Abstract
Purpose
To test whether angiotensin-converting enzyme inhibitor use is associated with decreased risk of community-acquired pneumonia in older adults.
Methods
We analyzed data from a nested case-control study of community-dwelling, immunocompetent adults aged 65–94 within an integrated healthcare delivery system. Cases of ambulatory and hospitalized pneumonia from 2000–2003 were identified from International Classification of Disease, version 9, codes and validated using medical record review. Controls were matched to cases by age, sex and calendar year. Using health plan pharmacy data, we defined current use as filling ≥ 2 prescriptions during the 180 days prior to the case's diagnosis date. We calculated standardized doses per day using World Health Organization defined daily doses. Multivariable conditional logistic regression estimated adjusted odds ratios (ORs) for pneumonia in relation to angiotensin-converting enzyme inhibitor use, adjusting for comorbidity, functional and cognitive status, and other covariates from medical record review and pharmacy data.
Results
Current use of angiotensin-converting enzyme inhibitors was seen in 23% (242/1039) of cases and 21% (433/2022) of controls. Lisinopril accounted for 95% of prescriptions. The OR for pneumonia comparing current use to no current use was 0.99 (95% confidence interval [CI] 0.83–1.19). The OR for use of more than 2 standardized daily doses per day was 1.39 (95% CI 0.93–2.06) compared to no current use.
Conclusions
Angiotensin-converting enzyme inhibitor use is not associated with reduced pneumonia risk in community-dwelling older adults.
Keywords: pneumonia, angiotensin-converting enzyme inhibitors, case-control studies, antihypertensive agents
INTRODUCTION
Pneumonia is common and can have devastating consequences in older adults. With influenza, it is the 8th leading cause of death in the US.1 Apart from smoking, few modifiable risk factors have been identified, and there are no interventions consistently shown to decrease risk. A low-cost, safe intervention to reduce pneumonia risk would be highly desirable.
Pneumonia is often caused by clinically silent aspiration of oropharyngeal contents. Angiotensin-converting enzyme (ACE) inhibitors could decrease pneumonia risk through their main mechanism of action. By blocking angiotensin-I-converting enzyme, ACE inhibitors increase levels of substance P and bradykinins in the respiratory tract. These substances stimulate the cough reflex2 and thus could decrease aspiration. Estimates for the prevalence of cough in ACE inhibitor users range from 5 to 20%.2 A meta-analysis of randomized trials reported an estimate of 10.6%.3 It is not known whether ACE inhibitors lower the cough threshold in all users, with only some becoming symptomatic, or only in a susceptible subgroup. If the former, the potential for ACE inhibitors to prevent pneumonia would be considerably greater. In a small study of normal volunteers, captopril shifted the dose-response curve for cough in response to inhaled capsaicin, an irritant.4 Overall, the impact of ACE inhibitors on the cough threshold in the general population of users is not well understood.
Because of the mechanisms described above, it has been hypothesized that ACE inhibitors could prevent pneumonia. One randomized trial5 and seven observational studies6–12 have examined this question with conflicting results. Results from the randomized trial showed 20% lower pneumonia risk in the ACE inhibitor group compared to placebo, but this difference was not statistically significant. Thus, this study could not definitively answer the question of whether ACE inhibitor use prevents pneumonia.
In observational studies, van de Garde et al. found an association with decreased risk in people with diabetes7 but not in other people.6 In a younger population, Mukamal et al. observed a slightly higher pneumonia risk among people using lipophilic ACE inhibitors (e.g. ramipril, quinapril, or fosinopril; OR 1.15, 95% CI 1.03–1.28) compared to nonusers but no altered risk in those using hydrophilic ACE inhibitors such as lisinopril.9 Most studies analyzed administrative data without validating pneumonia outcomes or confounders. Such data provide imperfect measures of pneumonia13,14 and of confounders such as chronic heart and lung disease.15–17 The resulting biases could obscure a true association or, due to residual confounding, produce a spurious finding even if no true association exists.
We examined the relationship between ACE inhibitor use and pneumonia using data from a prior case-control study18 in which pneumonia diagnoses were validated and confounders were ascertained through medical record review.
METHODS
Source population
These analyses used data from a nested case-control study conducted at Group Health (GH), an integrated healthcare delivery system in Washington State, USA. The study’s primary aim was to examine risk of community-acquired pneumonia in relation to receipt of influenza vaccine.18 We relied on the original study for its population and measures of outcomes and confounders and supplemented these with computerized pharmacy data about ACE inhibitor dispensings. This research was approved by the GH Human Subjects Review Committee with a waiver of consent.
