Abstract
Impaired consciousness in epilepsy has a significant negative impact on patient quality of life, yet is difficult to study objectively. Here we develop an improved prospective Responsiveness in Epilepsy Scale (RES-II) and report initial results compared to the earlier version of the scale (RES). RES-II is simpler to administer and includes both verbal and nonverbal test items. We evaluated 75 seizures (24 patients) with RES and 34 seizures (11 patients) with RES-II based on video-EEG review. The error rate per seizure by test administrators improved markedly from a mean of 2.01±0.04 with RES to 0.24±0.11 with RES-II. Performance during focal seizures showed a bimodal distribution, corresponding to the traditional complex partial vs. simple partial seizure classification. We conclude that RES-II has improved accuracy and testing efficiency compared to the original RES. Prospective objective testing will ultimately lead to a better understanding of the mechanisms of impaired consciousness in epilepsy.
Keywords: consciousness, epilepsy, focal seizures, partial seizures, generalized tonic-clonic seizures, behavioral testing
Introduction
Consciousness is a multidimensional construct, incorporating both subjective experience and objective external manifestations. One can also describe these dimensions in terms of the level and content of consciousness, and the neuroanatomical and behavioral correlates of each of these. [1–3] Anesthesia, sleep, minimally conscious state, and epileptic seizures, among other states, all partially disrupt the “consciousness system” and impact the normal manifestations of consciousness, yet preserve some elements of responsive behavior. [4–8] As different behaviors and abilities may be independently affected in each of these disorders, systematic behavioral testing can be highly informative.
Impaired consciousness in epilepsy is particularly difficult to study prospectively compared to other disorders of consciousness, due to its unpredictable and transient nature. [9] We previously developed a standardized testing battery, the Responsiveness in Epilepsy Scale (RES), designed to rapidly assess behavior during seizures in an objective, prospective fashion. [10] RES was based on the JFK Coma Scale, a comprehensive, validated tool for the assessment of impaired consciousness. [11] While RES was successful in its ability to obtain the behavioral data of interest, it was somewhat cumbersome to administer, prompting the current revision. In this study we describe the development and use of a revised version of the scale, “RES-II”, and demonstrate its improved accuracy and efficiency compared to the previous version. In addition, we describe initial results of testing in a group of patients with the new instrument.
Methods
92 adult and pediatric patients were recruited into this study. Methods for the experimental setting, subject recruitment, tester training, test administration and data acquisition for the first 68 patients were as described in detail in (Yang et al. 2012). Briefly, epilepsy inpatients undergoing video/EEG monitoring were tested using the standard RES battery, and results scored by concordance of two reviewers. Identical methods were used for the next group of 25 patients (one patient overlapped both groups), except that a revised version of the Responsiveness in Epilepsy Scale was employed during the period from September 2011 through April 2012. (“RES-II”, see Supplemental Data S1, discussed below) All procedures were approved by the Yale University Human Investigations Committee.
Development of the RES-II
We previously demonstrated that RES, a standardized behavioral testing battery, could be used to prospectively assess responsiveness during epileptic seizures. This aim was successful in spite of the inherent difficulty of performing rapid, interactive testing with a patient unpredictably experiencing an epileptic seizure. Based on this experience, we sought to improve both the reliability and efficiency of testing.
The original scale was adaptive in nature, such that the “level” of questioning depended on the success on previous questions. It was designed in this fashion to quickly shift to questions that would yield the most useful information during the limited timeframe of an epileptic event. However, doing so required an on-the-fly evaluation of responses, which often delayed rapid progression through the questions, limiting the amount of data collected for each event. This also was a source of error, as occasionally the tester would jump to an incorrect level of questioning. Finally, data analysis was cumbersome because different questions were asked of each subject, requiring data to be reported in an abstracted, summary fashion (“consciousness score”).
In designing the new scale, we sought to eliminate these issues by removing the stratification of question items, instead reducing the scale to a sequence of ten items asked repeatedly during each seizure. The exception is an 11th item, (application of strong nailbed pressure to test response to a painful stimulus) which is only asked once in the case that a subject failed to give any response to any of the 10 primary items. Although the use of this item does require a dynamic assessment of prior responses, this is an easily-discernible criteria and this approach restricts the administration of this potentially unpleasant test to rare instances when the patient is deeply unresponsive.
