Abstract
Relapse to smoking is common after initial abstinence with pharmacotherapy and behavioral support and represents a major clinical challenge. Although mechanisms underlying relapse to smoking have not been elucidated, preclinical studies suggest that glutamate receptors may be involved. We sought to test a selective antagonist of the glycine coag-onist site on the glutamate N-methyl-d-aspartate receptor, GW468816, for prevention of relapse in recently abstinent smokers. To do so, we enrolled 264 healthy female smokers in an open 8-week smoking cessation intervention with behavioral therapy and a standard dose of transdermal nicotine replacement therapy with taper and additional gum or lozenge as needed for nicotine withdrawal symptoms. Ninety-eight participants achieved 7-day point prevalence abstinence and were randomized into a 5-week double-blind, placebo-controlled, relapse-prevention trial of GW468816 (200 mg/d) and then followed for 60 days after randomization. There was no effect of treatment on abstinence rates at the end of treatment (χ2 [1, n = 96] = 0.168, p = 0.838), on the rates of relapse (χ2 [1, n = 98] = 0.031, p = 1.000) or lapse (χ2 [1, n = 62] = 0.802, p = 0.423), or on time to relapse (χ2 [1, n = 98) = 0.001, p = 0.972). No significant relationships were detected between plasma GW468816 concentrations and abstinence, time to relapse, or self-reported craving. In conclusion, despite promising preclinical data that support the use of a selective NMDA glycine site antagonist for prevention of relapse to smoking, we observed no effect of GW468816 on relapse or lapse rates, time to relapse, or craving compared to placebo.
Keywords: nicotine, smoking cessation, NMDA, glutamate, relapse prevention, glycine
Dependence on tobacco-derived nicotine accounts for more than 440,000 deaths, 5.1 million years of potential life lost, and $96.8 billion in productivity losses annually in the United States.1,2 The health impact of tobacco dependence on health is on the rise, whereas there were 100 million deaths worldwide in the 20th century caused by tobacco. If current trends continue, there will be 1 billion in the 21st century.3 Although effective pharmacotherapies exist that double or triple cessation rates when combined with behavioral therapy,4,5 relapse rates are very high, with sustained 1-year abstinence rates of less than 25% among those who attained initial abstinence, even with the best treatment available.6-12 Thus, we face a critical need to develop relapse prevention treatments that help smokers to sustain abstinence.
Nicotine dependence, like other addictions, is a chronic relapsing disorder. Although neurobiologic mechanisms underlying relapse to smoking have not been elucidated, it is known that relapse is associated with postquit craving, postquit negative affect, depressed mood, hyper-reactivity to environmental cues associated with smoking use, and stress.13-17 Effective relapse prevention treatment may require unique behavioral and pharmacological interventions that differ from those currently available for smoking cessation.
Nicotine acts as an agonist at presynaptic and postsynaptic receptors on both dopaminergic and glutamatergic neurons in the neural circuitry involved in motivational, emotional, and cognitive processes relevant to goal-directed behaviors such as drug seeking, and recent work suggests a role for glutamate and the ionotropic N-methyl-d-aspartate (NMDA) glutamate receptor in mediating relapse to drug use.18-30 N-methyl-d-aspartate receptors require binding of both glycine and glutamate for activation. N-methyl-d-aspartate antagonists acting at nonglycine sites on the receptor block chronic effects of opiates, alcohol, psychomotor stimulants, and nicotine.31-35 However, significant adverse effects on learning and motor coordination limit the therapeutic application of this approach, and glycine site antagonists offer an alternative to moderate NMDA receptor function that are better tolerated.36-38 A tetrahydroquinolinic derivative family of compounds active as competitive antagonists at the strychnine-insensitive glycine site on the NMDA receptor was developed by GlaxoSmithKline (GSK).39 One of these compounds, GW468816 (Fig. 1, compound 2a on Di Fabio et al39), has shown potential efficacy for prevention of relapse to nicotine-seeking behavior in short- and long-term preclinical abstinence models (GSK reports VR2000/00020/00 and VR2000/00026/00). In these studies, GW468816 reduced nicotine-cue and nicotine-priming–induced reinstatement of drug use after a period of abstinence in a rat model of nicotine self-administration without inducing motor side effects, suggesting potential efficacy under conditions known to trigger relapse to smoking. GW468816, in a range of oral doses (0.01–1 mg/kg), also demonstrated efficacy at reducing craving and withdrawal symptoms in preliminary human studies in abstinent smokers (GSK report VM2002/00022/00, study RES11015, 2002).