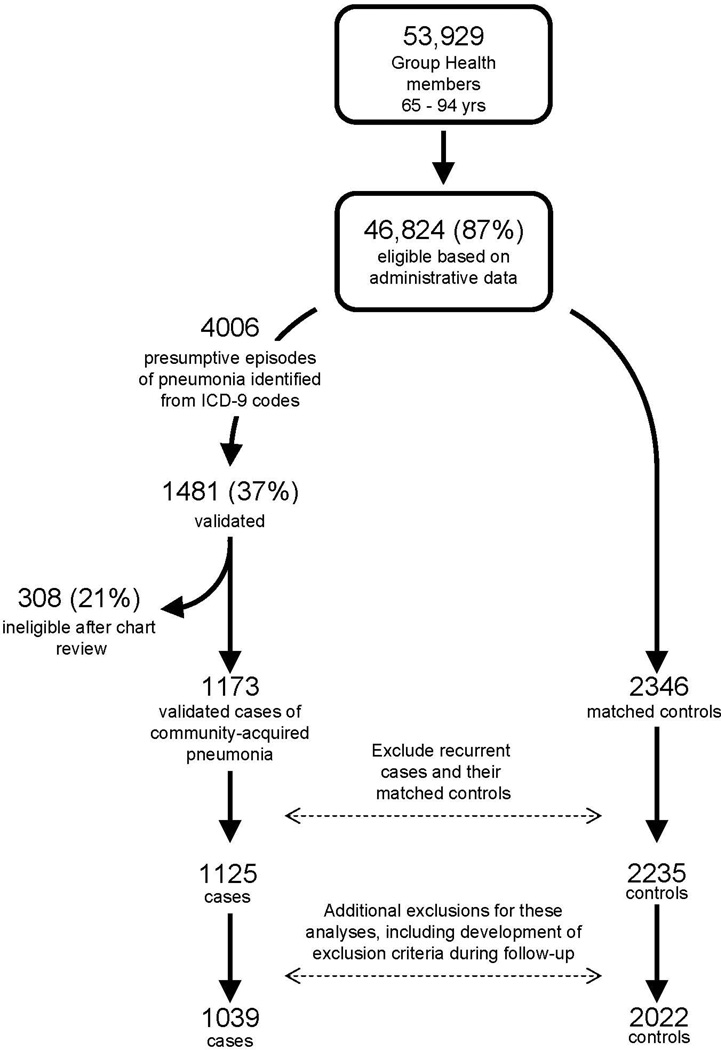
The study population included non-immunosuppressed, community-dwelling GH members age 65–94 with at least 2 years of continuous enrollment. Eligibility was determined from computerized pharmacy, laboratory, and utilization data and confirmed by medical record review. We excluded subjects who were immunosuppressed, defined as having serious cancer, recent cancer treatment, or severe chronic kidney disease, or receiving certain immunosuppressive medications or medications for human immunodeficiency virus (see Online Appendix Table 1). The original study focused on influenza vaccination, which typically occurs in the autumn, and thus study eligibility was initially determined as of September 1 in each study year (2000, 2001 and 2002). In the current study we sought to examine ACE inhibitor use immediately before pneumonia onset, which could have occurred months later. Health conditions developing after September 1 but before pneumonia onset could have influenced the decision to prescribe ACE inhibitors and risk of pneumonia. To address this potential source of confounding, we extended the time period during which eligibility was assessed, excluding subjects who developed certain conditions during follow-up (see Online Appendix Table 1). Figure 1 shows the flow of subjects through the study.
Figure 1.
Selection of cases and controls. For these analyses, we excluded additional subjects from the original study (86 cases and 213 controls) for the following reasons: 61 cases and 17 controls were found to have developed an exclusion criterion during follow-up (after September 1 of their study year). Additional people had used an immunosuppressive medication during baseline (23 cases, 29 controls). Two cases were discovered on further review to have been hospital-acquired. The other 167 controls were dropped because their matched case was excluded for one of the above reasons.
Selection of cases and controls
The methods for validating pneumonia diagnoses have been described previously.19 Briefly, we identified presumptive cases using International Classification of Disease, version 9 (ICD-9) codes from inpatient and outpatient encounters and validated them through medical record review. For many people, a chest radiograph report was available. These people were judged to have pneumonia if the report described a parenchymal infiltrate not known to be chronic. Ambulatory cases with no radiograph report available were considered not validated and excluded from the study. For hospitalized cases with no radiograph report (primarily those hospitalized outside GH), we reviewed hospital records. These cases were judged to have community-acquired pneumonia if the final physician assessment was that pneumonia caused the illness present at admission. Cases of hospital-acquired pneumonia and those due to massive aspiration (e.g. related to a seizure, stroke, or near-drowning) were excluded. We distinguished hospital-acquired from community-acquired pneumonia based on medical record review, specifically, whether the treating clinician indicated that pneumonia was present at admission (community-acquired) or developed during the hospitalization (hospital-acquired.)
Cases treated in an outside emergency department were considered valid, by default, because we found that these records were scant and our standard criteria could not be applied. In our final sample, 86% of cases were validated based on chest radiograph and 11% based on other medical records, and the remaining 3% (36 cases) were those considered valid by default. The date of pneumonia diagnosis was defined as the date of the first positive chest radiograph, hospital admission, or emergency department visit, respectively.
Because of the aims of the original study, cases were identified from September 1 until the end of influenza season in each study year.18 If subjects had multiple pneumonia episodes, only the first was included. Cases were assigned an index date corresponding to the date of pneumonia diagnosis. Up to two controls were selected for each case using incidence density sampling, matched on age, sex, and calendar year. This required controls to have survived and remained enrolled in GH through their matched case’s diagnosis date. Controls were assigned the same index date as their matched case. The final sample size was determined by the number of validated pneumonia cases occurring during the study period.
