Abstract
Purpose
Rotavirus (RV) is the leading cause of morbidity and mortality in children under 5 years of age worldwide. This study assessed the role of RV as a cause of gastroenteritis (GE)-associated hospitalization in children, generating baseline information to evaluate the potential impact of the RV vaccine in reducing RVGE disease burden in the Kingdom of Bahrain.
Methods
This single, pediatric hospital-based surveillance study was conducted over a period of 12 months beginning April 1, 2006. A total of 314 children aged under 5 years and hospitalized due to GE were enrolled in the study, following collection of written informed consent from parents/guardians. Stool samples were tested for the presence of RV using enzyme immunoassay, and a random subset of RV-positive samples was further genotyped using reverse transcriptase-polymerase chain reaction and reverse hybridization assay.
Results
Of 314 enrolled children, 239 were included in the final analysis. RV was detected in 107 children (44.8%), mostly in the 6–23 months age group (82/107; 76.6%). RVGE occurred throughout the year, with the highest proportion occurring during April (26/42; 61.9%). G1P[8[ was the most commonly detected RV strain (10/17; 58.8%) in the limited number of samples analyzed. Vomiting and severe RVGE were more commonly observed in RV-positive than RV-negative children before hospitalization (P = 0.0008 and 0.0204, respectively).
Conclusion
In our study, RV accounted for over 40% of GE-associated hospitalizations and particularly affected children under 2 years of age. These data will serve as a baseline for assessing the potential changes in the epidemiology of RV disease and for evaluating the potential impact of the introduction of RV vaccination.
Keywords: rotavirus, gastroenteritis, epidemiology, Kingdom of Bahrain
Introduction
Rotavirus (RV) is the primary etiological agent causing severe gastroenteritis (GE) in children. In 2008, RV accounted for approximately 453,000 (420,000–494,000) worldwide deaths and 36% of diarrheal hospitalizations among children under 5 years of age.1,2 Each year, approximately 65,000 deaths can be attributed to RV in 22 countries of the Eastern Mediterranean Region,3 with RV representing an important cause of severe diarrhea amongst around 102,000 children aged under 5 years in the Kingdom of Bahrain.4–6
In comparison with improvements in hygiene and sanitation, vaccination is an effective public health intervention, capable of controlling RV disease.7 Since 2006, the World Health Organization (WHO) has recommended two live, oral RV vaccines, RotaTeq® (Merck & Co, Inc, Whitehouse Station, NJ, USA) and Rotarix™ (Glaxo SmithKline Vaccines),8 both of which demonstrate good safety, efficacy, and effectiveness profiles in large-scale clinical trials and in reallife settings in many regions globally.9–17
In 2008, Rotarix™ was introduced into the Kingdom of Bahrain’s national immunization program as a two-dose schedule at 2 and 4 months of age.4,18 However, since this time, the coverage rate for at least one of the doses has fluctuated from 17%–87%.4 In order to assess the public health benefits of introducing the RV vaccine, its impact on RV-associated morbidity and mortality needs to be monitored.19 This study was therefore undertaken to generate baseline information on RVGE disease burden, which could be used as a reference for the interpretation of post-vaccine RV disease trends. These data would allow health care providers and decision makers to design appropriate future plans and assess the benefits of RV vaccination in reducing the burden of severe RVGE.
This hospital-based surveillance study assessed the role of RV in causing GE-associated hospitalization in children under 5 years; evaluated the age and seasonal distribution of RV-associated hospitalization; identified prevalent RV types; and assessed outcomes and associated treatment.
Materials and methods
Study design
This single, referral pediatric hospital-based surveillance study was conducted at the Salmaniya Medical Complex (SMC), the Kingdom of Bahrain, Manama over 12 months beginning April 1, 2006. SMC is a multispecialty secondary and tertiary government-referral hospital serving approximately 90% of children aged under 5 years in the Kingdom of Bahrain.20,21 The study design was based on the WHO 2002 generic protocol for hospital-based surveillance to estimate the burden of RVGE in children.22
The study was conducted in accordance with Good Clinical Practice guidelines, the Declaration of Helsinki, and all necessary local regulatory requirements. The study was approved by the local ethics committee.
