Summary
The aim of our study was to determine the etiology of nosocomial infections, their changes over a period of five years (2007-2011), and the measures for control of infections and antimicrobial resistance in the Burns Clinic of the N.I. Pirogov University Multi-Profile Hospital for Active Treatment and Emergency Medicine, Sofia, Bulgaria. The medical records for all the patients and the database of the “Clinical Microbiology and Surveillance of Infections” National Information System were reviewed and analyzed to identify the microbial pathogens isolated in our burns Clinic. The three most frequent nosocomial pathogens were S. aureus, A. baumannii and P. aeruginosa. In order to control effectively nosocomial infections, a system of anti-infective and anti- microbial resistance measures has been developed and routinely implemented in our Clinic since 2008. Since 2009, thanks to this system, there has been a significant decrease in the rates of multi-resistant Staphylococcus aureus strains. Although at present the incidence of the nosocomial infections in our burns clinic is lower than in neighboring countries, several important infection control issues still need to be solved. We mainly rely on updating and strengthening the existing anti-infective system in order to control the spread of multi-drug resistant organisms, such as A. baumannii, extended spectrum beta-lactamase-producing Enterobacteriaceae, and carbapenem-resistant P. aeruginosa.
Keywords: nosocomial infection, burns, multi-drug-resistant organisms
Abstract
Les Auteurs de cette étude se sont proposé de déterminer l’étiologie des infections nosocomiales, leur évolution sur une période de cinq ans (2007-2011), et les mesures de contrôle des infections et la résistance antimicrobienne auprès de la Clinique des Brûlures de l’Hôpital Universitaire Multi-Profil pour le Traitement Actif et la Médecine d’Emergence N.I. Pirogov, Sofia, Bulgarie. Ils ont examiné et analysé les dossiers médicaux de tous les patients et la base données du Système National d’Information pour “La Microbiologie Clinique et la Surveillance des Infections” dans le but d’individuer les pathogènes microbiens isolés dans la Clinique des Brûlures. Les trois agents pathogènes nosocomiaux les plus communs étaient S. aureus, A. baumannii et P. aeruginosa. Afin de contrôler efficacement les infections nosocomiales, un système de mesures de résistance anti-infectieuse et anti-microbienne a été développé et mis en oeuvre systématiquement dans notre clinique depuis 2008. Depuis 2009, grâce à ce système, les Auteurs ont constaté une diminution significative des taux des souches multi-résistantes de Staphylococcus aureus (SMRS). Même si l’incidence des infections nosocomiales dans notre Clinique des Brûlures est inférieure à ceux des pays voisins, plusieurs questions importantes de contrôle des infections doivent encore être résolus, il faut encore résoudre divers problèmes importants du contrôle des infections. Les Auteurs mettent continuellement à jour et renforcent le système anti-infection existant afin de contrôler la diffusion des organismes multi-résistants, tels que A. baumannii, les Enterobacteriaceae productrices de ß-lactamases à spectre élargi (BLSE) et P. aeruginosa résistant au carbapénem.
Introduction
Nosocomial infections (NI) are common in burn patients due to the typical features of the disease: loss of the first line of defense against microbial invasion; presence of devitalized, avascularized tissue that provides a favorable environment for microbial growth; alterations in the specific and nonspecific components of the immune system; gastrointestinal translocation; and extended hospitalization and multiple invasive diagnostic and therapeutic procedures.1,2 Prolonged courses of antibiotics, often in combination result in selection of multidrug resistant nosocomial strains which belong mainly to three bacterial species: Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii.3-6 Complex reasons, but mostly the widespread use of third generation cephalosporins in the last decade, have led to the emergence of new nosocomial pathogens in burns - the extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, which were uncommon until recently.2,7,8 All these multi-drug-resistant organisms (MDRO) may cause infections which are particularly challenging to treat as there are few antimicrobial agents still effective to combat them.9 In terms of new antibiotics, the pipeline is virtually dry, especially for Gram-negative bacteria. Efforts should therefore be directed toward preserving the activity of the available antimicrobials, combating antimicrobial resistance, and providing effective infection control measures for prevention of colonization and infection of patients with MDRO strains.10
The aim of our study was to determine the etiology of nosocomial infections, their changes over a period of five years (2007-2011), and the measures for control of infections and antimicrobial resistance in the Burns Clinic of the N.I. Pirogov University Multi-Profile Hospital for Active Treatment and Emergency Medicine, Sofia, Bulgaria.