Exposure measurement
Information about ACE inhibitor use came from GH’s computerized pharmacy data which include medication name, strength, quantity, date of dispensing, route of administration, and days supplied. In prior studies, 96% of GH members in this age group have reported filling all or almost all of their prescriptions at GH pharmacies.20 We included only orally administered ACE inhibitors. We defined subjects as “current users” if they filled 2 or more prescriptions within the 180 days prior to index date. Subjects not meeting this criterion were defined as nonusers.
Among current users, we estimated their expected number of standard doses used per day (SDD) based on their most recent ACE inhibitor dispensing prior to index date. To do this, we multiplied the number of pills dispensed by the strength of the drug and divided by the days supplied, yielding an expected number of milligrams used per day. We converted these to SDD using the Defined Daily Dose categorization scheme from the World Health Organization.21 Conversion factors are shown in Online Appendix Table 2. We categorized subjects’ SDD as < 1, 1 to 2, or > 2 SDD.
Other covariates
All covariates were ascertained by manual review of medical records, except for prescription medication use and utilization (described further below). Medical record reviews focused on the 2 year period prior to September 1 of each year (“baseline period”) and were conducted in the same manner for cases and controls. Covariates included: general demographic characteristics; health conditions hypothesized to be associated with pneumonia risk (chronic heart and lung disease, diabetes, stroke, dementia, and others); and severity measures for those conditions (Table 1). For example, for chronic obstructive pulmonary disease (COPD), severity measures were long-term oral corticosteroid use (receiving oral steroids to be taken at least 3 times per week for at least 3 months), home oxygen use, the presence of any measurement of forced expiratory volume in 1 second (FEV1), and recent COPD hospitalization. Medical record reviews also gathered information about functional status including need for assistance with ambulation or bathing, use of home health services, and whether a subject was ever described as frail by medical personnel. For cognitive status, abstractors noted whether participants had received a dementia diagnosis. GH automated pharmacy data provided information about medications expected to be associated with pneumonia risk (e.g. use of bronchodilators, corticosteroids, and furosemide.) The number of outpatient visits in the 1 year prior to September 1 of each year was obtained from automated utilization data.
Table 1.
Characteristics of pneumonia cases and controls
| Pneumonia cases (N=1,039) |
Controls (N=2,022) |
|
|---|---|---|
| Characteristic* | n (%)† | n (%)† |
| Median age (IQR), years‡ | 77 (71 – 82) | 77 (71 – 82) |
| Male‡ | 525 (50.5) | 1030 (50.9) |
| Case validated from: | ||
| Radiograph report | 963 (85.6) | NA |
| Other medical records | 126 (11.2) | NA |
| Valid by default§ | 36 (3.2) | NA |
| Median BMI (IQR), kg/m2† | 26.5 (23.4 – 30.6) (N=736) |
27.3 (24.5 – 30.6) (N=1341) |
| Median weight (IQR), kg | 74.5 (62.7 – 86.8) | 76.4 (65.0 – 87.7) |
| Median height (IQR), cm† | 168 (160 – 175) (N=737) |
168 (160 – 175) (N=1347) |
| Current smoker | 94/1027 (9.2) | 101/1972 (5.1) |
| Asthma | 185 (17.8) | 158 (7.8) |
| Chronic obstructive pulmonary disease (COPD) |
324 (31.2) | 227 (10.4) |
| Hospitalized for COPD | 50 (4.8) | 14 (0.7) |
| Home oxygen use | 83 (8.0) | 18 (0.9) |
| Long term oral corticosteroid use for lung disease |
28 (2.7) | 6 (0.3) |
| FEV1 measured | 166 (16.0) | 78 (3.9) |
| Congestive heart failure (CHF) | 192 (18.5) | 141 (7.0) |
| Hospitalized for CHF | 33 (3.2) | 16 (0.8) |
| Ejection fraction measured | 72 (6.9) | 35 (1.7) |
| Myocardial infarction | 126 (12.1) | 199 (9.8) |
| Coronary revascularization | 145 (14.0) | 224 (11.1) |
| Other heart conditions | 411 (39.6) | 640 (31.7) |
| Hypertension | 479 (46.1) | 949 (46.9) |
| Stroke | 87 (8.4) | 140 (6.9) |
| Swallowing disorder leading to aspiration | 13 (1.3) | 9 (0.4) |
| Alcoholism | 16 (1.5) | 22 (1.1) |
| Diabetes mellitus | 172 (16.6) | 300 (14.8) |
| Diabetes mellitus with complications | 97 (9.3) | 163 (8.1) |
| Dementia | 48 (4.6) | 68 (3.4) |
| At least one functional impairment | 191 (18.4) | 232 (11.5) |
| Requires assistance bathing | 22 (2.1) | 15 (0.7) |
| Requires assistance walking | 184 (17.7) | 221 (10.9) |
| Any use of home health services | 150 (14.4) | 125 (6.2) |
| Frail║ | 84 (8.1) | 58 (2.9) |
| Bronchodilator use¶ | 138 (13.3) | 51 (2.5) |
| Furosemide use¶ | 231 (22.2) | 206 (10.2) |
| Inhaled corticosteroid use¶ | 244 (23.5) | 159 (7.9) |
| Insulin or oral hypoglycemic use¶ | 114 (11.0) | 188 (9.3) |
| Oral corticosteroid use¶ | 262 (25.2) | 191 (9.4) |
| Received pneumococcal vaccine** | 951 (91.5) | 1846 (91.3) |
| Received influenza vaccine†† | 630 (60.6) | 1245 (61.6) |
| Number of outpatient visits, median (IQR)‡‡ | 11 (6 – 17) | 8 (5 – 14) |
Abbreviations: BMI, body mass index; IQR, interquartile range; FEV1, forced expiratory volume in 1 second; GH, Group Health.
