ischemia is a stimulus for the imbalance between Th17 vs Tregs or if there are other maternal or paternal factors leading to this dysregulation of immune factors.
B Lymphocytes
T cells divide and secrete interleukins and cytokines, such as TNF-α and IL-6, and assist other immune cells, such as B lymphocytes in their routine function including production of all forms of immunoglobulin (28,33,34). For full activation of B lymphocytes interaction with activated T lymphocyte via the CD4 receptor and several costimulatory molecules must occur. Two mechanisms whereby B lymphocytes are stimulated to produce antibody exist (28,32,33). The pathway of antibody production referred to as the T cell dependent pathway is the route for immunological memory that results in a second more robust antibody response to the same antigen. This pathway is mediated through macrophages, dendritic cells and T Lymphocytes of the T helper 1 lineage which secrete cytokines such as TNF-α, IL-1, IL-6 and IL-8, all of which are elevated in preeclamptic women. (20,21,24,25,27).
Angiotensin II type-1 receptor autoantibody, AT1-AA, have been shown to be produced by all women with reductions in placental blood flow examined thus far and production of the autoantibody is not terminated upon delivery of the fetus and placenta (35). It is unknown how long or to what extent these women continue to produce AT1-AA or if it plays a role in the development of cardiovascular disease which is prevalent later in life for peeclamptic women. Defining the pathway of AT1-AA production will aid in understanding it’s reoccurrence in preeclamptic women. Chronic overexpression of the AT1-AA via activated T cell dependent mechanisms could mediate degeneration in renal function leading to gradual increases in arterial pressure among preeclamptics.
Endothelial Dysfunction and Preeclampsia
The maternal vascular endothelium appears to be an important target of factors that are triggered in preeclampsia (1, 2, 4, 10, 11). Endothelium-derived relaxing and contracting factors play an important role in the regulation of vascular resistance and blood pressure. When abnormalities in the production or action of these factors occur, the vasculature is predisposed to vasoconstriction, leukocyte adherence, oxidation, and vascular inflammation (1, 2, 4). Markers of endothelial dysfunction are often elevated weeks prior to observance of clinical manifestations and therefore, may serve as predictors of the syndrome in women that develop preeclampsia (5–9).
Potential Mediators of Endothelial Dysfunction
Nitric Oxide (NO)
NO production is significantly elevated in normal pregnancy. Experimental studies also suggest that NO production plays an important role in the cardiovascular adaptations of pregnancy (11, 12, 36–40). Since NO is an important physiological vasodilator in normal pregnancy, NO deficiency during preeclampsia has been suggested to play a role in the disease process (36–43). While numerous studies indicate chronic NOS inhibition in pregnant rats produces hypertension associated with peripheral and renal vasoconstriction, proteinuria, intrauterine growth restriction, and increased fetal morbidity (41–44) it is unclear whether a NO deficiency occurs in women with preeclampsia. Much of the uncertainty in this area of research originates from the difficulty in directly assessing the activity of the NO system in the clinical setting. Recently, Noris et al (41) suggested that L-arginine depletion, caused by arginase II overexpression, may orient nitric oxide synthase toward oxidant species in the preeclamptic placenta. In addition, McCord and colleagues (40) reported that a relative deficiency of arginine in peripheral blood mononuclear cells may favor superoxide and peroxynitrite production and contribute to oxidative and nitrosative stress in preeclampsia. In a study by Conrad and colleagues (36), under conditions that were carefully monitored to reflect endogenous production and not dietary intake, there was no evidence for a decrease in NO production by measure of plasma or urinary excretion of nitrite and nitrate. Previous investigators have assessed the activity of the NO system in response to placental ischemia and cytokine excess in pregnant rats (42–45). Placental ischemia in pregnant rats has no effect on urinary nitrite/nitrate excretion relative to control pregnant rats, however, basal and stimulated release of nitric oxide from isolated vascular strips were significantly lower in the placental ischemic rat (42–44)
Oxidative Stress
In disease states of oxidative stress, an imbalance of pro-oxidant and anti-oxidant forces results in endothelial dysfunction, either by direct actions on the vasculature or through vasoactive mediators (45–50). During preeclampsia, oxidative stress may result from interactions between the maternal component which may include preexisting conditions such as obesity, diabetes, and hyperlipidemia, and/or the placental component which may involve secretion of lipid peroxides (45–50). Oxidative stress may mediate endothelial cell dysfunction and contribute to the pathophysiology of preeclampsia as indicated by the increased pro-oxidant activity along with decreased anti-oxidant protection in preeclampsia. A decrease in Superoxide dismutase (SOD) levels and reduced SOD activity has been reported in neutrophils and placentas of women with preeclampsia (45) illustrating an important link between the maternal and placental components.
In addition, several important anti-oxidants are significantly decreased in women with preeclampsia. Vitamin C, vitamin A, vitamin E, β-carotene, glutathione levels, and iron-binding capacity are lower in the maternal circulation of women with preeclampsia than women with a normal pregnancy (45–50). Due to low plasma vitamin C concentrations in preeclamptic women, investigators suggested that a combination of vitamins C and E may be a promising prophylactic strategy for prevention of preeclampsia (49,50). However, a recent multi-center clinical trial showed that antioxidant supplementation with vitamins C and E during pregnancy did not reduce the risk of intrauterine growth restriction, preeclampsia or death in nulliparous women (49). Furthermore, in one study supplementation with vitamin C and vitamin E increased the incidence of low birthweight babies thus indicating the use of high dose vitamin C and vitamin E does not appear to be justified during preeclampsia (50).
NAD(P)H oxidases are an important source of superoxide in neutrophils, vascular endothelial cells, and cytotrophoblast. Increased expression of NAD(P)H oxidase subunits have been reported in both trophoblast and placental vascular smooth muscle cells in placental tissue of women with preeclampsia (48). Moreover, higher placental NAD(P)H oxidase activity has been reported in women with early-onset preeclampsia as compared with those with late-onset of disease which is consistent with the concept that early-onset preeclampsia is more dependent on placental dysfunction than the later-onset disease (45–50).
Although there is evidence that oxidative stress occurs in preeclampsia, it remains unclear whether reduced placental perfusion serves as a stimulus for increased reactive oxygen species (ROS) or whether oxidative stress is a contributing factor in the reductions of uterine perfusion observed in preeclampsia. Furthermore, the importance of ROS in mediating the renal and cardiovascular abnormalities associated with placental ischemia is unknown. Our laboratory recently reported that chronic reductions in uterine perfusion pressure (RUPP) in pregnant rats results in cardiovascular and renal abnormalities similar to those found in preeclamptic women (24,25,51). We showed that placental ischemia results in the generation of oxidative stress in the placenta and a decrease in the innate antioxidant capacity in the plasma and the renal cortex in the pregnant rat. In addition, chronic treatment with tempol, a superoxide dismutase mimetic, attenuated the hypertension associated with placental ischemia in RUPP pregnant rats (51). Although the results from this study did not determine the potential role of oxidative stress to contribute to impaired placental perfusion during preeclampsia, the study demonstrated a clear role for placental ischemia as a cause of oxidative stress, which in turn contributes to the hypertension associated with RUPP.