The ten primary testing items themselves consist of many of the same questions used in RES-I including tests of orientation, receptive and expressive language, visual processing, motor praxis, basic sensorimotor responses, and visual tracking. (see RES-II protocol, Supplemental Data S1, online) However, two new items were added to meet another goal in designing RES-II: the greater use of non-verbal prompts and/or items eliciting non-verbal responses. For example, item 7 is the command “wave hello” wherein the action is demonstrated and a non-verbal wave back is sought. This is in contrast to item 5 in which the verbal command “touch your nose” is given, but the action is not demonstrated. This new sequence of items retains the diverse set of cognitive and sensorimotor functions we wish to test during seizures, but greatly improves the accuracy and efficiency of the process. The new scale retains the testing performed after a patient returns to baseline; this involves memory recall for information presented at seizure onset, as well as postictal motor testing.
Data analysis
Within 24 hours of any seizure tested with RES, each response was scored on a scale of 0 to 4 (see Supplemental Data S1, Online) based on review of video recordings. Scores were determined by the agreement of two reviewers, one of whom was the person who performed the testing. We have previously established high inter-rater and intra-rater reliability on performance scores. [10] Timing of all test items and responses was determined relative to seizure onset and end, and separate analyses were performed for the ictal and postictal periods. All seizure onset times are reported as whichever occurred first between electrographic or behavioral onset and all offset times as whichever occurred last between electrographic or behavioral offset based on review of video/EEG data.
Testing errors were defined as the administrator asking a question out of sequence. For example, if a testing item was completely skipped, or if the order of testing was incorrect, this was considered an error. We did not analyze more minor errors such as slight differences in the language used for questions or commands. Results were analyzed by Chi-squared or Student’s t-test. Bonferroni correction was used to adjust significance for multiple comparisons. Corrected significance threshold was p < 0.05. All values are reported as mean ± standard error of the mean.
Results
Demographics and testing (RES cohort)
68 subjects were recruited into the first phase of the study utilizing the original RES scale; some patient data from this phase has been previously reported [10]. A total of 75 seizures were captured from 24 of the 68 subjects (10M, 14F; mean age 36.2; 20 right-handed) (see Supplemental Table S2, online). 15 of these subjects underwent scalp EEG alone, 3 underwent scalp EEG plus ictal single photon emission computed tomography (SPECT), and 6 patients underwent intracranial EEG. The mean seizure duration was 101±17 seconds, and average duration of testing was 304±58 seconds. The first question was asked an average of 46.0±6.5 seconds after seizure onset.
Demographics and testing (RES-II cohort)
25 subjects were recruited into the recent phase of the study utilizing the RES-II scale. One subject had previously participated in testing with the original RES scale. A total of 34 seizures were captured from 11 of those subjects (7M, 4F; mean age 36.7; all right-handed) (see Supplemental Table S2, online). 5 of these subjects underwent scalp EEG alone, 5 underwent scalp EEG plus ictal single photon emission computed tomography (SPECT) study, and one patient underwent intracranial EEG. The mean seizure duration was 139±24 seconds, and average duration of testing was 225±26 seconds. The first question was asked an average of 16.0±2.0 seconds after seizure onset.
Comparison of RES vs RES-II
We compared data from RES and RES-II (Table 1). A total of 1,277 questions (36% ictal, 64% postictal) were asked with the revised scale, vs 2,372 (23% ictal, 77% postictal) with the previous scale. Slightly more questions were asked per seizure (37.6±4.0 with RES-II vs 31.6±3.0 with RES). With the use of the new RES-II scale, the average time required to ask one question during each seizure was 6.7 ±0.6 seconds, similar to or slightly faster than the 8.5±0.8 seconds per question with the original RES (p=0.12). In spite of this pace, there was a nearly ten-fold reduction in the average number of errors made per seizure during administration of the revised scale (0.24±0.11 with RES-II vs 2.01±0.36 with RES; p<0.01). All errors involved the administrator asking either the wrong question or asking a question in the wrong order. We also compared the error rates in terms of complete seizures with or without any errors (Table 1). There was a significant decrease in seizures with errors from nearly 50% with RES to about 15% with RES-II (χ2=11.8, p<0.001).
Table 1.
Comparison of RES and RES-II
| RES | RES-II | p value | |
|---|---|---|---|
| Time per question (sec) | 8.5 ± 0.8 | 6.7 ± 0.6 | 0.12 |
| Time from sz onset until testing start (sec) | 46.0 ± 6.5 | 16.0 ± 2.0 | <0.01 |
| Errors per sz | 2.01 ± 0.36 | 0.24 ± 0.11 | <0.01 |
| Szs with errors, % (szs with errors/total szs) | 49.3% (37/75) | 14.7% (5/34) | <0.01 |
We also observed significant improvement between the two cohorts in other aspects of the data which was not related to the content of the testing scale. Approximately 2,030 hours of RES monitoring were completed, resulting in an average of approximately 27.1 hours of RES monitoring required to capture one seizure. While only approximately 240 hours of RES-II monitoring were completed, this resulted in an average of 7.1 hours of monitoring required to capture one seizure, a significant improvement in efficiency. Additionally, the period of time between seizure onset and initiation of testing decreased from 46.0±6.5 to 16.0±2.0 (p<0.01) seconds, and the percent of seizures in which testing was only able to commence after seizure offset improved from 34% to 3%. (χ2=8.79, p<0.01) We attribute these improvements to optimization of resource management and data collection practices resulting from experience with collecting this type of data over the course of more than 3 years, although use of the simplified testing scheme in RES-II may also have contributed.