All characteristics are defined as of September 1 and are defined for the 2 year baseline period unless otherwise stated. Except as stated below, all covariates were measured by manual review of medical records.
Results are provided as n (%) unless otherwise stated. Less than 5% of people had missing values for all characteristics except for BMI, which was missing for 29% of cases and 34% of controls, and height, missing for 29% of cases and 33% of controls.
Matching variable used in selection of controls.
Cases were considered valid by default if they were diagnosed with pneumonia at an outside emergency department and no GH radiograph report was available; see Methods.
Ever described as frail by medical personnel (from chart review).
Defined from computerized pharmacy data as filling at least one prescription during the 2 year baseline period.
Any history of receiving pneumococcal vaccine.
Receipt of the current year’s influenza vaccine prior to index date.
Number of visits in 1-year baseline, from GH automated utilization data.
Analysis
We summarized characteristics of pneumonia cases and controls by calculating counts and proportions for categorical variables and medians and interquartile ranges for continuous variables. We also examined associations between these characteristics and ACE inhibitor use among control subjects.
For our primary analysis, we used multivariable conditional logistic regression to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for pneumonia risk, comparing current ACE inhibitor users to nonusers. We selected potential confounders and precision variables to include in the model a priori, guided by review of the literature and clinical plausibility. Variables included were: asthma, COPD, history of COPD hospitalization, use of home oxygen, long-term steroid use for lung disease, whether FEV1 was ever measured, presence of a swallowing disorder, congestive heart failure (CHF), history of CHF hospitalization, whether ejection fraction was ever measured, smoking status, stroke, myocardial infarction, coronary revascularization, other heart conditions, hypertension, diabetes with or without complications, number of outpatient visits in 1-year baseline, prior receipt of pneumococcal vaccine or current year’s influenza vaccine, need for assistance with ambulation or bathing, any use of home health services, dementia, ever described as frail, and use of inhaled bronchodilators, inhaled corticosteroids, oral corticosteroids, furosemide, digoxin, or insulin. We did not adjust for use of statins or acid-suppressing medications because our prior studies indicated no association between use of these medications and pneumonia risk.22,23
We also examined pneumonia risk according to ACE inhibitor daily dose among current users. We also conducted analyses in subgroups stratified by potential indication for ACE inhibitor use, defined as for primary or secondary prevention. The secondary prevention group was people with a history of CHF, stroke, myocardial infarction, coronary revascularization, or other heart disease, whereas the primary prevention group was individuals with none of these conditions. Because of the matched study design, some cases and controls had to be excluded from these analyses because they were discordant with others from their matched set with regard to these indications. We also conducted analyses separately for ambulatory and hospitalized pneumonia, to examine the association between ACE inhibitor use and pneumonia severity.
We conducted several sensitivity analyses. First, we examined an alternate definition of “current use”, in which use was defined as current when the expected duration of the most recent ACE inhibitor dispensing included the index date (multiplying days supplied by 125% to allow for imperfect compliance). Second, we defined nonusers more stringently by excluding possible users (that is, analyzing them as a separate group). Possible use was defined as having at least one ACE inhibitor dispensing in the past 12 months but not meeting our primary definition of “current use” (2 fills in 6 months.) Third, to explore the impact of covariate adjustment, we repeated our analyses using a more limited set of covariates (the “parsimonious” model), those for which adjustment altered the ACE inhibitor-pneumonia OR by 10% or more. Adjustment variables in this model were CHF, history of CHF hospitalization, and furosemide use (as well as matching variables). We also explored adding weight as a covariate in our primary model. Fourth, we conducted analyses to address the confounding which could arise if a recent hospitalization led to initiation of an ACE inhibitor and also increased pneumonia risk. We repeated our primary analyses excluding people who initiated ACE inhibitor use in the 90 days prior to index date and also adjusted for hospitalization in this time window.
Analyses were carried out using SAS software, version 9.2 (SAS Institute, Incorporated, Cary, North Carolina, United States).
RESULTS
These analyses included 1039 pneumonia cases and 2022 controls. Approximately half were women, and the median age was 77 (Table 1). Cases tended to have more comorbid illnesses than controls, and the severity of these illnesses was greater among cases. Cases also had more functional impairment.
Almost all ACE inhibitor dispensings (95.4%) were for lisinopril, a hydrophilic ACE inhibitor (see Online Appendix Table 2, which shows the number of prescriptions and days’ supply for specific ACE inhibitors dispensed to study subjects). Among current users, the median days’ supply received in the 180 days prior to index date was 176 days (interquartile range, 150 to 180.) Current use of ACE inhibitors among control subjects was associated with chronic cardiovascular disease including CHF, coronary artery disease, and history of myocardial infarction and coronary revascularization (Table 2). Current users were also more likely than nonusers to have hypertension, diabetes, and functional impairments.
Table 2.
