Abstract
Since nanomaterials are a heterogeneous group of substances used in various applications, risk assessment needs to be done on a case-by-case basis. Here the authors assess the risk (hazard and exposure) of a glass cleaner with synthetic amorphous silicon dioxide (SAS) nanoparticles during production and consumer use (spray application). As the colloidal material used is similar to previously investigated SAS, the hazard profile was considered to be comparable. Overall, SAS has a low toxicity. Worker exposure was analysed to be well controlled. The particle size distribution indicated that the aerosol droplets were in a size range not expected to reach the alveoli. Predictive modelling was used to approximate external exposure concentrations. Consumer and environmental exposure were estimated conservatively and were not of concern. It was concluded based on the available weight-of-evidence that the production and application of the glass cleaner is safe for humans and the environment under intended use conditions.
Keywords: Amorphous silica, SiO2, nanotechnology, nanomaterial, consumer product
Introduction
With growing expectations towards the benefits of nanotechnology, an increasing concern with respect to potential health and environmental risks, in particular those of nanomaterials, can be observed in the general public and the scientific community (Borm et al. 2006b; Kreyling et al. 2006; Krug & Wick 2011; Nel et al. 2006; Royal Academy of Engineering 2004). Various suggestions have been put forward to define the term “nanomaterial” (EU 2011; International Organization for Standardization 2008; SCENIHR 2010) usually addressing dimensions below 100 nm.
General concerns are expressed that due to the small size of nanoparticles and their corresponding large specific surface area a greater biological reactivity may lead to negative health implications. Specific concerns have been raised that nanoparticles with a fibrous shape and a high biopersistence may have asbestos-like hazardous properties due to their dimensions (Donaldson et al. 2010; Poland et al. 2008). Moreover, the role and importance of a potential translocation of nanoparticles from the primary site of entry to secondary organs and the resulting effects caused by enhanced biodistribution is a matter of debate (Borm et al. 2006a; Kreyling et al. 2006; Oberdörster 2009). Acknowledging the heterogeneity of this class of materials, the European Commission's Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) suggested that the risk assessment of nanoparticles should be carried out on a case-by-case basis by using a staged approach, including specific hazard identification and exposure analysis (SCENIHR 2007).
To the authors' knowledge, only few case studies have been published demonstrating the risk assessment for the use of nanomaterials in specific products or applications (e.g. Dekkers et al. 2011; DuPont 2007). Here the authors provide a specific risk assessment for colloidal synthetic amorphous silicon dioxide (SAS) in a glass cleaner formulation intended for consumer use in Europe, which was conducted for different life cycle steps with a special focus on the use by consumers and the potential effects on humans and the environment.
The variety of silicon dioxide specifications has been described elsewhere (ECETOC 2006). Amorphous silicon dioxide also exists in nature, for example as diatomite, and differs in its morphology from the crystalline form. It is assumed that the major load of silicon dioxide in natural surface water originates from geogenic and weathering processes (Miretzky et al. 2001) and is subject to significant seasonal variations.
SAS has been in human use for decades, for example as a food additive (E 551) without any limitation for a certain particle size (EU 2008a), but also as filler in the rubber industry, as auxiliary material in paper and textile industry or as anticaking agent for various drug preparations.
Materials and methods
Description of the raw material: colloidal SAS
The raw material used in the formulation of the glass cleaner is a colloidal dispersion of pure discrete spherical uncoated silicon dioxide particles (30% (w/w), CAS 7631-86-9, EINECS 231-545-4) in water. According to the supplier, the dispersion is electrostatically stabilised by small amounts of sodium hydroxide (0.55% as Na2O titrated value) (EKA Chemicals AB 2003). Residues of individual trace metals are below 40 ppm, except for Al (140 ppm). The material characterisation is summarised in Table I. Shape and particle size of the colloidal SAS were investigated by transmission electron microscopy (TEM) with a Philips CM 12 microscope (Philips, Eindhoven, The Netherlands). The raw material was sprayed on a copper grid coated with a Formvar® film (spray preparation). The micrographs were taken with 120 kV in a bright field mode. In addition, particle size distribution was investigated by dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern Instrument Ltd., Malvern, UK). The distribution is expressed in vol%. After applying the raw material on a glass slide, particles were investigated by atomic force microscopy (AFM) with a Nanoscope III microscope (Vecco Digital Instruments, Inc. Santa Barbara, CA, USA). Measurements were made in none contact tapping mode (amplitude).
Table I.
Characterisation and physico-chemical properties of the raw material: colloidal SAS.
| Properties | Description | Reference |
|---|---|---|
| Molecular formula | SiO2 | |
| Molecular weight* | 60.08 g/mol | |
| Morphology | Amorphous | EKA Chemicals AB, 2010 |
| Appearance | Transparent liquid | EKA Chemicals AB, 2003 |
| Density | 1.2 g/cm³ (20°C) | EKA Chemicals AB, 2003 |
| pH | 10 | EKA Chemicals AB, 2003 |
| Particle size | 9 nm (monodiperse) | EKA Chemicals AB, 2003 |
| Particle size distribution | 10–20 nm (TEM) 4–40 nm (DLS) |
See characterisation |
| Surface charge | Negative | |
| Surface area | 360 m²/g | EKA Chemicals AB, 2003 |
| Coating | None | |
| Log Pow* | 0.53 | US EPA, 2008 |
| Vapour pressure | 2300 Pa | EKA Chemicals AB, 2010 |
| Water solubility | 120 mg/l | Alexander et al. (1954) |
*Values relate to the monomeric structure; DLS, dynamic light scattering; SAS, synthetic amorphous silicon dioxide; TEM, transmission electron microscopy.
Glass cleaner formulation and packaging
It has previously been shown that application of particle dispersions on hard ceramic surfaces can lead to self-organisation of the nanoparticles resulting in enhanced drainage, increased drying speed and reduced re-soiling compared with the conventional technology (Dreja et al. 2004). These effects were elaborated for the development of a glass cleaning product for consumer use. Colloidal SAS is added to the formulation in order to modify the glass surface. During drying the hydroxyl groups of the colloidal SAS can react by forming siloxane bonds linking the particles together or to the surface, thus leading to enhanced hydrophilicity. With this modification the water film drains fast and homogeneously resulting in improved cleanliness.
Suitable surfactants, solvents and other cleaning components are also contained but cannot be fully disclosed due to intellectual property rights. General information can be obtained under the US Patent US7745383 (Dreja et al. 2010) or the German Patent Application DE102004019022A1 (Dreja et al. 2005). The colloidal silicon dioxide is applied with 0.3% (w/w) in the formulation resulting in a concentration of 0.09% (w/w) of silicon dioxide. The product is packed and marketed in a trigger bottle. One spray shot has a volume of about 2 ml, which corresponds to roughly 2 g of the product.
Risk assessment strategy
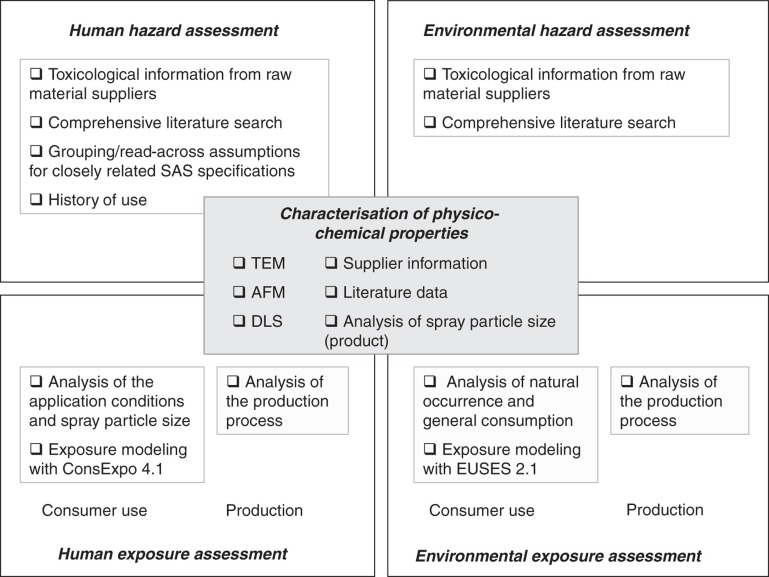
Figure 1 provides an overview of the methodological approach and the tools applied in the present assessment. A central element is the physico-chemical characterisation of the substance. The assessment of its toxicological and ecological properties is based on various data sources including a comprehensive literature review and read-across analyses. Exposure assessment includes the production process and the application by consumers for each the human and environmental assessment. The elements are described in more detail in the following chapters (Literature search and review, Determination of the particle size distribution of the glass cleaner aerosol, Modelling of consumer exposure using the software ConsExpo 4.1, Modelling of environmental exposure using the software EUSES 2.1 and the result section).
Figure 1.
Overview on the methodological approach and building blocks of the risk assessment. Methods mentioned in detail indicate experimental analyses performed in this case study.
