Abstract
Background
Asthma remains a serious global health challenge. Poor control of asthma symptoms is due in part to incorrect use of oral inhaler devices that deliver asthma medications, such as poor inhalation technique or use of a metered dose inhaler (MDI) after the recommended number of doses is expelled.
Objective
To review published research on the potential for patients to overestimate or underestimate the amount of asthma rescue medication in MDIs without integrated dose-counting mechanisms.
Methods
We searched PubMed and EMBASE using search terms “dose counter and asthma” and “dose counter and metered dose inhaler” for English language publications up to July, 2012, with a manual search of references from relevant articles.
Results
Up to 40% of patients believe they are taking their asthma medication when they actually are activating an empty or nearly empty MDI. Device design makes it impossible for an MDI to cease delivering drug doses at an exact point, and the number of actuations in an MDI may be twice the nominal number of recommended medication doses. Once the recommended number of medication doses is expelled, remaining actuations deliver decreasing concentrations of active medication and increasing concentrations of propellants and excipients. This phenomenon, called “tail-off,” is particularly problematic when medications are formulated as suspensions, as are rescue medications to control acute bronchospasm. Reliable inhalation of rescue medication could reduce asthma-related morbidity.
Conclusion
By helping to ensure that patients receive accurate metered doses of asthma rescue medication to relieve bronchoconstriction, dose counters may help to improve asthma management.
Keywords: asthma, beta agonists, dose counter, MDI, metered dose inhaler
Introduction
Asthma is a chronic inflammatory condition of the airways, characterized by limited airflow and punctuated by acute symptoms related to hyperresponsiveness to a variety of “triggers,” such as allergens or fumes (1). Chronic inflammation of the airways can lead to persistent alterations in airway structure, including sub-basement membrane fibrosis, mucus hypersecretion, injury to epithelial cells, smooth muscle hypertrophy, and angiogenesis (2). Asthma symptoms include wheezing, dyspnea, coughing, and chest tightness associated with broad but variable airflow obstruction in the lungs (3). During acute asthma events, symptom severity may range from mild to life-threatening, the latter being due to severe bronchospasm, airway edema, impaired gas exchange, and ultimately, respiratory failure.
Asthma continues to be a serious global health challenge. Worldwide, an estimated 300 million people suffer from the disease and prevalence is increasing (4–7). Of the 25 million Americans with asthma, 12 million experience acute symptoms (8) and asthma was linked to 3447 deaths (about 9 per day) in 2007 (5). Asthma symptoms account for about 500,000 hospitalizations and nearly 2 million emergency room visits per year in the United States (6).
Asthma-associated morbidity remains high despite improvements in diagnosis and the availability of comprehensive national and international clinical practice guidelines for managing the disease (2,3,9). Avoiding triggers is an important first step in asthma management, but may require lifestyle changes that patients find difficult or unacceptable (e.g., giving away a family pet), and adherence to lifestyle modifications is poor (1,5).
If lifestyle changes do not successfully prevent and control asthma symptoms, pharmacologic therapy can reduce the frequency and severity of asthma exacerbations, and reverse airflow obstruction during acute attacks (2). Most asthma medications are delivered as orally inhaled products in order to achieve local effects in the lung and to minimize systemic adverse effects. Inhaled asthma medications are categorized into two general classes: long-term control medications (also known as preventive or maintenance medications), which are taken regularly to achieve and maintain control of persistent asthma, and rapid-acting drugs (also known as rescue medications) taken as needed to provide prompt reversal of acute airflow limitation and relieve bronchospasm. Onset of action of inhaled drugs for rescue from acute bronchospasm is approximately 5–10 minutes. Rescue medications are typically short-acting β2-adrenergic agonists (SABAs), such as albuterol, but may be a long-acting beta agonist with rapid onset of action, such as formoterol (10). Bronchodilators provide relief of bronchoconstriction by relaxing bronchial smooth muscle and functionally enlarging the luminal diameter of the airways. This decreases airflow obstruction so that breathing becomes less labored. SABAs are delivered via wet nebulization or metered dose inhaler (MDI) (1). MDIs may provide better clinical outcomes and fewer adverse effects compared with nebulizers (11); however, nebulizers are useful for young children, older adults, and for patients who are unable to use an MDI.