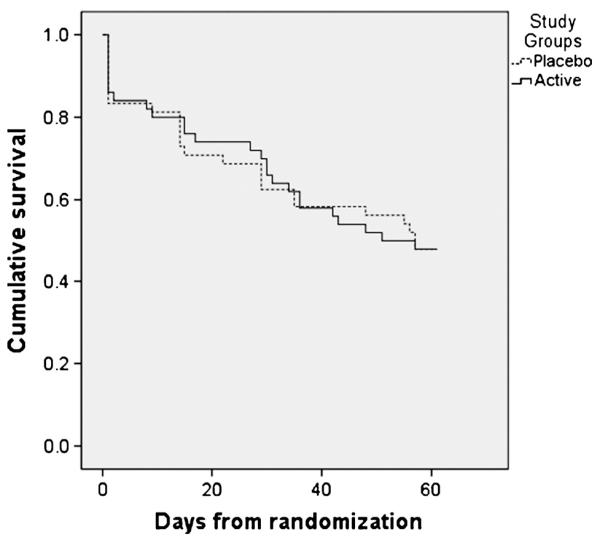
FIGURE 1.
Kaplan-Meier survival curves for the active and placebo groups.
To test the hypothesis that glycine-dependent modulation of NMDA receptors may reduce relapse, we conducted a 5-week double-blind, placebo-controlled trial of GW468816 in recently abstinent adult female smokers. Our primary hypotheses were that compared with those assigned to placebo, smokers assigned to GW468816 would (1) achieve a higher rate of 7-day point prevalence abstinence at the end of the 5-week relapse prevention phase, and (2) demonstrate a significantly longer time to relapse over a 60-day follow-up.
MATERIALS AND METHODS
Study Population and Recruitment
The Institutional Review Board of Massachusetts General Hospital approved study procedures, which were conducted in accordance with the Declaration of Helsinki. Eligible participants were women, aged 18 to 65 years, inclusive, who smoked 10 or more cigarettes per day for the prior 6 months, had either expired air carbon monoxide (CO) of greater than 10 ppm or saliva cotinine concentration greater than 30 ng/mL, and met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for nicotine dependence. Women of childbearing potential were required to have a negative pregnancy test before receiving the first dose of study medication and to agree to use an approved form of contraception from the day of the first dose of study medication until 90 days after the last dose of study medication. Potential participants were excluded who had unstable medical illness, investigational drug use in the prior 30 days, drug use disorder other than nicotine and caffeine or major depressive episode within the prior 6 months, lifetime diagnosis of organic mental disorder, bipolar disorder, or psychotic disorder, elevation over 1.5 times upper limit of normal value of liver enzymes, history of multiple adverse drug reactions, concurrent use of a statin, or if they had been unresponsive to an adequate course of nicotine replacement therapy (NRT) in the prior month. Males were excluded pending further preclinical study of the effect of GW468816 on spermatogenesis.
Assessments
Screening assessments included medical history, medications, age of initiation of daily smoking, duration, and frequency of tobacco use were assessed; and the structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Fagerstrom test for nicotine dependence (FTND),40 and Hamilton Rating Scale for Depression (HAM-D).41 Smoking behavior was assessed at screen and at each study visit with self-report and biochemical confirmation with either expired CO during NRT treatment or urinary cotinine after discontinuation of NRT. Seven-day point prevalence abstinence was defined as self-report of smoking no cigarettes in the prior 7 days, confirmed by expired air carbon monoxide (CO) of less than 10 ppm. Carbon monoxide was measured in end-expiratory air after a 15-second breath hold using a portable Bedfont Smokerlyzer III (Kent, UK). Lapse was defined as any smoking, even a puff, after initial abstinence. Relapse was defined as smoking 7 or more cigarettes in the prior week. The primary outcomes were time to relapse and 7-day point prevalence abstinence rates at the end of the randomized phase.
Other assessments included the Tiffany Questionnaire of Smoking Urges Brief (QSU-B) to assess craving,42 the 17-Item Hamilton Rating Scale for Depression (HAM-D 28),43 the State Trait Anxiety Inventory (STAI),44 the Positive and Negative Affect Scale (PANAS-X),45 and the Wisconsin Smoking With-drawal Scale.46 Presence, severity, and duration of adverse events were assessed at each visit by self-report and classified according to the NCI Common Terminology Criteria for Adverse Events (CTCAE v4.0).