Literature search and review
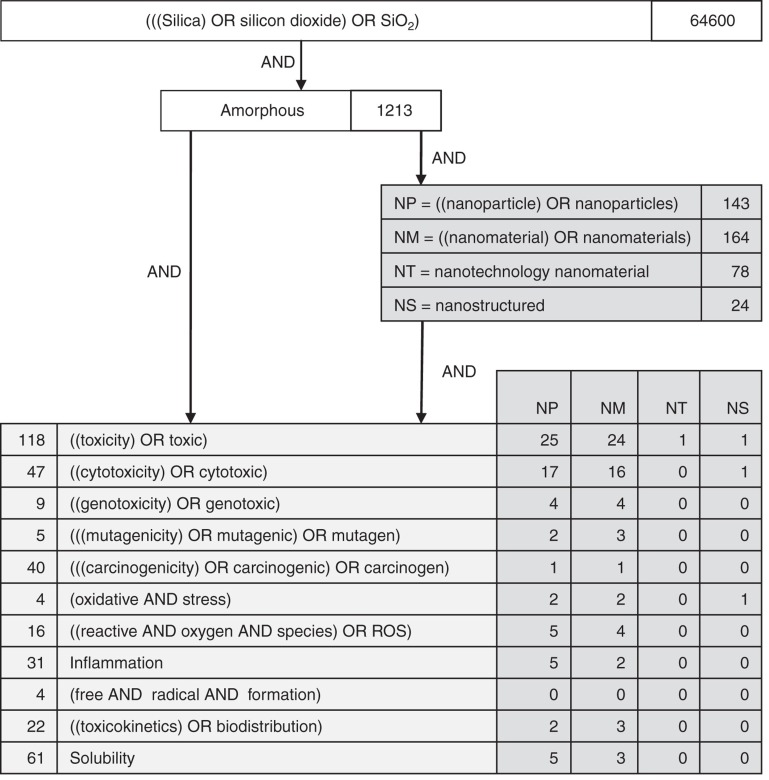
In addition to information provided by the raw material supplier, a literature search on the toxicological and ecological properties of SAS was performed in various databases and internet sources (PubMed, Medline, IUCLID) in February 2011 (Figure 2). Articles were selected by screening the titles and/or abstracts for relevant toxicological and ecological information on SAS. Additional references were identified through cross-references and searches on specific topics, resulting in 18 most relevant articles including some comprehensive reviews that were selected for detailed review based on well-documented materials and procedures.
Figure 2.
Literature search for toxicological information: the search terms and links between search terms are given as well as the number of hits.
Determination of the particle size distribution of the glass cleaner aerosol
Particle size distribution of the aerosol droplets was measured. For this purpose, six trigger bottles of the glass cleaner were examined in quintuplicate, three with open sieve at the trigger (resulting in a spray) and three with closed sieve (resulting in a foam) (see results, Table II, “Formulation 1”). An independent repeat was conducted with another six bottles of another batch (see results, Table II “Formulation 2”). The studies were conducted in compliance with the Principles of Good Laboratory Practice (EU 2004).
Table II.
Aerosol droplet size distribution of the glass cleaner.
| Test item no. | Dv1 (10%) (µm) | Dv1 (50%) (µm) | Dv1 (90%) (µm) | Cv2 (%) at 3.97 µm | Cv2 (%) at 10.44 µm |
|---|---|---|---|---|---|
| Formulation 1: spray/open sieve | |||||
| 1 | 30.933 | 82.06 | 181.96 | 0.00 | 0.82 |
| 2 | 39.69 | 113.34 | 201.98 | 0.00 | 0.46 |
| 3 | 32.28 | 90.55 | 188.35 | 0.00 | 0.68 |
| Mean | 34 | 95 | 191 | 0.0 | 0.7 |
| Formulation 1: foam/closed sieve | |||||
| 4 | 56.03 | 126.61 | 202.75 | 0.00 | 0.19 |
| 5 | 55.30 | 128.98 | 206.88 | 0.00 | 0.21 |
| 6 | 54.23 | 125.83 | 205.62 | 0.00 | 0.22 |
| Mean | 55 | 127 | 205 | 0.0 | 0.2 |
| Formulation 2: spray/ open sieve | |||||
| 1 | 29.433 | 83.96 | 185.08 | 0.00 | 0.84 |
| 2 | 29.57 | 80.47 | 186.01 | 0.00 | 0.88 |
| 3 | 23.99 | 60.69 | 161.88 | 0.00 | 1.52 |
| Mean | 28 | 75 | 178 | 0.0 | 1.1 |
| Formulation 2: foam/ closed sieve | |||||
| 4 | 52.87 | 126.11 | 205.25 | 0.00 | 0.22 |
| 5 | 57.33 | 128.91 | 207.12 | 0.00 | 0.19 |
| 6 | 51.67 | 127.93 | 205.03 | 0.00 | 0.25 |
| Mean | 54 | 128 | 206 | 0.0 | 0.2 |
1Dv (xx %): average aerosol droplet diameter at a given percentage (xx) of the cumulative volume, for example a value of Dv (10%) = 30.93 µm for the test item no. 1 (open sieve) means that 10% of all droplets (volume share) had diameters <30.93 µm; 2Cv: average cumulative volume at a given droplet diameter, for example a value of Cv (at 10.44 µm) = 0.82% for the test item no. 1 (open sieve) means that 0.82% of all droplets (volume share) had diameters <10.44 µm; 3Numbers are an average of five measurements.
Equipment and set-up
The particle size analyser Spraytec RTS 5006 with workstation (Malvern Instruments GmbH, Germany) was used with the following settings: laser wavelength: 670 nm; focal length: 100 nm; distance of dispersion unit from optical path/detector: 60 nm/150 nm; mode: flash mode; acquisition rate: 1 Hz (measurement per second), duty cycle: 0% (single scan); data acquisition period: 5000 ms; trigger: transmission 95%. According to specifications, the instrument can detect particles down to a size of 500 nm.
Measurement
Background measurements were performed and fulfilled the specified requirements (transmission >1500 and signal intensity for each detector ring <50). Samples were manually sprayed into the laser stream for 5 s in a 90° angle (approx. 20 strokes). The particle size distribution was recorded. Results are reported as average particle diameters at given percentages (10%, 50% and 90%) of the cumulative volume and as cumulative volume at particle diameters of 3.97 and 10.44 µm
Modelling of consumer exposure using the software ConsExpo 4.1
Consumer exposure was modelled with the software ConsExpo 4.1. (2008, Rijksinstituut voor Volksgezondheid en Milieu). Where applicable, default parameter values were used (selection of glass cleaner from the defaults database: product databases: cleaning and washing; product categories: miscellaneous; scenarios: application: spraying). In the following cases parameters were adapted: weight fraction compound: 0.1%; application frequency: 3/day (corresponds to cleaning of windows in three rooms), spraying away from exposed person; spray duration 30 s (sum per room); exposure duration in one room: 10 min; room size 58 m³; mass generation rate: 2 g/s (1 stroke per second); ventilation rate (as worst-case): 0.5/h; particle distribution median: 100 µm (default value), particle distribution coefficient of variation 0.6 fraction (default value); inhalation cut-off diameter: 100 µm (to be comparable with the workplace exposure limit of SAS which refers to total inhalable dust); uptake fraction 1 (= 100% of the substance in the air will be inhaled, worst-case assumption); inhalation rate: 32.9 m³/day (default based on light exercise of a person with a body weight of 60 kg). The result is given in the output section of the software as inhalation point estimate. To account for accidental spraying towards a person, one parameter was modified (tick box at “spraying towards exposed person”, cloud volume 0.125 m³) and the air concentration calculated separately.
Modelling of environmental exposure using the software EUSES 2.1
Environmental exposure is modelled by the European Union System for the Evaluation of Substances (EUSES), a computer tool (v. 2.1). It is based on the European technical guidance documents for risk assessment of new and existing substances and biocides (EU 2008b). Due to the variety of applications the emissions were assumed to enter wide dispersive into the environment and not in form of a few single local point sources. Explanations for tonnages used will be given in Chapter 34.3 (Exposure assessment for the consumer use of the glass cleaner).
Results: risk assessment
The risk of colloidal SAS in a glass cleaner intended for consumer use is assessed. Taking the identified hazardous properties and the exposure information into account and applying a weight-of-evidence approach, a final conclusion for the production and use of the cleaner is drawn. The evaluation is also put into perspective with the widespread occurrence and use of silicon dioxides.
Characterisation and physico-chemical properties of the raw material and comparison with other SAS types
Characterisation
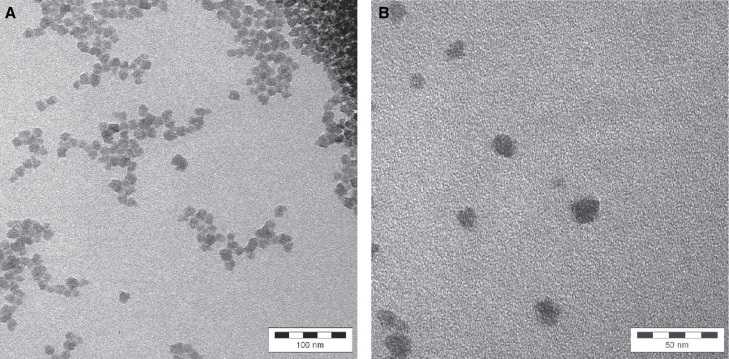
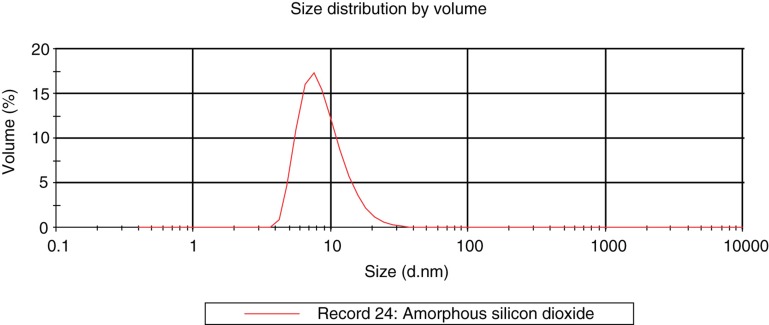
Particle size, size distribution, shape and aggregation state of the colloidal SAS were investigated by TEM (Figure 3) and DLS (Figure 4). Particles occur as individual objects of spherical shape, with many particles being aggregated or agglomerated. The stated particle size of 9 nm could be confirmed with the detected size ranging from about 10 to 20 nm as analysed by TEM and 4 to 40 nm by DLS.
Figure 3.
Analysis of size, shape and aggregation state of SAS particles in the raw material by transmission electron microscopy. A. Besides single particles, the state of aggregation and agglomerate on can be seen with lower resolution (1:250,000). The whole bar is 100 nm and one segment of it is 20 nm. B. With higher resolution, the size of the individual particles becomes more visible (1:560,000). The whole bar is 50 nm and one segment of it is 10 nm.