Among other factors, patient nonadherence to therapy is an important contributor to poor asthma control. While often volitional, nonadherence can also be inadvertent when prescribed medications are taken improperly. For example, patients may think they are taking their asthma medication when they actually are activating a nearly empty or an altogether empty MDI to deliver orally inhaled asthma medication (12). MDIs deliver a limited number of effective medication doses, as listed in the prescribing information for each product. After the manufacturer-recommended number of doses is expelled, the MDI will continue to actuate many more times (13). Accurately assessing the doses in an MDI is critically important for bronchodilating medications used for “rescue” from acute asthma symptoms.
This literature review describes the growing evidence that people commonly—and significantly—overestimate (or less commonly, underestimate) the remaining amount of active asthma rescue medication in MDIs without dose counters, that the techniques used to “guesstimate” whether a recue MDI is effectively empty are unreliable, and how having a rescue MDI with an integrated dose counter mechanism can improve the health and quality of life of patients with asthma.
Methods
We searched EMBASE and MEDLINE English literature up to July, 2012 with search terms “dose counter and asthma” and “dose counter and metered dose inhaler.” When pertinent articles were identified, we searched for relevant references used in those papers. For environmental reasons, inhalers with chlorofluorocarbon (CFC) propellant were no longer sold in the US from 2009, and the propellant in current MDIs is hydrofluoroalkane (HFA); however, much of the research reviewed here was conducted on MDIs with CFC propellant.
Results
Activating a nearly empty or an altogether empty MDI to deliver orally inhaled asthma medication appears to be a common occurrence when patients use an MDI without a dose counter (12,14,15). MDIs contain more drug formulation than the labeled number of drug doses to ensure dosing consistency in each actuation, up to the labeled number (16). Short of recording every actuation, there is no accurate and practical way to gauge the remaining number of effective doses in an MDI without the addition of a dose-counting mechanism.
MDI Design Contributes to Dosing Errors
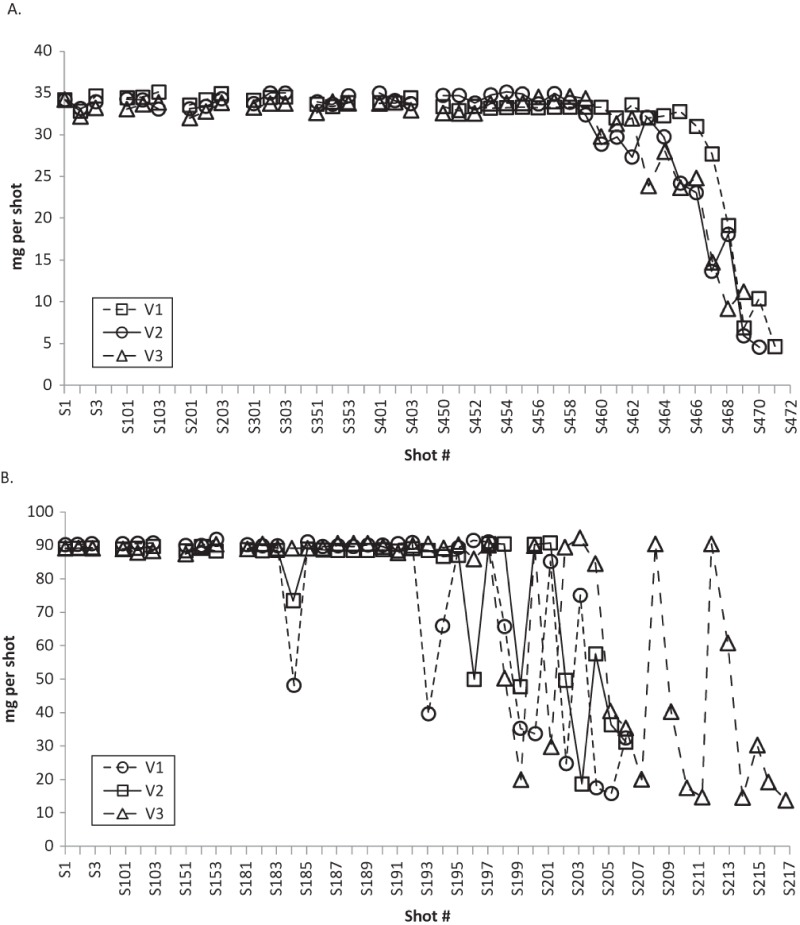
The invention of the metered-dose valve led to development of MDIs in the 1950s (13). The dosing and performance and, consequently, drug efficacy may be directly dependent on the design of the MDI. MDIs have 3 main components: the canister containing the drug formulation, the metering valve, which determines the quantity of formulation dispensed upon actuation, and an actuator (mouthpiece) which directs the aerosol into the patient’s lungs. In addition to the bronchodilating drug, the formulation contains a liquefied gas propellant (HFA) and may contain nonactive excipients. With current valve designs, it is not possible for an MDI to cease delivering drug doses completely at an exact point. MDIs continue to deliver a spray, which may not be within the labeled specifications for the active drug, for up to twice the nominal number of recommended doses. In one study, MDIs with CFC propellant had, on average, 86% more actuations than the labeled dose number, and MDIs with HFA propellant had 52% more (17). Importantly, the amount of drug in those additional actuations is variable; propellant and excipients form up to 99% of an asthma drug formulation (16). With continued use beyond the recommended number of doses, drug delivery per actuation becomes inconsistent and unpredictable, with the amount of active drug eventually becoming negligible, a phenomenon known as “tail-off” (13,18). Thus, after the recommended number of doses, the MDI may appear to be delivering a therapeutic spray when, in fact, it is not. Tail-off may be rapid (e.g., within 5 actuations), or erratic, requiring 