Abstract
Purpose
Central nervous system plasticity is essential for normal function, but can also reinforce abnormal network behavior, leading to epilepsy and other disorders. The role of altered ion channel expression in abnormal plasticity has not been thoroughly investigated. Nav1.6 is the most abundantly expressed sodium channel in the nervous system. Because of its distribution in the cell body and axon initial segment, Nav1.6 is crucial for action potential generation. The goal of the present study was to investigate the possible role of changes in Nav1.6 expression in abnormal, activity-dependent plasticity of hippocampal circuits.
Methods
We studied kindling, a form of abnormal activity-dependent facilitation. We investigated: 1. sodium channel protein expression by immunocytochemistry and sodium channel mRNA by in situ hybridization, 2. sodium current by patch clamp recordings, and 3. rate of kindling by analysis of seizure behavior. The initiation, development, and expression of kindling in wild type mice were compared to Nav1.6 +/− medtg mice, which have reduced expression of Nav1.6.
Results
We found that kindling was associated with increased expression of Nav1.6 protein and mRNA, which occurred selectively in hippocampal CA3 neurons. Hippocampal CA3 neurons also showed increased persistent sodium current in kindled animals compared to sham-kindled controls. Conversely, Nav1.6 +/− medtg mice resisted the initiation and development of kindling.
Discussion
These findings suggest an important mechanism for enhanced excitability, in which Nav1.6 may participate in a self-reinforcing cycle of activity-dependent facilitation in the hippocampus. This mechanism could contribute to both normal hippocampal function, and to epilepsy and other common nervous system disorders.
Keywords: epilepsy, kindling, hippocampus, persistent sodium current, LTP
Introduction
Network plasticity in the central nervous system is crucial for normal memory and learning, but it can also participate in the reinforcement of abnormal activity patterns such as epilepsy, chronic pain, and psychiatric disorders. Kindling is a well established model of abnormal activity-dependent plasticity, in which repetition of a weak stimulus over time leads to long-term facilitation, and ultimately, to prolonged seizures in response to mild stimulation (Goddard, 1967; Morimoto et al., 2004). The mechanisms of enhanced excitability in kindling have been extensively studied, but are not fully understood (Morimoto et al., 2004). Voltage-gated sodium channels are crucial for action potential generation and cellular excitability, and may show activity-dependent modulation (Offord and Catterall, 1989; Sashihara et al., 1996; Waxman, 2000). Prior work has shown that mutations in voltage-gated sodium channels can lead to genetic forms of epilepsy (Mulley et al., 2005; Berkovic et al., 2006). However, the potential role of altered sodium channel expression in acquired epilepsy and other forms of abnormal activity-dependent plasticity has not been thoroughly investigated.
Nav1.6 is the most abundantly expressed sodium channel in the adult central nervous system (Goldin, 2001). The neuronal distribution of Nav1.6 includes the cell body and initial segment (Goldin, 2001; Jenkins and Bennett, 2001), critical for action potential generation and repetitive firing (Yue et al., 2005; Astman et al., 2006). Prior investigation of network plasticity demonstrates a role for changes in synaptic function, such as altered neurotransmitter release or effects on receptors (Lissin et al., 1998; O’Brien et al., 1998; Turrigiano et al., 1998; Kandel, 2004; Lynch, 2004), but less attention has been given to changes in membrane excitability, such as those related to altered sodium channel expression. Because of the known role of the hippocampus in both normal and abnormal network plasticity (Lothman et al., 1991a; Lynch, 2004; Morimoto et al., 2004; Barco et al., 2006), we examined changes in sodium channel expression in this brain region, to determine if altered sodium channel expression may contribute to changes in network excitability. Since the mechanisms of network plasticity in epilepsy are shared by other conditions, investigation of epileptogenesis can yield general insights into abnormal network plasticity (Cain, 1989; Rogawski and Loscher, 2004a).
The acquisition of abnormal network plasticity in epileptogenesis occurs through several stages, including initiation, development, and stable expression (White, 2002). We found that once established, the long-term facilitation seen in kindling is associated with increased expression of Nav1.6 sodium channel protein and mRNA in hippocampal CA3 neurons. This change was also associated with an increase in persistent sodium current, known to participate in repetitive firing and enhanced excitability (Yue et al., 2005). Conversely, reduced expression of Nav1.6 in medtg heterozygote knockout mice (Burgess et al., 1995), caused a marked resistance to the initiation and development of kindling. These findings suggest that Nav1.6 plays an important role in all stages of abnormal central nervous system plasticity. Therefore, reduced Nav1.6 expression could be a promising therapeutic target for epilepsy and other central nervous system disorders.
Materials and Methods
Animals and Surgery
All procedures were in full compliance with approved institutional animal care and use protocols. Initial experiments were performed in rats, and final experiments in mice, because a mouse strain with reduced Nav1.6 expression was available. For initial experiments, we used male Sprague-Dawley rats (Harlan, Inc. Indianapolis, IN) weighing 175–200g, using recording and stimulating electrodes implanted in the amygdala as described previously (Blumenfeld et al., 2007). We later used male heterozygote Nav1.6 +/− medtg mice and male WT Nav1.6 +/+ littermates bred on a C57BL6 background (Burgess et al., 1995). All mice were genotyped by tail clipping and PCR amplification with primers specific for the transgene (Burgess et al., 1995; Kohrman et al., 1995). Implantation of electrodes in mice was performed at age 1–2 months (approximately 30g) under ketamine (30mg/kg), xlyazine (6mg/kg), and acepromazine (1mg/kg) anesthesia. A bipolar stainless steel Teflon-coated twisted pair electrode (Plastics One Inc, Roanke, VA; Part # 8IMS3333BXXE) was implanted stereotactically in the right amygdala (AP −2.0, ML 3.0, SI −4.6), (Franklin and Paxinos, 2001). The ground electrode was wrapped around a stainless steel screw (Small Parts Inc, Miami Lakes, FL; Part # MX-000120-01B) placed on the left side of the skull, and an additional screw was implanted for stability. Craniotomy holes were covered with superglue, followed by cranioplastic cement (Henry Schein Inc, Indianapolis, IN; Lang Jet Denture Repair Acylic, Part #1255710). Position of electrodes was confirmed histologically at the conclusion of recordings.