Figure 4.
Particle size distribution of the raw material by dynamic light scattering.
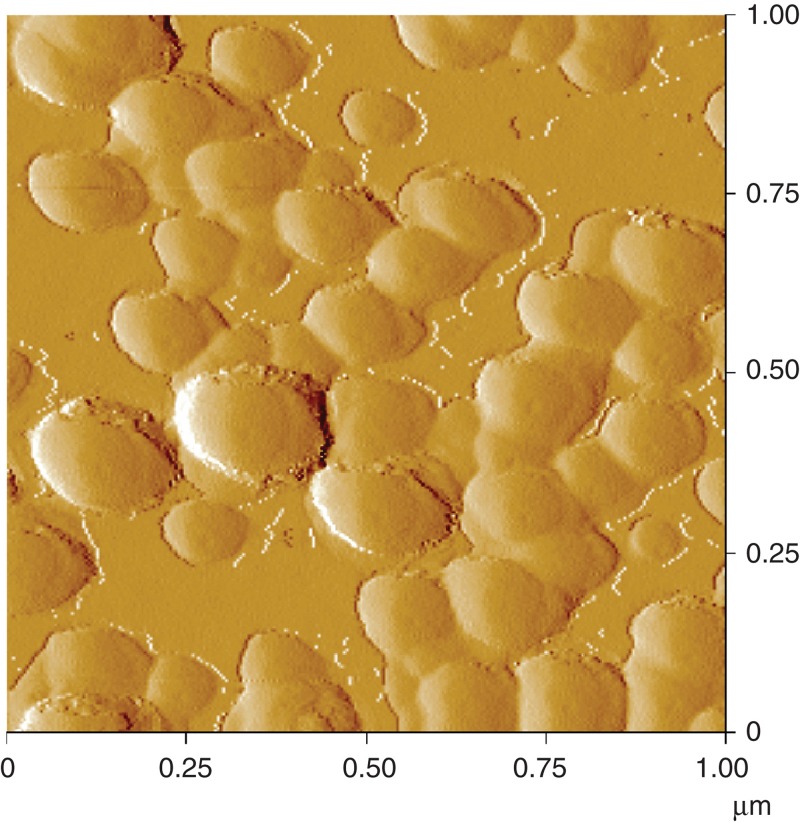
In order to investigate the appearance of the colloidal SAS on the glass surface, the surface topography was measured by AFM. A rough structure with spherical elevations could be observed for the surface treated with the colloidal SAS. The elevations comprise a size range of about 60–200 nm (Figure 5). From the distorted edges it is concluded that the primary particles of colloidal SAS agglomerated on the glass surface which resulted in larger assemblies.
Figure 5.
Analysis of the colloidal SAS applied on a glass slide by atomic force microscopy.
Physico-chemical properties and comparison with other SAS types
Physico-chemical properties of SAS are also specified in Table I. Specific properties of the colloidal SAS contained in the raw material were compared with properties of other SAS types which have been investigated and assessed before. Although different specifications of commercial SAS exist due to variations in the production processes, similarities in composition and in physico-chemical properties have previously justified read-across assumptions in a category approach for hazard characterisation (CIR 2009; OECD 2004; ECETOC 2006; Fruijtier-Pölloth 2012). For the purpose of the present risk assessment particle size, surface properties and solubility of SAS are described in more detail.
Particle size and surface properties
Commercial SAS usually have particle sizes between 1 and 350 µm referring to aggregates and agglomerates with primary particle sizes in the order of a few nanometres (AGS 2006; OECD 2004). The primary particles of SAS have not only recently been determined to be at the nanoscale but have been in that size already in the past. Although primary particles usually aggregate and agglomerate, it is very likely that single particles have been present in the studies conducted for the various types of SAS though the specifications mostly have not been reported in such detail. Colloidal SAS consists of primary particles and aggregates thereof in the liquid system which typically agglomerate irreversibly on drying (ECETOC 2006). This is in accordance with the investigations applying AFM.
SAS are hydrophilic if not surface-modified. This is also the case for the colloidal SAS in the current application. Silanol groups on the surface are neutralised with caustic soda for reasons of stabilisation resulting in a relatively inert structure. Regarding the specific surface area, the agglomerated powders of pyrogenic or precipitated SAS, for which most of the toxicological data have been generated, have a specific surface area measured by BET (Brunauer, Emmett and Teller) method comparable with a dried colloidal SAS sample (ECETOC 2006). For the colloidal SAS used in the glass cleaner, it seems therefore unlikely that hazardous effects specifically related with the particle surface differ from the well-investigated hydrophilic SAS.
Solubility
Dissolution of solid silicon dioxide to monosilicic acid follows the overall equation: SiO2 + 2 H2O ⇆ Si(OH)4. The solubility of amorphous and crystalline silicon dioxide has been investigated and is reflected in the report on SAS published by ECETOC (2006). Whereas quartz exhibited a low solubility of 5 mg/l, dissolved SAS had a saturation concentration of 2.0 mmol/l (120 mg/l) at 25°C (ECETOC 2006; Roelofs & Vogelsberger 2004). Early experiments on solubility of different SAS forms revealed a steady state equilibrium solubility of SAS powder (specific surface 240 m2/g) reached within 20 days in distilled water. The solubility reached 140 mg/l free monomeric silicon dioxide in solution determined by spectrophotometry of an at least 3 g/l nominal powder concentration (Alexander et al. 1954). The authors also investigated solubility of alkaline and acid colloidal SAS and silicon dioxide gels. The occurrence of monomeric silicon dioxide in solution was found to slightly increase with increasing pH. Hence, solubility increased from acid to alkaline environment from 100 to 150 mg/l and was thus well comparable with the solubility of silicium dioxide powder. In more recent experiments, pyrogenic and precipitated types of SAS (surface area 192–376 m²/g (BET), size of primary particles 7–14 nm) have been dissolved under biomimetic conditions in a buffer system and in simulated extracellular lung fluid at 37°C (pH 7.4, suspension of 1 g/l). Analytical measurements were done using the molybdic acid method referenced in the publication. The experimental and theoretical investigations demonstrated that particles with smaller size (larger surface area) reached higher maximum silicon dioxide concentrations (2.7 mmol/l). For all SAS types, the saturation concentration was reached within several hours (∼50 h). The authors concluded that small amounts of silicon dioxide nanoparticles should be quickly dissolved in a biological environment such as extracellular lung fluid (Roelofs & Vogelsberger 2004).
Summary of physico-chemical properties and possible implications for hazard assessment
All types of SAS share the same chemical composition, a primary particle size in the nanometre scale and a strong tendency to form aggregates and agglomerates in a dry state as also demonstrated for the raw material described here. Moreover, free silanol groups at the surface of all those types of non-modified SAS render them hydrophilic. Size, state of aggregation and surface characteristics largely determine the properties of SAS and justify treating the different types as one substance. With regard to their behaviour, there are indications that colloidal SAS dissolves in biological fluids (Roelofs & Vogelsberger 2004). Therefore, it can be assumed that the toxicological profile of various forms of SAS is comparable. This has been confirmed by a review paper on the mode of action of SAS published recently (Fruijtier-Pölloth 2012). Based on these considerations, most of the published data (ECETOC 2006; OECD 2004) on hazardous effects of SAS are considered to apply also to the hazard assessment of the colloidal SAS used in the glass cleaner.
Hazard assessment of SAS
Toxicological profile
The toxicological profile of SAS has been described in various summary reports (CIR 2009; DFG 1989; ECETOC 2006; OECD 2004). It has been evaluated as food additive for its safety by the Joint FAO/WHO Expert Committee on Food Additives (WHO 1974) and, moreover, has a long history of safe use in different applications.
Since the described glass cleaner involves colloidal SAS, the focus has been on data related to this morphology. It must be noted that crystalline silicon dioxide (quartz), for which pulmonary diseases like silicosis, chronic bronchitis and lung cancer are well known (IARC 1997), has significantly different properties. An initial literature search (Figure 2) with the search terms “silica” OR “silicon dioxide” OR “SiO2” resulted in 64,600 hits, demonstrating a huge amount of available data but not yet differentiated for the various existing forms. Narrowing down the search to amorphous silicon dioxide forms, nanoscale material and specific end points largely reduces the number of hits. Evidence shows that the overall toxicity of SAS is low. SAS is not classified as dangerous following the criteria of the EU Regulation on the “Classification, Labelling and Packaging of Substances and Mixtures” (EU 2008c; ECETOC 2006; EKA Chemicals AB 2010).
Toxicokinetics
Regarding adsorption, deposition and elimination of SAS it has been reported that there is little accumulation in the body, as this substance is eliminated by dissolution in the lung fluid, excreted in the faeces or eliminated via urine in animals and humans (OECD 2004). Although no skin penetration data for pure SAS are available, data for silicon dioxide-coated particles indicate that penetration through skin for such particles is unlikely (Gamer et al. 2006). This might be due to the chemical composition and surface properties. The hydrophilic character of uncoated SAS does not favour penetration. It could be demonstrated by in vivo experiments with SAS and subcutaneous application in rats that even if penetration happened, the substance would be eliminated up to approx. 97% within 6 weeks (pyrogenic SAS, 30, 40 or 50 mg as dispersion in water or 0.5% Tween or as dry powder), meaning that it is subject to dissolution and removal from the body (ECETOC 2006). The skin is thus not considered a relevant portal of entry of hydrophilic SAS. This is also supported by various publications on skin penetration of other nanoparticles, mainly zinc and titanium nanoparticles, some coated with silicon dioxide or with hydrophobic material, which suggest that such particles do not penetrate through the stratum corneum and epidermis and do not reach living tissue in the deeper regions of the skin (Butz 2009; Gamer et al. 2006; Mavon et al. 2007; Pfluecker et al. 2001).