10–20 actuations before the canister is finally empty of its drug contents, depending on several factors, including the valve design (13). MDIs with valves that have formulation “fill holes” at the base of the retaining chamber have more rapid and less erratic tail-off (Figure 1A) than MDIs with valves with fill holes at higher levels (Figure 1B). If the height of the liquid formulation falls below the fill hole, at the time of actuation there may be incomplete filling of the chamber (13). Tail-off is particularly problematic when the medication delivered by the MDI is formulated as a suspension rather than a solution. All short-acting asthma rescue medications currently available in an MDI are formulated as suspensions. Suspensions comprise micronized drug substance suspended in propellant and other excipients. If the drug substance adheres to the walls of the container or valve components, dose delivery and particle size distribution could be inconsistent (19). For this reason, shaking a rescue inhaler before actuation is an important part of correct MDI use.
Figure 1.—
Tail-off characteristics of asthma medications delivered by MDIs with different valve placement. Panel A shows tail-off from 3 separate MDIs with fill holes located at the base of the retaining cup that allow formulation to enter the valve (valve-down orientation). Panel B shows more erratic tail-off characteristics of 3 separate MDIs with valve design in which the fill holes are located at higher levels relative to the base of the retaining cup (Adapted from Schultz (13)).
Estimating Medication in an MDI with No Dose Counter
Studies conducted to assess the accuracy of asthma patients’ ability to gauge doses remaining in their MDIs have consistently shown high error rates (14,15,20,21). As noted, the only way to reliably determine the number of remaining doses in an MDI with no dose counting feature is by carefully and consistently tracking each dose, then subtracting it from the labeled number of doses. However, many people are not even aware of the recommended number of drug doses in their MDIs. In a study conducted to investigate how asthma sufferers determined when to replace their MDIs, 54% did not know the maximum number of actuations recommended by the manufacturer for their particular inhaler (15). Even when recommended dosing is known, patient records or diary entries of doses taken are rarely kept. In a study by Ogren et al., only 8% of patients kept any record of their MDI actuations (15). Most patients did not receive instructions from their health care providers to keep a count of doses taken; in a survey conducted by Sander et al., only 36% of respondents reported having been instructed to keep track of the number of MDI-delivered doses of their asthma medications (14).
Other methods by which patients estimate remaining medication in their MDIs are not reliable (13–15). Estimations of whether an MDI requires replacement based on the weight of the inhaler, or on the force, sounds, and taste of the actuation, are often made based on the volume of the remaining propellants and excipients and not on the remaining doses of active medication meeting therapeutic specifications (15,17). In a study by Rubin and Durotoye of pediatric asthma patients, 72% of the children (or their parents) reported using an MDI until they could no longer “hear” the MDI make a sound when it was actuated (17). Similarly, in the study by Sander and colleagues, 1 in 5 surveyed patients reported that they assessed their albuterol inhaler to be empty “when it stopped spraying,” unaware that propellant continues to spray long after the albuterol has run out (14). Many patients report shaking their MDIs to assess the amount of drug remaining in them (15,20,22). In one study, patients who adopted this method overestimated remaining medication by about 40 doses (21). Accordingly, 84% of MDIs evaluated in this study had been used well past the recommended number of actuations.