Material and methods
Study setting
This retrospective study was conducted at the Burns Clinic of the N.I. Pirogov University Multi-Profile Hospital for Active Treatment and Emergency Medicine (UMHATEM), Sofia, the largest center for treatment of burn patients and reconstructive surgery in Bulgaria. The clinic has 60 beds, ten of which are available for intensive care unit (ICU) patients. The policy of the Clinic is to admit: 1) any patient with = 1% total body surface area (TBSA) burns if there are full-thickness burn areas; 2) diabetic and other immunocompromised patients with any degree of burn. The data were obtained for a 5-yr period (1 January 2007 to 31 December 2011). In order to determine the isolated microbial pathogens in the clinic, the medical records for all the patients and database of the “Clinical Microbiology and Surveillance of Infections” National Information System were reviewed and analyzed. All patients admitted to the Clinic were eligible for the study, if they met the criteria for NI defined by the Centers for Disease Control (CDC), Atlanta.11 According to these criteria, an NI is defined as an infection that was neither present nor incubating at the time of the patient’s admission but had its onset during hospitalization.
Assessment and management of burn patients
The superficial burns treatment protocol includes wound debridement and application of the modern hydrocolloid, hydrofiber, silicone, alginate and polyurethane dressings or conventional dressings coated with paraffin or chlorhexidine. Dressings with powerful antiseptic effect and with ability to stimulate epithelialization are used in the treatment of deep dermal burns, especially if also large. Faster clearing of devitalized tissue and prophylaxis of local infections accelerate the healing processes in the wound, provide donor sites, and shorten treatment time. The treatment protocol of deep burns includes early surgical excision and single-stage autografting or multi-stage surgical excisions with application of temporary biological covering. This approach removes necrotic tissues and rapidly promotes wound closure. Until 2004 silver sulphadiazine (SSD) was the standard agent for topical treatment of superficial and deep burns in the Clinic. Since 2004 ActicoatTM(Smith&Nephew) dressing with silver nanocrystalline coating has been used routinely to treat deep dermal and full-thickness burn wounds, recipient grafts, and donor sites. ActicoatTM is applied for pre-operative treatment of the wounds and for dressings of grafted sites, excised areas, and donor sites.
Appropriate precautions were followed in cases of direct contact with the patient, such as wearing gowns, gloves, masks, etc. Hands were disinfected with appropriate disinfectant (according to the hospital disinfection procedure).
An antibiotic for surgical prophylaxis (cefuroxime) was given to burn patients who underwent broad excisional surgery. Empiric antimicrobial treatment (according to our antimicrobial stewardship protocol) was started in cases of inhalation injury or of clinical and laboratory data for bloodstream infection (i.e. high fever or hypothermia, disorientation, circulatory embarrassment, dark discoloration in previously clean wounds, early and rapid eschar separation, subcutaneous bleeding and increasing edema in surrounding areas, and very high or very low white blood cell counts). Patients with identified pathogens were treated with an antimicrobial agent, adjusted according to the isolate’s susceptibility.
Microbiological procedures
Samples from the burn wound were collected from all patients at the time of admission and thereafter when clinical signs of local infection appeared. Sub-eschar tissue biopsy for quantitative culture was taken when appropriate. Two consecutive blood cultures (BactAlert, bio- Merieux), urine and central line tips, were obtained from patients with fever or with clinical features of sepsis. Endotracheal aspirates were collected in patients with inhalation injury, intubation , and suspected ventilation-associated pneumonia. Throat, nasal, and rectal swabs were taken from all patients on admission and once weekly thereafter for MRSA and other pathogen carriage screening tests. The patients’ database was updated only if the samples showed changes of the antimicrobial susceptibility or if new microbial organisms emerged.