Study population
Children aged under 5 years hospitalized for GE (defined as the occurrence of diarrhea [≥3 looser than normal stools/day] with or without ≥2 vomiting episodes/24 hours19) and whose parents/guardians provided written informed consent were included. Children were excluded if GE had started >12 hours after hospitalization (possible nosocomial infections). In accordance with the WHO guidelines, children hospitalized more than once due to new GE episodes were considered as separate cases on each occasion.
Demographic and clinical assessment
Parental questionnaires were used to solicit information regarding each child’s demographic and GE symptoms. Data on clinical signs were obtained by reviewing the medical records.
RV diagnosis was performed using the WHO-defined GE criteria.19 The severity of RVGE was assessed using the Vesikari Clinical Severity Scoring Manual.23,24 A score of ≥11 was considered severe RVGE.
Stool sample handling and laboratory analysis
Stool samples were collected on admission and tested on-site for the presence of RV using enzyme immunoassay ([EIA] Premier™ Rotaclone®; Meridian Bioscience, River Hills Drive, OH, USA).25 A random subset of RV-positive samples was further genotyped (G and P types) using reverse transcriptase-polymerase chain reaction and reverse hybridization assay at DDL Diagnostic Laboratory, The Netherlands, as previously described.26
Statistical analyses
The proportion of RV-attributable diarrheal hospitalizations was calculated with exact 95% Clopper–Pearson confidence intervals (CIs)27 using the following formula:
Demographic characteristics, symptoms of GE, duration of hospitalization, treatment, and outcome at discharge were tabulated by RV status. Seasonality and severity of RVGE and distribution of RV G and P types were also described. Data were summarized in frequency tables, with percentages for categorical variables and mean, median, and standard deviation for continuous variables. Lastly, chi-square and Fisher’s exact tests were used to analyze the association between the clinical characteristics and RV status. All statistical analyses were descriptive/exploratory and performed using Statistical Analysis System (SAS) software (v 9.2; SAS Institute Inc, Cary, NC, USA).
Results
Demographic characteristics
Overall, 314 children were approached, of whom 75 were excluded as they did not meet the predefined eligibility criteria. The median age of the 239 children included in the final analysis was 13.0 months (range 1.0–56.0 months); 145 (60.7%) children were male, and the majority (99.6%) lived in the area served by the study hospital. Most GE-associated hospitalizations occurred in children aged 6–23 months (169/239, 70.7%; Figure 1).
Figure 1.
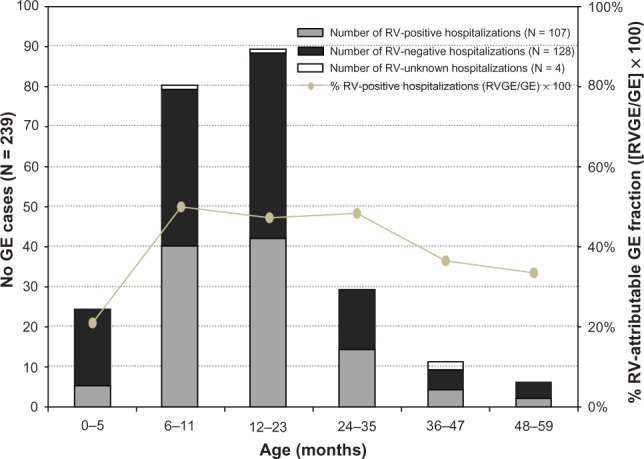
Age distribution of GE- and RV-attributable fraction of GE (Na = 239).
Note: aTotal number of children included in the final analysis.
Abbreviations: GE, gastroenteritis; RV, rotavirus.
RV distribution
In the final analysis, 107 children (44.8%) were RV-positive; 128 (53.5%) were RV-negative; and four (1.7%) had unknown RV status as their stool samples were not collected. The proportion of RV-attributable GE hospitalizations was highest in children aged 6–23 months (82/107; 76.6%; Figure 1).
RVGE was observed throughout the year, with no definite seasonal distribution. The maximum number of RVGE cases were recorded during April 2006 (26/42; 61.6%), followed by June and November 2006 (8/14 and 4/7, respectively; both 57.1%; Figure 2).
Figure 2.