Kindling and Recording Procedure
Animals were given a one week recovery period after surgery. Recording and stimulation were performed with an A-M Systems (Carlsborg, WA) Microelectrode AC Amplifier (Model # 1800, Version 7.0) and Isolated Pulse Stimulator (Model # 2100, Version 6.0). The recording amplifier was custom modified by adding a relay to automatically switch between recording and stimulating modes. The stimulus train consisted of square biphasic (1ms each phase) pulses at 60Hz, with train duration of 1s. For each animal, afterdischarge threshold (Racine, 1972b) was determined by titrating the current starting at 80μA for rats or 40μA for mice, and increasing the current by 20μA with interstimulus interval ≥ 60s until an afterdischarge lasting ≥ 3s was observed on the EEG in the amygdala contacts (Greenwood et al., 1989; Kelly et al., 1999). The threshold stimulus for each animal was then repeated twice daily, with an interstimulus interval of at least 60 minutes (Racine et al., 1972; Racine, 1972b, a; Kelly et al., 1999). EEG signals were recorded via commutator (Plastics One, Inc.). Recording procedures for rats were described previously (Blumenfeld et al., 2007). Similarly, for mice band pass frequency filter settings were 1–1000 Hz. Signals were digitized at a sampling rate of 2.5 kHz with a Cambridge Electronic Design Power 1401 (Cambridge, UK), and viewed using Spike 2 (CED, Cambridge, UK) to measure seizure duration. Seizure behavioral severity was rated based on the Racine scale (Racine, 1972a). Animals were considered fully kindled when they had three consecutive Racine class 5 seizures (Racine, 1972a), and stimulation was then stopped. The control sham-kindled animals were implanted and handled identically to the kindled animals except that the sham-kindled controls always received a 1μA stimulus. Each kindled animal was paired with a sham-kindled animal that underwent an identical number of stimulation and recording sessions. Perfusion of animals for histology or sacrifice of animals for patch clamp electrophysiology was performed 14 days after the last stimulus.
Collection of tissue for histology
Euthanasia was performed using Nembutal (80 mg/kg i.p.) in rats, or ketamine/xylazine (80/5 mg/kg i.p.) in mice. Animals were then perfused with 0.01M phosphate-buffered saline (PBS) followed by cold 4% buffered paraformaldehyde. Brains from perfused animals were immediately removed, postfixed and cryoprotected in 30% sucrose/1M PBS. Tissue harvesting was done under RNAse-free conditions. The same animals were used for the immunocytochemistry and in situ hybridization experiments by using alternate 10 μm slices for the two techniques. Specimens from kindled and sham-kindled control animals were processed together under identical conditions.
Immunocytochemistry
Ten-micron coronal sections of the cerebral hemispheres at the level of the hippocampus were cut and incubated in blocking solution (5% normal goat serum and 1% bovine serum albumin in PBS) containing 0.1% Triton X-100 and 0.02% sodium azide at room temperature for 30 minutes, and then incubated with a sodium channel subtype-specific antibody to Nav1.1 (residues 465–481, 1:100 dilution, Alomone, Jerusalem), Nav1.2 (residues 467–485, 1:100 dilution, Alomone), and Nav1.6 (residues 1042–1061), (1:100, Alomone, Jerusalem) overnight at 4°C. Slides were washed in PBS and then incubated with goat anti-rabbit IgG-Cy3 (1:2000, Amersham, New Jersey). Immunofluorescence signal was detected using fluorescein illumination (emission wavelength 516–565 nm). Specificity of the Nav1.6 antibody has been tested previously in our laboratory with Nav1.6 −/− med mouse homozygotes, which showed no significant staining (Black et al., 2002).
In situ hybridization
Ten-micron coronal sections of the cerebral hemispheres at the level of the hippocampus were deproteinized with proteinase K (2.5 mg/ml) and acetylated with 0.25% acetic anhydride and 0.1M triethanolamine and were incubated in hybridization buffer (50% formamide/10% dextran sulfate/20mM TRIS HCl pH 7.5, 5mM EDTA/0.3M NaCl/0.2% SDS/500 mg/ml yeast tRNA/1x Denhardt’s solution/10mM DTT, containing DIG-UTP labeled sodium channel riboprobe (1.0 ng/ml) for 12 hours at 60 °C. After rinsing in 2x SSC/50% formamide and RNase solution in 0.5x SSC, sectionswere transferred into buffer 1 (100mM TRIS·HCl, pH 7.5/150 mMNaCl), incubated in alkaline phosphatase-labeled anti-DIG antibody(dilution, 1:500 in buffer 1) overnight at 4°C, and then reacted ina chromogen solution containing 4-nitroblue tetrazolium chloride(NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) in buffer (100 mM TRIS·HCl, pH 9.5/10 mM NaCl/50 mM MgCl2) for three hours at room temperature. DIG-labeled antisense and sense riboprobes for Nav1.6 (nucleotides 6461–6761) were synthesized from each cDNA by RT-PCR. Sense riboprobes yielded no signals on in situ hybridization (data not shown).
Analysis of in situ and immunocytochemistry data
Results from identical regions and layers of hippocampus in kindled and sham-kindled control animals were processed in parallel. Quantitative microdensitometry was performed with a Nikon Eclipse TE300 microscope using IPLab software (Scanalytics). The quantification process was done with the experimenter blinded to the identity of the experimental group. Signal intensities were determined by outlining individual hippocampal neurons from both hemispheres. Only neurons with distinct borders whose nuclei fell within the plane of section were analyzed. Approximately 100 neurons were analyzed per region of hippocampus (CA1, CA3, dentate gyrus) per animal. Hippocampal pyramidal neurons in CA1 and CA3 were distinguished by morphology and were used for analysis in all animals, making it unlikely that different populations of neurons were stained in kindled vs. sham-kindled controls. Analysis of neurons from left versus right hippocampus showed no significant difference in level of expression within each group of animals, so these data were combined. Mean immunofluorescence of neurons from each region of hippocampus from each set of animals was compared using one-way ANOVA with post-hoc Fisher’s least significant difference analysis with Bonferroni adjustment for multiple comparisons. An alpha level of 0.05 was used as a threshold for statistical significance. All data are presented as mean ± SE.
Cell dissociation for voltage clamp
For cell dissociation of adult animals, methods were adapted from Kay and Wong (Kay and Wong, 1986). Mice were deeply anesthetized with halothane and rapidly decapitated. The brain was quickly removed while cooling in ice cold sucrose solution (in mM): 87 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 glucose, 75 sucrose, 7 MgCl2, 0.5 CaCl2, bubbled with 95% O2/5% CO2. 400 μm thick coronal slices were cut with a microslicer DTK-1000 zero 1 (DSK, Dosaka EM Co., Kyoto-shi, Japan), while cooling with ice cold 95%O2/5%CO2 bubbled sucrose solution. The rostral hippocampus was identified and excised from the slices under a microscope. Tissue blocks corresponding to area CA3 were carefully microdissected and saved using an ophthalmic scalpel (Feather Safety Razor Co., LTD. Medical Division, Osaka, Japan). After incubation at room temperature for 1.5 h in O2 bubbled PIPES solution (in mM: 120 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 PIPES, 25 Glucose, pH 7.0 (NaOH), 275 mOsmol) with 1 mg/ml trypsin, cells were washed for 15 min in enzyme free PIPES solution, in which they were stored until needed (no longer than 5 h). Directly before recording, tissue blocks were transferred into recording solution (see below) and neurons were isolated with the help of iridectomy scissors. No pasteur pipettes were used for trituration.