Plasma samples for GW468816 concentration were taken predose at study visits 9, 11, and 13 frozen and stored at −20°C until transferred to World Wide Bioanalysis, DMPK, GlaxoSmithKline, UK for analysis. The GW468816 serum analytic method was based on protein precipitation with acetonitrile followed by high performance liquid chromatography with tandem mass spectrometric detection analysis. Using a 50-KL aliquot of human plasma, the lower limit of quantification for GW468816 was 0.1 μg/mL, with a higher limit of quantification of 100 μg/mL. Quality control samples (QC) prepared at 3 different concentrations and stored with study samples were analyzed with each batch of samples against separately prepared calibration standards. For the analysis to be acceptable, no more than one third of the QC results were to deviate from the nominal concentration by more than 15%, and at least 50% of the results from each QC concentration should be within 15% of nominal. The applicable analytical runs met all predefined run acceptance criteria for GW468816 (GSK report VD2004/00181/00).
Interventions
After consent and screening assessments listed above, eligible study participants initiated open-label NRT patch of 21 mg/d for 4 weeks, 14 mg/d for 2 weeks, and 7 mg/d for 2 weeks plus prn nicotine gum or lozenge for craving at a dose of up to 10 mg/d for the first 4 weeks, 6 mg/d for the next 2 weeks, and 4 mg/d for the final 2 weeks and weekly 15-minute individual, manualized, supportive behavioral interventions designed to encourage smoking cessation and to prevent relapse. Behavioral visits focused on proper use of NRT, strategies for the quit date and for coping with urges to smoke, and education on withdrawal symptoms. Participants were asked to stop smoking and start NRT 1 week after the baseline visit. Participants not achieving at least 1 week of continuous abstinence by the end of the open phase (visit 7) were tapered off NRT and discontinued from the study.
Participants who met criteria for 7-day point prevalence abstinence at study visit 8 were eligible to enter the 5-week relapse prevention phase and were randomly assigned to receive double-blind GW468816, 200 mg orally, or identical placebo. Study medications were given concurrently with NRT for the last week of the NRT taper. Subjects who completed the relapse prevention phase entered the follow-up phase.
Analysis
To confirm randomization success, the GW468816 and placebo groups were compared on measures that may influence likelihood of relapse, including severity of nicotine dependence, nicotine withdrawal symptoms, craving, and negative affect. χ2 tests then compared biochemically confirmed 7-day point prevalence abstinence and the occurrence of relapse or lapse during the relapse prevention phase between the study groups (GW468816 versus placebo). Kaplan-Meier survival analysis assessed time (days) to relapse during the 60-day period after randomization. The incidence of adverse events was also compared via χ2 analysis. A final analysis examined plasma concentrations of GW468816 by outcome in the 29 subjects selected for pharmacokinetic analysis. A t test assessed the difference in plasma concentrations of GW468816 by the past week’s smoking status, both measured at visit 13, and a Kaplan-Meier survival analysis assessed difference in time to relapse across tertiles of plasma GW468816 concentration. Plasma concentrations at visits 9, 11, and 13 were assessed for correlation with craving as assessed with the “urge to smoke” subscale of the Wisconsin Smoking Withdrawal Scale and both factors of the Tiffany QSU. An intent- to-treat approach was used for analyses, including all subjects who were randomized regardless of study completion (n = 98). The sole exception is for the test of lapse rates by study group; a completer analysis was conducted for lapse rates as information on lapse status was available only for those who completed the relapse prevention phase (n = 63). An attrition analysis revealed no differences between those who were retained for the 60-day follow-up versus those lost to follow-up in age, education, or expired air CO at recruitment. Those who dropped out were treated as relapsed in the outcomes analysis. An alpha level of 0.05 was used for all analyses.
With a sample size of 98 and alpha level of 0.05, the survival analysis had 80% power to detect a difference in time to relapse between the study groups, assuming a hazard ratio (ie, the difference in the proportions of relapsed subjects per group) of 2.24 and a median time to relapse of 60 days in the placebo group (ie, assuming a relapse rate of 50% over 60 days in the placebo group).47
RESULTS
Participant Disposition and Characteristics
A total of 292 female smokers from the Boston area who responded to media advertisements completed the informed consent process and were screened, and 264 enrolled in the singlesite, open-label, smoking cessation phase with NRT (visits 1–8). Subjects achieving 1 week or longer of abstinence at the end of this phase (n = 98) were enrolled in the 5-week randomized, parallel group comparison of GW468816 (n = 50) or placebo (n = 48) for prevention of relapse to smoking (visits 9–13; Table 1). Participants were on average 48 years old, with 14 years of education; 85% were white, 44% were married, and 56% were employed full time, and were moderately to severely nicotine dependent. The groups were well matched on assessments of craving, withdrawal symptoms at randomization, and depressive and anxiety symptoms. No significant group differences were observed in the demographic, smoking, withdrawal, or psychiatric variables.