Subcultures were performed using standard operating procedures on sheep blood agar, MacConkey, chocolate agar (Merk), and Chromagar Candida (Becton Dickinson) and incubated for 24-48 h at 37 °C. For biochemical identification of the microbial isolates, routine methods were applied and the mini-Api and Vitek-2 Compact (bio- Merieux) systems were used. Antimicrobial susceptibility was determined by disk diffusion method (DDM) according to the latest available guidelines of the Clinical Laboratory Standards Institute (CLSI).12 Discs with 30 mkg cefoxitin (Becton Diskinson) were used to distinguish between methicillin-resistant staphylococci (MRS) and methicillin- susceptible staphylococci (MSS). In addition, oxacillin screen agar (bioMerieux) was utilized for detection of metyhicillin-resistant S. aureus (MRSA). S. aureus ATCC 25923 was included as a reference strain for quality control. The initial screening for ESBL production was carried out according to CLSI guidelines.12 Also, the DDMconfirmatory method was applied using the following disk pairs: cefotaxime 30 mkg - cefotaxime/clavulanic acid 30/10 mkg and ceftazidime 30 mkg-ceftazidime/clavulanic acid 30/10 mkg (Becton Dickinson). The Gram-negative isolates were classified as multi-drug resistant (MDR) if they were resistant to more than three classes of antimicrobial drugs.
Infection control
In order to control effectively nosocomial infections and especially to control and eradicate MDROs in the burns clinic, the local antimicrobial stewardship committee has developed a system of measures according to CDC guidelines13 and the local data of antimicrobial resistance of nosocomial pathogens. The system includes the following:
Antimicrobial stewardship protocol with three levels of restriction for prescribing antimicrobials; local guide to surgical prophylaxis and empiric antimicrobial therapy; determining the primary and alternative regimens for patients with severe infections and pathogen still unknown.
Local treatment: routine use of ActicoatTM for dressing burn wounds, recipient grafts and donor sites.
Standard and transmission control: a protocol for proper hand hygiene according to recent CDC recommendations and contact precautions for patients colonized or infected with MDROs.
Education: regular clinical-laboratory meetings and educational seminars aiming at enhancing staff knowledge on antimicrobial stewardship and infection control.
Decolonization only for S. aureus/MRSA carriers - both patients and staff (as MDR Gram-negative decolonization remains an unresolved issue): nasal decolonization with mupirocin ointment, oral antibiotics at discretion and 4% chlorhexidine baths for patients at least twice a week.
Environmental measures: thorough cleaning; control of disinfection and sterilization procedures: every three months for the ICU and twice per year for the other departments.
This system of anti-infective and anti-microbial resistance measures has been routinely implemented in the clinic since 2008.
Statistical analysis
Comparisons across groups were performed with the chi-squared test.
A p-value of <0.05 was considered significant.
Results
During the study period, 5894 patients were admitted to the Burns and Plastic Surgery Clinic in Sofia. The demographics data are presented in Table I.
Table I. Demographics of patient data.

Table II presents the total number of patients admitted and microbiologically tested, the number of patients with nosocomial isolates, and the number of isolated nosocomial strains (duplicate isolates are not included) during the study period. The type of specimens from which the nosocomial strains were isolated is not considered in this study. Some patients were infected/colonized with more than one pathogen and had more than one isolate in this population.
Table II. Total number of patients and of isolated nosocomial strains.
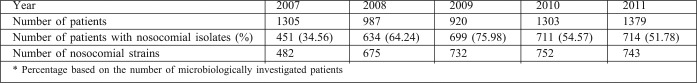
As can be seen, the incidence of patients with NI increased almost two-fold in 2008 in comparison with 2007 (the difference is significant, p<0.05) and reached its peak level in 2009 (p<0.05, in comparison between 2009 and 2008). Afterwards, in 2010 it significantly decreased (p<0.05) and in 2011 about half of the microbiologically tested patients acquired at least one NI during hospitalization, significantly more than at the beginning of the study period (p<0.05). The total number of patient-days in 2011 was 70094, with 743 NI for the year, The NI rate in 2011 was therefore 10.6 per 1000 patient-days.
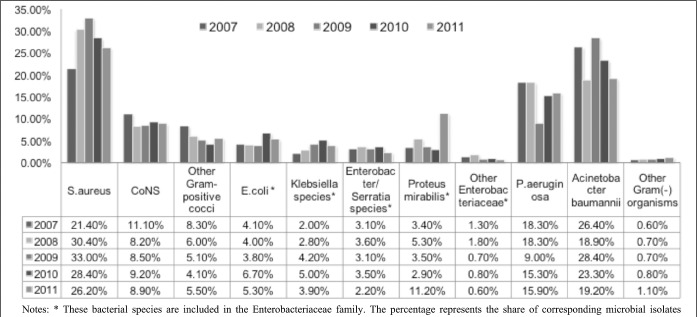
The spectrum of nosocomial pathogens isolated from clinical specimens in burn patients during the study period is presented in Fig. 1.