Patch Clamp Electrophysiology
Whole-cell voltage-clamp recordings (Hamill et al., 1981) were performed on acutely dissociated CA3 pyramidal neurons. Pyramidal cells were chosen according to their distinct anatomical features. When isolated from the tissue block they clearly showed a triangularly shaped soma and an apical dendrite, and some still had basal dendrites attached. By their size and anatomical features they were clearly distinguishable from other hippocampal cells. Recordings were performed using an EPC-9 amplifier (HEKA electronics, Lambrecht/Pfalz, Germany) and fire polished 1–1.5 MΩ electrodes (World Precision Instruments, Inc, Sarasota, FL, USA). The pipette solution contained (in mM): 140 CsF, 10 NaCl, 1 EGTA, and 10 HEPES (pH 7.38, CsOH). To obtain adequate clamp control, the extracellular sodium concentration was reduced to 25 mM. The extracellular bath contained (in mM): 20 NaCl, 100 CholineCl, 15 Glucose, 25 TEA, 10 Hepes, 1 MgCl2, 1 CaCl2, 0.1 CdCl2, pH 7.38 (NaOH; final sodium concentration after pH adjustment: 25 mM), 296 mosmol/l. All recordings were conducted at room temperature (~21 °C). The pipette potential was adjusted to zero before seal formation, and the voltages were not corrected for liquid junction potential. Capacity transients were cancelled, and series resistance was compensated by 65–95%. Leakage current was subtracted digitally online using hyperpolarizing potentials applied after the test pulse (P/4 procedure). We did not use TTX. Currents were acquired at a sampling rate of 20 kHz and filtered at 10 kHz and 2.9 kHz in series using Pulse software (HEKA electronics, Lambrecht/Pfalz, Germany). For current density measurements, the currents were divided by the cell capacitance, as read from the amplifier. Mean capacitances for the dissociated CA3 neurons in each group of mice were as follows: sham-kindled WT: 7.11 ± 0.43 pF; kindled WT: 6.72 ± 0.52 pF; sham-kindled Nav 1.6 med +/−: 6.63 ± 0.39 pF; kindled Nav 1.6 med +/−: 7.44 ± 0.64 pF. Typical peak currents under these conditions were 8 to 10 nA, allowing good voltage clamp control. Standard current-voltage (I-V) families were obtained at predetermined times from establishing cell access using 60 ms pulses from a holding potential of −120 mV to a range of potentials (−110 to +40 mV) with 5 to 10 seconds between pulses. All data are presented as mean ± SEM.
Results
Changes in Nav1.6 and sodium current with the expression of kindling
We studied changes in sodium channel protein levels, mRNA, sodium current, and behavioral seizure severity in kindled animals, compared to sham kindled controls. Behavioral kindling is known to persist for long periods of time once induced (McIntyre et al., 2002). Therefore, to study changes related to the stable expression of kindling we examined sodium channel protein, mRNA, and current two weeks after kindling was established. To determine changes in protein expression and mRNA selectively in the cell body region we used immunocytochemistry and in situ hybridization, studying a relatively large number of neurons to enable quantitative analysis (see Material and Methods for details). Initial pilot experiments were performed in rats, because this is the species in which kindling was originally described (Goddard, 1967). The pilot experiments showed a selective increase in Nav1.6 expression in kindled rat hippocampal CA3 pyramidal neurons compared to controls (see Supplementary Data 1, online). Nav1.1 and Nav1.2, the other main sodium channels normally expressed in the adult central nervous system (Goldin, 2001), showed no significant changes in protein expression with kindling (Supplementary Data 1, online). We did not study other sodium channel subtypes. Following these initial results, subsequent experiments focused on Nav1.6, and were performed in mice instead of rats, because of the availability of a mouse transgenic model with reduced Nav1.6 (Burgess et al., 1995; Kearney et al., 2002).
In kindled mice, like in rats, we found increased expression of voltage-gated sodium channel Nav1.6 protein in the hippocampus (Figure 1). The increases in Nav1.6 protein expression were seen selectively in hippocampal CA3 pyramidal cells (Figure 1 B, C, F). Similarly, kindling produced increases in Nav1.6 mRNA in CA3 neurons (Figure 1 D–F). CA3 neurons give rise to widespread commissural and longitudinal association fibers, making them well placed to amplify abnormal paroxysmal activity that has exceeded the gating capacity of the upstream dentate gyrus (Lothman et al., 1991a).
Figure 1. Kindling increases Nav1.6 protein expression and mRNA in CA3 pyramidal cells.
A. Section through mouse hippocampus showing regions used for analysis of changes in mRNA and protein expression with kindling. Hippocampal neurons are stained by in situ hybridization for Nav1.6. B–C. Examples of Nav1.6-specific immunolabeling showing increased Nav1.6 protein in CA3 of kindled (C) compared to sham-kindled control (B) mice. D–E. Examples of in situ hybridization showing increased Nav1.6 mRNA in CA3 of kindled (E) compared to sham-kindled control (D) mice. F. Quantification of optical density changes in CA3 across all animals for regions shown in B–E. Nav1.6 protein (B,C) and mRNA (D,E) was significantly increased in CA3 region (red bars) of kindled compared to control animals (*, p < 0.05; ANOVA with post-hoc Fisher’s least significant difference analysis, Bonferroni corrected; n=12 kindled animals, 12 sham-kindled controls). CA1 and dentate gyrus (DG) (yellow and blue bars, respectively) showed no significant changes. Images shown in B–E were equivalently enhanced (ie, the identical brightness and contrast enhancements were made to each picture) to help demonstrate the differences in a way that would be clear in a printed format. Actual mean optical density values for examples shown were 22.74 (B), 41.07 (C), 49.61 (D) 85.28 (E). For quantification (F), raw unenhanced images were used. Scale bars in B and D are 50 microns.
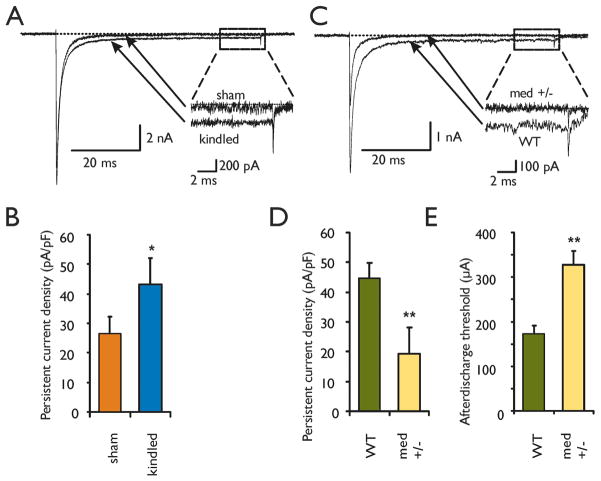
Following depolarization and action potential generation, a fraction of sodium current can persist, leading in some cases to repetitive action potential firing and abnormal excitability (Segal and Douglas, 1997; Su et al., 2001). Nav1.6 is capable of generating persistent sodium current (Smith et al., 1998; Maurice et al., 2001; Rush et al., 2005). To determine if the increased expression of Nav1.6 in kindling is associated with functional changes in sodium current, we next performed voltage clamp recordings from isolated CA3 neurons in kindled and in sham-kindled adult mice. We observed increased persistent sodium current density in CA3 neurons from kindled mice (mean 43.2 ± 8.8 pA/pF SEM; n=30) compared to sham-kindled controls (26.4 ± 4.4 pA/pF; n = 40; p < 0.05, two-tailed t-test) (Figure 2 A–B). Quantification of persistent sodium current was done at −20mV since at more negative values sodium channels may not be fully activated and leak current could contaminate the results, and at more positive values potassium and other channels would contaminate the results. Current density vs. voltage curves also demonstrated a maximal effect of kindling on persistent sodium current near −20mV (see Supplementary Data 2, online). Kindling did not affect the properties of the peak sodium current, including peak current density, voltage dependence or kinetics of activation/inactivation (see Supplementary Data 2 online). The persistent current density represents a small percentage of the peak current density (3.2 ± 0.9 % for kindled, and 2.3 ± 0.5 % for sham controls). However, because enhanced persistent sodium current is known to promote action potential burst firing and enhanced excitability (Segal and Douglas, 1997; Waxman, 2000; Su et al., 2001), these findings suggest that abnormal increases in Nav1.6 expression may be an important mechanism for activity-dependent facilitation, as occurs in kindling. While these results demonstrate increased Nav1.6 and persistent sodium current with kindling expression, the next question was whether Nav1.6 contributes to the enhanced excitability seen with kindling initiation and development.
