When evaluating a patient with anemia in whom inflammation is present, the physician typically observes the mean corpuscular volume and then requests biochemical analyses to determine the serum iron and ferritin levels, total iron-binding capacity, and transferrin saturation (calculated as serum iron level divided by the total iron-binding capacity, expressed as a percentage). If the transferrin saturation is less than 10% and the ferritin level is less than 30 ng per milliliter, iron deficiency is diagnosed as the cause of the anemia. If the transferrin saturation is low (15%) and the ferritin level is high (>200 ng per milliliter), inflammatory block is generally diagnosed.
The reason why the mean corpuscular volume is often normal (in 70% of cases1) and anemia relatively mild in patients with inflammation is a clinical enigma, because iron delivery to the marrow is limited — as indicated by the low transferrin saturation. In fact, iron delivery to the marrow should be even less when inflammation is present than when there is iron deficiency, since the total iron-binding capacity (an indirect measure of the transferrin level) is low in inflammatory states. A recent study by Zhang et al.2 appears to solve this mystery as well as addressing an enigma regarding hepcidin and iron absorption in the gastrointestinal tract.
Hepcidin is the key regulator of iron homeostasis. 3 The body has no proven means to modulate iron excretion; its iron content is controlled through modulation of iron acquisition and storage. In patients with iron deficiency, iron absorption is increased, as is the release of iron from macrophages. When the iron level is high, absorption decreases and the release of iron from macrophages is inhibited. Hepcidin regulates these adaptive processes by controlling the surface expression of the iron export protein ferroportin (FPN1 [also known as SLC40A1]) on duodenal enterocytes and on iron-recycling macrophages. Hepcidin binds ferroportin, leading to the phosphorylation and ultimate degradation of ferroportin. 4 Hepcidin synthesis is decreased by iron deficiency, most anemias, and tissue hypoxia and increased by iron excess and inflammation. In patients with inflammation, an abundance of hepcidin should lead to poor uptake of dietary iron from the gastrointestinal tract, iron sequestration in macrophages, little iron recycling to the erythron for red-cell production, and microcytic anemia. This pathophysiology is termed inflammatory block.
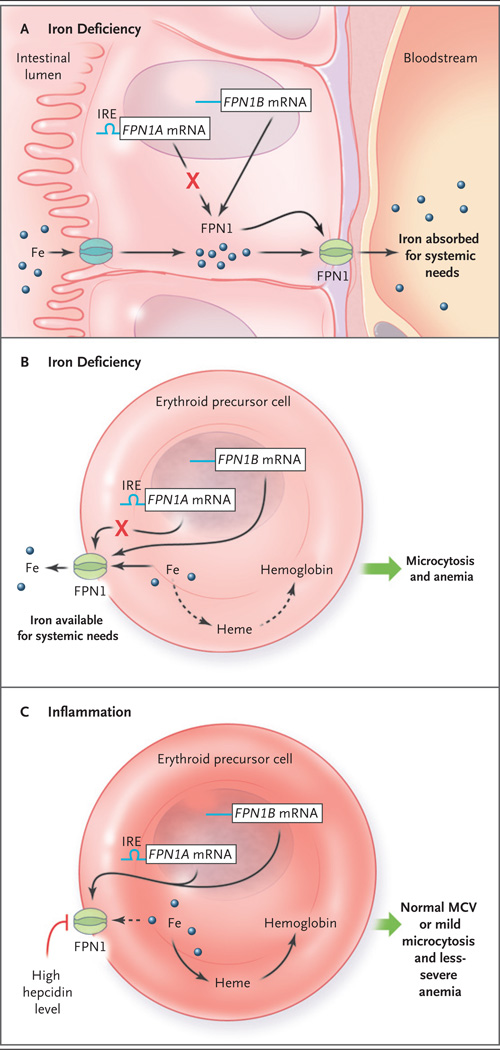
When investigating the regulation of iron uptake in the gastrointestinal tract, Zhang et al. discovered an alternative RNA transcript encoding ferroportin: FPN1B. In contrast to the previously described transcript (FPN1A), FPN1B lacks an ironresponsive element in its 5′ untranslated region, and therefore its translation continues when the cell’s iron content is low. This ensures that dietary iron uptake (that is, iron efflux from the enterocyte into the circulation) is maintained when a person is deficient in iron (Fig. 1A). Ostensibly, duodenal enterocytes act altruistically and can prioritize the systemic need for iron over their own need for cellular iron.