Performance on RES-II
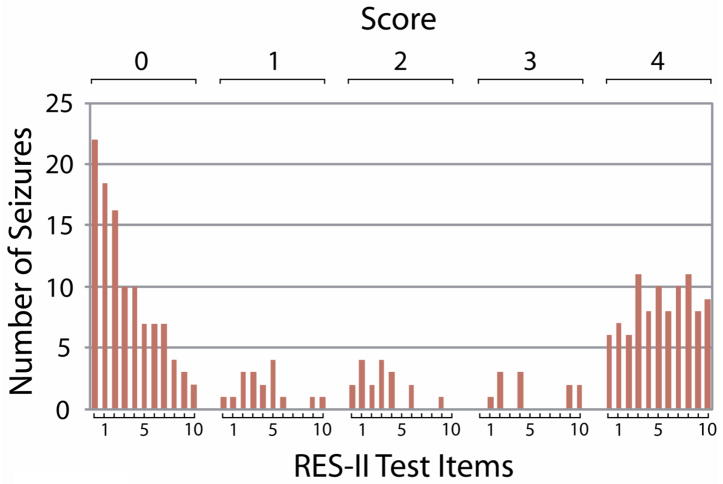
Although only one generalized seizure was tested, obvious impairment was present: all 22 ictal questions administered during this seizure received the lowest possible score of “0”, indicating no response at all. Partial seizures comprised the vast majority of seizures tested (n=33) and showed a more variable pattern of performance. Interestingly, performance scores in partial seizures showed a bimodal distribution (Figure 1). For example, for the first question asked (ictal memory 1A), 22 of 33 seizures received the minimum score of “0” (no response whatsoever) and 6 of 33 subjects received the maximum score of “4” (able to correctly repeat the time of day). Only 5 seizures showed one of the three intermediate scores of impairment. Similarly, intermediate scores were also less common for the other testing items (Figure 1).
Figure 1.
Bimodal distribution of scores on RES-II testing. The initial cycle of testing is shown for all partial seizures (excluding post-ictal values). Since the mean seizure duration was 139s and each item took 6.7s on average (with 11 items total), typically just over one iteration of testing was completed for each seizure during the ictal period. Scores show a bimodal distribution, with the large majority receiving a score of either “0” (no response whatsoever) or “4” (normal, unimpaired response). This pattern is similar to that seen in data using the original RES. [10]
To investigate this further, we examined the timecourse of responses in partial seizures with initial impairment (score 0) vs. those with spared performance (score 4) on the first question asked (Figure 2). For partial seizures with initial impairment, impaired responsiveness tended to continue for later testing items as well, while seizures with initial spared performance maintained good performance at later times. These findings are in agreement with Yang et al. and suggest that focal (partial) seizures can often be separated relatively cleanly into those with vs. without overall impaired responsiveness. [10]
Figure 2.
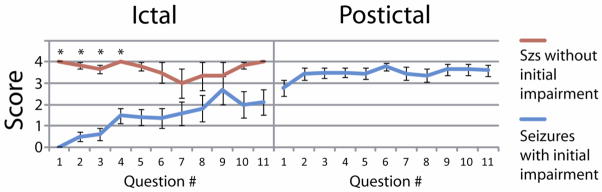
RES-II performance over the initial 11 test items for partial seizures that scored either a “0” or “4” on the initial question. Seizures that showed initial impairment tended to remain impaired through the course of the seizure, yet seizures with initial spared performance showed relatively good performance throughout. Seizures with impaired performance recovered in the postictal period. Note that “Question #” refers to how many questions have been asked since seizure onset or offset, not specifying which RES-II item was performed. In the ictal period, the first question was always Memory 1A (asking patient to repeat the current time aloud). In the post-ictal period however the first question asked varied because seizure offset occurred at an unpredictable moment during testing and administration of the test items continued in sequence. (*= p<0.05, with Bonferroni correction)
Discussion
This study demonstrates that behavioral testing of responsiveness during epileptic seizures is substantially more accurate and efficient utilizing the revised version of the Responsiveness in Epilepsy Scale. The overall administrator error rate decreased nearly 10-fold compared to the previous version, and there is a trend in the data showing reduced time required for each question during the ictal period. As the training and testing methods remained identical, as did many of the items themselves, we attribute these improvements to the simpler testing protocol and the removal of the need for on-line assessment of patient responses.