Figure 2. Kindling increases persistent sodium current in CA3 neurons.
A. Representative voltage clamp current traces from kindled and sham-kindled control CA3 pyramidal neurons. Inset shows magnification of the last 10 ms of the depolarizing pulse (to −20mV from −120mV holding potential), demonstrating increased persistent inward sodium current with kindling. B. Group data showing increased persistent sodium current density in kindled (mean 43.2 ± 8.8 pA/pF SEM; n=30 cells in 5 animals) vs. sham kindled control hippocampal CA3 neurons (26.4 ± 4.4 pA/pF; n = 40 cells in 5 animals; * p < 0.05, two-tailed t-test) at −20mV. C. Representative current traces from Nav1.6 +/− medtg heterozygotes and Nav1.6 +/+ WT littermate CA3 neurons. Inset shows magnification of the last 10 ms of the depolarizing pulse (to −20mV from −120mV holding potential), demonstrating reduced persistent sodium current in Nav1.6 +/− knockout heterozygote. Peak current is also reduced in the Nav1.6 +/− mice. D. Group data showing reduced persistent sodium current density in Nav1.6 +/− heterozygotes (19.4 +− 0.6 pA/pF; n=18 cells in 7 animals) vs. WT hippocampal CA3 neurons (44.8 +− 0.5 pA/pF; n = 19 cells in 4 animals; ** p < 0.01, two-tailed t-test). E. Afterdischarge threshold was increased in Nav1.6 +/− knockout (328 ± 30 μA; n=20 animals) compared to WT control littermates (173 ± 18 μA; n=21 animals; ** p=0.0001, two-tailed t-test).
Reduced Nav1.6 inhibits the initiation and development of kindling
If Nav1.6 is important for abnormal activity-dependent plasticity, we hypothesized that reduced Nav1.6 should inhibit the initiation and development of kindling. To test this, we used Nav1.6 +/− heterozygote medtg mice, which have reduced expression of Nav1.6, but no obvious phenotypic abnormalities (Burgess et al., 1995; Kearney et al., 2002). Homozygote medtg mice die by age P19. Adult heterozygote Nav1.6 +/− medtg mice have reduced peak and persistent sodium current density compared to Nav1.6 +/+ (WT) littermates (Figure 2 C–D). For kindling to occur, spontaneous electrical activity called an afterdischarge must follow the stimulus (Goddard, 1967; Morimoto et al., 2004). The afterdischarge is, therefore, critical to kindling initiation. We measured afterdischarge threshold in each animal at the start of kindling. In Nav1.6 +/− mice, nearly twice as much stimulus current was needed to produce an afterdischarge (328 ± 30 μA; n=20) compared to WT controls (173 ± 18 μA; n=21; p=0.0001, two-tailed t-test) (Figure 2 E). This suggests that reduced Nav1.6 expression and sodium current in Nav1.6 +/− mice leads to reduced network excitability in vivo, and to reduced susceptibility for abnormal activity such as afterdischarge generation.
While these findings demonstrate that Nav1.6 +/− mice resist the initiation of kindling, we were also interested in determining if the development of kindling occurs more slowly in Nav1.6 +/− mice. Prior work has shown that when sodium current is reduced, for example by medications, the afterdischarge threshold is increased (Ehle, 1980; Rundfeldt et al., 1990; Lothman et al., 1991b; Morimoto et al., 1997). Of note, if a sufficiently large current is used to produce afterdischarges even with sodium channel blockers, the rate of kindling development is unaffected (Ehle, 1980; Albertson et al., 1984; Post et al., 1984; Weiss and Post, 1987). However, reduced channel expression (e.g. in Nav1.6 +/− mice) may affect kindling development differently from sodium channel blocking medications. To test whether Nav1.6 +/− mice resist the development of kindling, we used the threshold stimulus for each animal so that reliable afterdischarges were produced. With this approach, we found that Nav1.6 +/− mice kindle more slowly than WT littermates (Figure 3). The seizure severity was persistently lower in Nav1.6 +/− mice compared to WT mice given the same number of stimuli (Figure 3A). Full kindling (3 consecutive class 5 seizures) was achieved after 27 ± 2 (mean ± SEM) stimuli in WT mice. However, after 27 stimuli, most Nav1.6 +/− mice still had only class 1 or 2 seizures (Figure 3A). Full kindling of Nav1.6 +/− did occur, but required 65 ± 6 stimuli, significantly longer than in WT (p< 0.00001, two-tailed t-test). In addition, it took significantly more stimuli to reach each stage of kindling for Nav1.6 +/− mice compared to WT (Figure 3B). These findings suggest that Nav1.6 +/− mice resist both the initiation (higher afterdischarge threshold) and development (slower kindling rate) of abnormal network plasticity.
Figure 3. Nav1.6 +/− mice kindle more slowly than WT littermates.
A. Behavioral severity of seizures (mean Racine score) increases more slowly in Nav1.6 +/− mice than in WT controls. Full kindling (3 consecutive class 5 seizures) is achieved after 27 ± 2 (mean ± SEM) stimuli in WT but requires 65 ± 6 stimuli in Nav1.6 +/− mice (p< 0.00001, two-tailed t-test). B. Number of stimuli needed to achieve each stage of kindling for the first time. Nav1.6 mice require more stimuli to reach each stage of kindling compared to WT controls (overall group difference MANOVA, F=26.71, p=0.00002; Individual two-tailed t-tests with Bonferroni correction ** p=0.01, *** p<0.001). The plot in (A) did not include stimuli for which <3 animals remained (seen only towards end of kindling). For A and B, n= 20 Nav1.6 +/− animals, and n=21 WT animals.