It has been suggested that floating an inhaler in water, known as a “float test,” can provide a measure of the inhaler’s useful contents (20). However, no universal flotation status accurately reflects when a device has reached the maximum recommended number of actuations (15). Moreover, this test is not only unreliable since some inhalers will float when they are full, it may damage the MDI by obstructing the metering valve (15–17).
Practical Experience
In practice, patients who are not tracking rescue medication doses often either discard an MDI that may still contain acceptable metered doses of drug, or continue to use a product that may no longer contain the labeled within-specification dosage. The former is wasteful and expensive and the latter is potentially dangerous (16). Holt and colleagues asked patients receiving asthma treatment to return their MDIs, when deemed empty, to their physicians (21). Of 109 returned MDIs, 11% of “empty” MDIs still contained more than 20% (>40 doses) of the recommended metered doses (Table 1) (21). Sander et al. reported that more than half (53%) of the asthma patients they surveyed who used an inhaled bronchodilator (N = 342) refilled their bronchodilator prescriptions more frequently than recommended in national guidelines (2,14). A bronchodilator prescription typically requires refill only a few times a year, yet almost 20% of patients reported refilling their inhaler at least once a month. The authors speculated that the excessive number of bronchodilator refills might be due, in part, to throwing away partially used inhalers to forestall the prospect of finding an inhaler empty during an acute asthma event (14).
Table 1.—
Discrepancies in patient perceptions of the number of doses of rescue medication remaining in an MDI with no dose counter. Seventeen patients with asthma who regularly use an MDI estimated the number of salbutamol doses remaining in MDIs that had been partially emptied to different degrees (Adapted from Holt et al. (21)).
| Actual number of remaining salbutamol doses in MDIs which had been partially emptied | 40 doses | 30 doses | 20 doses | 10 doses | 0 doses |
|---|---|---|---|---|---|
| Range of patient estimations of the number of doses in the MDI (min–max) | 0–180 | 0–190 | 0–140 | 0–180 | 0–80 |
| Mean difference between the actual and the estimated number of doses in the MDI | 76.8 | 81.0 | 63.0 | 54.8 | 23.6 |
| Proportionate overestimations of the remaining doses in the MDI | +36.8% | +51.0% | +43.0% | +44.8% | +23.6% |
While discarding an MDI with effective doses remaining is both costly and wasteful, relying on rescue medication from a depleted MDI during acute bronchospasm can be life-threatening. In the study by Holt and colleagues, collected MDIs labeled to contain 200 salbutamol doses were used, on average, to deliver 224 actuations (21). Two separate studies found that more than 40% of asthma patients replaced their MDI when it was completely empty and over 20% said they could “never tell” when their MDI was running out of medication (22,23). In the Sander et al. study, 87 asthmatic patients—25% of those surveyed—discovered that their albuterol inhaler was empty when needed for relief from acute asthma symptoms, and of them, 8% had to call for emergency assistance (14). Most of these 87 patients (82%) considered their MDI empty only when nothing came out of it, making it likely that they were inhaling only propellant for many doses, thereby increasing their risk of prolonged bronchoconstriction and airflow limitation requiring urgent care.
Patients Prefer Dose Counters
When asked, many patients report that not knowing how much medication is left in their MDIs makes them anxious about receiving a subtherapeutic dose or no medication at all (22,24). Dose counters are, therefore, likely to alleviate patient anxiety about running out of asthma medication in emergency situations. Thus, it is not surprising that patient satisfaction studies consistently show wide acceptance and approval of dose counters on MDIs (22–24). Patients rate dose counters among the top 5 best features of their MDI (14,23).
In addition to relieving anxiety, reliable knowledge of when to replace an MDI can improve asthma management and, in turn, improve patients’ quality of life (25). Because they are preferred by patients (22), adherence to prescribed asthma treatment might also improve. Moreover, physician inspection of dose counters at office visits may provide a means of evaluating patient adherence to treatment and present an opportunity to discuss appropriate use of the MDI and proper inhalation technique.
Discussion
In 2003, the US Food and Drug Administration issued a Guidance to Industry emphasizing the importance of (but not requiring) integrated dose counters or dose indicators on MDIs for orally inhaled medications targeted to the lungs (16). Dose indicators that rely solely on a color coded display or indicator symbol to signal when the MDI is nearing the end of its recommended doses are less precise than dose counters that use a numeric display (Figure 2). Typically, discarding an MDI is recommended when the dose counter reads “0” or by the expiration date, whichever comes first. The expiration date for a rescue inhaler is generally 1 year after the prescription is filled (26) or after the foil pouch is opened (27). At present, the only available albuterol rescue inhalers with integrated dose counting mechanisms are ProAir® HFA (Teva Pharmaceutical Industries, Ltd.) (26) and Ventolin® HFA (GlaxoSmithKline) (27).