Figure 1. Models of Iron Processing in Cases of Iron Deficiency and Inflammation.
Panel A shows the duodenal enterocyte in the irondeficient state, in which translation of the ferroportin FPN1A messenger RNA (mRNA) transcript (blue line) is repressed (as mediated through its iron-responsive element [IRE]) but levels of FPN1B mRNA transcripts (blue line) — the existence of which was recently described by Zhang et al.2 — increase, and thus the level of the FPN1 protein is maintained. Because the hepcidin level is low, iron is efficiently exported, through FPN1, to the systemic circulation. Erythroid precursors function similarly to enterocytes in the context of iron deficiency (Panel B). FPN1 remains on the cell surface, and iron is exported out of the erythroid precursor for systemic needs. This results in a decreased heme level, and in turn, decreased hemoglobin synthesis, reflected clinically as microcytosis and anemia. In the context of inflammation (Panel C), interleukin-6 and other cytokines stimulate (by means of an iron-independent mechanism) hepatocytes to secrete high levels of hepcidin. Hepcidin triggers FPN1 degradation, blocking iron export from erythroid precursors (dashed arrow), resulting in a relative preservation of hemoglobin synthesis, mean corpuscular volume (MCV), and red-cell production.
A surprise from these investigations is that erythroid precursors also express FPN1B. Screening of tissue samples revealed FPN1B only in duodenal cells and erythroid precursors. It is unclear why erythroid precursors might export iron when hemoglobin makes up more than 90% of the protein content of the mature red cell. Zhang et al. hypothesize that hepcidin expression allows for the communication of systemic iron deficiency (through ferroportin) to the erythroid marrow, as well as the duodenum, so that the erythron can divert some iron to critical, systemic needs (in myoglobin, cytochromes, mitochondrial iron–sulfur clusters, and enzyme synthesis). This would increase the likelihood that iron requirements of nonerythroid cells are met. It will be important for the authors or others to quantify the expression and location of ferroportin in erythroid precursors and demonstrate that the amount of iron exported is sufficiently high to be of physiological relevance.
Although Zhang et al. did not discuss this possibility, their observations might also explain the discrepant findings in patients with iron deficiency (Fig. 1B) and those with inflammatory block (Fig. 1C). In the context of inflammation, the hepcidin level is high, and ferroportin, including the ferroportin present on erythroid precursors, is degraded. Consequently, iron is retained within the erythroid precursor, which maintains the mean corpuscular volume (or at least results in a less dramatically decreased volume) and protects against anemia (Fig. 1C). This mechanism prioritizes red-cell production for oxygen delivery when this is needed to fight inflammatory processes such as chronic infection (e.g., tuberculosis) and thus has an evolutionary rationale. Alternatively, when iron is deficient, the hepcidin level is low,5 ferroportin is present on erythroid precursors, and some iron is exported, leading to red cells with a low mean corpuscular volume and more severe anemia (Fig. 1B).
Clinical situations are often more complex than Figure 1 suggests: iron-deficient erythropoiesis and high hepcidin levels can coexist in a patient. Examples of this complexity are severe inflammatory processes such as juvenile rheumatoid arthritis and genetic disorders such as iron-refractory iron deficiency anemia. In patients with these complexities, the persistently high hepcidin level impairs the absorption of dietary iron, ultimately leading to iron deficiency. Patients with active systemic lupus erythematosus and concurrent aspirin- associated gastrointestinal bleeding (resulting in iron loss) have a similar physiology. When iron delivery to the erythron is sufficiently low, fewer and smaller red cells will be made, despite hepcidin- mediated compensation.
The work of Zhang et al. confirms the intricate nature of the role of hepcidin in iron homeostasis and shows that the interplay of checks and balances is more complicated than previously understood. More gratifyingly, it may explain observations that have been confusing.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Means R. Anemia of chronic disease. In: Young NS, Gerson SL, High KA, editors. Clinical hematology. Philadelphia: Mosby Elsevier; 2006. [Google Scholar]
- 2.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9:461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 5.Theurl I, Aigner E, Theurl M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]