Endothelin
Another endothelial-derived factor that may play a role in preeclampsia is the vasoconstrictor, endothelin-1 (ET-1). While some studies have reported no significant changes in circulating levels of ET-1 during moderate forms of preeclampsia, a possible role for ET-1 as a paracrine or autocrine agent in preeclampsia remains worthy of consideration (25,52,53,). Since ET-1 is released towards the vascular smooth muscle in a paracrine fashion, changes in plasma levels of ET may not reflect its local production. Indeed, this is one of the reasons why it has been difficult to ascertain whether preeclampsia is associated with altered ET production. Local synthesis of ET has been assessed in preeclamptic women and investigators have found preproendothelin mRNA to be elevated in a variety of tissues (52,53). Because of the limitations of clinical studies the importance of locally produced ET in the pathophysiology of preeclampsia remains unclear. Therefore, several animal studies assessing the role of ET-1 and the utility of selective ET type A receptor antagonists on hypertension in response to placental ischemia have been performed.
Alexander et al. examined the role of ET-1 in mediating the hypertension in placental ischemic RUPP rat model of preeclampsia (54). Renal expression of preproendothelin was significantly elevated in both the medulla and the cortex of pregnant RUPP rats with chronic reductions in uterine perfusion pressure compared with control pregnant rats. Chronic administration of the selective ETA receptor antagonist, ABT627 markedly attenuated the hypertension in pregnant RUPP rats. In contrast, ETA receptor blockade had no significant effect on blood pressure in the normal pregnant animal. These findings suggest that ET-1 plays a major role in mediating the hypertension produced by chronic reductions in uterine perfusion in pregnant rats.
In addition, sera from pregnant rats exposed to chronic RUPP increases ET-1 production by cultured endothelial cells (55). The exact mechanism linking enhanced renal production of ET-1 to placental ischemia in pregnant rats or in preeclamptic women is unknown. One potential mechanism for enhanced ET-1 production is via transcriptional regulation of the ET-1 gene by TNF-α. TNF-α is elevated in preeclamptic women and has been implicated in the disease processes (20–22).
Arachidonic acid metabolites
While significant alterations in the balance of prostacyclin and thromboxane production occur in women with preeclampsia, the importance of arachidonic acid metabolites in the pathophysiology of this disease has yet to be fully elucidated (11,12,56). Experimental studies in animals have attempted to determine the role of AA metabolites in response to placental ischemia. Increases in systemic arterial pressure produced by acute placental ischemia in pregnant dogs can be prevented by thromboxane receptor antagonism (57). While urinary excretion of thromboxane B2 is increased in response to placental ischemia in pregnant rats, acute administration of a thromboxane receptor antagonists failed to alter blood pressure (58). In contrast, inhibition of cytochrome P450 enzymes with 1-aminobenzotriazole (ABT) attenuated the hypertension, increased renal vascular resistance, 20-HETE formation and CYP4A expression in the renal cortex normally observed in the placental ischemic pregnant rat (59).
Angiogenic factors
Considerable clinical evidence has accumulated that preeclampsia is strongly linked to an imbalance between pro-angiogenic such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) and anti-angiogenic factors such as soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin (sEng) in the maternal circulation (60–68). Studies have shown that both plasma and amniotic fluid concentrations of sFlt-1 are increased in preeclamptic patients, as well as placental sFlt-1 mRNA (60–68).
Maynard et al. (63) reported several years ago that exogenous administration of sFlt-1 into pregnant rats via adenovirus mediated gene transfer resulted in increased arterial pressure and proteinuria, and decreased plasma free VEGF and PlGF concentrations similar to that observed in the preeclamptic patients. Subsequently, similar observations using adenovirus transfection have been reported in the mouse (64). Recently, Li and coworkers showed that VEGF infusion attenuates the increased blood pressure and renal damage observed in pregnant rats over-expressing sFlt-1 (65). Thus, this study suggests that sFlt-1 and alterations in angiogenic factors may contribute to the clinical symptoms observed in preeclampsia; however, these observations did not shed any light on the mechanisms whereby sFlt-1 over-expression occurs in preeclampsia. Other investigators have recently demonstrated that uteroplacental ischemia increased plasma and placental sFlt-1 and this is associated with decreased VEGF and PlGF in the late gestation pregnant rat (66). Similarly, Makris and colleagues have reported uteroplacental ischemia increases sFlt-1 in the baboon as well (67).
Investigators have shown placental ischemia to be a stimulus for sEndoglen as well. Endoglin is a component of the TGF-β receptor complex and is a hypoxia inducible protein associated with cellular proliferation and NO signaling. sEng is suggested to be anti-angiogenic as it is thought to impair TGF-β1 binding to cell surface receptors. Venkatesha et al. (68) have shown that sEng inhibits in vitro endothelial cell tube formation to a similar extent as sFlt-1. Further, the authors reported that adenoviral mediated increase of sFlt-1 and sEng in concert exacerbated the effects of either factor alone. The dual overexpression resulted in fetal growth restriction, severe hypertension and nephritic range proteinuria in pregnant rats (68). Thus, there is compelling experimental evidence that compliments clinical observations that sEng is an important factor in the pathogenesis of preeclampsia.
Renin-Angiotensin System (RAS) and AT1-AA
During normal pregnancy, plasma renin concentration, renin activity, and angiotensin II (Ang II) levels are all elevated, yet vascular responsiveness to ANG II appears to be reduced (57,58). In contrast, during preeclampsia there appears to be a marked increase in the sensitivity to Ang II (57, 58). Although the mechanisms underlying these observations remain unclear, there is growing evidence to suggest that dysregulation of the tissue-based and circulating RAS may be involved in the pathophysiology of preeclampsia (57–61).
Recent studies in preeclamptic women demonstrate increased circulating concentrations of an agonistic autoantibody to the angiotensin type 1 receptor (AT1-AA) (70–74). In addition to being elevated during preeclampsia, the AT1-AA has also been reported to be increased in postpartum women. Hubel and colleagues demonstrated that the AT1-AA does not regress completely after delivery and that the increase in AT1-AA correlated with insulin resistance and sFlt-1 (35). The importance of AT1-AA after preeclampsia, especially in the context of increased cardiovascular risk, remains to be determined.