Fig. 1. Spectrum and rate of nosocomial pathogens isolated in burn patients.
S. aureus has been the pathogen with the highest incidence rate in the clinic since 2008, but it has shown a decreasing rate trend. After 2007 Acinetobacter baumannii had the second incidence rate, followed by P. aeruginosa.
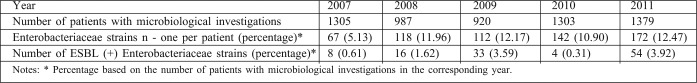
The incidence of Enterobacteriaceae significantly increased in 2008 in comparison with 2007 (p<0.05) and remained on this level till the end of the study period (Table III ). However, the share of ESBL-producing strains increased significantly in 2008 in comparison with 2007 (p<0.05) and in 2009 in comparison with 2008 (p<0.05), significantly dropped in 2009 in comparison with 2008 (p<0.05) and again significantly increased in 2011, in comparison both with 2010 (p<0.05) and with the beginning of the study period (p<0.05), reaching nearly 1/3 of all Enterobacteriaceae isolates.
Table III. Changes in the incidence of Enterobacteriaceae and of ESBL-producing strains over the years.
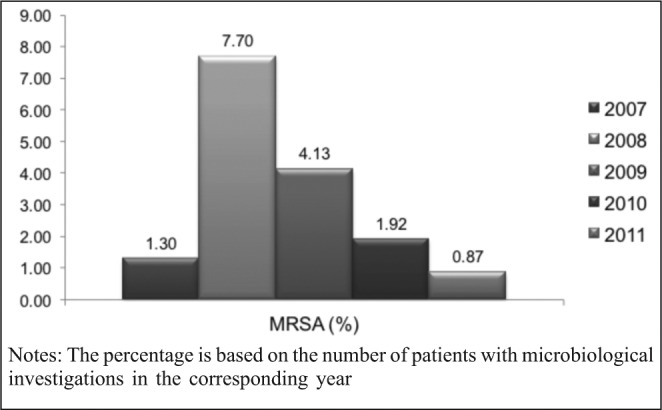
The MRSA rate significantly increased in 2008 in comparison with 2007 (p<0.05), then significantly dropped in 2009 in comparison with 2008, also dropped in 2010 in comparison with 2009 and in 2011 in comparison with 2010 (p<0.05 for all compared groups). The incidence of MRSA in 2011 in comparison with 2007 became lower, albeit without statistical significance (p>0.05)
During the study period, as many as 163 nasal swabs from the staff and 5148 from the patients were screened for S. aureus carriage. S. aureus was isolated in 9.2% of staff nasal swabs and two cases of MRSA carriage were established. S. aureus nasal carriage was found in 22.2% of the patients. MRSA was isolated from single admission swabs but 12.4% of the patients acquired MRSA during hospitalization. Decolonization was successful in all the cases.
The next two figures (Fig. 3,4) present the in vitro susceptibility of the Gram-negative pathogens with the highest incidence rate in the Burns Clinic. The changes through the years are not considered in this study.
Fig. 3. Susceptibility of A. baumannii to antimicrobial drugs.
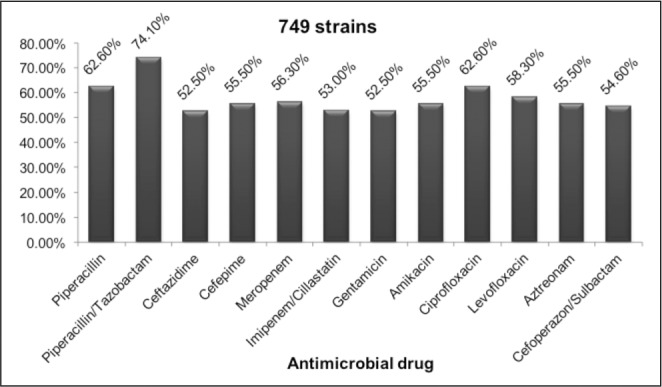
Fig. 4. Susceptibility of A. baumannii to antimicrobial drugs.