Characteristics associated with use of ACE inhibitors among controls
| Current users* (N=433) |
Nonusers* (N=1589) |
|
|---|---|---|
| Characteristic† | n (%)† | N (%)† |
| Age, median (IQR) | 77 (71 – 81) | 77 (71 – 82) |
| Male | 255 (58.9) | 775 (48.8) |
| BMI, median (IQR)‡ | 28.2 (25.3 – 31.6) (N=317) |
27.0 (24.3 – 30.1) (N=1024) |
| Median weight (IQR), kg | 80.0 (67.7 – 90.9) | 75.0 (64.5 – 86.4) |
| Median height (IQR), cm‡ | 170 (160 – 178) (N=318) |
168 (158 – 175) (N=1029) |
| Current smoker | 21/420 (5.0) | 80/1552 (5.2) |
| Asthma | 42 (9.7) | 116 (7.3) |
| Chronic obstructive pulmonary disease (COPD) | 45 (10.4) | 167 (10.5) |
| Hospitalized for COPD | 2 (0.5) | 12 (0.8) |
| Home oxygen use | 6 (1.4) | 12 (0.8) |
| Long term oral corticosteroid use for lung disease |
1 (0.2) | 5 (0.3) |
| FEV1 ever measured | 21 (4.8) | 57 (3.6) |
| Congestive heart failure (CHF) | 54 (12.5) | 87 (5.5) |
| Hospitalized for CHF | 8 (1.8) | 8 (0.5) |
| Ejection fraction measured | 14 (3.2) | 21 (1.3) |
| Myocardial infarction | 80 (18.5) | 119 (7.5) |
| Coronary revascularization | 89 (20.6) | 135 (8.5) |
| Other heart conditions | 209 (48.3) | 431 (27.1) |
| Hypertension | 333 (76.9) | 616 (38.8) |
| Stroke | 50 (11.5) | 90 (5.7) |
| Swallowing disorder leading to aspiration | 1 (0.2) | 8 (0.5) |
| Alcoholism | 2 (0.5) | 20 (1.3) |
| Diabetes mellitus | 132 (30.5) | 168 (10.6) |
| Diabetes mellitus with complications | 76 (17.6) | 87 (5.5) |
| Dementia | 15 (3.5) | 53 (3.3) |
| At least one functional impairment | 65 (15.0) | 167 (10.5) |
| Requires assistance bathing | 4 (0.9) | 11 (0.7) |
| Requires assistance walking | 62 (14.3) | 159 (10.0) |
| Any use of home health services | 30 (6.9) | 95 (6.0) |
| Frail§ | 19 (4.4) | 39 (2.5) |
| Bronchodilator use║ | 10 (2.3) | 41 (2.6) |
| Furosemide use║ | 79 (18.2) | 127 (8.0) |
| Inhaled corticosteroid use║ | 46 (10.6) | 113 (7.1) |
| Insulin or oral hypoglycemic use║ | 93 (21.5) | 95 (6.0) |
| Oral corticosteroid use║ | 34 (7.9) | 157 (9.9) |
| Received pneumococcal vaccine¶ | 406 (93.8) | 1440 (90.6) |
| Received influenza vaccine** | 270 (62.4) | 975 (61.4) |
| Number of outpatient visits, median (IQR)†† | 10 (6 – 15) | 8 (5 – 13) |
Abbreviations: BMI, body mass index; IQR, interquartile range; FEV1, forced expiratory volume in 1 second.
Current use is defined as receiving ≥2 fills of an ACE inhibitor within 180 days prior to index date. Nonusers did not meet this criterion.
All characteristics are defined as of September 1, and the time period of interest is the 2 year baseline period unless otherwise stated. Except as stated below, all covariates were measured by manual review of medical records.
Less than 5% of cases and controls had missing values for all characteristics except for BMI, which was missing for 27% (116/433) of current users and 36% (565/1589) of nonusers, and height, missing for 27% (115/433) of current users and 35% (560/1589) of nonusers.
Ever described as frail by medical personnel (from chart review).
Defined from computerized pharmacy data as filling at least one prescription for a medication in this class during the 2 year baseline period.
Any history of receiving pneumococcal vaccine.
Receipt of the current year’s influenza vaccine prior to index date.
Number of visits in 1-year baseline, from GH automated utilization data.