Acute toxicity, irritation and skin sensitisation
The acute oral and dermal toxicity of SAS is low (LD50, oral, rat > 2000 mg/kg bw, LD50, dermal, rabbit > 5000 mg/kg) (OECD 2004). Following inhalation exposure of rats to the highest technically feasible concentrations of 140 to ∼ 2000 mg/m3 SAS, no lethal effects were observed (OECD 2004). SAS is not considered a skin or eye irritant (ECETOC 2006; OECD 2004). There is no evidence of skin sensitisation caused by SAS in workers over decades of practical experience (OECD 2004). In a guinea pig sensitisation study of silicon dioxide no effects were observed (CIR 2009). Based on its structure and physico-chemical properties it is not expected that SAS causes skin sensitisation (ECETOC 2006). The information on acute toxicity, irritation and skin sensitisation is also in agreement with data provided by the supplier (EKA Chemicals AB 2010).
Repeated dose toxicity
Systemic toxicity after repeated application of SAS could not be detected in various studies (ECETOC 2006; OECD 2004). The inhalation of respirable particles of SAS produced a time- and dose-related local inflammation response of the lung tissue in animal studies (OECD 2004). However, it was observed in experimental investigations that these responses are transient and reversible after termination of exposure and during the recovery periods, presumably due to a better lung clearance and elimination based on an increased solubility compared with quartz (Arts et al. 2007; Johnston et al. 2000; Merget et al. 2002; Warheit et al. 1995). The comparison of different forms of SAS (pyrogenic, precipitated and gel, agglomerates of 1–4 µm in the test atmospheres) in a 5-day inhalation study revealed that differences were limited and confined to the first-day post-exposure (Arts et al. 2007). In a subchronic 13 weeks inhalation toxicity study with SAS and quartz dust in rats, the SAS forms (precipitated and pyrogenic, mean primary particle sizes of 12 and 18 nm, size distribution of agglomerates between 1 and 120 µm) resulted in a qualitatively comparable effect and a complete clearance from the lungs after exposure in contrast to the crystalline quartz (Reuzel et al. 1991). Another comparison of the effects of SAS including the colloidal form (mass median aerodynamic diameter 3–4 µm) in short-term inhalation experiments with rats support these findings (Warheit et al. 1995). Fruijtier-Pölloth (2012) summarised that none of the SAS forms, including colloidal nanosized particles, were shown to bioaccumulate and all disappear within a short time from living organisms by physiological excretion mechanisms with some indications that the smaller the particle size, the faster the clearance is.
There is no evidence of long-term respiratory health effects in workers employed in manufacturing of SAS (ECETOC 2006). In Germany, the occupational exposure limit for the inhalation of SAS is 4 mg/m3 total dust for 8 h (AGS 2006). Like with other particulate matter of small size, inhalation of larger quantities may lead to health effects as observed with other poorly soluble, inert ultrafine dusts. Overload effects can occur via deposition, and subsequently inflammatory processes can take place in the lung. At concentrations up to 100 mg/m³ as measured at a production and packaging site, no adverse effects could be observed with persons who were exposed to varying concentrations of SAS dust at the workplace and who were regularly checked for their health status over 12 years (DFG 1989). Moreover, no risk of teratogenicity is to be expected within the observance of the occupational exposure limit. With regard to reproduction, investigations in experimental animals led to the conclusion that prolonged exposure to SAS is not expected to harm the reproductive performance or embryonic/fetal development (OECD 2004). Although there is some uncertainty with regard to substance characterisation in the available studies, the conclusion was drawn by the Organisation for Economic Co-operation and Development (OECD) based on the available weight-of-evidence.
Genotoxicity and carcinogenicity
As reviewed by OECD (2004) and by ECETOC (2006), the overall evidence from existing studies indicates that SAS is not genotoxic and unlikely to be carcinogenic in humans. In particular, SAS does not induce mutations either in vitro or in vivo in standard methods (OECD 2004). This is also in agreement with data provided by the supplier (EKA Chemicals AB 2010). Colloidal SAS has been investigated in a Comet assay with 3T3-L1 fibroblasts and as a result, no significant genotoxicity was observed under the described test conditions (Barnes et al. 2008). Based on negative results in long-term oral feeding studies of rats and mice and based on epidemiological experience, there is no evidence of a carcinogenic potential arising from exposure towards SAS (OECD 2004).
Investigations addressing specific properties of nanoparticles
As reviewed recently (Napierska et al. 2010; Jin et al. 2009), several studies explicitly addressing effects discussed as relevant for nanotoxicity, like increased cytotoxicity, inflammation, oxidative stress or formation of reactive oxygen species, have been conducted with various specifically designed amorphous silicon dioxide nanoparticles, sometimes explicitly described as “nanosized”. Some of these materials are very similar to the colloidal SAS assessed in this study; others have altered properties due to a different morphology (e.g. mesoporous silicon dioxide) or chemical modifications (surface coatings). Overall, the toxicity of SAS nanoparticles in these studies seems to be low in vitro and in vivo (Jin et al. 2009). However, a couple of in vitro studies indicated that specific SAS nanoparticles were able to exert altered effects in cellular systems. It was assumed that the surface properties of the silicon dioxide particles contribute decisively to the observed biological effects; limitations of the original studies were mainly caused by insufficient substance characterisation and use of non-validated test protocols (Napierska et al. 2010). In addition, such tests were mostly performed with cancer cell lines which are deviating significantly from the normal physiological situation and are therefore connected with uncertainty regarding their relevance for human health and need careful interpretation. Investigations with primary cells or in vivo studies, which would allow an extrapolation to the physiological situation in humans with less uncertainty, are published less frequently. The authors and others have shown previously that responses of primary cells (e.g. oxidative stress) can be considerably different compared with cell lines (Albrecht et al. 2009). Most relevant to the risk assessment are studies that directly compare in vitro with in vivo effects, or include valid controls and/or benchmark substances to allow for a better judgment on the physiological relevance to humans and to provide a solid data base to correlate doses and effects.
As reviewed in a recent study, the mode of action of the group of SAS has been analysed by in vitro models and mechanistic studies (Fruijtier-Polloth 2012). It was concluded that although physical and chemical interactions of SAS with cell surfaces can result in signalling responses and inflammatory responses after exposure to high doses, there is no evidence for a novel, hitherto unknown mode of action.
Ecotoxicological profile
The acute toxicity of different types of SAS (functionalised and non-functionalised surfaces, <5 µm particle size) against diverse aquatic organisms was reported by ECETOC (2006) to be in the range of ≈1000 and ≈10,000 mg/l, respectively, showing that no significant aquatic toxicity was determined. Because no specific information about the particle size of the material tested is provided, these data are primarily considered as supportive for an environmental risk assessment. Notably, this range is also congruent with information from the supplier of the colloidal SAS used in the glass cleaner (EKA Chemicals AB 2010).
In literature, some studies are available that focus on toxicity to environmentally relevant organisms, such as bacteria, plants, daphnia and fish (e.g. Heinlaan et al. 2008; Hund-Rinke & Simon 2006; Oberdörster et al. 2006). The ecotoxicological properties are largely influenced by the surface properties of the material and environmental factors such as pH, organic carbon content and ionic strength of the medium.
The inhibitory effect of silicon dioxide nanoparticles (advertised particle size 14 nm, actual particle size in suspension 205 nm) on the multiplication of the bacteria Escherichia coli and Bacillus subtilis was studied by Adams et al. (2006). Multiplication of B. subtilis was inhibited by 84 ± 9.9% at 2000 mg/l and 99 ± 1.8% at 5000 mg/l after 14–20 h incubation, while the inhibition was lower for E. coli (48 ± 8.5% at 5000 mg/l). However, inhibition was obtained in culture media optimised for the multiplication and might not be representative for natural systems with other influencing factors.
Van Hoecke et al. (2008) studied the uptake of colloidal SAS by algae by electron microscopy and found that it is adsorbed to the algal surface but not internalised. The opposite was observed by Fujiwara et al. (2008) who found SAS particles incorporated into the test organisms. In parallel, they found strongly size-dependent effect concentrations in the low gram/litre range. However, due to the dissolution processes of SAS it can be anticipated that the particles will not be stable within the cell over time.
Acute and chronic ecotoxicity of SAS against algae were studied extensively (Van Hoecke et al. 2011; Van Hoecke et al. 2008; Wei et al. 2010). The green algae Scenedesmus obliquus was exposed to silicon dioxide particles of 10–20 mm diameter and showed low toxicity responses on growth rate with an ErC20 of 388 mg/l after 72 h (216 mg/l after 96 h) Van Hoecke et al. (2008) studied ecotoxicity of commercial colloidal SAS in suspensions (particle size: 12.5–27 nm) against Pseudokirchneriella subspicata and found 10-fold higher toxicity responses at ErC20 of 37 mg/l (no observed effect concentration (NOEC) = 22 mg/l). The effects were correlated with a significant decrease of chlorophyll content (Van Hoecke et al. 2008; Wei et al. 2010). It was therefore suggested that the toxic effects were related to the large specific surface area of the nanoparticles and hence an inhibitory effect due to light shading. It should further be taken into consideration that colloidal SAS is able to bind earth alkaline ions by ion exchange mechanisms (Karami 2009; Suda et al. 1999). The depletion of essential nutrients (e.g. magnesium) may therefore also be a factor explaining the observed toxicity of colloidal SAS on chlorophyll contents (Van Hoecke et al. 2008; Wei et al. 2010). A summary of algal toxicity data is shown in Table III.
Table III.
Summary of literature data on the algal toxicity of SAS.
| References | Particle size (nm) | EC20 | NOEC | Remark |
|---|---|---|---|---|
| Wei et al. (2010) | 10–20 | 48 h 144 mg/l 72 h 388 mg/l 96 h 216 mg/l |
Concentration-dependent chlorophyll reduction | |
| Van Hoecke et al. (2008) | 12.5 | 72 h 20 mg/l | Plateau toxicity 50% growth reduction | |
| 27 | 72 h 28.8 mg/l | |||
| Van Hoecke et al. (2011) | 22 | 48 h 9.9 mg/l (pH 7.6) | 4.6 | |
| 48 h 218.9 mg/l (pH 7.4) | 100 | 4.7 mg C/l NOM | ||
| Fujiwara et al. (2008) | 5, 26, 78 |
EC50
96 h 8000 mg/l 96 h 71,000 mg/l 96 h 91,000 mg/l |
Faint of chlorophyll colour |
NOEC, no observed effect concentration; NOM, natural organic matter; SAS, synthetic amorphous silicon dioxide.