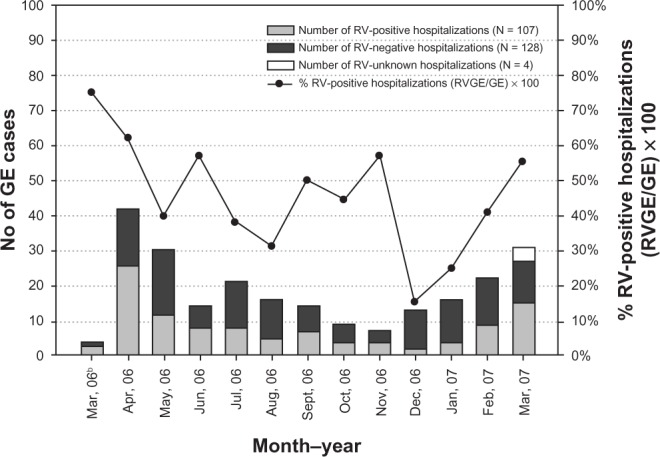
Seasonal distribution of GE and RVGE hospitalizations (Na = 239).
Notes: All four unknown RV cases were observed in March 2007 and were excluded from the final analysis. aTotal number of children included in the final analysis; bfor four cases enrolled in the study in April 2006, the hospital admission dates were between March 29 and 31, 2006; hence, the data for March 2006 is not representative of the entire month of March.
Abbreviations: GE, gastroenteritis; RV, rotavirus.
Clinical characteristics
Before hospitalization, 94.4% (101/107) of RV-positive children and 83.6% (107/128) of RV-negative children had experienced a severe episode of GE. This association was found to be statistically significant (P = 0.0204; Table 1).
Table 1.
Clinical characteristics by RV status (Na = 239)
| Symptoms and signs | Nb (percentage of cases)
|
P-value | |
|---|---|---|---|
| RV-positive (Nc = 107) | RV-negative (Nc = 128) | ||
| Severity of GE before hospitalization | |||
| Mild (1–6) | 0 (0.0) | 1 (0.8) | 0.0204^,* |
| Moderate (7–10) | 6 (5.6) | 20 (15.6) | – |
| Severe (≥11) | 101 (94.4) | 107 (83.6) | – |
| Symptoms before hospitalization | |||
| Diarrhea | 107 (100.0) | 128 (100.0) | – |
| Vomiting | 104 (97.2) | 108 (84.4) | 0.0008^,* |
| Fever | 63 (58.9) | 78 (60.9) | 0.2015# |
| Degree of dehydration before hospitalization | |||
| None | 2 (1.9) | 11 (8.6) | 0.0796^ |
| Mild/moderate | 102 (95.3) | 114 (89.1) | – |
| Severe | 3 (2.8) | 3 (2.3) | – |
| Duration of diarrhea before hospitalization (days) | |||
| 1–4 | 84 (78.5) | 99 (78.0) | 0.5718# |
| 5 | 7 (6.5) | 5 (3.9) | – |
| ≥6 | 16 (15.0) | 23 (18.1) | – |
| Treatment received before hospitalization | |||
| Oral rehydration | 40 (37.4) | 31 (24.2) | 0.0461#,* |
| Intravenous rehydration | 30 (28.0) | 22 (17.2) | 0.0954# |
| Antibiotics | 38 (35.5) | 48 (37.5) | 0.7530# |
| Duration of hospitalization (days) | |||
| Mean (SD) | 3.8 (1.99) | 4.3 (2.72) | – |
| Treatment received during hospitalization | |||
| Oral rehydration | 1 (0.9) | 0 (0.0) | 0.4553^ |
| Intravenous rehydration | 106 (99.1) | 128 (100) | 0.4553^ |
| Antibiotics | 34 (31.8) | 60 (46.9) | 0.0186#,* |
| Outcome at discharge | |||
| Recovered | 106 (99.1) | 125 (97.7) | – |
| Ongoing GE | 0 (0.0) | 1 (0.8) | – |
| Transferred to another hospital | 0 (0.0) | 2 (1.6) | – |
| Unknownd | 1 (0.9) | 0 (0.0) | – |
Notes: Four children were excluded from the final analysis as their RV status was not determined. P-values calculated only for categorical and clinically significant variables, wherever possible.
Total number of children included in the final analysis;
number of children with the corresponding disease characteristic;
number of children in each category;
RV status unknown for four children from whom stool samples were not collected.
statistically significant P-value (for 0.05 alpha level).
chi-square P-value to test the association between the clinical symptoms and RV status.