The difference in kindling rates between Nav1.6 +/− and WT controls was not due to failure to produce afterdischarges in Nav1.6 +/− mice, since the percentage of stimuli causing afterdischarges was similar in the two groups (82.9 ± 3.7% of stimuli produced afterdischarges in Nav1.6 +/− mice; n=20; 90.0 ± 3.0% produced afterdischarges in WT controls; n=21). Duration of afterdischarges (seizures) was reduced in Nav1.6 +/− mice by 10 to 30% at each stage of kindling (Figure 4). Seizure duration increased monotonically with seizure behavioral severity, as has been reported previously (Racine, 1972b; Blumenfeld et al., 2007), but duration increased more slowly in the Nav1.6 +/− mice (Figure 4).
Figure 4.

Seizure durations were shorter in Nav1.6 +/− mice compared to WT littermates. Mean seizure durations were shorter at stages 1 through 5 of kindling in Nav1.6 +/− mice (** p<0.01, ***p<0.0001, two tailed t-tests with Bonferroni correction). Combining across all Racine stages, overall seizure durations were significantly shorter in Nav1.6 +/− mice (17.5 ± 0.2s; mean ± SEM) compared to WT (21.8 ± 0.5s; P < 0.0001, two-tailed t-test; n=1033 seizures in 20 Nav1.6 +/− heterozygotes; n=507 seizures in 21 WT animals).
Although more stimuli were needed to produce kindling in Nav1.6 +/− mice (Figure 3), if stimulation was continued long enough to finally produce kindling, then Nav1.6 +/− mice showed selective upregulation of Nav1.6 expression in hippocampal CA3 neurons similar to control animals (Figure 5). We also observed a trend towards increased persistent sodium current density in CA3 neurons from kindled Nav1.6 +/− mice; however, the increase did not reach statistical significance at −20 mV (see Supplementary Data 2, and Supplementary Data 3, online). These finding suggest that although Nav1.6 +/− animals resist the development of kindling, if sufficient stimulation is given to establish kindling, then Nav1.6 upregulation still occurs, and could contribute to abnormal network plasticity.
Figure 5. When Nav1.6 +/− mice are fully kindled, there is an increase in Nav1.6 protein and mRNA in CA3 neurons, similar to WT (Figure 1).

A–B. Examples of Nav1.6-specific immunolabeling showing increased Nav1.6 protein in CA3 pyramidal cells of kindled Nav1.6 +/− mice (B) compared to sham-kindled Nav1.6 +/− (A) mice. C–D. Examples of in situ hybridization showing increased Nav1.6 mRNA in CA3 neurons of kindled Nav1.6 +/− mice (D) compared to sham-kindled Nav1.6 +/− (C) mice. E. Quantification of optical density changes in CA3 neurons across all animals for regions shown in A–D. Nav1.6 protein (A,B) and mRNA (C,D) was significantly increased in CA3 region (red bars) of kindled compared to sham-kindled animals (*, p < 0.05; ANOVA with post-hoc Fisher’s least significant difference analysis, Bonferroni corrected; n=12 kindled animals, 12 sham-kindled controls). CA1 and dentate gyrus (DG) (yellow and blue bars, respectively) showed no significant changes. Images shown in A–D were equivalently enhanced (ie, the identical brightness and contrast enhancements were made to each picture) to help demonstrate the differences in a way that would be clear in a printed format. Actual mean optical density values for examples shown were 22.31 (A), 39.15 (B), 54.02 (C), 87.69 (D). For quantification (E), raw unenhanced images were used. Scale bars in A and C are 50 microns.
Discussion
We have found that kindling increases Nav1.6 and persistent Na current in hippocampal CA3 neurons, and that reduced Nav1.6 inhibits this form of abnormal activity-dependent plasticity. These results suggest an important role for altered expression of voltage-gated sodium channels in the abnormally enhanced excitability seen in epilepsy and other chronic disorders of the nervous system. In keeping with this, several common medications for epilepsy, chronic pain, and psychiatric disorders act by blocking voltage-gated sodium channels (Rogawski and Loscher, 2004a, b).
Prior work has shown that voltage-gated sodium channels are capable of activity dependent changes (Offord and Catterall, 1989; Sashihara et al., 1996; Waxman, 2000). In addition, increased expression of voltage-gated sodium channels has been observed in several disorders of the peripheral and central nervous system, including epilepsy (Lombardo et al., 1996; Bartolomei et al., 1997; Waxman, 2000; Waxman et al., 2000; Bertram, 2003; Klein et al., 2004). Mutations in voltage-gated sodium channels are known to cause several genetic forms of epilepsy (Mulley et al., 2005; Berkovic et al., 2006). However, the role of activity-dependent changes in sodium channel expression in acquired epilepsy, and in related forms of abnormal central nervous system plasticity warrant further study. Prior investigation of normal and abnormal network plasticity has, so far, emphasized changes in synaptic function, such as altered neurotransmitter release, effects on receptors, or ultrastructural changes in synaptic connections (Lissin et al., 1998; O’Brien et al., 1998; Turrigiano et al., 1998; Lynch, 2004; Morimoto et al., 2004). There is some evidence that ion channel modulation can also alter signaling in the nervous system (Byrne and Kandel, 1996; Johnston et al., 2003). In particular, activity-dependent changes in intrinsic membrane excitability leading to action potential burst firing may be crucial for the abnormal plasticity seen in epilepsy and related disorders (Rogawski and Loscher, 2004b, a; Yue et al., 2005). The present study directly demonstrates that repeated activity, which leads to abnormally enhanced excitability, is capable of producing an increase in Nav1.6 in the hippocampus. Other mechanisms certainly participate in the abnormal activity-dependent plasticity which occurs in kindling (Morimoto et al., 2004). However, the central role of voltage-gated sodium channels in action potential generation, and of Nav1.6 in the persistent sodium current (Segal and Douglas, 1997; Goldin, 2001; Maurice et al., 2001; Su et al., 2001), make this channel well suited to play a major role in abnormal activity-dependent plasticity. Nav1.6 is abundantly expressed in the central nervous system, particularly in the cell body and initial segment (Goldin, 2001; Jenkins and Bennett, 2001), where it is colocalized with ankyrin (Jenkins and Bennett, 2001). Nav1.6 contributes a persistent sodium current in this region, critical for action potential generation and repetitive firing (Yue et al., 2005; Astman et al., 2006). CA3 neurons are important for hippocampal network plasticity (Traub et al., 1989; Yang et al., 2008). Therefore, the changes in persistent sodium current we observed in hippocampal CA3 neurons during kindling, are well poised to elicit large changes in network excitability (Lothman et al., 1991a), and may play an important role in the expression of kindling and other forms of abnormal network plasticity.
Modulation of other ion channels likely also plays an important role in activity-dependent changes in epilepsy, and should be investigated further. Although our preliminary results in a rat model did not show changes in hippocampal Nav1.1 or 1.2 with kindling, we cannot exclude the possible role of these channels or others including Nav1.3 in abnormal network plasticity. Indeed, prior work has shown altered expression of other Na+ channel isoforms (Nav1.1, Nav1.2 and Nav1.3) in the hippocampus, both in human epilepsy (Lombardo et al., 1996; Whitaker et al., 2001) and in rodent epilepsy models (Chen et al., 2004; Yu et al., 2006). Although we did not study Nav1.3 here, altered expression of this channel has been demonstrated to play a role hyperexcitability in pathological states including epilepsy (Yu et al., 2006; Hains and Waxman, 2007). In addition, much recent work suggests that altered expression of hyperpolarization gated cation channels may also participate in epileptogenesis (Jung et al., 2007; Blumenfeld et al., 2008; Richichi et al., 2008).