Figure 2.—

Integrated dose counter on a metered-dose inhaler. Shown here is a ProAir® metered-dose inhaler (manufactured by Teva Pharmaceutical Industries, Ltd., Horsham, PA).
If there is a “down-side” to integrated dose counting mechanisms on MDIs, it may be that they are more expensive to produce than MDIs without them. After 2008, generic albuterol inhalers stopped being made because they contained CFC propellant (it is unknown when generic albuterol inhalers may be available again). The economic burden of asthma management is already considerable. In 2009, the estimated annual expenditure related to health care and lost productivity due to asthma was more than $20 billion according to the National Heart, Lung, and Blood Institute (28). Nevertheless, dose counters may reduce health care costs by decreasing asthma-related morbidity and attendant medical costs (14,17). For example, inhalation of rescue medication rather than propellant to control acute bronchospasm and quell acute asthma symptoms may avoid the cost of an emergency room visit (29).
Conclusion
Although there have been relatively few studies conducted to assess the use of MDIs, they consistently show patients frequently overestimate the amount of active asthma rescue medication in MDIs without dose counters. Dose counters provide the only accurate and practical method of ascertaining the remaining number of effective doses in an MDI. By ensuring that patients do not use a rescue MDI beyond the recommended number of actuations and that they are receiving the appropriate metered dose of asthma medication, dose counters can improve asthma management and potentially decrease asthma-related morbidity and mortality, and improve patients’ quality of life. For these reasons, integrated dose counting mechanisms should be required on MDIs that deliver asthma rescue medication.
Acknowledgments
We thank Mark Lepore, MD, Teva Global Respiratory Research and Development, Teva Pharmaceuticals, Frazer, PA, for his careful review and helpful suggestions during manuscript development.
Declaration of interest
Drs. Conner and Buck are employed by Teva Pharmaceutical Industries, Ltd., Kansas City, Missouri. Sheila Truten, Medical Communication Company, Inc., Wynnewood, PA, provided editorial assistance during manuscript development.
References
- 1.Kim H, Mazza J. Asthma. Allergy Asthma Clin Immunol. 2011;7(Suppl 1):S2. doi: 10.1186/1710-1492-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. http://www.ncbi.nlm.nih.gov/books/NBK7232/ National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Heart, Lung, and Blood Institute. NIH Publication 07–4051. 2007. Available at. Accessed June 14, 2012.
- 3.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FizGerald M, Gibson P, Ohta K, O’Byrne P, Pdersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 4. http://www.lung.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf American Lung Association. Trends in asthma morbidity and mortality. July, 2011. Available at. Accessed June 14, 2012.
- 5. http://www.cdc.gov/VitalSigns/Asthma/ Asthma in the US. Growing every year. Available at. Accessed February 6, 2012.
- 6. http://www.epa.gov/asthma/pdfs/asthma_fact_sheet_en.pdf United States Environmental Protection Agency. Asthma facts. 2011; EPA-402-F-04-019. Available at. Accessed June 14, 2012.
- 7. http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx Asthma statistics. Available at. Accessed February 6, 2012.