TABLE 1.
Clinical Characteristics
| Placebo (n = 48) |
GW468816 (n = 50) |
|
|---|---|---|
| Age | 46.5 (10.4) | 49.3 (10.5) |
| CO, ppm | 16.2 (8.9) | 15.4 (6.9) |
| Weight, lbs | 159.2 (25.7) | 155.9 (38.1) |
| FTND total | 4.9 (1.9) | 4.9 (1.7) |
| Pack years | 26.5 (19.4) | 29.2 (22.5) |
| T-QSU total | 21.0 (8.7) | 22.2 (9.5) |
| T-QSU factor 1 | 4.9 (2.4) | 5.3 (2.4) |
| T-QSU factor 2 | 4.0 (1.6) | 4.9 (2.5) |
| Wisconsin Smoking Withdrawal total |
54.6 (9.3) | 55.6 (10.2) |
| Snaith-Hamilton total | 22.3 (5.1) | 22.1 (5.1) |
| HAMD 17 total | 2.3 (2.2) | 3.3 (3.4) |
| Barratt Impulsiveness Scale total | 60.8 (10.1) | 61.1 (6.9) |
| Life events total | 127.7 (120.3) | 104.4 (69.3) |
| SSEQ total | 710.4 (377.3) | 782.4 (400.2) |
| PANAS negative subscale | 30.4 (14.6) | 31.1 (12.3) |
| PANAS positive subscale | 32.9 (8.2) | 35.6 (6.6) |
| PANAS fear subscale | 17.9 (8.8) | 19.3 (8.3) |
| PANAS hostility subscale | 20.0 (9.8) | 18.0 (7.4) |
| PANAS guilt subscale | 20.1 (9.4) | 20.9 (9.0) |
| PANAS sadness subscale | 16.3 (8.2) | 15.3 (7.0) |
| STAI-State total | 32.9 (5.7) | 36.4 (7.9) |
Of the 98 participants who completed the open phase with at least 7 days of abstinence and were randomized, 69 (70.4%) completed at least 3 weeks of the randomized phase, 63 (64.3%) attended all 5 visits in the randomized phase, and 41 (41.9%) were followed for 60 days. Among the 57 subjects who dropped out before the 60-day follow-up, a 3 × 2 χ2 test revealed no between-group differences among participants who dropped out between 0 and 7 days, 8 and 35 days, or 36 and 60 days after randomization (χ2 [2, n = 57] = 0.874, p = 0.650). Per our analytic plan, subjects who discontinued the study prematurely were considered to have relapsed.
Effect of GW468816 on Abstinence
Fifty-two subjects (54.2%) met criteria for a 7-day point prevalence abstinence at the end of the relapse prevention phase of the trial, whereas 42 (42.8%) relapsed during the 5-week phase. Twenty-seven subjects (56.3%) in the active group and 25 (52.1%) in the placebo group were abstinent in the last 7 days of the relapse prevention phase (χ2 [1, n = 98] = 0.168, Fisher exact p = 0.838). Twenty-one subjects in each group (42.0% in the active group vs 41.7% in the placebo group) relapsed during the relapse prevention phase (χ2 [1, n = 98] = 0.031, Fischer exact p = 1.000). Restricting the analysis to only those subjects who completed the full relapse prevention phase (n = 63) did not affect these results. In this subgroup for whom complete data on lapses were available, 20 subjects (32.3%) reported a lapse during the relapse prevention phase, including 9 (27.3%) in the active group and 11 (37.9%) in the placebo group (χ2 [1, n = 63] = 0.802, Fisher exact p = 0.423).
Over the 60 days after randomization, the relapse rate was 52.0% (n = 51). Figure 1 shows Kaplan-Meier analysis survival curves. No group differences were observed in the time to relapse (χ2 = 0.001, N = 98, p = 0.972). Among those who relapsed, the mean (SD) days to relapse was 20.8 (18.3) in the GW468816 group and 20.6 (20.3) in the placebo group. Restricting the analysis to those subjects who were followed for the full 60-day period (n = 41) did not affect results.