Modulating Nav1.6 expression is a promising target for selective therapy. This is particularly true in light of our finding that reduced Nav1.6 expression significantly retards both the initiation and development of worsening seizures in the kindling model. The development of epilepsy is thought to depend on a self-reinforcing cycle of structural and functional changes → abnormal neuronal activity → additional structural and functional changes (White, 2002). It may be possible to interrupt this cycle, for example, by reducing expression of Nav1.6. We found that, in agreement with studies of medications that reduce sodium current (Ehle, 1980; Rundfeldt et al., 1990; Lothman et al., 1991b; Morimoto et al., 1997), reduced sodium current in Nav1.6 +/− mice is associated with an increased afterdischarge threshold and thus resistance to the initiation of kindling. Interestingly, unlike sodium channel blocking medications which do not affect the rate of kindling development (Ehle, 1980; Albertson et al., 1984; Post et al., 1984; Weiss and Post, 1987), we observed a marked slowing of the rate of kindling development in Nav1.6 +/− mice. Prior studies have shown that the rate of kindling development can be reduced by a number of modulatory factors including rat strain (McIntyre et al., 1999; McIntyre et al., 2002), altered immediate early gene expression (Cain et al., 1995; Watanabe et al., 1996; Elmer et al., 1997; Potschka et al., 2002), neurotrophic factors (Kokaia et al., 1995; Reibel et al., 2000), neuropeptides (Binaschi et al., 2003; Reibel et al., 2003), glutamate blockers (Cain et al., 1988; Sato et al., 1988; Kodama et al., 1999; Rogawski et al., 2001), and noradrenaline (Bengzon et al., 1990). Interestingly, unlike Nav1.6 +/− mice, many of these factors slow kindling development, but do not affect the afterdischarge threshold (Bengzon et al., 1990; Cain et al., 1995; Kokaia et al., 1995; Watanabe et al., 1996; Elmer et al., 1997; McIntyre et al., 2002; Binaschi et al., 2003). Thus, independent effects have been observed previously on kindling initiation (e.g. sodium channel blocking medications) and development (e.g. neuromodulators). In contrast, the present results show that reduced Nav1.6 inhibits both kindling initiation and development, and that Nav1.6 is also upregulated during kindling expression. These findings suggest an important role for Nav1.6 at all stages of abnormal activity-dependent plasticity.
The finding that reduced Nav1.6 expression retards kindling has important mechanistic and therapeutic implications. Since excitation can increase Nav1.6 expression, and Nav1.6 is permissive for further increases in excitability, our results suggest a model in which Nav1.6 may participate in a self-reinforcing cycle of abnormally enhanced network activity (White, 2002). Future work will be needed to determine if similar self-reinforcing modulation of sodium channel expression could operate in other brain regions, and in other forms of abnormal plasticity. Recent studies in a different form of epilepsy in a rat model, have shown that upregulation of sodium channels parallels the development of seizures (Klein et al., 2004), and can be suppressed by prolonged treatment beginning at an early stage of development (Blumenfeld et al., 2008). Current treatments for epilepsy are aimed at blocking seizures, for example through medications which reduce sodium current (Rogawski and Loscher, 2004b). However, these treatments only block seizures temporarily, and do not alter the development or expression of the underlying epileptic disorder (Duncan et al., 2006). The present findings suggest a promising new therapeutic strategy: medication or gene therapy to reduce expression of a specific voltage-gated sodium channel subtype. It will be crucial to further investigate the safety and efficacy of such treatment strategies before human trials can be attempted, particularly since haploinsufficiency of the Nav 1.6 gene in one human family was associated with ataxia and cognitive impairment (Trudeau et al., 2006). Interestingly, in concordance with our findings in kindling, Nav1.6 mutations were recently shown to confer seizure resistance in other mouse models including severe myoclonic epilepsy of infancy, and flurothyl- and kainic acid (KA)-induced seizures (Martin et al., 2007). It is not known if reduced expression of Nav1.6 is associated with resistance to epilepsy in humans, however, this should be investigated in ongoing large scale investigations of human genes that influence seizures susceptibility (Jacobs et al., 2001). With further study, interrupting the cycle of abnormal excitability and selective changes in voltage-gated sodium channel expression may become an important modality for treating epilepsy and other nervous system disorders.
Supplementary Material
Acknowledgments
We thank Miriam Meisler for providing Nav1.6 +/− medtg mice to establish a breeding colony. We also thank Steven A. Siegelbaum for helpful comments on the manuscript, Anthony M. Rush for technical advice in dissociating hippocampal neurons and electrophysiology setup, Akash Shah for initial work on mouse kindling methods, Chhitij Bashyal and Matthew Vestal for verifying histology, and Pam Zwinger for mouse genotyping. This work was supported by NIH R01 NS049307 and by Betsy and Jonathan Blattmachr (HB), the Epilepsy Foundation of America and UCB Pharma, Inc. (AL), and the Department of Veterans Administration Rehabilitation Research and Development Service (SGW). We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Albertson TE, Joy RM, Stark LG. A pharmacological study in the kindling model of epilepsy. Neuropharmacology. 1984;23:1117–1123. doi: 10.1016/0028-3908(84)90227-2. [DOI] [PubMed] [Google Scholar]
- Astman N, Gutnick MJ, Fleidervish IA. Persistent sodium current in layer 5 neocortical neurons is primarily generated in the proximal axon. J Neurosci. 2006;26:3465–3473. doi: 10.1523/JNEUROSCI.4907-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Gastaldi M, Massacrier A, Planells R, Nicolas S, Cau P. Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain. J Neurocytol. 1997;26:667–678. doi: 10.1023/a:1018549928277. [DOI] [PubMed] [Google Scholar]
- Bengzon J, Kokaia M, Brundin P, Lindvall O. Seizure suppression in kindling epilepsy by intrahippocampal locus coeruleus grafts: evidence for an alpha-2-adrenoreceptor mediated mechanism. Exp Brain Res. 1990;81:433–437. doi: 10.1007/BF00228137. [DOI] [PubMed] [Google Scholar]
- Berkovic SF, Mulley JC, Scheffer IE, Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trends in Neurosci. 2006;29:391–397. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Bertram EH. How Epilesy Changes Sodium Channels. Epilepsy Curr. 2003;3:72–73. doi: 10.1046/j.1535-7597.2003.03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binaschi A, Zucchini S, Bregola G, Rodi D, Mazzuferi M, Reinscheid RK, Simonato M. Delayed epileptogenesis in nociceptin/orphanin FQ-deficient mice. Neuroreport. 2003;14:825–827. doi: 10.1097/00001756-200305060-00009. [DOI] [PubMed] [Google Scholar]