Interestingly, the AT1-AA appear to be responsible for a variety of effects in several different tissues ranging from increased intracellular Ca++ mobilization to monocyte activation and stimulation of IL-6 production from mesangial cells (70–75). Another effect that has recently been attributed to the AT1 receptor is stimulation of sFlt-1 expression from trophoblast cells (70). Additional mechanisms whereby the AT1-AA mediates increases in blood pressure is by activating the ET-1 system as well as stimulating placental oxidative stress (76, 77)
While these findings potentially implicate AT1-AA as a central mediator of several pathways in preeclampsia, both the specific mechanisms that lead to excess production and the mechanisms whereby AT1-AA increases blood pressure during pregnancy remain unclear. Our laboratories recently provided evidence demonstrating that placental ischemia in pregnant rats is associated with increased circulating levels of the AT1-AA (77). While these findings indicate that reduced placental perfusion may be an important stimulus for AT1-AA production, the fact that AT1-AA are present in patients with pathological uterine artery Doppler independent of preeclampsia suggests that AT1-AA may not the primary cause of preeclampsia (76–78)
Role of cytokines in mediating blood pressure response to placental ischemia
While inflammatory cytokines such as IL-6 and TNF alpha have been reported to be elevated in preeclamptic women, the importance of these cytokines in mediating the cardiovascular and renal dysfunction in response to placental ischemia during pregnancy has yet to be fully elucidated (10,11,18,20,24,24,27). It is becoming increasingly evident that proinflammatory cytokines such as IL-6 and TNF alpha interact with important blood pressure regulatory systems such as the renin-angiotensin system (RAS), sympathetic nervous system, endothelial and angiogenic factors (24,24,27). We reported that serum levels of TNF alpha and IL-6 are elevated in RUPP rats and chronic infusion of TNF alpha or IL-6 into pregnant rats increases arterial pressure and decreases renal plasma flow and glomerular filtration rate (24,25,27). These findings indicate that TNF alpha and IL-6 may play a role in mediating the hypertension and impaired renal hemodynamics observed in response to placental ischemia in pregnant rats.
Interaction between endothelin and inflammatory cytokines
Endothelial damage is a known stimulus for endothelin (ET-1) synthesis, therefore, increases in the production of ET-1 and activation of ETA receptors may participate in the pathophysiology of hypertension during preeclampsia.. As stated previously the exact mechanism linking enhanced renal production of ET-1 to placental ischemia in pregnant rats or in preeclamptic women is unknown. Our laboratories recently examined the potential role for TNF alpha as a stimulus for increased ET-1 during pregnancy (25). Chronic infusion of TNF alpha in pregnant rats, at a rate to mimic plasma levels (2–3 fold increase) observed in women with preeclampsia significantly increased in blood pressure. The increase in arterial pressure produced by TNF alpha in pregnant rats is associated with significant increases in local production of ET-1 in the kidney, placenta, and vasculature. Moreover, the increase in mean arterial pressure in response to TNF alpha is completely abolished in pregnant rats treated with an ETA receptor antagonist (25). Subsequently, our laboratory examined the role of endogenous TNF alpha in mediating the increase in ET-1 and arterial pressure in response to placental ischemia in RUPP rats. The soluble TNF alpha receptor, Enbrel, was administered on day 18 of gestation to normal pregnant and to RUPP pregnant rats (81). Neither blood pressure nor circulating TNF alpha in normal pregnant rats was altered by administration of Enbrel (81). In contrast, MAP was significantly lower in RUPP rats when circulating TNF alpha levels were significantly decreased by administration of Enbrel. Again we demonstrated ET-1 transcript, via real time PCR, to be significantly elevated in cortices, medulla and placenta of RUPP rats as compared to normal pregnant rats. In this study we found ET-1 transcript lowered, albeit not significantly, in the cortex, medulla, and the placenta of RUPP rats treated with Enbrel thus supporting a role for TNF alpha stimulation of local ET-1 production. Furthermore, we demonstrated that ET-1 protein secretion from cultured endothelial cells exposed to serum from RUPP rats was significantly decreased with administration of Enbrel to the experimental culture media as well as administration Enbrel to RUPP rats in vivo. These results support a potential role of TNF-α to orchestrate endothelial activation or dysfunction either directly or by some intermediary pathway. Although these data provide support linking ET-1 production via TNF alpha stimulated pathways with hypertension in response to reductions in uterine perfusion pressure in pregnant rats, they also indicate the importance of additional factors in stimulating ET-1 as a mediator of hypertension during pregnancy.
Interaction between Angiogenic Factors and Inflammatory Cytokines
The balance between pro- and anti-angiogenic factors has recently become an area of interest in cancer, autoimmune and preeclamptic research. While there are numerous factors recognized as important for angiogenesis, the VEGF system has gained the most attention with respect to preeclampsia [60–67]. Recent evidence illustrates the importance of VEGF in the regulation and maintenance of blood pressure and renal function during various forms of hypertension and/or renal injury (82–84), including pregnancy induced hypertension (PIH) and preeclampsia [85,86]. In contrast to cytokine mediated hypertension during pregnancy, increased serum sFlt-1 produced hypertension in non-pregnant animals suggesting that the effects of sFlt-1 on the vasculature were direct and not restricted to pregnancy.
While there is increasing evidence to support the concept that elevated levels of sFlt-1 play an important role in the pathogenesis of preeclampsia, much less is known about mechanisms that result in its dysregulated expression. Nevo et al. recently suggested that hypoxia may be a primary stimulus for excess placental production of sFlt-1 based on evidence from pregnancies at high altitude pregnancies and from in vitro cytotrophoblast primary cultures exposed to hypoxia [87]. These data suggest that poor trophoblast invasion might lead to a hypoxic placenta and ultimately to pathogenic levels of sFlt-1.
Another factor that may contribute to altered regulation of angiogenic factors may be inflammatory cytokines. Cytokines have been shown to increase angiogenic factors and promote angiogenesis in chronic systemic diseases such as rheumatoid arthritis (RA) [88,89] and in cancer, the relationship between inflammation and dysregulation of angiogenic factors in pregnancy is unknown. The elevation of inflammatory mediators that occurs with preeclampsia may prevent VEGF-induced angiogenesis by stimulating sFlt-1. Girardi and colleagues stimulated monocytes with products of the complement cascade and found it to directly trigger sFlt-1. Subsequently the authors demonstrated that inhibition of complement activation blocked increases in sFlt-1 and rescued pregnancies in a mouse model of immunologically mediated perimplantation pregnancy loss [19]. In addition recent studies have found that the cytokine granulocyte-macrophage colony stimulating factor increases sFlt-1 expression in monocytes and inhibits angiogenesis in mice [89]. Monocyte derived sFlt-1 also inhibits endothelial cell migration and tube formation [89]. Collectively, these studies support a role for various immune mechanisms as contributors to pregnancy loss and fetal growth restriction. Further studies addressing the role of monocyte activation will be essential in determining their importance as mediators of angiogenic dysregulation and abnormal placental development during preeclampsia.