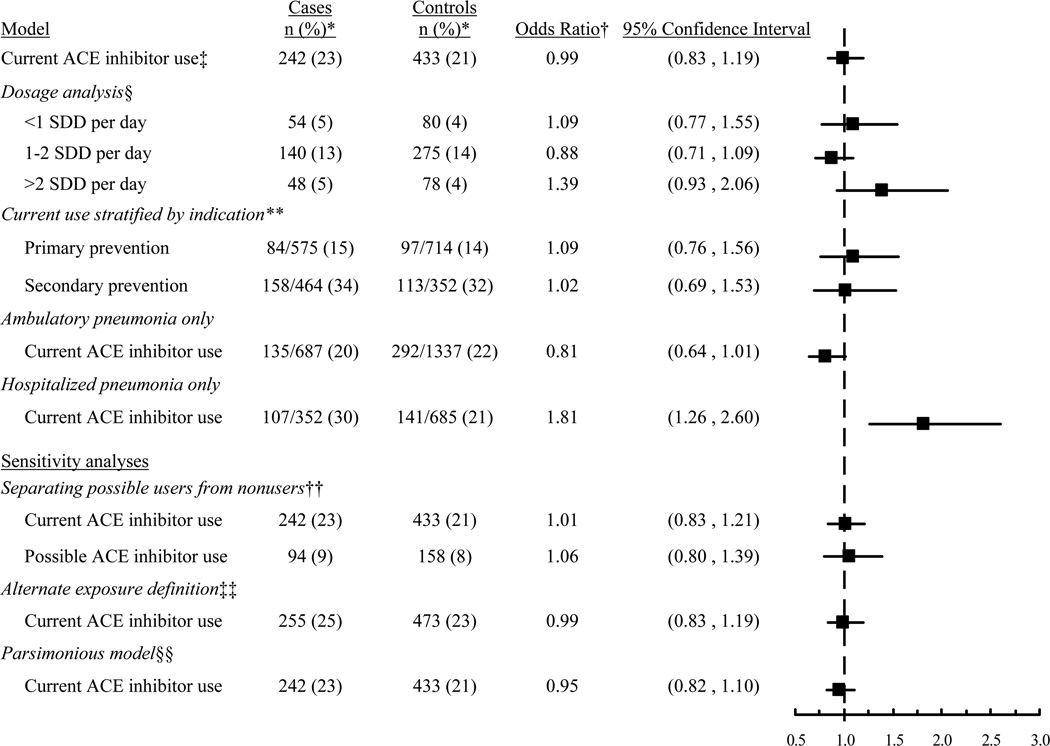
Among pneumonia cases, 242/1039 (23.3%) were current ACE inhibitor users, compared to 433/2022 (21.4%) controls (adjusted OR 0.99, 95% CI 0.83 to 1.19, compared to nonuse; Table 3). There was no evidence of decreasing risk with increasing daily dose (p=0.15). Compared to nonuse, the adjusted OR for pneumonia for current use of <1 SDD was 1.09 (95% CI 0.77 to 1.55); for 1 to 2 SDD, 0.88 (95% CI 0.71 to 1.09); and for > 2 SDD, 1.39 (95% CI 0.93 to 2.06). Results were similar in analyses stratifying on the basis of indication for primary or secondary prevention, but analyses for ambulatory vs. hospitalized cases yielded somewhat different results, with a higher risk of hospitalized pneumonia seen in current ACE inhibitor users (Figure 2). Other sensitivity analyses yielded results consistent with the main analysis (Figure 2). Results also did not change after excluding people initiating ACE inhibitor use within 90 days prior to index date nor adjusting for weight or hospitalization in the 90 days prior to index date.
Table 3.
Risk of community-acquired pneumonia in relation to use of ACE inhibitors
| Pneumonia Cases |
Controls | Odds ratio (95% CI) | ||
|---|---|---|---|---|
| (N=1,039) | (N=2,022) | Adjusted for matching variables† |
Further adjusted‡ |
|
| ACE inhibitor use* | n (%) | n (%) | ||
| Current user | 242 (23.3) | 433 (21.4) | 1.12 (0.97, 1.29) | 0.99 (0.83, 1.19) |
| Nonuser | 797 (76.7) | 1589 (78.6) | 1.00 (Ref.) | 1.00 (Ref.) |
Abbreviations: CHF, congestive heart failure; CI, confidence interval; FEV1, forced expiratory volume in 1 second.
Current use is defined as receiving ≥2 fills of an ACE inhibitor within 180 days prior to index date. Nonusers did not meet this criterion.
Adjusted only for age, sex, and index date.
Adjusted for matching variables above and for asthma, chronic obstructive pulmonary disease (COPD) and history of COPD hospitalization, use of home oxygen, long-term oral corticosteroid use for lung disease, whether FEV1 was ever measured, presence of a swallowing disorder, congestive heart failure (CHF) and history of CHF hospitalization, whether ejection fraction was ever measured, smoking status, stroke, myocardial infarction, coronary revascularization, other heart conditions, hypertension, diabetes with or without complications, number of outpatient visits in 1-year baseline, receipt of pneumococcal influenza vaccine, need for assistance with ambulation or bathing, use of home health services, dementia, ever described as frail, and use of the following medications: inhaled bronchodilators, inhaled corticosteroids, oral corticosteroids, furosemide, digoxin, or insulin.
Figure 2.
Risk estimates for the association between current ACE inhibitor use and risk of pneumonia. *The denominator is 1039 for cases and 2022 for controls, except where otherwise stated. The denominators for analyses stratified by indication and care setting (ambulatory vs. hospitalized) are different because these analyses only included the subset of cases and controls meeting the specified criteria. †Adjusted for matching variables (age, sex, index date), comorbidities, and functional and cognitive status, except where stated. Referent group is non-users. ‡Defined as filling ≥ 2 prescriptions in the 180 days prior to index date. §Standardized daily doses (SDD) estimated from most recent ACE inhibitor dispensing using World Health Organization definitions. **Secondary prevention was defined as having a history of congestive heart failure, stroke, myocardial infarction, coronary revascularization, or other heart disease. Primary prevention is use in the absence of these conditions. ††”Possible use” was defined as having at least 1 ACE inhibitor fill in the past 12 months but not meeting criteria for current use. In this analysis, “nonusers” were those with no ACE inhibitor fills in the past 12 months. ‡‡Current use was defined as having a dispensing that, based on days supply, would have lasted beyond the index date (days supply multiplied by 1.25 to allow for imperfect adherence.) §§Adjusted for matching variables (age, sex, index date) and only those covariates for which adjustment resulted in >10% change in the main exposure odds ratio (see Methods).