- Black JA, Renganathan M, Waxman SG. Sodium channel Na(v)1.6 is expressed along nonmyelinated axons and it contributes to conduction. Brain Res Molecular Brain Res. 2002;105:19–28. doi: 10.1016/s0169-328x(02)00385-6. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Rivera M, Vasquez JG, Shah A, Ismail D, Enev M, Zaveri HP. Neocortical and thalamic spread of amygdala kindled seizures. Epilepsia. 2007;48(2):254–262. doi: 10.1111/j.1528-1167.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse CP, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia. 2008 doi: 10.1111/j.1528-1167.2007.01458.x. In press--available online early at http://www.blackwell-synergy.com/loi/epi. [DOI] [PMC free article] [PubMed]
- Burbidge SA, Dale TJ, Powell AJ, Whitaker WR, Xie XM, Romanos MA, Clare JJ. Molecular cloning, distribution and functional analysis of the NA(V)1.6. Voltage-gated sodium channel from human brain. Brain Res Mol Brain Res. 2002;103:80–90. doi: 10.1016/s0169-328x(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant ‘motor endplate disease’. Nat Genet. 1995;10:461–465. doi: 10.1038/ng0895-461. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DP. Long-term potentiation and kindling: how similar are the mechanisms? Trends in Neurosci. 1989;12:6–10. doi: 10.1016/0166-2236(89)90146-x. [DOI] [PubMed] [Google Scholar]
- Cain DP, Desborough KA, McKitrick DJ. Retardation of amygdala kindling by antagonism of NMD-aspartate and muscarinic cholinergic receptors: evidence for the summation of excitatory mechanisms in kindling. Exp Neurol. 1988;100:179–187. doi: 10.1016/0014-4886(88)90210-5. [DOI] [PubMed] [Google Scholar]
- Cain DP, Grant SG, Saucier D, Hargreaves EL, Kandel ER. Fyn tyrosine kinase is required for normal amygdala kindling. Epilepsy Res. 1995;22:107–114. doi: 10.1016/0920-1211(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, Chen Y, McEwen DP, O’Malley HA, Bharucha V, Meadows LS, Knudsen GA, Vilaythong A, Noebels JL, Saunders TL, Scheuer T, Shrager P, Catterall WA, Isom LL. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Ehle AL. Effects of phenytoin on amygdaloid kindled seizures in the rat. Electroencephalogr Clin Neurophysiol. 1980;48:102–105. doi: 10.1016/0013-4694(80)90049-8. [DOI] [PubMed] [Google Scholar]
- Elmer E, Kokaia M, Ernfors P, Ferencz I, Kokaia Z, Lindvall O. Suppressed kindling epileptogenesis and perturbed BDNF and TrkB gene regulation in NT-3 mutant mice. Exp Neurol. 1997;145:93–103. doi: 10.1006/exnr.1997.6478. [DOI] [PubMed] [Google Scholar]
- Fisahn A. Kainate receptors and rhythmic activity in neuronal networks: hippocampal gamma oscillations as a tool. J Physiol. 2005;562:65–72. doi: 10.1113/jphysiol.2004.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 2. San Diego, Calif.; London: Academic; 2001. [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Ann Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Greenwood RS, Meeker R, Sullivan H, Hayward JN. Kindling in spontaneous hypertensive rats. Brain Res. 1989;495:58–65. doi: 10.1016/0006-8993(89)91217-1. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Sodium channel expression and the molecular pathophysiology of pain after SCI. Progress in Brain Res. 2007;161:195–203. doi: 10.1016/S0079-6123(06)61013-3. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jacobs MP, Fischbach GD, Davis MR, Dichter MA, Dingledine R, Lowenstein DH, Morrell MJ, Noebels JL, Rogawski MA, Spencer SS, Theodore WH. Future directions for epilepsy research. Neurology. 2001;57:1536–1542. doi: 10.1212/wnl.57.9.1536. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL. Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci. 2003;358:667–674. doi: 10.1098/rstb.2002.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2004;24:475–522. doi: 10.1007/s10540-005-2742-7. [DOI] [PubMed] [Google Scholar]
- Kay AR, Wong RK. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986;16:227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Kearney JA, Buchner DA, De Haan G, Adamska M, Levin SI, Furay AR, Albin RL, Jones JM, Montal M, Stevens MJ, Sprunger LK, Meisler MH. Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6) Hum Mol Genet. 2002;11:2765–2775. doi: 10.1093/hmg/11.22.2765. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Battye RA, McIntyre DC. Cortical spreading depression reversibly disrupts convulsive motor seizure expression in amygdala-kindled rats. Neuroscience. 1999;91:305–313. doi: 10.1016/s0306-4522(98)00656-3. [DOI] [PubMed] [Google Scholar]
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. doi: 10.1016/j.brainres.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Kodama M, Yamada N, Sato K, Kitamura Y, Koyama F, Sato T, Morimoto K, Kuroda S. Effects of YM90K, a selective AMPA receptor antagonist, on amygdala-kindling and long-term hippocampal potentiation in the rat. Eur J Pharmacol. 1999;374:11–19. doi: 10.1016/s0014-2999(99)00295-2. [DOI] [PubMed] [Google Scholar]
- Kohrman DC, Plummer NW, Schuster T, Jones JM, Jang W, Burgess DL, Galt J, Spear BT, Meisler MH. Insertional mutation of the motor endplate disease (med) locus on mouse chromosome 15. Genomics. 1995;26:171–177. doi: 10.1016/0888-7543(95)80198-u. [DOI] [PubMed] [Google Scholar]
- Kokaia M, Ernfors P, Kokaia Z, Elmer E, Jaenisch R, Lindvall O. Suppressed epileptogenesis in BDNF mutant mice. Exp Neurol. 1995;133:215–224. doi: 10.1006/exnr.1995.1024. [DOI] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. Mossy fiber sprouting as a potential therapeutic target for epilepsy. Curr Neurovasc Res. 2004;1:3–10. doi: 10.2174/1567202043480242. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci U S A. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo AJ, Kuzniecky R, Powers RE, Brown GB. Altered brain sodium channel transcript levels in human epilepsy. Brain Res Molecular Brain Res. 1996;35:84–90. doi: 10.1016/0169-328x(95)00194-w. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, 3rd, Stringer JL. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991a;37:1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Williamson JM, VanLandingham KE. Intraperitoneal phenytoin suppresses kindled responses: effects on motor and electrographic seizures. Epilepsy Res. 1991b;9:11–18. doi: 10.1016/0920-1211(91)90042-e. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum Mol Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre DC, Kelly ME, Dufresne C. FAST and SLOW amygdala kindling rat strains: comparison of amygdala, hippocampal, piriform and perirhinal cortex kindling. Epilepsy Res. 1999;35:197–209. doi: 10.1016/s0920-1211(99)00012-1. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Poulter MO, Gilby K. Kindling: some old and some new. Epilepsy Res. 2002;50:79–92. doi: 10.1016/s0920-1211(02)00071-2. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Sato H, Sato K, Sato S, Yamada N. BW1003C87, phenytoin and carbamazepine elevate seizure threshold in the rat amygdala-kindling model of epilepsy. Eur J Pharmacol. 1997;339:11–15. doi: 10.1016/s0014-2999(97)01347-2. [DOI] [PubMed] [Google Scholar]