Most recently our laboratory has demonstrated sFlt-1 secretion from cultivated RUPP leukocytes to be significantly increased compared to leukocytes isolated from normal pregnant rats (90). In addition we have recently shown that chronic, moderate increases in TNF-α, stimulates the overexpression of sFlt-1 during pregnancy (91). Furthermore, placental explants collected from TNF-α induced hypertensive pregnant rats, secreted excess sFlt-1 implicating the placenta as one potential source of this anti-angiogenic factor. Finally, we have demonstrated that like TNF-α induced hypertension, TNF-α stimulated sFlt-1 can be attenuated by blockade of the angiotensin II type 1 receptor indicating a potential role of AT1-AA, in addition to inflammatory cytokines to stimulate sFlt-1 during pregnancy.
Interaction between the Renin-Angiotensin System and Cytokines
The renin angiotensin system (RAS) is one of the most powerful endocrine systems involved in the regulation of arterial pressure. In addition to its renal, vascular, and central effects, there is now evidence supporting a pro-inflammatory mechanism for Ang II in the progression of hypertension. Ang II not only enhances the synthesis of TNF alpha and IL-6 from immune cells but it also appears that IL-6 may play an important role in mediating the hypertension produced by angiotensin II in mice [10,11,69,72,73]. Whether cytokines directly activate the renin-angiotensin system is not yet clear.
Our recent data shows that IL-6 treated pregnant rats had significantly higher plasma renin activity when compared to control pregnant animals [27]. Although this effect of IL-6 on plasma renin activity could potentially lead to enhanced vasoconstriction, reduced sodium excretory function, and hypertension; the quantitative importance of the renin-angiotensin system in mediating the in vivo effects of IL-6 during pregnancy is unknown and remains to be an important area of investigation. Our laboratory has reported that in addition to increased PRA infusion of IL-6 stimulates production of AT1-AA in pregnant rats but not in virgin rats (92). Furthermore hypertension in response to IL-6 was attenuated with an AT1 receptor antagonist (92).
In addition, chronic elevation of TNF alpha in pregnant rats was also associated with increased production of the AT1-AA (77). Moreover, we found that the hypertension in response to placental ischemia in pregnant rats and in response to chronic infusion of TNF alpha in pregnant rats was markedly attenuated by antagonism of the AT1 receptor. Collectively, these novel findings indicate that placental ischemia and TNF-α and IL-6 are important stimuli of AT1-AA production during pregnancy and that activation of the AT1 receptor appears to play an important role in these forms of hypertension in pregnant rats.
Interaction between AT1-AA, ET-1, anti-angiogenic factors and oxidative stress
One mechanism whereby AT1-AA increase arterial pressure in pregnant rats is activation of the endothelin system (76). In this study we demonstrated that increasing levels of AT1-AA to levels observed in preeclamptic women and in placental ischemic rats, increases mean arterial pressure (MAP) in pregnant rats by activation of the endothelin system. We report that infusion of purified rat AT1-AA, isolated from serum collected from a pregnant transgenic rat cross overproducing components of the renin angiotensin system into pregnant rats from day 12 to day 19 of gestation, increased serum AT1-AA, blood pressure, and tissue levels of preproendothelin. Finally, we report that AT1-AA-induced hypertension in pregnant rats was attenuated by either oral administration of the AT1 receptor antagonist losartan or an ET type A receptor antagonist. In addition, the increase in endothelin transcript in response to AT1-AA induced hypertension was abolished by administration of an AT1 receptor antagonist.
Another mechanism whereby AT1-AA increases arterial pressure in pregnant rats is by stimulating placental oxidative stress (93,94). Previous work has shown that the AT1-AA stimulates ROS through NADPH oxidase in vitro (93,94). In addition, we have shown that chronic AT1-AA increase ROS in the placenta of pregnant rats (94). Furthermore we recently demonstrated a role for oxidative stress in the hypertension produced by placental ischemia in pregnant rats (51). We recently reported that malondialdehyde, MDA, superoxide, and myeloperoxidase activity, markers of oxidative stress, are increased nearly twofold in the placentas of RUPP rats compared to the NP controls, this is in agreement with what is observed in placentas from preeclamptic women. Finally, we have recently shown that tempol, an SOD mimetic acting to scavenge ROS, has beneficial effects in RUPP hypertension (51). To test the hypothesis that scavenging ROS would prevent the hypertension observed in the AT1-AA infused rats, we pretreated rats with tempol and found that it markedly attenuated the increase in arterial pressure and placental oxidative stress indicating the importance of AT1-AA induced ROS in mediating hypertension during pregnancy (94).
Previous studies by Xia and Kellems et al demonstrated AT1-AA from preeclamptic women induces sFlt-1 production via AT1R and calcineurin/nuclear factor of activated T-cells signaling (95). The authors demonstrated by injecting the IgG or affinity-purified AT1-AA from women into pregnant mice caused hypertension, proteinuria, glomerular endotheliosis, placental abnormalities, IUGR and elevated sFlt-1. The onset of these symptoms were prevented by AT1R antagonist or an AT1-AA neutralizing seven-amino-acid epitope binding peptide. Most recently, in agreement with the Xia laboratory, we have confirmed that AT1-AA infusion increased blood pressure and plasma and placental sFlt-1 in pregnant rats (91). Furthermore chronic AT1-AA infusion increased circulating sEng during pregnancy but had no effect on placental sEng indicating an additional cellular source for this anti-angiogenic factor.
While these studies suggest a potential interaction between AT1-AA and sFlt-1, a clear association between AT1-AA, sFlt-1 and the disease in women had never been fully established. Much uncertainty about this relationship was only heightened by recent clinical studies by Stepan et al., who found that while most preeclamptic patients expressed high sFlt-1 and the AT1-AA, in a population of patients characterized by reduced uterine perfusion and no other pregnancy complications, there was no association between the AT1-AA and sFlt-1(96,97). In these cases sFlt-1 was not elevated when AT1-AA was frequently present. Most recently, Xia and colleagues clearly demonstrate that the titer of AT1-AA not only correlate to the severity of the disease but that there was a strong correlation between AT1-AA activity to sFlt-1 in severe preeclamptics (95).
These findings concur with previous experimental studies demonstrating that AT1-AA induces features of preeclampsia, however, many unanswered questions still exist. While we have reported that placental ischemia and inflammatory cytokines are important stimuli for AT1-AA, the antigenic stimulus for AT1-AA production is still unknown. Moreover, the pathway of production of the AT1-AA, such as T cell dependent verses T cell independent, has yet to be elucidated. Furthermore, it is unclear how early in gestation the onset of AT1-AA production occurs. Studies inhibiting the production of the AT1-AA in pregnant animal models of preeclampsia are also necessary to advance our understanding of the pathophysiological role of the autoantibody during pregnancy. A better understanding of the pathophysiology of AT1-AA production in preeclampsia may lead to novel therapeutic targets for the treatment of the disease and /or a marker for predicting patient risk of developing preeclampsia.