Among subjects assigned to GW468816, there was no difference in plasma GW468816 concentration at week 13 in those who met the criteria for 7-day point prevalence abstinence at that time (mean [SD], 13.8 [11.35]) and those did not (mean [SD], 11.9 [9.7]; t [21] = −0.305, p = 0.763). A KaplanMeier analysis revealed no difference in days to relapse during the relapse prevention phase across tertiles of plasma GW468816 concentration (χ2 [2, n = 98] = 0.297, p = 0.862).
Adverse Events
Three serious adverse events (SAEs) were reported during the relapse prevention phase, 2 SAEs in the subjects assigned to GW468816 (facial edema due to sunburn and non-small cell adenocarcinoma of the lung) and one SAE in a subject in the placebo arm (hospitalization for diverticulitis). None were thought to be related to study treatment. Analyses of adverse events (AEs) were conducted at the subject level, with 1.34 (1.41) AEs reported per person in the GW468816 group and 1.67 (1.86) events per person in the placebo group. χ2 analyses were conducted to determine whether there was a significant group difference in AEs reporting and are presented in Table 2.
TABLE 2.
Adverse Events
| Placebo |
GW468816 |
Total |
|||||
|---|---|---|---|---|---|---|---|
| Adverse Events Reported by 10% or More of Participants on GW468816 |
(n = 48) |
(n =50) |
(n = 98) |
||||
| n | % | n | % | n | % | χ2 (P)* | |
| Nausea | 1 | 2.1 | 6 | 12.0 | 7 | 7.1 | 3.631 (.11) |
| Insomnia | 6 | 12.5 | 7 | 14.0 | 13 | 13.3 | 0.048 (1.0) |
| Adverse event by systems | |||||||
| Cardiovascular | 2 | 4.2 | 3 | 6.0 | 5 | 5.1 | 0.170 (1.0) |
| General/administration site | 7 | 14.6 | 8 | 16.0 | 15 | 15.3 | 0.038 (1.0) |
| Gastrointestinal | 11 | 22.9 | 14 | 28.0 | 25 | 25.5 | 0.333 (.65) |
| Hematological | 1 | 2.1 | 1 | 2.0 | 2 | 2.0 | 0.001 (1.0) |
| Metabolic | 1 | 2.1 | 2 | 4.0 | 3 | 3.1 | 0.303 (1.0) |
| Musculoskeletal | 6 | 12.5 | 6 | 12.0 | 12 | 12.2 | 0.006 (1.0) |
| Nervous system | 15 | 31.3 | 12 | 24.0 | 27 | 27.6 | 0.645 (0.50) |
| Respiratory | 13 | 27.1 | 6 | 12.0 | 19 | 19.4 | 3.565 (0.08) |
| Dermatological | 2 | 4.2 | 4 | 8.0 | 6 | 6.1 | 0.626 (0.68) |
| Special senses | 2 | 4.2 | 1 | 2.0 | 3 | 3.1 | 0.387 (0.61) |
| Urogenital/reproductive | 2 | 4.2 | 0 | — | 2 | 2.0 | 2.127 (0.24) |
Pearson χ2 statistics, with Fisher exact P values.
There were no changes in laboratory assessments of total bilirubin, direct bilirubin, total protein, or gamma glutamyl transferase in those assigned to receive GW468816 from randomization (visit 8) to the end (visit 13) of the 5-week relapse prevention phase as analyzed with paired-samples t tests. None of the mean laboratory measurements were outside of the reference range.
Finally, there were no significant correlations between plasma GW468816 concentrations and concurrent assessments of craving or nicotine withdrawal symptoms. Use of nonparametric Spearman rank correlation tests, conducted to verify the results in the face of skewed variable distributions, did not alter the analysis. The mean plasma concentration of GW468816 achieved by the subjects assigned to GW468816 were 14.69 (9.33) μg/mL at week 1, 15.70 (9.80) μg/mL at week 3, and 13.43 (10.90) μg/mL at week 5.