DISCUSSION
ACE inhibitor use was not associated with reduced pneumonia risk in this study of community-dwelling older people. There was no pattern of decreasing risk with higher daily dose. Results were unchanged in many sensitivity analyses.
These findings are consistent with some but not all prior studies. Van de Garde observed no association in an observational study of the general population6 but in a second study, he observed a decreased risk with ACE inhibitor use among diabetics.7 Our sample size precluded subgroup analyses limited to diabetics, but we did not find decreased pneumonia risk in people using ACE inhibitors for secondary prevention. Examining a younger population, Mukamal et al. found no association of pneumonia risk with use of hydrophilic ACE inhibitors (including lisinopril, the most common medication in our population).9 In contrast, Myles et al. reported decreased risk of pneumonia in current ACE inhibitor users (OR 0.75, 95% CI 0.65 to 0.86, compared to nonuse).8 All of these studies relied on automated data, which do not perfectly measure either pneumonia or potential confounders. In prior studies, administrative data had a sensitivity of 65–80% for pneumonia13,14 and positive predictive value (PPV) around 80%14 compared to clinical assessment or medical record review. In addition, we have found that administrative data have a sensitivity of about 80% and PPV from 73–80% for important confounders including chronic lung and heart disease. Thus, for the studies discussed above, results could represent a true finding or bias due to misclassification of outcome status or confounders.
Several prior studies observed a decreased risk associated with ACE inhibitor use in Asian populations,5,11,12 suggesting that racial or ethnic differences (including genetic differences) could play a role. For instance, in secondary analyses of data from a randomized trial, Ohkubo et al. found that among Asian participants, the OR for ACE inhibitor use and pneumonia was 0.53 (95% CI, 0.33 to 0.86), while among non-Asian participants it was 0.95 (95% CI, 0.71–1.27).5 Another important factor may be that two of these studies11,12 examined the association in institutionalized older people (mean age >80) with a high prevalence of comorbidity, including half or more with dementia, in whom aspiration is likely to be relatively common. Results may differ for this vulnerable population compared to the general population. It has also been suggested that ACE inhibitor effects may differ by ACE deletion genotype. In actuality, results about the impact of genotype are conflicting. Takahashi et al. observed an association between ACE inhibitor use and pneumonia only among people with the II or ID genotype (OR 0.42, 95% CI 0.18 to 0.98) and none in those with the DD genotype (OR 0.71, 95% CI 0.20 to 2.5).12 In contrast, Ohkubo found no significant association for any genotype.5 Thus, it remains unclear why the results of prior studies are discrepant.
We carried out analyses separately for ambulatory and hospitalized cases of pneumonia to examine whether the association of ACE inhibitors with pneumonia varies by pneumonia severity. The analyses limited to hospitalized pneumonia cases yielded unexpected findings that differed noticeably from our other analyses (OR for current ACE inhibitor use 1.81, 95% CI 1.26 to 2.60). The reason for this elevated risk with current ACE inhibitor use is not clear. We are not aware of any prior studies reporting higher pneumonia risk with ACE inhibitor use. This finding may reflect the fact that comorbid conditions that are indications for ACE inhibitor use (such as diabetes and CHF) may influence the decision to admit a person with pneumonia to the hospital. Alternatively, given the number of secondary and sensitivity analyses that we performed, this result may simply reflect a spurious finding.
Study strengths include validation of pneumonia cases and measurement of potential confounders from medical records, which are more accurate than the automated data used in most prior studies.13–17 Limitations include possible residual confounding due to unmeasured characteristics. There could be misclassification of exposure if people did not take dispensed medications or used medications not prescribed to them. One medication, lisinopril, accounted for 95% of dispensings in our population, and so our results may not apply to other ACE inhibitors. We are not aware of information suggesting that ACE inhibitors differ in their effect on substance P and bradykinins, the mechanism hypothesized for protection against pneumonia. We studied a predominantly white population, so our results may not be generalizable to other groups.
Because of the substantial burden posed by pneumonia in older adults, it would be desirable to identify an inexpensive, readily available and safe intervention to decrease risk. Some prior studies raised hope that ACE inhibitors might be such an intervention. Our results do not support this hypothesis. In future studies, it may useful to examine the association between ACE inhibitor use and pneumonia in special populations, such as people with known swallowing disorders, nursing home residents, or other racial/ethnic groups. Taken together with prior studies, our results suggest that at this time, it would be premature to conduct randomized trials of ACE inhibitors to prevent pneumonia.
Supplementary Material
Key points.
Angiotensin-converting enzyme (ACE) inhibitors could prevent pneumonia by heightening the cough reflex, decreasing aspiration.
Prior studies yielded inconsistent results and also relied on automated data, and so inaccurate measurement could bias results.
In this study with validated outcomes and confounders, ACE inhibitor use was not associated with lower pneumonia risk (adjusted odds ratio 0.99, 95% confidence interval 0.83–1.19). Risk was not lower with higher daily dose.
It would be premature to conduct randomized trials of ACE inhibitors to prevent pneumonia.