Supplier information of the colloidal SAS used in glass cleaner indicates the highest ecotoxic potential against algae (EKA Chemicals AB 2010). Hence, it appears adequate to rely on algae for the derivation of a predicted no effect concentration for the aquatic environment (PNECaqua) because this is the only trophic level for which data on the ecotoxicity of well-characterised colloidal SAS for aquatic organisms are available. Due to different test designs, data on algal toxicity show very diverse values ranging from NOEC and EC20 values between about 10 mg/l up to far more than 1000 mg/l. For the purpose of this risk assessment a weight-of-evidence approach has been chosen. Taking into account that the toxicity was significantly reduced by natural organic material under natural conditions a reasonable worst-case acute toxicity to algae (ErC50) is higher than 100 mg/l. By applying a safety factor of 1000 a conservative PNECaqua of 0.1 mg/l is derived based on acute studies.
Exposure assessment for the production of the glass cleaner
General aspects
The glass cleaner formulation is a liquid product which is produced in a batch process with a volume of ten tons per batch. On average 6–10 batches are produced per day on approximately 50 days per year. As the nanoparticles make up 0.09% (w/w) of one batch, the amount of silicon dioxide handled at the production facility can be calculated to be 90 kg/day and 4.5 tons/year, respectively. The raw material is provided as liquid dispersion and is pumped into the batch. All formulation steps take place in closed process operations.
Human exposure
No release of nanoparticles from the closed system is expected to occur at any step of the formulation assuming well maintenance, no opening of the system during the process and exclusion of accidental release situations. Thus, exposure via the inhalative, dermal or oral route is negligible for workers and does not need to be assessed in more detail. As a precaution, general workplace safety measures (protective clothing) are applied and the production is managed under a certified quality management system with regular inspections ensuring the fulfilment of all requirements.
Environmental exposure
According to the generic emission estimates of the sector organisation of the cleaning product manufacturers, 0.2% of the raw materials (and thus of colloidal SAS) is emitted to the environment due to regular cleaning of mixing and packaging equipment. The washings are released to the wastewater. The emissions of SAS from production of the glass cleaner therefore amount to 0.18 kg/day. Taking into account the chemical properties of SAS and the abundance of silicon dioxide in the environment, the amount released by the glass cleaner production can be neglected.
During recent years, the fate of silicon dioxide nanoparticles has been studied in both, the wastewater and soil compartments (Chang et al. 2007; Jarvie et al. 2009; Lecoanet et al. 2004; Wiesner et al. 2009). Similar to its ecotoxic behaviour, the overarching conclusion of all these studies is that the fate and mobility of a nanoparticle is significantly influenced by its surface properties (e.g. coating material, surface charges, etc.). The stability of SAS in solution strongly depends on pH, ionic strength and the natural silicon dioxide concentration in the environment.
While silicon dioxide nanoparticles coated with non-ionic surfactants are efficiently removed during sewage treatment (>75%), non-coated particles almost quantitatively passed the sewage treatment without removal. Jarvie et al. (2009) and Chang et al. (2007) found that the 1–5 nm particle fraction of non-functionalised silicon dioxide nanoparticles were effectively coagulated by treatment with polyaluminium. An elimination of about 50% was observed. For quantitative exposure estimation (EUSES model), it is conservatively assumed that the sewage treatment eliminates 10% of SAS. The estimate for PEClocal for the production amounts to approximately 10 µg/l.
Exposure assessment for the consumer use of the glass cleaner
General description of use conditions
The average use of glass cleaners in German households has been investigated in a market research study (Henkel AG & Co. KGaA 2005). It was shown that most frequently windows are cleaned approximately three to four times per year. For consumers' convenience, glass cleaners are offered as sprays in trigger bottles. Consumers usually spray the formulation onto the dirty surface with a few strokes. In doing so the spray aerosol is directed away from the human body. Cleaning cloths are used to manually wipe the windows.
As there is sedimentation of the larger sized droplets, only smaller droplets remain in the spray mist in the air around the consumer. As a worst-case scenario, it can be assumed that the product is applied in a small and enclosed room with a low ventilation rate. Usually, however, the ventilation rate is expected to be high due to opening of the windows. The cleaning cloths are usually put in the washing machine after a few to several cleaning events. Alternatively, cloths may be disposed with household waste. Empty plastic bottles are recommended to be recycled or they are disposed of as household waste.
Human exposure
During the cleaning event, the consumer is exposed towards the spray atmosphere and towards the product remaining on a cleaning cloth. This might result in a possible inhalative and dermal exposure with the product and the raw materials contained. Moreover, an indirect oral exposure cannot be excluded due to a possible migration of the substances from the surface of a cleaned kitchen countertop to non-packaged food. Accidental exposure can happen during unintended use resulting in oral or dermal exposure (detailed calculations not shown). As skin penetration of SAS is unlikely based on current knowledge (chapter 3.2.1 (Hazard assessment of SAS)), skin contact will not lead to systemic exposure. Regarding the current application the most important exposure route is via the lung.
Inhalation exposure may occur when the silicon dioxide contained in aerosol droplets reach the breathing zone of consumers during the actual use of the product. Measurements of size distribution of the aerosol droplets containing the complete product formulation were performed in order to evaluate which fractions of the aerosol are inhalable and which are respirable. Results of the size distribution measurements of aerosol droplets generated during the use of a glass cleaner formulation are shown in Table II (two independent repeats). Measurements were performed by laser diffraction analysis with open and closed sieve at the trigger because consumers can be exposed towards a spray aerosol (open sieve) and foam aerosol (closed sieve).
With the spray function 50% of the aerosol droplets (volume share) had a droplet size smaller than 95 µm for Formulation 1 and 75 µm for Formulation 2. The mean fraction of aerosol droplets with a size of not more that 10 µm was 0.7% (average cumulative volume) for Formulation 1 and 1.1% (average cumulative volume) for Formulation 2. No droplets with a diameter below 4 µm could be detected for both formulations.
With the foam function the situation was comparable. Fifty percent of the aerosol droplets (volume share) had a droplet size smaller than 127 µm for Formulation 1 and 128 µm for Formulation 2. The mean fraction of aerosol droplets with a size of not more that 10 µm was 0.2% (average cumulative volume) for both formulations. No droplets with a diameter below 4 µm could be detected.
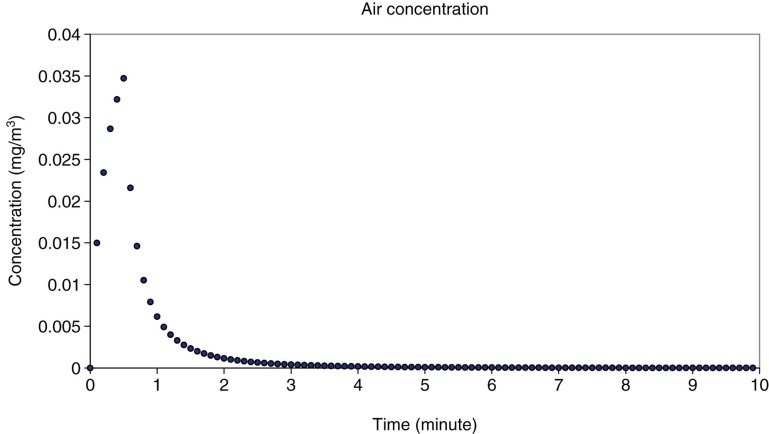
Besides determination of the droplet size, the airborne concentration of SAS during one spray event was modelled with the software ConsExpo®. It takes into account the concentration of non-volatiles in the product and the droplet sizes which determine how long particles stay in the air. The calculation of the indoor inhalation exposure was done for the spraying process because it is to be assumed that the amount of SAS released to the air is higher for this event compared with the application of foam. Under the assumptions mentioned above (intended use), a mean concentration of 0.002 mg/m³ of SAS in the surrounding breathing zone of the consumer during one cleaning event was calculated as point estimate. The peak concentration was calculated to be 0.035 mg/m³ shortly after spraying the product on the surface and rapidly decreased towards zero after 3 min exposure time (Figure 6). The air concentration calculated for an accidental setting (spraying towards exposed person) resulted in 0.044 mg/m³.
Figure 6.
Calculated air concentration of SAS during consumer exposure towards the glass cleaner spray.
The exposure concentration related to a frequent use of the cleaner, for example in professional settings, was not calculated separately as the number of exposure events increases while the exposure concentration of SAS in the surrounding remains the same.
Based on physico-chemical properties of the chemical substance, it is assumed that there will be no release of isolated nanoparticles from the glass into the surrounding air in case of a later mobilisation of dirt.
Environmental exposure
Of the worldwide consumed SAS (pyrogenic and precipitated) which was reported to account for >500,000 tons in 2002 (OECD 2004) the colloidal SAS comprises only a small fraction. Quantitative data on colloidal SAS use in Western Europe are provided in the Chemicals Handbook up to the year 2007 (Lauriente & Yokose 2008). While the total amount used is reported to be 18,000 tons/year mainly representing applications in the pulp and paper, textile and beverage industry, the uses for domestic cleaning purposes are not explicitly mentioned due to minor significance. An exact number of the fraction released to the aquatic environment is not known. However, because of its functional role in the above-mentioned industrial applications it is assumed that the majority of SAS does not enter the environment but remains in the processes. Hence, as a conservative estimation it is assumed that in maximum about 10% of the SAS reported in total will eventually enter the environment leading to an amount of 1800 tons/year.