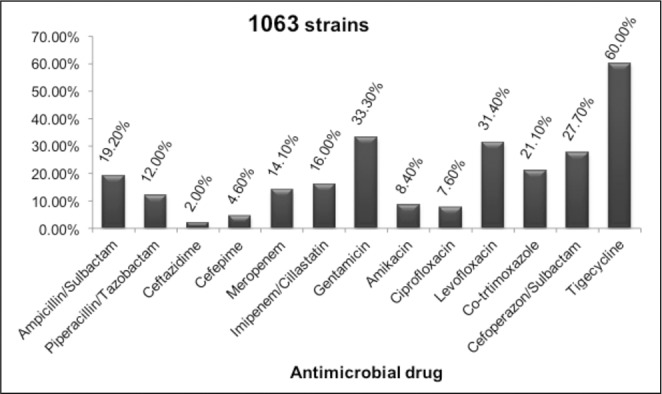
More than half of the strains were susceptible to all the anti-Pseudomonas drugs tested. The most in vitro active one was Piperacillin/Tazobactam, however the rate of carbapenem-resistant isolates was high: 43.7-47.0%. A molecular analysis of the resistant mechanisms in A. baumannii isolates has not been carried out so far, but is imminent.
The susceptibility of A. baumannii to antimicrobials was extremely low. Most of the strains were MDR which posed serious therapeutic problems. The rate of the carbapenem- resistant isolates reached 82.7-84.6%. In the last year a new drug was introduced to the hospital and the country, the glycilcycline tigecycline. In the first trimester of the year nearly 100% of A. baumannii strains were susceptible in vitro susceptible to this drug but non-susceptible isolates gradually emerged. At present, 40% of the strains reveal intermediate susceptibility. There are still no resistant isolates.
In total 743 environmental specimens were taken and tested during the study period. Fifty-five nosocomial stains were isolated from them (7.4%), mainly A. baumannii, Enterobacteriaceae, staphylococci and P. aeruginosa. A. baumannii prevailed (14 isolates) and was found in dressing rooms, patients’ surroundings, medical equipment and other places, while A. baumannii was found in sinks and bathtubs. Single ESBL(+) Enterobacteriaceae strains were isolated in 2010 and 2011.
Fig. 2. Changes in the rates of MRSA during the study period.
Discussion
Infection can lead to deterioration of the wound healing process and severe systemic complications and is the leading cause of morbidity and mortality in patients with burns. Because of the change in burn therapy from a non-operative to an operative approach in our Burns Clinic, mortality in burn patients has dramatically dropped and functional outcomes have improved over the past 20 years. Due to the considerable improvements in surgical and intensive care for burn victims, it is now estimated that 75% of the mortality following thermal injures is related directly to infections. Therefore, knowledge of the bacteriology of burns is of prime importance to fast and clinically sound therapeutic decisions in critically ill burn patients. 5
Our investigation revealed nosocomial infection rate of 10.6 per 1000 patient days in 2011 which was less than the rate published by Oncul O. et al. (18.2) and Alp E et al. (14.7).1,14
The three most frequent nosocomial pathogens in our Burns Clinic for the study period were S. aureus, A. baumannii and P. aeruginosa. Almost all publications identify these three bacterial species as the main ones in the spectrum of nosocomial infections in burns, albeit with a difference in their order of frequency. Many of the published data indicate the leadership of S. aureus/MRSA as a nosocomial pathogen but in most Asian and Arab countries A. baumannii still prevails. These differences may be due to different local conditions, such as climate, topical and systemic treatment regimens, sampling procedures, infection prevention protocols as well as the study period.5 According to data for Turkey, published by Oncul O. et al.,1 the prevailing one is A. baumannii with quite higher rates than ours – more than half of isolates (57%), followed by A. baumannii (21%), with rates similar to ours, and S. aureus (14%), with lower rates than ours. Interestingly, the incidence of MRSA in their unit is similar to ours in 2009, but since then we have achieved a significant decrease. Alaghehbandan R. et al.,15 Azimi L. et al.,3 Ozkurt Z. et al.,4 and Nasrabadi B. and Hajia M.16 have published similar data. A. baumannii is the most frequent Gram-negative isolate in the majority of burn center, but in some clinics (including our own) it dropped to second place after MDR-A. baumannii.6,14,17 However, in many cases the isolation of A. baumannii was due to room contamination rather than infection.18 The increasing incidence of ESBL-producing Enterobacteriaceae in our burns clinic is worrisome. The decrease in their rate in 2010 was mainly due to the change in protocol that restricted the use of third generation cephalosporins, but afterwards it increased again (as this measure on its own is not enough to control their spread). ESBL-producing strains cause problems in other burn units around the world.4,7,8
A. baumannii and P. aeruginosa are noted for their intrinsic resistance to antibiotics and for their ability to acquire genes encoding resistance determinants. Data for antimicrobial resistance of A. baumannii published by other authors for patients with burns are similar to ours.3,4,14-16,19 Strains producing metalo-beta-lactamases (MBLs) are an emerging threat causing burn wound infections with increased mortality and morbidity and with a potential to spread rapidly resulting in outbreaks and epidemics.20 We therefore sent to the Bulgarian Reference Laboratory five carbapenem-resistant A. baumannii strains, isolated in 2011, for molecular analysis in order to determine their mechanisms of resistance and the possible involvement of MBL enzymes.