Fisher’s exact test P-value to test the association between the clinical symptoms and RV status.
Abbreviations: GE, gastroenteritis; RV, rotavirus; SD, standard deviation.
Before hospitalization, vomiting and dehydration were more commonly observed in RV-positive children, with vomiting being significantly associated with RV-positive status (P = 0.0008; Table 1). The mean (standard deviation) duration of hospitalization was 4.1 (±2.41) days, regardless of RV status.
Intravenous (IV) rehydration therapy was administered to 28.0% (30/107) of RV-positive and 17.2% (22/128) of RV-negative children in the emergency room before hospitalization. Almost all children with known RV status (234/235; 99.6%) required IV rehydration therapy during hospitalization. By discharge, most children (231/235; 98.3%) had recovered. No deaths occurred during the study period (Table 1).
Of 107 RV-positive samples, a random subset of 17 were typed. Wild-type G1P[8[ was the most commonly detected RV strain in ten samples (58.8%), and G2P[4[, G2P[8[, and G9P[8[ were each detected in two samples. Clinical material was unavailable from one child for RV typing (Figure 3).
Figure 3.
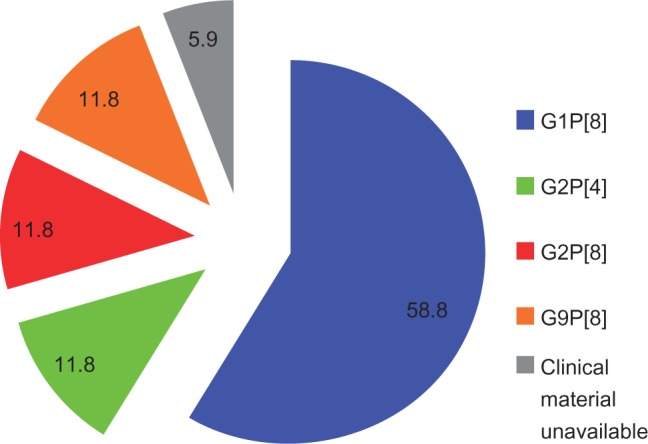
RV type distribution (Na = 17).
Note: aNumber of samples typed.
Abbreviation: RV, rotavirus.
Discussion
This hospital-based surveillance study indicated RV as a major cause of GE in children under 5 years of age in the Kingdom of Bahrain and accounts for 44.8% of GE hospitalizations. This proportion is consistent with previous reports for RVGE hospitalizations (16%–61%) in Middle Eastern and North African countries28 and is comparable with more recent estimates of the proportion of RVGE from Iran (40.0%),29 Turkey (53.0%),30 Saudi Arabia (39.9%),31 and Oman (49.0%);32 in each of these cases, the same WHO generic protocol for assessing RVGE burden in children aged under 5 years was used.22 These results are also consistent with the worldwide estimates (40.0%) of RVGE hospitalizations among children aged under 5 years.33 Nevertheless, our findings are higher than previous reports from the Kingdom of Bahrain in 1990 (13.9%)5 and 2002 (20.8%),6 and could reflect differences in subject screening and the improved sensitivity of the EIA kit we used, which exhibits 100% sensitivity and 97% specificity to RV.25
Importantly RVGE-related hospitalizations were highest amongst children under 2 years of age, as previously observed in Middle Eastern countries such as Iran, Saudi Arabia, and Jordan.29,31,34
As was also observed in earlier studies conducted in Europe35 and Saudi Arabia,31 we found that the severity of GE-related clinical characteristics depended on the RV status of the child. The reporting of vomiting in our study was significantly higher in RV-positive than RV-negative children, which is consistent with earlier reports.23,36,37
We found the seasonal distribution of RV to be similar to that previously seen in this region, with RVGE occurring throughout the year.28–32 While RV exhibits marked seasonal variation in Europe and other regions with a temperate climate,36 seasonality is less marked in regions with warmer climates;28–32 RVGE appears to peak during cooler and drier periods in the Middle East.38
In our study, G1P[8[ was the most commonly detected RV type in the limited number of samples analyzed, followed by G9P[8[, G2P[8[, and G2P[4[. Indeed, G1P[8[ was also the most commonly detected RV type in recent studies carried out in Turkey,30 Saudi Arabia,31 and other Middle Eastern countries.28 In Iran, G4P[8[ was the most common RV type,29 and a more diverse strain pattern was noted in Oman.39 Recent WHO surveillance data across the Eastern Mediterranean region revealed considerable strain diversity among 1,290 RV-positive samples characterized from children under 5 years of age who had been hospitalized with RVGE.33 G2P[4[ was the most commonly identified RV type, accounting for 24.0% of all cases, followed by G1P[8[ (17%) and G9P[8[ (5.0%).33