DISCUSSION
We found no evidence that the selective NMDA glycine site antagonist, GW468816, dosed at 200 mg/d for 5 weeks, reduced relapse or improved smoking outcomes in recently abstinent female smokers who quit smoking using NRT. Whereas median plasma concentration of 14.3 μg/mL in the group randomized to GW468816 suggests overall good medication compliance, plasma drug concentrations were not correlated with smoking outcomes: 7-day point prevalence abstinence at the end of treatment, relapse or lapse during the relapse prevention phase, time to relapse, or subjective report of the urge to smoke. These results, coupled with self-report of excellent adherence to study medication, suggest a lack of efficacy of GW468816 for prevention of relapse to smoking despite preclinical evidence suggesting promise for this mechanism and this compound for this indication. The paucity of adverse effects, particularly CNS effects, suggests that it is possible that the selected dose was not adequate to generate the expected CNS receptor binding.
Potential confounding factors such as failed randomization and abnormal patterns of relapse after smoking cessation intervention were explored, and no differences in demographic characteristics, psychiatric measures, or dropout rates by treatment group were found. The relapse rate of 42.8% over 5 weeks with behavioral support and 52% over 60 days is relatively low compared with relapse at 30 days (64%) after unaided cessation (Powell et al48) but comparable to the relapse rate after treatment discontinuation reported in other studies.9,10
Limitations
The present study tested the relapse prevention efficacy of a single dose of GW468816, 200 mg/d. Although dose modeling indicated that this dosage would result in efficacious plasma concentrations, group differences with respect to AEs were not evident, raising the possibility that GW468816 at 200 mg/d exerts little CNS effect, and a dose ranging trial may have provided a more definitive result. This study was limited to women, and because sex differences in the smoking cessation process have been identified, it is unknown whether the effect of GW468816 differs in male smokers. To address these limitations, future studies with GW468816 could consider using a dose-finding design and inclusion of male smokers if safety data allow. It is also possible that GW468816 may be effective in smoking cessation rather than relapse prevention only, since the current trial only tested a relapse prevention hypothesis for those smokers who quit smoking using NRT. However, 2 unpublished studies aiming to test 1-week abstinence in smokers willing to quit failed to differentiate GW468816 from placebo while showing significant effects on craving attenuation (GSK document VM2002/00013/01, study GL310001, and HM2004/00172/00, study GL3102034). Another limitation to the study may have been power, as the detectable hazard ratio with 80% power for 98 subjects was 2.24. Because the rate of relapse was equivalent between the groups, the likelihood that the study failed to detect a significant effect of medication is low.
In summary, despite promising preclinical and early clinical data suggesting that GW468816, at the dose tested, would have efficacy in preventing relapse to smoking through reducing activation at the glycine site of NMDA glutamate receptors, the study found no evidence that GW468816, 200 mg/d, reduces relapse to smoking in recently abstinent female smokers. Furthermore, recent preclinical work suggests that increasing activity at the strychnine-insensitive glycine binding site of NMDA receptors, either through agonist or inhibition of reuptake, may prevent reinstatement of nicotine use, suggesting that compounds that increase rather than decrease activity at the glycine site of the NMDA receptor may have efficacy for reducing relapse to smoking.49
Acknowledgments
This study was funded by National Institute of Health grant U01DA019378 and by research product and grant support from GlaxoSmithKline (Drs Evins, Kaufman, and Fava).
GlaxoSmithKline conducted the analysis of plasma concentrations of GW468816. Funding sponsors had no further role in data collection, data analysis, interpretation of findings, the writing of the research report, or in the decision to submit the manuscript for publication.
Footnotes
AUTHOR DISCLOSURE INFORMATION
Dr Fava has received research support from: Abbott Laboratories; Alkermes, Inc; Aspect Medical Systems; AstraZeneca; BioResearch; BrainCells Inc; Bristol-Myers Squibb; Cephalon, Inc; CeNeRx BioPharma; Clinical Trials Solutions, LLC; Clintara, LLC; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc; Euthymics Bioscience, Inc; Forest Pharmaceuticals, Inc; Ganeden Biotech, Inc; GlaxoSmithKline; Icon Clinical Research; i3 Innovus/Ingenix; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; NARSAD; NCCAM; NIDA; NIMH; Novartis AG; Organon Pharmaceuticals; PamLab, LLC; Pfizer Inc; Pharmavite® LLC; Photothera; Roche; RCT Logic, LLC; Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc; Synthelabo; Wyeth-Ayerst Pharmaceuticals, Inc; Transcept Pharmaceuticals, Inc; and Vanda Pharmaceuticals, Inc.