We believe that the main achievement of our system of control measures was the significant decrease in the rates of MRSA strains. We consider the extensive use of ActicoatTM wound dressings and the decolonization practices applied to the carriers to have made the greatest contribution to these results. The ActicoatTM dressing ensures a perfect antimicrobial barrier to critical colonization by killing a broad spectrum of microbial organisms without inducing resistance. We realized that after application of this particular dressing the MRSA incidence decreased and we adopted the decolonization procedures as part of our Clinic’s routine practice. The benefit of decolonization of the MRSA carriers was undoubtedly proven.21,22 Of course, all the other measures such as hand hygiene protocol, antimicrobial stewardship, contact precautions, etc., are also very important.
Although the incidence of NI in our clinic is lower than in our neighboring countries,1,14 there are several important infection control issues that we face at present: 1) the MDR A. baumannii strains; 2) carbapenem-resistant A. baumannii strains; and 3) ESBL-producing Enterobacteriaceae.
In order to solve the existing problems, we rely mainly on updating and strengthening compliance with the existing system of anti-infective control measures:
Antimicrobial stewardship. The use of carbapenems to treat A. baumannii and A. baumannii infections should be restricted because of the high levels of resistance to them, while the use of piperacillin/tazobactam, especially for Pseudomonas infections, should be encouraged. Tigecyclin should be included in the highest level of the restricted antimicrobial drugs in order to preserve its activity.
It is necessary to bolster contact precautions for patients colonized/infected with MDROs. Since it is not always possible to place the patient in a single room, ‘cohorting’, gathering in the same room of infected/colonized patients with the same MDRO, should be arranged.
Environmental measures. In addition to cleaning reinforcement, we also use linen and clothing impregnated with substances with antimicrobial activity to prevent the colonization of newcomers to the clinic with nosocomial pathogens.
Conclusion
The three most frequent nosocomial pathogens in the UMHATEM Pirogov Burns Clinic are S. aureus, A. baumannii and P. aeruginosa. A significant decrease in the rates of MRSA strains has been achieved since 2009, thanks to the infection control system that has been routinely implemented since 2008. Although at present the incidence of NI in our clinic is lower than in our neighboring countries, several important infection control issues still need to be solved. We rely mainly on updating and strengthening the existing system of anti-infective and anti-microbial resistance measures in order to control the spread of MDROs such as A. baumannii, ESBL-producing Enterobacteriaceae, and carbapenem-resistant P. aeruginosa.
Acknowledgments
The Authors thank the staff of the N.I. Pirogov Burns and Plastic Surgery Clinic, Laboratory of Clinical Microbiology, UMHATEM, the staff of the National Reference Laboratory of Staphylococci, NCIPD, Sofia, Bulgaria, and Mr Tanio Tanev for statistical support.