The main limitation of our study was that, although the study was designed to estimate the incidence of RV-attributable GE-hospitalization, the denominator of births was unavailable to match the numerator data on hospitalization and the incidence could not be estimated. Our study was also limited by the inability to define the catchment area for RVGE cases or to obtain accurate birth cohort data for the catchment populations. The proportion of GE hospitalizations could not be estimated with certainty since data on the number of screened subjects were not available. The strain diversity observed during the study has to be interpreted with caution since only a limited number of samples were analyzed and this may not warrant any statistically valid conclusions about the RV type distribution in this population. Furthermore, the study considered only hospitalized children with GE and did not include children treated for GE as outpatients or in emergency rooms. However, by considering only the more severe cases leading to hospitalization, our study helps understand the RV-attributable fraction of GE and can be related to the direct cost of hospitalization. Hence, our estimates provide a good representation of severe RVGE cases.
To our knowledge, this is the first hospital-based surveillance report on RV type distribution to include analyses of the clinical features and associated treatment in the Kingdom of Bahrain. The study was conducted according to the WHO generic protocol22 (comprising well-established case definitions and standard data collection), with typing of both RV G and P types undertaken in an accredited reference laboratory. Although the number of children enrolled was relatively small, this study was conducted in a large hospital serving approximately 90% of the area’s pediatric population, suggesting that our findings are likely to be representative of the Bahrain population.
Conclusion
RV accounted for 44.8% of GE hospitalizations over a 1-year period from 2006–2007 in children aged under 5 years in our study. Children under 2 years of age were particularly affected by RVGE. These data will help in estimating the number of RVGE cases in the Kingdom of Bahrain and serve as a robust baseline for assessing the potential impact of RV vaccination in reducing the burden of RVGE following its introduction into the national immunization program in 2008.
Author contributions
MA was the coordinating investigator, together with HZ, for the conduct of the study. SA was involved in the conception of the study. FS contributed to the study design and performed the statistical analysis. RD managed the study at GlaxoSmithKline Vaccines and contributed to the analysis and interpretation and critically reviewed the study report. All the authors contributed to the development of the article, revised every draft and approved the final version for submission. All the authors had full access to the data and the corresponding author had the final responsibility for the submission of the manuscript.
Acknowledgments
The authors thank Nada Riachi, Karin Hallez, and Aly Ziwar (all employed by the GlaxoSmithKline group of companies) for their contributions and monitoring support, and DDL Diagnostic Laboratory, The Netherlands for performing the genotyping assay. The authors also thank Devi Priya (employed by the GlaxoSmithKline group of companies) for medical writing, Abdelilah Ibrahimi (XPE Pharma and Science on behalf of GlaxoSmithKline Vaccines) for publication coordination, and Julia Donnelly (on behalf of GlaxoSmithKline Vaccines) for support in copy editing.
Footnotes
Disclosure
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analyses. GlaxoSmithKline Biologicals SA also paid all costs associated with the development and publication of the present manuscript. RD, FS, and SA are employees of the GlaxoSmithKline group of companies and RD has stock options. MA and HZ report no conflicts of interest in this work.