Dr Evins has received research support from Pfizer, Janssen, GSK, and Aspect Medical Systems, and honoraria from Pfizer, Janssen, GSK and Boehringer Ingelheim. Dr Mischoulon has received research support from Nordic Naturals, and Ganeden; royalties from Back Bay Scientific and Lippincott Williams & Wilkins; and honoraria from Pamlab, Nordic Naturals, and Reed Medical Education. Dr Rigotti has received research grant support from Pfizer and Nabi Biopharmaceuticals and is an unpaid consultant for Pfizer and Free and Clear. Drs Pachas, Janes, Urbanoski, Nino-Gomez, Loebl and Ms Carlini, Sousa, and Bentley have none to disclose.
REFERENCES
- 1.CDC Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb Mort Wkly Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- 2.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 3.WHO . WHO Report on the Global Tobacco Epidemic: The MPower Package. World Health Organization; Geneva, Switzerland: 2008. [Google Scholar]
- 4.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Garrett BE, Rose CA, Henningfield JE. Tobacco addiction and pharmacological interventions. Expert Opin Pharmacother. 2001;2:1545–1555. doi: 10.1517/14656566.2.10.1545. [DOI] [PubMed] [Google Scholar]
- 7.Hughes JR, Gulliver SB, Fenwick JW, et al. Smoking cessation among self-quitters. Health Psychol. 1992;11:331–334. doi: 10.1037//0278-6133.11.5.331. [DOI] [PubMed] [Google Scholar]
- 8.Brandon TH, Tiffany ST, Obremski KM, et al. Postcessation cigarette use: the process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 9.Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 10.Jorenby DE, Leischow SJ, Nides MA, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- 11.Hall SM, Humfleet GL, Reus VI, et al. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry. 2002;59:930–936. doi: 10.1001/archpsyc.59.10.930. [DOI] [PubMed] [Google Scholar]
- 12.Hays JT, Hurt RD, Rigotti NA, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Ann Intern Med. 2001;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 13.Kenford SL, Smith SS, Wetter DW, et al. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70:216–227. [PubMed] [Google Scholar]
- 14.Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 15.Janes AC, Pizzagalli DA, Richardt S, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiffman S, Ferguson SG, Gwaltney CJ. Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology (Berl) 2006;184:608–618. doi: 10.1007/s00213-005-0175-4. [DOI] [PubMed] [Google Scholar]
- 17.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist ly379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens DN. A glutamatergic hypothesis of drug dependence: extrapolations from benzodiazepine receptor ligands. Behav Pharmacol. 1995;6:425–446. [PubMed] [Google Scholar]
- 20.Trujillo KA. Effects of noncompetitive N-methyl-d-aspartate receptor antagonists on opiate tolerance and physical dependence. Neuropsychopharmacology. 1995;13:301–307. doi: 10.1016/0893-133X(95)00088-U. [DOI] [PubMed] [Google Scholar]
- 21.Trujillo KA, Akil H. Excitatory amino acids and drugs of abuse: a role for N-methyl-d-aspartate receptors in drug tolerance, sensitization and physical dependence. Drug Alcohol Depend. 1995;38:139–154. doi: 10.1016/0376-8716(95)01119-j. [DOI] [PubMed] [Google Scholar]
- 22.Balfour DJ. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus accumbens [corrected] Nicotine Tob Res. 2004;6:899–912. doi: 10.1080/14622200412331324965. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt WJ, Kretschmer BD. Behavioural pharmacology of glutamate receptors in the basal ganglia. Neurosci Biohav Rev. 1997;21:381–392. doi: 10.1016/s0149-7634(96)00044-9. [DOI] [PubMed] [Google Scholar]
- 24.Risso F, Parodi M, Grilli M, et al. Chronic nicotine causes functional upregulation of ionotropic glutamate receptors mediating hippocampal noradrenaline and striatal dopamine release. Neurochem Int. 2004;44:293–301. doi: 10.1016/s0197-0186(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 25.Kiba H, Jayaraman A. Nicotine induced c-fos expression in the striatum is mediated mostly by dopamine d1 receptor and is dependent on NMDA stimulation. Brain Res Mol Brain Res. 1994;23:1–13. doi: 10.1016/0169-328x(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 26.Levin E, Tizabi Y, Rezvani A, et al. Chronic nicotine and dizocilpine effects on regionally specific nicotinic and NMDA glutamate receptor binding. Brain Res. 2005;1041:132–142. doi: 10.1016/j.brainres.2005.01.104. [DOI] [PubMed] [Google Scholar]