The potential anti-inflammatory properties of progesterone supplementation for Preeclamptics
For preeclamptic patients the current treatment includes blood pressure control, seizure prophylaxis, and delivery of the fetus depending on gestational age and disease severity. There have been a host of agents suggested to be potential therapies for the prevention or treatment of preeclampsia, although none have proven effective. Recent studies have demonstrated administration of progesterone, specifically 17α-hydroxyprogesterone caproate (17 OHP) to be an effective agent for the prevention of recurrent preterm birth, however, literature on the role of progesterone and preeclampsia is sparse and conflicting. A recent review by Sammour et al. (2005) suggests that progesterone is a viable therapeutic agent for the treatment of preeclampsia. Interestingly, the exact mechanism whereby 17 OHP may improve vascular function and lower blood pressure is unclear, it is believed that its anti-inflammatory properties are responsible for the reduction in subsequent preterm delivery in multiparous women. Studies in humans elucidating the mechanism of action of progesterone and labor cite its potential role as an inhibitor of prostaglandins and inflammatory cytokines such as tumor necrosis factor-α (TNF-α). However, the potential use for progesterone for the treatment of hypertension in response to placental ischemia or inflammatory cytokines remains unknown.
In a recent study our laboratory demonstrated that 17 OHP blunted the hypertension in a model of placental ischemia in pregnant rats (99). We illustrated that in addition to having no deleterious outcome on pup weight, 17 OHP might exert anti-inflammatory effects to protect against endothelial cell activation and the release of the vasoconstrictor ET-1. Many studies have shown that inflammatory cytokines activate the endothelin system (25,81). In this study we demonstrated that administration of 17 OHP markedly decreased circulating TNF-α and IL-6 in response to placental ischemia. Furthermore, we demonstrated that administration of 17 OHP to RUPP rats is associated with decreased transcription of ET-1 in the renal cortices, thus suggesting one possible mechanism for the decrease in arterial pressure in RUPP rats receiving 17 OHP. However, administration of 17 OHP had no effect on ET-1 in placentas of RUPP rats (99).
Subsequently we designed a study to test the efficacy of progesterone as an anti-hypertensive treatment during TNF-α-induced hypertension mediated via activation of the ET-1 system (100). In this study we demonstrated that progesterone directly attenuates basal ET-1 production from endothelial cells in culture. Under control conditions, we found that TNF-α caused a dose-dependent increase in ET-1 production in human umbilical vein endothelial cells. The addition of progesterone attenuated these effects of TNF-α on ET-1 production. Thus, our in vitro findings indicate that progesterone has an important direct effect on endothelial cells to blunt TNF-α-stimulated ET-1 synthesis (100). In addition, we found that administration of 17-OHP to TNF-α-induced hypertensive pregnant rats decreased arterial pressure in response to TNF-α during pregnancy. As with our previous studies, we found that TNF-α-induced hypertension is associated with increased ET-1 transcript in both renal cortical and placental tissues. Administration of 17-OHP had no effect on placental ET-1 transcript but blunted the significant increase in ppET-1 in the renal cortices of TNF-α-induced hypertensive pregnant rats. Importantly, we again found no differences in pup weight of rats receiving 17-OHP compared to normal pregnant rats, indicating that the potential antihypertensive effect of 17-OHP was not deleterious to the fetus.
Although administration of 17 OHP effects renal ET-1 transcript and hypertension in response to placental ischemia, each of these studies illustrate the importance of other factors such as sFlt-1, AT1-AA, ROS, or decreased NO availability in the ischemic placenta that play a role in the pathophysiology of hypertension during pregnancy. Each of these factors could play a role in increased ET-1 levels in the placenta and may contribute to the remaining increase in blood pressure in the RUPP rats treated with 17 OHP. These factors were not evaluated in these studies, but could be the subject of further investigation into the role of 17 OHP as possible anti-hypertensive therapy in the setting of placental ischemia.
Conclusion
Preeclampsia is defined as new onset hypertension with protienuria during pregnancy. It is accompanied by widespread maternal vascular dysfunction and a chronic inflammatory response characterized by increase TNF alpha, IL-6, activated circulating immune cells and autoantibodies. Additionally, anti-angiogenic peptides are released inhibiting vascular remodeling essential for increased blood flow to the growing uteroplacental unit. Although these factors accompany the clinical syndrome of preeclampsia it is suggested that they are secondary to the maternal decrease in placental blood flow. Albeit, experimental evidence by ours and many other investigators have demonstrated the importance of these soluble factors to increase blood pressure and stimulate the production of such anti-angiogenic factors thereby eliciting a vicious cycle existing within the maternal vasculature as well as within the placental unit. Further investigation into the genesis of decreased uteroplacental perfusion and the stimulus of these placental factors is needed to further our understanding and therapeutic advancement for women and children affected by this devastating disease.
Figure 1. Potential role for immune activation in mediating the pathophysiology of hypertension during preeclampsia.
Immunomodulators stimulated in response to placental ischemia play an important role in the generation of ROS, production of sFlt-1 and enhanced ET-1 and ANG II sensitivity thereby contributing to the development of hypertension during pregnancy
Acknowledgments
This work was supported by NIH grant HL51971 78147 and AHA 0835472N.