References
- 1.Estimated rotavirus deaths for children under 5 years of age: 2008, 453 000 [webpage on the Internet] Geneva: World Health Organization; 2008Available from: http://www.who.int/immunization_monitoring/burden/rotavirus_estimates/en/Accessed May 17, 2013 [Google Scholar]
- 2.Rotavirus surveillance worldwide – 2009. Wkly Epidemiol Rec. 2011;86:174–176. [No authors listed] English, French. [PubMed] [Google Scholar]
- 3.Malek MA, Taleb N, Abu-Elyazeed R, et al. The epidemiology of rotavirus diarrhea in countries in the Eastern Mediterranean region. J Infect Dis. 2010;202(Suppl):S12–S22. doi: 10.1086/653579. [DOI] [PubMed] [Google Scholar]
- 4.Immunization profile – Bahrain [webpage on the Internet] Geneva: World Health Organization; Available from: http://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=BHRAccessed May 17, 2013 [Google Scholar]
- 5.Dutta SR, Khalfan SA, Baig BH, Philipose L, Fulayfil R. Epidemiology of rotavirus diarrhea in children under five years in Bahrain. Int J Epidemiol. 1990;19(3):722–727. doi: 10.1093/ije/19.3.722. [DOI] [PubMed] [Google Scholar]
- 6.Ismaeel AY, Jamsheer AE, Yousif AQ, Al-Otaibi MA, Botta GA. Causative pathogens of severe diarrhea in children. Saudi Med J. 2002;23(9):1064–1069. [PubMed] [Google Scholar]
- 7.Mrukowicz J, Szajewska H, Vesikari T. Options for the prevention of rotavirus disease other than vaccination. J Pediatr Gastroenterol Nutr. 2008;46(Suppl 2):S32–S37. doi: 10.1097/MPG.0b013e31816f79b0. [DOI] [PubMed] [Google Scholar]
- 8.Rotavirus vaccine and intussusception: report from an expert consultation. Wkly Epidemiol Rec. 2011;86:317–321. [No authors listed] English, French. [PubMed] [Google Scholar]
- 9.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Human Rotavirus Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 10.Vesikari T, Matson DO, Dennehy P, et al. Rotavirus Efficacy and Safety Trial (REST) Study Team Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 11.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Human Rotavirus Vaccine Study Group Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 12.Phua KB, Lim FS, Lau YL, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomized, double-blind, controlled study. Vaccine. 2009;27:5936–5941. doi: 10.1016/j.vaccine.2009.07.098. [DOI] [PubMed] [Google Scholar]
- 13.Vesikari T, Itzler R, Karvonen A, et al. RotaTeq, a pentavalent rotavirus vaccine: efficacy and safety among infants in Europe. Vaccine. 2010;28:345–351. doi: 10.1016/j.vaccine.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 15.de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhea in El Salvador: case-control study. BMJ. 2010;340:c2825. doi: 10.1136/bmj.c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justino MC, Linhares AC, Lanzieri TM, et al. Effectiveness of the monovalent G1P[8[ human rotavirus vaccine against hospitalization for severe G2P[4[ rotavirus gastroenteritis in Belém, Brazil. Pediatr Infect Dis J. 2011;30(5):396–401. doi: 10.1097/INF.0b013e3182055cc2. [DOI] [PubMed] [Google Scholar]
- 17.Snelling TL, Schultz R, Graham J, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis. 2009;49(3):428–431. doi: 10.1086/600395. [DOI] [PubMed] [Google Scholar]
- 18.Recommended immunization schedule for the expanded program on immunization, Bahrain [webpage on the Internet] Kingdom of Bahrain: Ministry of Health; Available from: http://www.moh.gov.bh/EN/HealthInformation/immunizations.aspx?print=trueAccessed May 17, 2013 [Google Scholar]
- 19.Generic Protocol for Monitoring Impact of Rotavirus Vaccination on Gastroenteritis Disease Burden and Viral Strains Geneva: World Health Organization; 2008Available from: http://whqlibdoc.who.int/hq/2008/WHO_IVB_08.16_eng.pdfAccessed May 17, 2013 [Google Scholar]
- 20.Salmaniya Medical Complex (SMC) [webpage on the Internet] Kingdom of Bahrain: Ministry of Health; 2013Available from: http://www.moh.gov.bh/en/HealthEstablishment/SMC.aspxAccessed May 17, 2013 [Google Scholar]