Acknowledgments
Funding: Dr. Dublin was funded by a Paul Beeson Career Development Award from the National Institute on Aging, grant K23AG028954, and by the Branta Foundation. The Beeson Award is also supported by the American Federation for Aging Research and the Hartford and Starr Foundations and Atlantic Philanthropies. Group Health Research Institute internal funds covered the data collection and analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Conflicts of interest: Dr. Nelson has done consulting work for Glaxo Smith Kline. Dr. Lisa Jackson has grant funding from Novartis, Sanofi Pasteur, Glaxo Smith Kline and Pfizer. Dr. Dublin received a Merck/American Geriatrics Society New Investigator Award. For the remaining authors, no conflicts were declared.
Prior presentation: This work was presented as a poster at the International Conference on Pharmacoepidemiology, Chicago, IL, August 15, 2011.
REFERENCES
- 1.Kochanek KD, Xu J, Murphy SL, et al. Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011;59:1–51. [PubMed] [Google Scholar]
- 2.Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med. 1992;117:234–242. doi: 10.7326/0003-4819-117-3-234. [DOI] [PubMed] [Google Scholar]
- 3.Bangalore S, Kumar S, Messerli FH. Angiotensin-converting enzyme inhibitor associated cough: deceptive information from the Physicians' Desk Reference. Am J Med. 2010;123:1016–1030. doi: 10.1016/j.amjmed.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Morice AH, Lowry R, Brown MJ, et al. Angiotensin-converting enzyme and the cough reflex. Lancet. 1987;2:1116–1118. doi: 10.1016/s0140-6736(87)91547-9. [DOI] [PubMed] [Google Scholar]
- 5.Ohkubo T, Chapman N, Neal B, et al. Effects of an angiotensin-converting enzyme inhibitor-based regimen on pneumonia risk. Am J Respir Crit Care Med. 2004;169:1041–1045. doi: 10.1164/rccm.200309-1219OC. [DOI] [PubMed] [Google Scholar]
- 6.van de Garde EM, Souverein PC, van den Bosch JM, et al. Angiotensin-converting enzyme inhibitor use and pneumonia risk in a general population. Eur Respir J. 2006;27:1217–1222. doi: 10.1183/09031936.06.00110005. [DOI] [PubMed] [Google Scholar]
- 7.van de Garde EM, Souverein PC, Hak E, et al. Angiotensin-converting enzyme inhibitor use and protection against pneumonia in patients with diabetes. J Hypertens. 2007;25:235–239. doi: 10.1097/HJH.0b013e328010520a. [DOI] [PubMed] [Google Scholar]
- 8.Myles PR, Hubbard RB, McKeever TM, et al. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18:269–275. doi: 10.1002/pds.1715. [DOI] [PubMed] [Google Scholar]
- 9.Mukamal KJ, Ghimire S, Pandey R, et al. Antihypertensive medications and risk of community-acquired pneumonia. J Hypertens. 2010;28:401–405. doi: 10.1097/HJH.0b013e3283330948. [DOI] [PubMed] [Google Scholar]
- 10.Etminan M, Zhang B, Fitzgerald M, et al. Do angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers decrease the risk of hospitalization secondary to community-acquired pneumonia? A nested case-control study. Pharmacotherapy. 2006;26:479–482. doi: 10.1592/phco.26.4.479. [DOI] [PubMed] [Google Scholar]
- 11.Okaishi K, Morimoto S, Fukuo K, et al. Reduction of risk of pneumonia associated with use of angiotensin I converting enzyme inhibitors in elderly inpatients. Am J Hypertens. 1999;12:778–783. doi: 10.1016/s0895-7061(99)00035-7. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi T, Morimoto S, Okaishi K, et al. Reduction of pneumonia risk by an angiotensin I-converting enzyme inhibitor in elderly Japanese inpatients according to insertion/deletion polymorphism of the angiotensin I-converting enzyme gene. Am J Hypertens. 2005;18:1353–1359. doi: 10.1016/j.amjhyper.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 13.van de Garde EM, Oosterheert JJ, Bonten M, et al. International classification of diseases codes showed modest sensitivity for detecting community-acquired pneumonia. J Clin Epidemiol. 2007;60:834–838. doi: 10.1016/j.jclinepi.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Aronsky D, Haug PJ, Lagor C, et al. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20:319–328. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
- 15.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–141. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 16.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002;40:675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Kieszak SM, Flanders WD, Kosinski AS, et al. A comparison of the Charlson comorbidity index derived from medical record data and administrative billing data. J Clin Epidemiol. 1999;52:137–142. doi: 10.1016/s0895-4356(98)00154-1. [DOI] [PubMed] [Google Scholar]
- 18.Jackson ML, Nelson JC, Weiss NS, et al. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JC, Jackson M, Yu O, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26:4947–4954. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. 4th edition. Vol. 234. Chichester: John Wiley & Sons; 2005. [Google Scholar]
- 21.Methodology WHOCCfDS. ATC/DDD Index 2012. [accessed January 6, 2012];2011 Dec 19; http://www.whocc.no/atc_ddd_index/
- 22.Dublin S, Jackson ML, Nelson JC, et al. Statin use and risk of community acquired pneumonia in older people: population based case-control study. BMJ. 2009;338:b2137. doi: 10.1136/bmj.b2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dublin S, Walker RL, Jackson ML, et al. Use of proton pump inhibitors and H2 blockers and risk of pneumonia in older adults: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:792–802. doi: 10.1002/pds.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.