REFERENCES
- 1.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- 2.Thadhani RI, Johnson RJ, Karumanchi SA. Hypertension During Pregnancy: A Disorder Begging for Pathophysiological Support. Hypertension. 2005;46:1250–1251. doi: 10.1161/01.HYP.0000188701.24418.64. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Gammill H. Insulin Resistance in Preeclampsia. Hypertension. 2006;47:341–342. doi: 10.1161/01.HYP.0000205123.40068.84. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JM, Gammill HS. Preeclampsia: Recent Insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 5.Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, Price K, Karumanchi SA, Valdes G. Endothelial Dysfunction: A Link Among Preeclampsia, Recurrent Pregnancy Loss, and Future Cardiovascular Events? Hypertension. 2007;49:90–95. doi: 10.1161/01.HYP.0000251522.18094.d4. [DOI] [PubMed] [Google Scholar]
- 6.Blaauw J, Graaff R, van Pampus MG, van Doormaal JJ, Smit AJ, Rakhorst G, Aarnoudse JG, Khan F, Belch JJF, Macleod M, Mires G. Changes in Endothelial Function Precede the Clinical Disease in Women in Whom Preeclampsia Develops in Response: Endothelial Function and Preeclampsia. Hypertension. 2006;47:e14–e15. doi: 10.1161/01.HYP.0000201448.69581.e2. [DOI] [PubMed] [Google Scholar]
- 7.Khan F, Belch JJF, Macleod M, Mires G. Changes in Endothelial Function Precede the Clinical Disease in Women in Whom Preeclampsia Develops. Hypertension. 2005;46:1123–1128. doi: 10.1161/01.HYP.0000186328.90667.95. [DOI] [PubMed] [Google Scholar]
- 8.Myers J, Mires G, Macleod M, Baker P. In Preeclampsia, the Circulating Factors Capable of Altering In Vitro Endothelial Function Precede Clinical Disease. Hypertension. 2005;45:258–263. doi: 10.1161/01.HYP.0000153461.58298.a4. 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of Maternal Endothelial Dysfunction with Preeclampsia. JAMA: The Journal of the American Medical Association. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 10.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia: Linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38(3 Pt 2):718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 11.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JM, Von Versen-Hoeynck F. Maternal Fetal/Placental Interactions and Abnormal Pregnancy Outcomes. Hypertension. 2007;49:15–16. doi: 10.1161/01.HYP.0000251523.44824.02. [DOI] [PubMed] [Google Scholar]
- 13.Sabour S, Franx A, Rutten A, Grobbee DE, Prokop M, Bartelink ML, van der Schouw YT, Bots ML. High Blood Pressure in Pregnancy and Coronary Calcification. Hypertension. 2007;49:813–817. doi: 10.1161/01.HYP.0000258595.09320.eb. [DOI] [PubMed] [Google Scholar]
- 14.Mello G, Parretti E, Marozio L, Pizzi C, Lojacono A, Frusca T, Facchinetti F, Benedetto C. Thrombophilia Is Significantly Associated With Severe Preeclampsia: Results of a Large-Scale, Case-Controlled Study. Hypertension. 2005b;46:1270–1274. doi: 10.1161/01.HYP.0000188979.74172.4d. [DOI] [PubMed] [Google Scholar]
- 15.Irgens HU, Reisater L, Irgens LM, Lie RT, Roberts JM. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study Pre-eclampsia and cardiovascular disease later in life: who is at risk? BMJ. 2001;323:1213–1217. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poston L. Endothelial Dysfunction in Preeclampsia. Pharmacological Reports. 2006;(58):69–74. [PubMed] [Google Scholar]
- 17.McMaster MT, Ahou Y, Fisher SJ. Abnormal Placentation and the Syndrome of Preeclampsia. Semin Nephrol. 2004;24(6):540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Satyam A, Sharma JB. Leptin, IL-10, and Inflammatory Markers (TNF-alpha, IL-6 and IL-8) in Preeclamptic, Normotensive Pregnant and Healthy Non-Pregnant Women. Am J Reprod. Immunol. 2007 Jul;58(1):21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 19.Girardi G, Yarilin D, Thurman JM, et al. Complement Activation Induces Dysregulation of Angiogenic Factors and Causes Fetal Rejection and Growth Restriction. JEM. 2006 Sept.203(9):2164–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- 22.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, Walker ID, Sattar N, Greer IA. Short- and Long-Term Changes in Plasma Inflammatory Markers Associated With Preeclampsia. Hypertension. 2004;44:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 23.Armanini D, Calo LA. Aldosterone, Inflammation, and Preeclampsia. Hypertension. 2005;45:e10. doi: 10.1161/01.HYP.0000157170.94539.ee. [DOI] [PubMed] [Google Scholar]
- 24.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension Produced by Reductions in Uterine Perfusion in the Pregnant Rat: Role of Tumor Necrosis Factor-{alpha} Hypertension. 2005a;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 25.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of Endothelin in Mediating Tumor Necrosis Factor-Induced Hypertension in Pregnant Rats. Hypertension. 2005b;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 26.Leik C, Walsh SW. Neutrophils Infiltrate Resistance-Sized Vessels of Subcutaneous Fat in Women With Preeclampsia. Hypertension. 2004;44:72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 27.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension Produced by Reductions in Uterine Perfusion in the Pregnant Rat: Role of Interleukin 6. Hypertension. 2006;48:711–716. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 28.Abbus A. In: Cellular and Molecular Immunology. 5. Lichtman A, editor. Philadelphia, Pennsylvania: Elsevier; [Google Scholar]
- 29.Wulff H, Pennington M. Targeting effector memory T-cells with Kv1.3 blockers. Curr Opin Drug Discov Devel. 2007 Jul;10(4):438–445. [PubMed] [Google Scholar]
- 30.Rutella S. Granulocyte colony-stimulating factor for the induction of T-cell tolerance. Transplantation. 2007 Jul 15;84(1) Suppl:S26–S30. doi: 10.1097/01.tp.0000269611.66517.bf. [DOI] [PubMed] [Google Scholar]
- 31.Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, Nanan R. Systemic Increase in the Ratio between Foxp3+ and IL-17-Producing CD4+ T Cells in Healthy Pregnancy but Not in Preeclampsia. J Immunol. 2009 Dec 1;183(11):7023–7030. doi: 10.4049/jimmunol.0901154. [DOI] [PubMed] [Google Scholar]
- 32.Heo YJ, Joo YB, Oh HJ, Park MK, Heo YM, Cho ML, Kwok SK, Ju JH, Park KS, Cho SG, Park SH, Kim HY, Min JK. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4(+) T cell population of rheumatoid arthritis patients. Immunol Lett. 2009 Nov 4; doi: 10.1016/j.imlet.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Hasegawa M, Fujimoto M, Takehara K, Sato S. Pathogenesis of systemic sclerosis: altered B cell function is the key linking systemic autoimmunity and tissue fibrosis. J Dermatol Sci. 2005 Jul;39(1):1–7. doi: 10.1016/j.jdermsci.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Panayi GS. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology (Oxford) 2005 May;44(Suppl 2):ii3–ii7. doi: 10.1093/rheumatology/keh616. [DOI] [PubMed] [Google Scholar]