References
- 1.Oncul O, Ulkur E, Acar E, et al. Prospective analysis of nosocomial infections in Burn Care Unit, Turkey. Indian J Med Res. 2009;130:758–64. [PubMed] [Google Scholar]
- 2.Kumar A, Kashyap B, Mishra S, et al. Bacteriological analysis and antibacterial resistance pattern in burn sepsis: An observation at a tertiary care hospital in east Delhi. Infectious Diseases in Clinical Practice. 2011;19:406–12. [Google Scholar]
- 3.Azimi L, Mtevalian A, Ebrahimzadeh Namvar A, et al. Nosocomial infections in burned patients in Motahari hospital, Tehran, Iran. Dermatol Res Pract. 2011;436952:1–4. doi: 10.1155/2011/436952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozkurt Z, Altoparlak U, Iba Yilmaz S, et al. Reducing hospital infection rates in the burn unit by adherence to infection control measures & a six-year experience. Turk J Med Sci. 2012;42:17–24. [Google Scholar]
- 5.Guggenheim M, Zbinden R, Handschin AE, et al. Changes in bacterial isolates from burn wound and their antibiograms: A 20-year study (1986-2005). Burns. 2009;35:553–60. doi: 10.1016/j.burns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Bayat A, Shaaban H, Dodgson A, et al. Implications for burns unit design following outbreak of multi-resistant Acinetobacter infection in ICU and Burns Unit. Burns. 2003;209:303–6. doi: 10.1016/s0305-4179(03)00011-1. [DOI] [PubMed] [Google Scholar]
- 7.Shi MM, Zhao DM, Wang Q, et al. Analysis of drug resistance and risk factors of Enterobacteriaceae in burn units. Zhonghua Shao Shang Za Zhi. 2010;26:199–201. [PubMed] [Google Scholar]
- 8.Zorgani A, Franka RA, Zaidi MM, et al. Trends in nosocomial bloodstream infections in a burn intensive care unit: An eight-year survey. Ann Burns Fire Disasters. 2010;23:88–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenblatt-Farrell N. The landscape of antibiotic resistance. Environmental Health Perspectives. 2009;117:A244–50. doi: 10.1289/ehp.117-a244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan M. Antimicrobial resistance in the European Union and the World.. Conference on Combating Antimicrobial Resistance: Time for action; Copenhagen, Denmark. March 2012. [Google Scholar]
- 11.Garner JS, Jarvis WR, Emori TG, et al. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 12.Performance standards for antimicrobial susceptibility testing. Twentieth Information Supplement. M100-S20. 2010. p. 30. [Google Scholar]
- 13.Siegel JD, Rhinehart E, Jackson M, et al. Healthcare infection practices committee. Management of multidrug-resistant organisms in health care settings. Am J Infect Control. 2007;35:S165–93. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Alp E, Coruh A, Gunay GK, et al. Risk factors for nosocomial infection and mortality in burn patients: 10 years of experience at a university hospital. J Burn Care Research. 2012;33:379–85. doi: 10.1097/BCR.0b013e318234966c. [DOI] [PubMed] [Google Scholar]
- 15.Alaghehbandan R, Azimi L, Rastegar Lari A, et al. Nosocomial infections among burn patients in Teheran, Iran: A decade later. Ann Burns Fire Disasters. 2012;25:3–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Nasrabadi B, Hajia M. Multidrug-resistant P. aeruginosa strains in Burn Center Hospital, Tehran, Iran. Health MED. 2012;6:403–6. [Google Scholar]
- 17.Nowak P, Paluchowska P, Budak A. Distribution of blaOXA genes among carbapenem-resistant Acinetobacter baumannii nosocomial strains in Poland. New Microbiol. 2012;35:317–25. [PubMed] [Google Scholar]
- 18.Herruso R, de la Crus J, Fernandez MJ, et al. Two consecutive outbreaks of Acinetobacter baumannii 1-a in a burn intensive care unit for adults. Burns. 2004;30:419–23. doi: 10.1016/j.burns.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Lamia T, Bousselmi K, Saida BR, et al. Epidemiological profile and antibiotic susceptibility of Pseudomonas aeruginosa isolates within the burned patient hospitalized in the intensive care burn unit. Tunis Med. 2007;85:124–7. [PubMed] [Google Scholar]
- 20.Yogeesha Babu KV, Vijayanath V, Niranjan HP, et al. Study of imipenem resistant metallo-beta-lactamase positive Pseudomonas aeruginosa from burns wound infections, environmental sources and impact of infection control measures in a burns care center. J Pure and Applied Microbiology. 2011;5:695–703. [Google Scholar]
- 21.Orsi GB, Falcone M, Venditti M. Surveillance and management of multi-drug resistant microorganisms. Expert Rev Antiinfect Ther. 2011;9:653–679. doi: 10.1586/eri.11.77. [DOI] [PubMed] [Google Scholar]
- 22.Chan JD, Dellit TH, Choudhuri JA, et al. Active surveillance cultures of methicillin-resistant Staphylococcus aureus as a tool to predict methicillin-resistant S. aureus ventilator-associated pneumonia. Crit Care Med. 2012;40:1437–42. doi: 10.1097/CCM.0b013e318243168e. [DOI] [PubMed] [Google Scholar]