- Mulley JC, Scheffer IE, Petrou S, Dibbens LM, Berkovic SF, Harkin LA. SCN1A mutations and epilepsy. Hum Mutat. 2005;25:535–542. doi: 10.1002/humu.20178. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation.[see comment] Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Offord J, Catterall WA. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron. 1989;2:1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- Post RM, Weiss SR, Pert A. Differential effects of carbamazepine and lithium on sensitization and kindling. Prog Neuropsychopharmacol Biol Psych. 1984;8:425–434. [PubMed] [Google Scholar]
- Potschka H, Krupp E, Ebert U, Gumbel C, Leichtlein C, Lorch B, Pickert A, Kramps S, Young K, Grune U, Keller A, Welschof M, Vogt G, Xiao B, Worley PF, Loscher W, Hiemisch H. Kindling-induced overexpression of Homer 1A and its functional implications for epileptogenesis. Eur J Neurosci. 2002;16:2157–2165. doi: 10.1046/j.1460-9568.2002.02265.x. [DOI] [PubMed] [Google Scholar]
- Racine R, Okujava V, Chipashvili S. Modification of seizure activity by electrical stimulation. 3. Mechanisms. Electroencephalogr Clin Neurophysiol. 1972;32:295–299. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. I. After-discharge threshold. Electroencephalogr Clin Neurophysiol. 1972a;32:269–279. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972b;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reibel S, Larmet Y, Le BT, Carnahan J, Marescaux C, Depaulis A. Brain-derived neurotrophic factor delays hippocampal kindling in the rat. Neuroscience. 2000;100:777–788. doi: 10.1016/s0306-4522(00)00351-1. [DOI] [PubMed] [Google Scholar]
- Reibel S, Benmaamar R, Le BT, Larmet Y, Kalra SP, Marescaux C, Depaulis A. Neuropeptide Y delays hippocampal kindling in the rat. Hippocampus. 2003;13:557–560. doi: 10.1002/hipo.10110. [DOI] [PubMed] [Google Scholar]
- Richichi C, Brewster AL, Bender RA, Simeone TA, Zha Q, Yin HZ, Weiss JH, Baram TZ. Mechanisms of seizure-induced ‘transcriptional channelopathy’ of hyperpolarization-activated cyclic nucleotide gated (HCN) channels. Neurobiol Dis. 2008;29:297–305. doi: 10.1016/j.nbd.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med. 2004a;10:685–692. doi: 10.1038/nm1074. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004b;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Kurzman PS, Yamaguchi SI, Li H. Role of AMPA and GluR5 kainate receptors in the development and expression of amygdala kindling in the mouse. Neuropharmacology. 2001;40:28–35. doi: 10.1016/s0028-3908(00)00112-x. [DOI] [PubMed] [Google Scholar]
- Rundfeldt C, Honack D, Loscher W. Phenytoin potently increases the threshold for focal seizures in amygdala-kindled rats. Neuropharmacology. 1990;29:845–851. doi: 10.1016/0028-3908(90)90159-o. [DOI] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashihara S, Greer CA, Oh Y, Waxman SG. Cell-specific differential expression of Na(+)-channel beta 1-subunit mRNA in the olfactory system during postnatal development and after denervation. J Neurosci. 1996;16:702–713. doi: 10.1523/JNEUROSCI.16-02-00702.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Morimoto K, Okamoto M. Anticonvulsant action of a non-competitive antagonist of NMDA receptors (MK-801) in the kindling model of epilepsy. Brain Res. 1988;463:12–20. doi: 10.1016/0006-8993(88)90521-5. [DOI] [PubMed] [Google Scholar]
- Segal MM, Douglas AF. Late sodium channel openings underlying epileptiform activity are preferentially diminished by the anticonvulsant phenytoin. J Neurophysiol. 1997;77:3021–3034. doi: 10.1152/jn.1997.77.6.3021. [DOI] [PubMed] [Google Scholar]
- Smith MR, Smith RD, Plummer NW, Meisler MH, Goldin AL. Functional analysis of the mouse Scn8a sodium channel. The J Neurosci: The Official Journal Of The Society For Neuroscience. 1998;18:6093. doi: 10.1523/JNEUROSCI.18-16-06093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Alroy G, Kirson ED, Yaari Y. Extracellular calcium modulates persistent sodium current-dependent burst-firing in hippocampal pyramidal neurons. J Neurosci. 2001;21:4173–4182. doi: 10.1523/JNEUROSCI.21-12-04173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Miles R, Wong RK. Model of the origin of rhythmic population oscillations in the hippocampal slice. Science. 1989;243:1319–1325. doi: 10.1126/science.2646715. [DOI] [PubMed] [Google Scholar]
- Trudeau MM, Dalton JC, Day JW, Ranum LP, Meisler MH. Heterozygosity for a protein truncation mutation of sodium channel SCN8A in a patient with cerebellar atrophy, ataxia, and mental retardation. J Med Genet. 2006;43:527–530. doi: 10.1136/jmg.2005.035667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons.[see comment] Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Johnson RS, Butler LS, Binder DK, Spiegelman BM, Papaioannou VE, McNamara JO. Null mutation of c-fos impairs structural and functional plasticities in the kindling model of epilepsy. J Neurosci. 1996;16:3827–3836. doi: 10.1523/JNEUROSCI.16-12-03827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. The neuron as a dynamic electrogenic machine: modulation of sodium-channel expression as a basis for functional plasticity in neurons. Philos Trans R Soc Lond B Biol Sci. 2000;355:199–213. doi: 10.1098/rstb.2000.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channels and their genes: dynamic expression in the normal nervous system, dysregulation in disease states(1) Brain Res. 2000;886:5–14. doi: 10.1016/s0006-8993(00)02774-8. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Post RM. Carbamazepine and carbamazepine-10,11-epoxide inhibit amygdala-kindled seizures in the rat but do not block their development. Clin Neuropharmacol. 1987;10:272–279. doi: 10.1097/00002826-198706000-00008. [DOI] [PubMed] [Google Scholar]
- Whitaker WR, Faull RL, Dragunow M, Mee EW, Emson PC, Clare JJ. Changes in the mRNAs encoding voltage-gated sodium channel types II and III in human epileptic hippocampus. Neuroscience. 2001;106:275–285. doi: 10.1016/s0306-4522(01)00212-3. [DOI] [PubMed] [Google Scholar]
- White HS. Animal models of epileptogenesis. Neurology. 2002;59:S7–S14. doi: 10.1212/wnl.59.9_suppl_5.s7. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Pan E, Xiong Z, McNamara JO. Zinc-mediated Transactivation of TrkB Potentiates the Hippocampal Mossy Fiber-CA3 Pyramid Synapse. Neuron. 2008 doi: 10.1016/j.neuron.2007.11.026. in press. [DOI] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy.[erratum appears in Nat Neurosci. 2007 Jan;10(1):134] Nature Neuroscience. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Yue C, Remy S, Su H, Beck H, Yaari Y. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. J Neurosci. 2005;25:9704–9720. doi: 10.1523/JNEUROSCI.1621-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.