- 35.Hubel CA, Wallukat G, Wolf M, Herse F, Rajakumar A, Roberts JM, Markovic N, Thadhani R, Luft FC, Dechend R. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension. 2007 Mar;49(3):612–617. doi: 10.1161/01.HYP.0000256565.20983.d4. [DOI] [PubMed] [Google Scholar]
- 36.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NO(x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO(x) diet. Am J Physiol. 1999;277:F48–F57. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 37.Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 39.Serrano NC, Casas JP, Diaz LA, Paez C, Mesa CM, Cifuentes R, Monterrosa A, Bautista A, Hawe E, Hingorani AD, Vallance P, Lopez-Jaramillo P. Endothelial NO Synthase Genotype and Risk of Preeclampsia: A Multicenter Case-Control Study. Hypertension. 2004;44:702–707. doi: 10.1161/01.HYP.0000143483.66701.ec. [DOI] [PubMed] [Google Scholar]
- 40.McCord N, Ayuk P, McMahon M, Boyd RCA, Sargent I, Redman C. System y+ Arginine Transport and NO Production in Peripheral Blood Mononuclear Cells in Pregnancy and Preeclampsia. Hypertension. 2006;47:109–115. doi: 10.1161/01.HYP.0000197952.22711.c4. [DOI] [PubMed] [Google Scholar]
- 41.Noris M, Todeschini M, Cassis P, Pasta F, Cappellini A, Bonazzola S, Macconi D, Maucci R, Porrati F, Benigni A, Picciolo C, Remuzzi G. L-Arginine Depletion in Preeclampsia Orients Nitric Oxide Synthase Toward Oxidant Species. Hypertension. 2004;43:614–622. doi: 10.1161/01.HYP.0000116220.39793.c9. [DOI] [PubMed] [Google Scholar]
- 42.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 43.Alexander BT, Llinas MT, Kruckeberg WC, Granger JP. L-arginine attenuates hypertension in pregnant rats with reduced uterine perfusion pressure. Hypertension. 2004;43:832–836. doi: 10.1161/01.HYP.0000119192.32360.a9. [DOI] [PubMed] [Google Scholar]
- 44.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35:367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- 45.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 46.Tsukimori K, Fukushima K, Tsushima A, Nakano H. Generation of Reactive Oxygen Species by Neutrophils and Endothelial Cell Injury in Normal and Preeclamptic Pregnancies. Hypertension. 2005;46:696–700. doi: 10.1161/01.HYP.0000184197.11226.71. [DOI] [PubMed] [Google Scholar]
- 47.Tsukimori K, Nakano H, Wake N. Difference in Neutrophil Superoxide Generation During Pregnancy Between Preeclampsia and Essential Hypertension. Hypertension. 2007;49:1436–1441. doi: 10.1161/HYPERTENSIONAHA.106.086751. [DOI] [PubMed] [Google Scholar]
- 48.Raijmakers MTM, Dechend R, Poston L. Oxidative Stress and Preeclampsia: Rationale for Antioxidant Clinical Trials. Hypertension. 2004;44:374–380. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 49.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS ACTS Study Group. Vitamins C and E and the Risks of Preeclampsia and Perinatal Complications. The New England Journal of Medicine. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 50.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 51.Sedeek M, Gilbert JS, LaMarca B, Shollok M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71:1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 53.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol. 1991;165:724–727. doi: 10.1016/0002-9378(91)90317-k. [DOI] [PubMed] [Google Scholar]
- 54.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 55.Roberts L, LaMarca B, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced Endothelin Synthesis by Endothelial Cells Exposed to Sera From Pregnant Rats With Decreased Uterine Perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 56.Walsh SW. Eicosanoids in preeclampsia. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2004;70:223–232. doi: 10.1016/j.plefa.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Woods LL. Importance of prostaglandins in hypertension during reduced uteroplacental perfusion pressure. Am J Physiol. 1989;257:R1558–R1561. doi: 10.1152/ajpregu.1989.257.6.R1558. [DOI] [PubMed] [Google Scholar]
- 58.Llinas MT, Alexander BT, Seedek M, Abram SR, Crell A, Granger JP. Enhanced thromboxane synthesis during chronic reductions in uterine perfusion pressure in pregnant rats. Am J Hypertens. 2002;15:793–797. doi: 10.1016/s0895-7061(02)02975-8. [DOI] [PubMed] [Google Scholar]
- 59.Llinas MT, Alexander BT, Capparelli MF, Carroll MA, Granger JP. Cytochrome P-450 Inhibition Attenuates Hypertension Induced by Reductions in Uterine Perfusion Pressure in Pregnant Rats. Hypertension. 2004;43:623–628. doi: 10.1161/01.HYP.0000117721.83371.9f. [DOI] [PubMed] [Google Scholar]
- 60.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential Changes in Antiangiogenic Factors in Early Pregnancy and Risk of Developing Preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 62.Lindheimer MD, Romero R. Emerging Roles of Antiangiogenic and Angiogenic Proteins in Pathogenesis and Prediction of Preeclampsia. Hypertension. 2007;50:35–36. doi: 10.1161/HYPERTENSIONAHA.107.089045. [DOI] [PubMed] [Google Scholar]
- 63.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GDV, Saade GR. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396. doi: 10.1016/j.ajog.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 65.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O'Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS. Recombinant Vascular Endothelial Growth Factor 121 Attenuates Hypertension and Improves Kidney Damage in a Rat Model of Preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert JS, Babcock SA, Granger JP. Hypertension Produced by Reduced Uterine Perfusion in Pregnant Rats Is Associated With Increased Soluble Fms-Like Tyrosine Kinase-1 Expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]
- 67.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–984. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 68.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 69.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol. 2005;288:F614–F625. doi: 10.1152/ajprenal.00410.2003. [DOI] [PubMed] [Google Scholar]
- 70.Xia Y, Ramin SM, Kellems RE. Potential Roles of Angiotensin Receptor-Activating Autoantibody in the Pathophysiology of Preeclampsia. Hypertension. 2007;50:269–275. doi: 10.1161/HYPERTENSIONAHA.107.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, Hering L, Muller DN, Luft FC, Staff AC. Dysregulation of the Circulating and Tissue-Based Renin-Angiotensin System in Preeclampsia. Hypertension. 2007;49:604–611. doi: 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- 72.Levesque S, Moutquin JM, Lindsay C, Roy MC, Rousseau F. Implication of an AGT Haplotype in a Multigene Association Study With Pregnancy Hypertension. Hypertension. 2004;43:71–78. doi: 10.1161/01.HYP.0000104525.76016.77. [DOI] [PubMed] [Google Scholar]
- 73.Mello G, Parretti E, Fatini C, Riviello C, Gensini F, Marchionni M, Scarselli GF, Gensini GF, Abbate R. Low-Molecular-Weight Heparin Lowers the Recurrence Rate of Preeclampsia and Restores the Physiological Vascular Changes in Angiotensin-Converting Enzyme DD Women. Hypertension. 2005a;45:86–91. doi: 10.1161/01.HYP.0000149950.05182.a3. [DOI] [PubMed] [Google Scholar]