As a conservative estimation the use of a glass cleaner formulation for consumer application with 0.09% (w/w) silicon dioxide will lead to a Europe wide release of less than 10 tons/year. This, therefore, represents less than 1‰ of the total annual consumption. However, to provide a conservative description of the substance emissions into the environment, EUSES exposure calculations on wide dispersive uses are based on a total of 1800 tons/year.
Considering the use in glass cleaners it may be assumed that the contained colloidal SAS is mainly adsorbed on the glass surface. Weathering processes may lead to dissolution and a potential rinse-off over time leading to some exposure to soil in the surrounding of a building and/or (indirectly) to the aquatic environment. It can be assumed that this way of exposure does not lead to a significant entry of nanoparticles in the soil environment. Another exposure path can be considered down the drain to wastewaters, eventually leading to emissions into natural rivers via the sewage systems and partly to soil via sewage sludge applications on agricultural soil. Due to the product application the exposure path to enter the aquatic environment is dominant.
Based on the assumptions made, a regional background predicted environmental concentration (PECregional) of SAS <1 µg/l is calculated. From the wide dispersive use of the glass cleaner a local concentration of SAS in surface water is calculated to be approximately 10 µg/l (PEClocal).
Currently, there are no accepted methods to screen for the bioaccumulation potential of nanoparticles. However, due to the dissolution behaviour of SAS in natural media no significant bioaccumulation potential may be assumed.
Risk assessment
Risk assessment regarding the production of the glass cleaner
Human risk assessment
The production of the glass cleaner is a wet process and production lines are contained. At the workplace exposure to colloidal SAS can be regarded as negligible, provided that there are no unintended leakages. It is controlled by suitable technical and general workplace safety standards which are applied as a matter of hygiene and responsible care. On the basis of a thorough consideration of exposure and taking into account the low toxicity of SAS, a risk is considered to be low at the production even in case of occasional contact to the skin, since skin penetration is not expected.
Environmental risk assessment
The PEClocal for production (10 µg/l) is considerably below the PNEC (0.1 mg/l). In addition, it is considerably below the naturally occurring concentrations of dissolved silicon dioxide in larger German rivers, which typically range between 1 and 10 mg/l (e.g. Ladwig 2012; Wind et al. 2008). The environmental risk from the production of the glass cleaner formulation with colloidal SAS is thus considered to be low.
Risk assessment regarding the consumer use of the glass cleaner
Human risk assessment (focus on inhalation)
The risk assessment for the inhalative route during application of the glass cleaner is demonstrated in detail as this is of highest relevance for the consumer.
As described before, the hazard of SAS in general is relatively low especially compared with quartz. It is well known that SAS does not lead to persistent toxicological effects in the lung as long as overload conditions regarding the amount of dust in the lungs are avoided. One major aspect is therefore that the exposure is also low.
In general, the fraction of total airborne particles which reaches the human airways depends on the properties of the particles, on air speed and direction close to the body, as well as on breathing rate and human physiology. The likeliness of inhalation of particles, their deposition, tissue reactions and exhalation differs strongly between individuals. Conventions have been set up for particle size-dependent sampling of airborne particles (CEN 1993). Those conventions are used to evaluate the likeliness of inhalation of airborne aerosol droplets depending on their diameter. Considering the distribution of droplet size as determined for the glass cleaner formulation about half of all aerosol particles (spray and foam) would not be inhalable according to the conventions (>100 µm). A fraction of 1.1% as maximum of the product sprayed and 0.2% of the foam have droplets with diameters below 10 µm (average cumulative volume). It can be predicted that 50% of those droplets might reach the thoracic region of the lungs (<10 µm). In this region, mucociliary clearance mechanisms are in place. It is very unlikely that droplets from the glass cleaner spray will be deposited in the alveolar region, as no droplets with a diameter below 4 µm have been detected in the samples analysed. Even if some individual droplets of this fraction were small enough to reach the alveoli and were not exhaled, SAS could be cleared by alveolar macrophages and by dissolution.
A typical particle size distribution of SAS has already in the past included a certain fraction of particles at the nanoscale (DFG 1989). From human experience it is known that even after repeated exposure towards SAS dust in a concentration up to 100 mg/m³, no negative health implications for workers in industry have been observed. As mentioned before, an occupational exposure limit of 4 mg/m³ has been derived for colloidal SAS for an exposure duration of 8 h and for the inhalable fraction of dust (defined for particles with a size below 100 µm) (AGS 2006; DFG 1989).
For a quantitative risk assessment for cleaning of windows the calculated amounts (0.002 mg/m³ during one cleaning event with a peak concentration of 0.035 mg/m³ in the first minute, and 0.044 mg/m³ in an accidental situation, all based on an inhalation cut-off diameter of 100 µm) are compared with the existing specific occupational exposure limit (4 mg/m³). The estimated concentrations are in the order of magnitude of 100 lower. Although this threshold has been set up for the workplace situation, it is considered to be sufficiently conservative also for the private household as it covers a timeframe of 8 h and the inhalable fraction. The usual duration of the cleaning process is much lower and accidental situations are exceptional.
As the colloidal SAS is not available in a dusty form, as aerosol droplets are larger than 4 µm in diameter and, thus, not regarded as respirable and as the concentration of SAS in the surrounding atmosphere of the person using the product is far below the threshold value assessed to result in no adverse health implications, it is concluded that the risk of applying the glass cleaner with colloidal SAS is very low.
Environmental risk assessment
In the current analysis the estimations were focused on the release of SAS particles into the aquatic environment due to its use in products. Although only a very small amount of colloidal SAS is used in hard surface cleaning products compared with other applications, a conservative assumption on total emissions was made that includes the application in domestic hard surface cleaning but also other applications leading to environmental exposure. Based on estimates the PEC of environmentally relevant SAS (PECregional 1 µg/l, PEClocal 10 µg/l) can be calculated to be much lower than the PNEC (0.1 mg/l). Further to that due to the natural dissolution and re-deposition processes of silicon dioxide, no long-term stability of SAS is anticipated under environmental conditions. Taking into account the uncertainties that remain and under the conservative assumptions made for tonnage, fate and ecotoxicity of colloidal SAS no risk is indicated for the aquatic environment.
Discussion
Risk assessment methodology
This study presents the human and environmental risk assessment of colloidal SAS (= amorphous silicon dioxide nanoparticles) in a glass cleaner. Regarding the appropriate methodology for risk assessment of nanomaterials there is an ongoing debate on how this topic can be best approached (Holsapple et al. 2005; SCENIHR 2009). It has been concluded by SCENIHR that there is currently no generally applicable paradigm for nanomaterial-specific hazard identification, and a case-by-case approach would be the appropriate choice for risk assessment (SCENIHR 2009). In addition, Baier-Anderson et al. (2007) have suggested a framework to address areas of incomplete or uncertain information by using reasonable assumptions leading to appropriate risk management practices. This approach is a general principle not limited to the risk assessment of nanomaterials and regulatory guidance has been provided previously reflecting the state-of-the-art in risk assessment (ECHA 2008, 2010b, c).
Metrics for nanomaterials
The metrics currently used in risk assessment are usually based on mass to express exposure and toxicological effects. In the area of nanotoxicology a scientific debate is ongoing on whether another metric like particle number or surface area should be added (Bouwmeester et al. 2011; Warheit et al. 2007). This is, for example, based on the observation of toxicological effects like inflammation, which are likely to be related to particle surface area (Donaldson & Tran 2002; Höhr et al. 2002). Although there are currently no definitive conclusions on the best metric, it is suggested that the physico-chemical description should be detailed to allow an expression of dose-response in the different metrics (Hankin et al. 2011). In the case of SAS, it needs to be considered that available data are partly reaching back to the 1960s/70s when dose was used to be expressed in mass and details on particle numbers or surface area were not given. As the substance is not a new nanomaterial but has always been in a nanostructured form, the existing risk assessments and exposure values are still valuable in the current discussion on nanoparticles. In a recent study (Fruijtier-Pölloth 2012), it is described that, in addition, none of the newly available data give any evidence for a novel, hitherto unknown mechanism of toxicity that may raise concerns with regard to human health or environmental risks. Thus, to allow for comparability, the mass-based approach is considered a valid option in this investigation.
Hazard identification
The OECD Working Party on Manufactured Nanomaterials has reviewed the available standard test guidelines for hazard identification and has shown that principally the test guidelines are suitable also for nanomaterials but might need modification in some cases (OECD 2011). This conclusion supports the validity of existing data on SAS as long as the study design and performance fulfil accepted and scientific standards.
It needs to be acknowledged that the studies referring to SAS often lack a detailed specification of the particle size distribution though it is known that a fraction of particles at the nanoscale is usually contained. An exact quantitative comparison between studies with different materials is therefore possible only to a limited extent. Since the parameters and methodologies for nanomaterial characterisation are getting more refined and standardised (Bouwmeester et al. 2011; NanoCare Project Partners 2009; Schulze et al. 2008), this situation is expected to improve in the future. The same holds true for kinetic analyses. Further research may provide more insight into size-dependent penetration properties of silicon dioxide through various biological barriers and dissolution as well as excretion kinetics. Some recent cell culture studies in the area of nanotoxicology are questionable regarding their physiological relevance. Critical factors are the inclusion of appropriate controls and benchmarks, selection of physiologically relevant concentrations, non-speculative interpretation of effects and development of standardised and validated procedures (Bouwmeester et al. 2011; NanoCare Project Partners 2009).
Exposure assessment
Regarding the tools for exposure assessment ConsExpo® and EUSES are widely accepted for regulatory purposes (ECHA 2010a, c). By applying default as well as worst-case assumptions exposure estimates were derived. The spray model was developed on the basis of the results of experimental work. Acknowledging the fact that some parameters are difficult to estimate, the reliability of the default values is categorised in the software documentation and taken into account. Although measured data would generally be preferred over modelled data to account for the most realistic situation, a well-established mechanism of exposure modelling allows quantifying exposure because the results are conservative, which means real conditions are overestimated. Measuring single airborne nanoparticles in experimental settings is still an issue for research. Approaches for workplace settings have been published (Kuhlbusch et al. 2011), however, it needs to be considered that an air concentration of SAS in private settings when applying the glass cleaner is much lower than potential dust concentrations at workplaces. Highly sensitive measurement devices and a reliable differentiation against the background particle burden would be needed, but are currently not available as standardised techniques.