- 21.Health System Organization Kingdom of Bahrain: Regional Health Systems Observatory-EMRO Available from: http://gis.emro.who.int/HealthSystemObservatory/PDF/Bahrain/Health%20system%20organization.pdfAccessed May 17, 2013
- 22.Generic Protocols for (i) Hospital-Based Surveillance to Estimate the Burden of Rotavirus Gastroenteritis in Children and (ii) a Community-Based Survey on Utilization of Health Care Services for Gastroenteritis in Children WHO, Generic Protocol, 2002 Vaccine and Biologicals Geneva: World Health Organization; 2002Available from: http://whqlibdoc.who.int/hq/2002/WHO_V&B_02.15.pdfAccessed May 17, 2013 [Google Scholar]
- 23.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22(3):259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 24.Lewis K.Vesikari Clinical Severity Scoring System Manual PATH2011Available from: http://www.path.org/publications/files/VAD_vesikari_scoring_manual.pdfAccessed May 17, 2013
- 25.Premier™ Rotaclone®. EIA for the Detection of Rotavirus in Human Fecal Samples Cincinnati, OH: Meridian Bioscience, Inc; Available from: http://www.meridianbioscience.com/Content/Assets/Files/2.13%20Rotavirus%20and%20Adenovirus%20Products/Package-Insert-Premier-Rotaclone.pdfAccessed May 17, 2013 [Google Scholar]
- 26.van Doorn LJ, Kleter B, Hoefnagel E, et al. Detection and genotyping of human rotavirus VP4 and VP7 genes by reverse transcriptase PCR and reverse hybridization. J Clin Microbiol. 2009;47(9):2704–2712. doi: 10.1128/JCM.00378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clopper CJ, Pearson ES. The use of confidence intervals illustrated in the case of the binomial. Biometrika. 1934;26(4):404–413. [Google Scholar]
- 28.Khoury H, Ogilvie I, El Khoury AC, Duan Y, Goetghebeur MM. Burden of rotavirus gastroenteritis in the Middle Eastern and North African pediatric population. BMC Infect Dis. 2011;11:9. doi: 10.1186/1471-2334-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eesteghamati A, Gouya M, Keshtkar A, et al. Sentinel hospital-based surveillance of rotavirus diarrhea in Iran. J Infect Dis. 2009;200(Suppl 1):S244–S247. doi: 10.1086/605050. [DOI] [PubMed] [Google Scholar]
- 30.Ceyhan M, Alhan E, Salman N, et al. Multicenter prospective study on the burden of rotavirus gastroenteritis in Turkey, 2005–2006: a hospital-based study. J Infect Dis. 2009;200(Suppl 1):S234–S238. doi: 10.1086/605056. [DOI] [PubMed] [Google Scholar]
- 31.Al Mazrou Y, Khalil M, Azhar E, et al. Hospital-based surveillance of the burden of rotavirus gastroenteritis among children aged <5 years in Saudi Arabia; 4th Europaediatrics; July 3–6, 2009; Moscow, Russia. [Google Scholar]
- 32.Al Awaidy SA, Bawikar S, Al Busaidy S, et al. Considerations for introduction of a rotavirus vaccine in Oman: rotavirus disease and economic burden. J Infect Dis. 2009;200(Suppl 1):S248–S253. doi: 10.1086/605339. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention (CDC) Rotavirus surveillance – worldwide, 2001–2008. MMWR Morb Mortal Wkly Rep. 2008;57(46):1255–1257. [PubMed] [Google Scholar]
- 34.Nafi O. Rotavirus gastroenteritis among children aged under 5 years in Al Karak, Jordan. East Mediterr Health J. 2009;16(10):1064–1069. [PubMed] [Google Scholar]
- 35.Forster J, Guarino A, Parez N, et al. Rotavirus Study Group Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics. 2009;123(3):e393–e400. doi: 10.1542/peds.2008-2088. [DOI] [PubMed] [Google Scholar]
- 36.Karadag A, Acikgoz ZC, Avci Z, et al. Childhood diarrhoea in Ankara, Turkey: epidemiological and clinical features of rotavirus-positive versus rotavirus-negative cases. Scand J Infect Dis. 2005;37(4):269–275. doi: 10.1080/00365540410020983. [DOI] [PubMed] [Google Scholar]
- 37.Uhnoo I, Olding-Stenkvist E, Kreuger A. Clinical features of acute gastroenteritis associated with rotavirus, enteric adenoviruses, and bacteria. Arch Dis Child. 1986;61(8):732–738. doi: 10.1136/adc.61.8.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al Baqlani S, Peenze I, Dewar J, et al. Molecular characterization of rotavirus strains circulating in Oman in 2005. J Infect Dis. 2010;202(Suppl):S258–S262. doi: 10.1086/653582. [DOI] [PubMed] [Google Scholar]