- 27.Wang LP, Li F, Shen X, et al. Conditional knockout of NMDA receptors in dopamine neurons prevents nicotine-conditioned place preference. PLoS One. 2010;5:e8616. doi: 10.1371/journal.pone.0008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenny PJ, Chartoff E, Roberto M, et al. Nmda receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain R, Mukherjee K, Balhara YP. The role of NMDA receptor antagonists in nicotine tolerance, sensitization, and physical dependence: a preclinical review. Yonsei Med J. 2008;49:175–188. doi: 10.3349/ymj.2008.49.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki Y, Jia Y, Niu R, et al. Nicotine exposure in vivo induces long-lasting enhancement of NMDA receptor-mediated currents in the hippocampus. Eur J Neurosci. 2006;23:1819–1828. doi: 10.1111/j.1460-9568.2006.04714.x. [DOI] [PubMed] [Google Scholar]
- 31.Schilstrom B, Nomikos GG, Nisell M, et al. N-methyl-d-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998;82:781–789. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- 32.Schenk S, Valadez A, Worley CM, et al. Blockade of the acquisition of cocaine self-administration by the NMDA antagonist mk-801 (dizocilpine) Behav Pharmacol. 1993;4:652–659. [PubMed] [Google Scholar]
- 33.Schilstrom B, De Villiers S, Malmerfelt A, et al. Nicotine-induced fos expression in the nucleus accumbens and the medial prefrontal cortex of the rat: role of nicotinic and nmda receptors in the ventral tegmental area. Synapse. 2000;36:314–321. doi: 10.1002/(SICI)1098-2396(20000615)36:4<314::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 34.Ranaldi R, French E, Roberts DC. Systemic pretreatment with mk-801 (dizocilpine) increases breaking points for self-administration of cocaine on a progressive-ratio schedule in rats. Psychopharmacology (Berl) 1996;128:83–88. doi: 10.1007/s002130050113. [DOI] [PubMed] [Google Scholar]
- 35.Kosowski AR, Cebers G, Cebere A, et al. Nicotine-induced dopamine release in the nucleus accumbens is inhibited by the novel ampa antagonist zk200775 and the nmda antagonist cgp39551. Psychopharmacology (Berl) 2004;175:114–123. doi: 10.1007/s00213-004-1797-7. [DOI] [PubMed] [Google Scholar]
- 36.Karcz-Kubicha M, Wedzony K, Zajaczkowski W, et al. NMDA receptor antagonists acting at the glycine b site in rat models for antipsychotic-like activity. J Neural Transm. 1999;106:1189–1204. doi: 10.1007/s007020050233. [DOI] [PubMed] [Google Scholar]
- 37.Morrow BA, Taylor JR, Roth RH. R-(+)-ha-966, an antagonist for the glycine/nmda receptor, prevents locomotor sensitization to repeated cocaine exposures. Brain Res. 1995;673:165–169. doi: 10.1016/0006-8993(94)01456-r. [DOI] [PubMed] [Google Scholar]
- 38.Shoaib M, Shippenberg TS, Goldberg SR, et al. Behavioral studies with the glycine partial agonist (+)-ha966 on cocaine-induced locomotor activity and reinforcement. Behav Pharmacol. 1995;6:568–576. [PubMed] [Google Scholar]
- 39.Di Fabio R, Alvaro G, Bertani B, et al. Chiral tetrahydroquinoline derivatives as potent anti-hyperalgesic agents in animal models of sustained inflammation and chronic neuropathic pain. Bioorg Med Chem Lett. 2007;17(5):1176–1180. doi: 10.1016/j.bmcl.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Heatherton TF, Kozlowski LT, Frecker RC, et al. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 42.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (qsu-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- 45.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 46.Welsch SK, Smith SS, Wetter DW, et al. Development and validation of the Wisconsin smoking withdrawal scale. Exp Clin Psychopharmacol. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 47.Schoenfeld D. Statistical considerations for clinical trials and scientific experiments. Available at: http://hedwig.mgh.harvard.edu/sample_size/size.html.
- 48.Powell J, Dawkins L, West R, et al. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology. 2010;212:537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- 49.Uslaner JM, Drott JT, Sharik SS, et al. Inhibition of glycine transporter 1 attenuates nicotine—but not food-induced cue-potentiated reinstatement for a response previously paired with sucrose. Behav Brain Res. 2010;207:37–43. doi: 10.1016/j.bbr.2009.09.035. [DOI] [PubMed] [Google Scholar]