Applicability of risk assessment approach
It can be argued that the risk assessment approach reported here is applicable for the colloidal SAS in the present application, because already under the conservative assumptions of the first tier and the available information on the substance no risk was indicated neither for man nor the environment. However, it should be kept in mind that such an approach may not necessarily be applicable to all nanomaterials as these are a very heterogeneous group of substances and are used in a variety of applications. From the current knowledge, the authors conclude that risk assessments for nanomaterials should, therefore, be done on a case-by-case basis.
Conclusions
This analysis takes into account information on human and environmental hazards and modelled exposure data based on state-of-the-art methodologies. It demonstrates that the use of colloidal SAS does not pose a risk for humans and the environment for the intended use in a glass cleaner formulation based on the available weight-of-evidence. The applied risk assessment strategy was built on conservative assumptions to allow for consideration of uncertainties. In summary, it proved to be a useful methodology for a reasonable risk evaluation of amorphous silicon dioxide nanoparticles in this case study.
Acknowledgements
The authors would like to thank Doris Gesterkamp and Elke Lehringer for assisting in the literature search and for editorial support; Michaela Berchter, Burkhard Eschen and Hans-Juergen Schwark for performing analytical measurements; Lothar Kintrup, Thomas Mueller-Kirschbaum, Roland Schroeder, Walter Sterzel, Johannes Tolls and Frederike Wiebel for general support and review of the manuscript. Funding source: The work was funded by Henkel AG & Co. KGaA.
Declaration of interest
The authors are employees of Henkel AG & Co. KGaA. The authors state that this did not influence the scientific objectiveness of the analysis.
References
- Adams LK, Lyon DY, Alvarez PJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- AGS TRGS 900 – Technische Regeln für Gefahrstoffe. Ausschuss für Gefahrstoffe – AGS-Geschäftsführung – BAuA. www.baua.de. 2006 Online. Available at. Accessed on 13 July 2011.
- Albrecht C, Scherbart AM, van Berlo D, Braunbarth CM, Schins RP, Scheel J. Evaluation of cytotoxic effects and oxidative stress with hydroxyapatite dispersions of different physicochemical properties in rat NR8383 cells and primary macrophages. Toxicol In Vitro. 2009;23:520–530. doi: 10.1016/j.tiv.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Alexander GB, Heston WM, Iler RK. The solubility of amorphous silica in water. J Phys Chem. 1954;58:453–455. [Google Scholar]
- Arts JH, Muijser H, Duistermaat E, Junker K, Kuper CF. Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3 months. Food Chem Toxicol. 2007;45:1856–1867. doi: 10.1016/j.fct.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Baier-Anderson C, Balbus J, Carberry J, Denison R, Doraiswamy K, Gannon J, et al. NANO Risk Framework. Environmental Defense – DuPont Nano Partnership. www.NanoRiskFramework.com. 2007 Online. Available at. Accessed on 29 June 2011. 1-102.
- Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J, Lesniak A, et al. Reproducible comet assay of amorphous silica nanoparticles detects no genotoxicity. Nano Lett. 2008;8:3069–3074. doi: 10.1021/nl801661w. [DOI] [PubMed] [Google Scholar]
- Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, et al. Research strategies for safety evaluation of nanomaterials, part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol Sci. 2006a;90:23–32. doi: 10.1093/toxsci/kfj084. [DOI] [PubMed] [Google Scholar]
- Borm PJ, Robbins D, Haubold S, Kuhlbusch T, Fissan H, Donaldson K, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006b;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester H, Lynch I, Marvin HJ, Dawson KA, Berges M, Braguer D, et al. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology. 2011;5:1–11. doi: 10.3109/17435391003775266. [DOI] [PubMed] [Google Scholar]
- Butz T. Dermal Penetration of Nanoparticles – What we know and what we don't. SöFW J10. 2009;135:30–34. [Google Scholar]
- CEN Workplace atmospheres. Size fraction definitions for measurement of airborne particles – EN 481:1993. Eur Committee Stand. 1993:1–9. [Google Scholar]
- Chang JS, Chang KL, Hwang DF, Kong ZL. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ Sci Technol. 2007;41:2064–2068. doi: 10.1021/es062347t. [DOI] [PubMed] [Google Scholar]
- CIR Cosmetic Ingredient Review; Washington, DC: 2009. Final Report of the Cosmetic Ingredient Review Expert Panel – Safety Assessment of Silica and Related Cosmetic Ingredients. [Google Scholar]
- Dekkers S, Krystek P, Peters RJ, Lankveld DX, Bokkers BG, van Hoeven-Arentzen PH, et al. Presence and risks of nanosilica in food products. Nanotoxicology. 2011;5:393–405. doi: 10.3109/17435390.2010.519836. [DOI] [PubMed] [Google Scholar]
- DFG 1989. Deutsche Forschungsgemeinschaft (DFG) – Amorphe Kieselsäuren. Toxikologisch-arbeitsmedizinische Begründungen von MAK-Werten (Maximale Arbeitsplatzkonzentrationen) ISBN 978-3-527-19030-0:1-26.
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Tran CL. Inflammation caused by particles and fibers. Inhal Toxicol. 2002;14:5–27. doi: 10.1080/089583701753338613. [DOI] [PubMed] [Google Scholar]
- Dreja M, Noglich J, Guckenbiehl B. Method for cleaning hard surfaces using a composition comprising a colloidal silica sol. US7745383 2010
- Dreja M, Noglich J, Josa J. Self-organized nano-particles for enhanced wetting of hard surfaces. Tenside Surf Det. 2004;41:180–186. [Google Scholar]
- Dreja M, Noglich J, Menke R, Plantikow P. Hydrophilierender Reiniger für harte Oberflächen. Patentblatt DE102004019022A1 2005
- DuPont DuPont™ Light Stabilizer Framework Example – Nanomaterial Risk Assessment Worksheet DuPont™ Light Stabilizer. http://www.edf.org/documents/6913_TiO2_Worksheet.pdf. 2007 Online. Available at. Accessed on 5 July 2011.
- ECETOC 2006. Synthetic Amorphous Silica (CAS No. 7631-86-9) – JACC REPORT No. 51. European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels ISSN-0773-6339-51.
- ECHA Guidance on information requirements and chemical safety assessment. Chapter R.10: Characterisation of dose [concentration]-response for environment. http://www.echa.europa.eu. 2008 Online. Available at. Accessed on 14 July 2011. 1-65.
- ECHA Guidance on information requirements and chemical safety assessment Chapter R.16: Environmental Exposure Estimation. http://www.echa.europa.eu. 2010a Online. Available at. Accessed on 21 July 2011. Ref.: ECHA-10-G-06-EN:1-59.
- ECHA Guidance on information requirements and chemical safety assessment. Chapter R.8: Characterisation of dose [concentration]-response for human health. http://www.echa.europa.eu. 2010b Online. Available at. Accessed on 14 July 2011. Ref.: ECHA-2010-G-19-EN:1-194.
- ECHA Guidance on information requirements and chemical safety assessment. Chapter R.15: Consumer exposure estimation. http://www.echa.europa.eu. 2010c Online. Available at. Accessed on 21 July 2011. Ref.: ECHA-10-G-03-EN:1-59.
- EKA Chemicals AB Product Data Sheet for amorphous silicon dioxide, aqueous colloidal dispersion 2003 [Google Scholar]
- EKA Chemicals AB Material Safety Data Sheet for amorphous silicon dioxide, aqueous colloidal dispersion 2010 [Google Scholar]
- EU Directive 2004/10/EC of the European Parliament and of the Council on the harmonisation of laws, regulations and administrative provisions relating to the application of the principles of good laboratory practice and the verification of their applications for tests on chemical substances (codified version) Official J Eu Union L. 2004;50:44–59. [Google Scholar]
- EU Commission Directive 2008/84/EC laying down specific purity criteria on food additives other than colours and sweeteners. Official J Eur Union L. 2008a;253:1–175. [Google Scholar]
- EU European Chemicals Bureau, Ispra, Italy. http://ihcp.jrc.ec.europa.eu/our_activities/health-env/risk_assessment_of_Biocides/euses. 2008b Online. Available at. Accessed on 29 July 2011.
- EU Regulation (EC) No. 1272/2008 of the European Parliament and of the Council on classification, labelling and packaging of substances and mixtures. Official J Eur Union L. 2008c;353:1–1354. [Google Scholar]
- EU Commission recommendation of 18 October 2011 on the definition of nanomaterial.(2011/696/EU) Official J Eur Union L. 2011;275:38–40. [Google Scholar]
- Fruijtier-Pölloth C. The toxicological mode of action and the safety of synthetic amorphous silica-A nanostructured material. Toxicology. 2012;294:61–79. doi: 10.1016/j.tox.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Suematsu H, Kiyomiya E, Aoki M, Sato M, Moritoki N. Size-dependent toxicity of silica nano-particles to Chlorella kessleri. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2008;43:1167–1173. doi: 10.1080/10934520802171675. [DOI] [PubMed] [Google Scholar]
- Gamer AO, Leibold E, van Ravenzwaay B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol In Vitro. 2006;20:301–307. doi: 10.1016/j.tiv.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hankin SM, Peters SAK, Poland CA, Foss Hansen S, Holmqvist J, Ross BL, et al. 2011. Specific Advice on Fulfilling Information Requirements for Nanomaterials under REACH (RIP-oN 2) – Final Project Report. British Standards Institution (BSI) RNC/RIP-oN2/FPR/1/FINAL:1-356.
- Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Henkel AG & Co. KGaA Quantitative Grundlagenstudie zur Fensterreinigung unpublished data 2005 [Google Scholar]
- Höhr D, Steinfartz Y, Schins R, Knaapen AM, Martra G, Fubini B, et al. The surface area rather than the surface coating determines the acute inflammatory response after instillation of fine and ultrafine TiO2 in the rat. Int J Hyg Environ Health. 2002;205:239–244. doi: 10.1078/1438-4639-00123. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Farland WH, Landry TD, Monteiro-Riviere NA, Carter JM, Walker NJ, et al. Research strategies for safety evaluation of nanomaterials, part II: toxicological and safety evaluation of nanomaterials, current challenges and data needs. Toxicol Sci. 2005;88:12–17. doi: 10.1093/toxsci/kfi293. [DOI] [PubMed] [Google Scholar]
- Hund-Rinke K, Simon M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Pollut Res Int. 2006;13:225–232. doi: 10.1065/espr2006.06.311. [DOI] [PubMed] [Google Scholar]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Silica. Int Agency Res Cancer (IARC) 1997;68:1–9. [Google Scholar]
- International Organization for Standardization 2008. Nanotechnologies – Terminology and definitions for nano-objects – Nanoparticle, nanofibre and nanoplate (ISO/TS 27687:2008(E)). In: ISO copyright office.
- Jarvie HP, Al-Obaidi H, King SM, Bowes MJ, Lawrence MJ, Drake AF, et al. Fate of silica nanoparticles in simulated primary wastewater treatment. Environ Sci Technol. 2009;43:8622–8628. doi: 10.1021/es901399q. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lohstreter S, Zhao JX. Toxicity of Spherical and Anisotropic Nanosilica. Nanomat Life Sci. 2009;2:221–244. [Google Scholar]
- Johnston CJ, Driscoll KE, Finkelstein JN, Baggs R, O'Reilly MA, Carter J, et al. Pulmonary chemokine and mutagenic responses in rats after subchronic inhalation of amorphous and crystalline silica. Toxicol Sci. 2000;56:405–413. doi: 10.1093/toxsci/56.2.405. [DOI] [PubMed] [Google Scholar]
- Karami A. Study on modification of colloidal silica surface with magnesium ions. J Colloid Interface Sci. 2009;331:379–383. doi: 10.1016/j.jcis.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Kreyling W, Semmler-Behnke M, Möller W. Health implications of nanoparticles. J Nanoparticle Res. 2006;8:543–562. [Google Scholar]
- Krug HF, Wick P. Nanotoxicology: An Interdisciplinary Challenge. Angew Chem Int Ed Engl. 2011;50:1260–1278. doi: 10.1002/anie.201001037. [DOI] [PubMed] [Google Scholar]
- Kuhlbusch TA, Asbach C, Fissan H, Gohler D, Stintz M. Nanoparticle exposure at nanotechnology workplaces: a review. Part Fibre Toxicol. 2011;8:22. doi: 10.1186/1743-8977-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladwig N. Kritische Betrachtung von Eutrophierungstendenzen in der inneren Deutschen Bucht. Berichte aus dem Forschungs- und Technologiezentrum Westküste der Universität Kiel. 2012;47:1–167. [Google Scholar]
- Lauriente DH, Yokose K. Chemical Economics Handbook. http://chemical.ihs.com/CEH/Public/Reports/766.4000/ 2008 Online. Available at: Accessed on 15 July 2011.
- Lecoanet HF, Bottero JY, Wiesner MR. Laboratory assessment of the mobility of nanomaterials in porous media. Environ Sci Technol. 2004;38:5164–5169. doi: 10.1021/es0352303. [DOI] [PubMed] [Google Scholar]
- Mavon A, Miquel C, Lejeune O, Payre B, Moretto P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacol Physiol. 2007;20:10–20. doi: 10.1159/000096167. [DOI] [PubMed] [Google Scholar]
- Merget R, Bauer T, Kupper HU, Philippou S, Bauer HD, Breitstadt R, et al. Health hazards due to the inhalation of amorphous silica. Arch Toxicol. 2002;75:625–634. doi: 10.1007/s002040100266. [DOI] [PubMed] [Google Scholar]
- Miretzky P, Conzonno V, Fernández Cirelli A. Geochemical processes controlling silica concentrations in groundwaters of the Salado River drainage basin, Argentina. J Geochem Explor. 2001;73:155–166. [Google Scholar]
- NanoCare Project Partners NanoCare – Health related Aspects of Nanomaterials – Final Scientific Report. Frankfurt. 2009:1–158. [Google Scholar]
- Napierska D, Thomassen LC, Lison D, Martens JA, Hoet PH. The nanosilica hazard: another variable entity. Part Fibre Toxicol. 2010;7:39. doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Oberdörster E, McClellan-Green P, Haasch M. Nanotechnology for Life Sciences. Weinheim: Wiley-VCH; 2006. Ecotoxicity of engineered nanomaterials. [Google Scholar]
- Oberdörster G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J Intern Med. 2009;267:89–105. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- OECD 2004. SIDS Initial Assessment Report on Synthetic amorphous Silica and Silicates – CAS 7631-86-9, 112945-52-5, 112926-00-8: Silicon Dioxide; CAS 1344-00-9: Silicic Acid, Aluminum Sodium Salt; CAS 1344-95-2: Silicic Acid, Calcium Salt. In.
- OECD Nanosafety at the OECD: The First Five Years 2006-2010. http://www.oecd.org. 2011 Online. Available at. Accessed on 14 July 2011. 1-16.
- Pfluecker F, Wendel V, Hohenberg H, Gartner E, Will T, Pfeiffer S, et al. The human stratum corneum layer: an effective barrier against dermal uptake of different forms of topically applied micronised titanium dioxide. Skin Pharmacol Appl Skin Physiol. 2001;14(Suppl 1):92–97. doi: 10.1159/000056396. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Reuzel PG, Bruijntjes JP, Feron VJ, Woutersen RA. Subchronic inhalation toxicity of amorphous silicas and quartz dust in rats. Food Chem Toxicol. 1991;29:341–354. doi: 10.1016/0278-6915(91)90205-l. [DOI] [PubMed] [Google Scholar]
- Roelofs F, Vogelsberger W. Dissolution Kinetics of Synthetic Amorphous Silica in Biological-Like Media and Its Theoretical Description. J Phys Chem B. 2004;108:11308–11316. [Google Scholar]
- Royal Academy of Engineering The Royal Society - Science Policy Section; London: 2004. Nanoscience and nanotechnologies: opportunities and uncertainties; pp. 1–127. [Google Scholar]
- SCENIHR Scientific Committee on Newly Identified and Emerging Health Risks. Opinion on the appropriateness of the risk assessment methodology in accordance with the technical guidance documents for new and existing substances for assessing the risks of nanomaterials. http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_010.pdf 2007 Available at.
- SCENIHR Scientific Committee on Newly Identified and Emerging Health Risks. Risk Assessment of Products of Nanotechnologies. http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_023.pdf. 2009 Online. Available at. Accessed on 5 July 2011.
- SCENIHR Scientific Committee on Newly Identified and Emerging Health Risks. Scientific Basis for the Definition of the Term “Nanomaterial”. http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_032.pdf. 2010 Online. Available at. Accessed on 5 July 2011.
- Schulze C, Kroll A, Lehr C-M, Schäfer UF, Becker K, Schnekenburger J, et al. Not ready to use – overcoming pitfalls when dispersing nanoparticles in physiological media. Nanotoxicology. 2008;2:51–61. [Google Scholar]
- Suda S-I, Tashiro T, Umegaki T. Synthesis of MgO-SiO2 and CaO-SiO2 amorphous powder by sol-gel process and ion exchange. J Non-Crystalline Solids. 1999;255:178–184. [Google Scholar]
- US EPA 2008. Estimation Programs Interface Suite™ for Microsoft® Windows, Version 4.0 (EPIWEB v4.0) (EPISuite v4.0) Washington, DC, USA.
- Van Hoecke K, De Schamphelaere KA, Ramirez-Garcia S, Van der Meeren P, Smagghe G, Janssen CR. Influence of alumina coating on characteristics and effects of SiO2 nanoparticles in algal growth inhibition assays at various pH and organic matter contents. Environ Int. 2011;37:1118–1125. doi: 10.1016/j.envint.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Van Hoecke K, De Schamphelaere KA, Van der Meeren P, Lucas S, Janssen CR. Ecotoxicity of silica nanoparticles to the green alga Pseudokirchneriella subcapitata: importance of surface area. Environ Toxicol Chem. 2008;27:1948–1957. doi: 10.1897/07-634.1. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Borm PJ, Hennes C, Lademann J. Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhal Toxicol. 2007;19:631–643. doi: 10.1080/08958370701353080. [DOI] [PubMed] [Google Scholar]
- Warheit DB, McHugh TA, Hartsky MA. Differential pulmonary responses in rats inhaling crystalline, colloidal or amorphous silica dusts. Scand J Work Environ Health. 1995;21(Suppl 2):19–21. [PubMed] [Google Scholar]
- Wei C, Zhang Y, Guo J, Han B, Yang X, Yuan J. Effects of silica nanoparticles on growth and photosynthetic pigment contents of Scenedesmus obliquus. J Environ Sci (China) 2010;22:155–160. doi: 10.1016/s1001-0742(09)60087-5. [DOI] [PubMed] [Google Scholar]
- WHO 1974. WHO (World Health Organization) Food Additives Series no. 5 – Silicon Dioxide and certain Silicates. Joint FAO/WHO Expert Committee on Food Additives.
- Wiesner MR, Lowry GV, Jones KL, Hochella MF, Jr, Di Giulio RT, Casman E, et al. Decreasing uncertainties in assessing environmental exposure, risk, and ecological implications of nanomaterials. Environ Sci Technol. 2009;43:6458–6462. doi: 10.1021/es803621k. [DOI] [PubMed] [Google Scholar]
- Wind T, Steber J, Tolls J. 50 Years of environmental monitoring at Henkel. Tenside Surf Det. 2008;45:144–152. [Google Scholar]