- 8.Sullivan PW, Ghushchyan VH, Slejko JF, Belozeroff V, Globe DR, Lin S-L. The burden of adult asthma in the United States: Evidence from the Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2011;127:363–369. doi: 10.1016/j.jaci.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Gould MK, Blanc PD, Miller DP, Kamath TV, Lee JH, Sullivan SD. Asthma control, severity, and quality of life: Quantifying the effect of uncontrolled disease. J Allergy Clin Immunol. 2007;120:396–402. doi: 10.1016/j.jaci.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Lee-Wong M, Chou V, Ogawa Y. Formoterol fumarate inhalation powder vs albuterol nebulizer for the treatment of asthma in the acute care setting. Ann Allergy Asthma Immunol. 2008;100:146–152. doi: 10.1016/S1081-1206(10)60423-1. [DOI] [PubMed] [Google Scholar]
- 11.Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;2:CD000052. doi: 10.1002/14651858.CD000052.pub2. April 19; doi: 10.1002/14651858.CD000052.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Rubin BK. What does it mean when a patient says, My asthma medication is not working? Chest. 2004;126:972–981. doi: 10.1378/chest.126.3.972. [DOI] [PubMed] [Google Scholar]
- 13.Schultz RK. Drug delivery characteristics of metered-dose inhalers. J Allergy Clin Immunol. 1995;96:284–287. doi: 10.1016/s0091-6749(95)70207-5. [DOI] [PubMed] [Google Scholar]
- 14.Sander N, Fusco-Walkert SJ, Harder JM, Chipps BE. Dose counting and the use of pressurized metered-dose inhalers: Running on empty. Ann Allergy Asthma Immunol. 2006;97:34–38. doi: 10.1016/s1081-1206(10)61366-x. [DOI] [PubMed] [Google Scholar]
- 15.Ogren RA, Baldwin JL, Simon RA. How patients determine when to replace their metered-dose inhalers. Ann Allergy Asthma Immunol. 1995;75:485–489. [PubMed] [Google Scholar]
- 16. http://www.fda.gov/cder/guidance/index.htm Guidance for Industry: Integration of dose-counting mechanisms into MDI drug products. Available at. Accessed June 14, 2012.
- 17.Rubin BK, Durotoye L. How do patients determine that their metered-dose inhaler is empty? Chest. 2004;126:1134–1137. doi: 10.1378/chest.126.4.1134. [DOI] [PubMed] [Google Scholar]
- 18.Hess DR. Aerosol delivery devices in the treatment of asthma. Respir Care. 2008;53:699–723. [PubMed] [Google Scholar]
- 19. http://www.fda.gov/downloads/Drugs/Guidances/ucm070573.pdf Guidance for Industry. Metered dose inhaler (MDI) and dry powder inhaler (DPI) drug products. Chemistry, manufacturing and controls. Available at. Accessed March 12, 2013.
- 20.Rickenbach MA, Julious SA. Assessing fullness of asthma patients’ aerosol inhalers. Br J Gen Pract. 1994;44:317–318. [PMC free article] [PubMed] [Google Scholar]
- 21.Holt S, Holt A, Weatherall M, Masoli M, Beasley R. Metered dose inhalers: A need for dose counters. Respirology. 2005;10:105–106. doi: 10.1111/j.1440-1843.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman RL, Sheth K, Lincourt WR, Locantore NW, Carranza-Rosenzweig J, Crim C. Real-world assessment of a metered-dose inhaler with integrated dose counter. Allergy Asthma Proc. 2006;27:486–492. doi: 10.2500/aap.2006.27.2921. [DOI] [PubMed] [Google Scholar]
- 23.Sheth K, Wasserman RL, Lincourt WR, Locantore NW, Carranza-Rosenzweig J, Crim C. Fluticasone propionate/salmeterol hydrofluoroalkane via metered-dose inhaler with integrated dose counter: Performance and patient satisfaction. Int J Clin Pract. 2006;60:1218–1224. doi: 10.1111/j.1742-1241.2006.01138.x. [DOI] [PubMed] [Google Scholar]
- 24.LaForce C, Weinstein C, Nathan RA, Weinstein SF, Staudinger H, Meltzer EO. Patient satisfaction with a pressurized metered-dose inhaler with an integrated dose counter containing a fixed-dose mometasone furoate/formoterol combination. J Asthma. 2011;48:625–631. doi: 10.3109/02770903.2011.587579. [DOI] [PubMed] [Google Scholar]
- 25.Pereira ED, Cavalcante AG, Pereira EN, Lucas P, Holanda MA. Asthma control and quality of life in patients with moderate or severe asthma. J Bras Pneumol. 2011;37:705–711. doi: 10.1590/s1806-37132011000600002. [DOI] [PubMed] [Google Scholar]
- 26.ProAir® HFA (albuterol sulfate) Inhalation Aerosol prescribing information. Teva Respiratory, LLC; Horsham, PA: Rev 03/2012. [Google Scholar]
- 27.Ventolin® HFA (albuterol sulfate) prescribing information. GlaxoSmithKline; Research Triangle Park, NC: Rev 10/2012. [Google Scholar]
- 28. http://www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf National Heart, Lung, and Blood Institute. Chartbook on Cardiovascular, Lung and Blood Diseases. Available at. Accessed February 1, 2012.
- 29.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]