- 74.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic Autoantibodies to the AT1 Receptor in a Transgenic Rat Model of Preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 76.Babbette LaMarca, Parrish Marc, Ray Lillian Fournier, Murphy Sydney R, Roberts Lyndsay, Gerd Wallukat Porter Glover, Wenzel Katrin, Cockrell Kathy, Martin James N, Jr, Michael J, Ryan, Dechend Ralf. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: Role of endothelin-1. Hypertension. 2009;54(4):905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.LaMarca BB, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Elevated agonistic autoantibodies to the angiotensin type 1 (AT1-AA) receptor in response to placental ischemia and TNF alpha in pregnant rats. Hypertension. 2008;52:7–11. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II Type 1 Receptor Agonistic Antibodies Reflect Fundamental Alterations in the Uteroplacental Vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 79.Giovanni Gadonski B, Lamarca Babbette D, Sullivan Elizabeth, et al. Hypertension produced by reductions in uterine perfusion in the pregnant rat: Role of Interleukin-6. Hypertension. Oct;48(4):711. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 80.Granger JP, LaMarca BBD, Cockrell K, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 81.LaMarca BB, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: Effect of TNF alpha blockade. Hypertension. 2008;52:1–5. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu A, Masuda Y, Mori T, et al. Vascular Endothelial Growth Factor165 Resolves Glomerular Inflammation and Accelerates Glomerular Capillary Repair in Rat Anti-Glomerular Basement Membrane Glomerulonephritis. Journal of the American Society of Nephrology. 2004;15:2655–2665. doi: 10.1097/01.ASN.0000141038.28733.F2. [DOI] [PubMed] [Google Scholar]
- 83.Miyamoto K, Kitamoto Y, Tokunaga H, et al. Protective effect of vascular endothelial growth factor/vascular permeability factor 165 and 121 on glomerular endothelial cell injury in the rat. Lab Invest. 2004;84:1126–1136. doi: 10.1038/labinvest.3700134. [DOI] [PubMed] [Google Scholar]
- 84.Hara A, Wada T, Furuichi K, et al. Blockade of VEGF accelerates proteinuria, via decrease in nephrin expression in rat crescentic glomerulonephritis. Kidney Int. 2006;69:1986–1995. doi: 10.1038/sj.ki.5000439. [DOI] [PubMed] [Google Scholar]
- 85.Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. Journal of Maternal-Fetal and Neonatal Medicine. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 86.Krysiak O, Bretschneider A, Zhong E, et al. Soluble Vascular Endothelial Growth Factor Receptor-1 (sFLT-1) Mediates Downregulation of FLT-1 and Prevents Activated Neutrophils From Women With Preeclampsia From Additional Migration by VEGF. Circulation Research. 2005;97:1253–1261. doi: 10.1161/01.RES.0000194324.29363.82. [DOI] [PubMed] [Google Scholar]
- 87.Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–R1093. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maruotti N, Canatore FP, Crivellato E, et al. Angiogenesis in Rheumatoid Arthritis. Histol Histopathol. 2006;(21):667–566. doi: 10.14670/HH-21.557. [DOI] [PubMed] [Google Scholar]
- 89.Eubank TD, Roberts R, Galloway M, et al. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity. 2004 Dec;21(6):831–842. doi: 10.1016/j.immuni.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tam Tam K, Wallace K, Rutland S, Mott K, Weimer A, Martin JN, Jr, LaMarca B. PLACENTAL ISCHEMIA TRIGGERS IMMUNE ACTIVATION AS LEUKOCYTE OVERPRODUCTION OF SFLT-1: A STEP IN THE PATHOGENESIS OF PREECLAMPSIA?. Presented at the 30th Annual Meeting: The Pregnancy Meeting Society of Maternal Fetal Medicine; February. 2, 2010; Chicago IL. [Google Scholar]
- 91.Parrish M, Murphy SR, Keiser S, Fournier L, Dechend R, Martin JN, Granger JP, LaMarca B. Soluble fms-like tyrosine-1 (sFlt-1) production is enhanced during hypertension in response to agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA) and Tumor Necrosis Factor- alpha (TNF-A) during pregnancy. Presented at the Central Association of Obstetrics and Gynecology meeting; Oct 26–28; Maui, Hawaii. [Google Scholar]
- 92.Speed J, Fournier L, Cockrell K, Dechend R, Granger J, LaMarca B. IL-6 induced hypertension in pregnant rats is associated with production agonistic autoantibodies to the angiotensin II type I receptor. Presented at Experimental Biology; April 5 2009; New Orleans, LA. [Google Scholar]
- 93.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 94.Parrish M, Fournier L, Arany M, Weimer A, Cockrell K, Dechend R, Martin JN, Jr, LaMarca B. Hypertension in response to agonistic autoantibodies to the angiotensin II type I receptor (AT1-AA): role of reactive oxygen species (ROS). Presented at the 30th Annual Meeting: The Pregnancy Meeting Society of Maternal Fetal Medicine; February. 2 2010; Chicago IL. [Google Scholar]
- 95.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin Receptor Agonistic Autoantibody Is Highly Prevalent in Preeclampsia. Correlation With Disease Severity. Hypertension. 2009 Dec 7; doi: 10.1161/HYPERTENSIONAHA.109.140061. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stepan H, Walther T. Questionable Role of the Angiotensin II Receptor Subtype 1 Autoantibody in the Pathogenesis of Preeclampsia. Hypertension. 2007;50:e3. doi: 10.1161/HYPERTENSIONAHA.107.091421. [DOI] [PubMed] [Google Scholar]
- 97.Stepan H, Faber R, Wessel N, Wallukat G, Schultheiss HP, Walther T. Relation between Circulating Angiotensin II Type 1 Receptor Agonistic Autoantibodies and Soluble fms-Like Tyrosine Kinase 1 in the Pathogenesis of Preeclampsia. J Clin Endocrinol Metab. 2006;91:2424–2427. doi: 10.1210/jc.2005-2698. [DOI] [PubMed] [Google Scholar]
- 98.Sammour MB, El-Kabarity H, Fawzy MM, Schindler AE. Prevention and treatment of pregnancy-induced hypertension (preeclampsia) with progestogens. J Steroid Biochem Mol Biol. 2005;97(5):439–440. doi: 10.1016/j.jsbmb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 99.Veillon Edward W, Jr. MD, Keiser Sharon D, MD, Parrish Marc R, MD, Bennett William, PhD, Cockrell Kathy, Fournier Lillian, Granger Joey P, PhD, Martin James N, Jr. MD, Lamarca Babbette., PhD 17 OHP blunts the hypertensive response associated with Reductions in Uterine Perfusion Pressure in pregnant rats. Journal of Obstetrics and Gynecology. 2009;201:324–329. doi: 10.1016/j.ajog.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keiser Sharon D, MD, Veillon Edward W, MD, Parrish Marc R, DO, Bennett William, PhD, Cockrell Kathy, Fournier Lillian, Granger Joey P, PhD, Martin James N, Jr, MD, Lamarca Babbette., PhD Effects of 17-hydroxyprogesterone on tumor necrosis factor-alpha-induced hypertension during pregnancy. Am J Hyp. 2009 doi: 10.1038/ajh.2009.149. Epub 19704104. [DOI] [PMC free article] [PubMed] [Google Scholar]