At the same time, patterns of impairment observed using the previous scale were generally reproduced with data from the revised scale. The single generalized tonic-clonic seizure showed complete RES impairment. Partial seizures showed a bimodal pattern of deficits, and were generally classifiable as those without any impairment, or those with significant impairment persisting for the duration of the seizure. This dissociation supports the previous observation that partial seizures can be separated based on the presence of impaired or preserved responsiveness, corresponding to the traditional categories of “complex partial” and “simple partial” seizures. [10]
Objective assessment of impaired consciousness during seizures is difficult to make using traditional methods. In future work, a direct comparative analysis of the current method of ictal assessment and other instruments could be of benefit. [12, 13] Previous approaches have relied predominantly on introspective and/or retrospective data. [9, 12, 13] Non-standardized or retrospective assessment is particularly problematic with regards to issues such as the similar perception of amnesia and unconsciousness, or confounders of altered consciousness such as aphasia or ictal speech. [2, 9] Prospective assessment of prolonged epileptic events such as non-convulsive status epilepticus have been made using neuropsychological testing over the course of 10–15 minutes, however the large majority of seizures are both unpredictable and too brief for these methods. [14] One study demonstrated prospective testing of responsiveness performed by family members and hospital staff, but this approach is greatly limited by the inability to directly and accurately compare responses to the diversity of non-standardized prompts. [15] Other attempts at prospective testing have been limited to verbal prompts by a remote observer, which tend to limit testing to language-dependent prompts. [16] Arthuis and colleagues developed the “consciousness seizure scale” which has the advantage of using both verbal and non-verbal responses, as well as patients’ memory of the event, to classify seizures as those with or without altered consciousness. [13] However, this scale is not based on standardized prospective testing.
We believe that many of these difficulties have been overcome with RES-II, and that performing prospective, standardized testing is ideal for describing a seizure’s impact on elements of consciousness, at least those which are externally observable and affect behavior. Impaired consciousness in epilepsy has a major negative impact on quality of life, and a better understanding is necessary to progress towards improved treatment for these patients. [17, 18]
One limitation of RES-II is that the scale still requires administration by a trained, nearby tester. Other limitations of the present study primarily stem from the modest number of seizures tested. In addition, even with prospective testing there are inherent limitations in the ability of any testing paradigm to assess the internal experiential aspects of consciousness. As has been discussed by others in detail, patients may have conscious experiences that they cannot communicate either during or after seizures due to motor, language or memory impairment. [2, 19] However, recent work suggests that impaired consciousness in epilepsy is most often due to a deficit in the level of consciousness, i.e. dysfunction in the overall degree of arousal, rather than focal motor or language deficits; and preliminary imaging and electrophysiological data support this view. [20] While we have performed behavioral testing (both RES-I and RES-II) on a number of patients with simultaneous SPECT imaging or intracranial EEG, additional data will be required to draw firmer conclusions regarding the relationship between specific anatomic structures and impairment of the level and content of consciousness.
In conclusion, we have demonstrated that RES-II greatly reduces some of the weaknesses of the previous version, and is an effective standardized scale for assessing ictal impairment. This type of objective assessment may be helpful for future studies detailing impairment related to dysfunction of specific anatomic structures. We believe that the prospective and interactive design of this scale is an improved paradigm for objectively assessing how consciousness is impaired during seizures.
Supplementary Material
Supplemental Data S1. Responsiveness in Epilepsy Scale—Revised (RES-II)
Patients #1–24 tested with RES, patients #25–35 tested with RES-II. aBased on overall EEG of all seizures, not just seizure occurring during RES. Abbreviations: HC=hippocampus, R=right, L=left, O=occipital, T=temporal, P=parietal, F=frontal, Sup=superior, Lat=lateral, Inf=inferior, Bi=bilateral, H=hemisphere, GTC=secondarily generalized tonic-clonic, sz=seizure, Pt=patient.
Highlights.
We describe a revised version of the Responsiveness in Epilepsy Scale (RES-II).
RES-II was found to be more accurate and efficient to administer.
Ictal performance corresponded to traditional seizure classifications.
RES-II is a robust instrument for objective, prospective study of ictal behavior.
Acknowledgments
This work was supported by the Patrick and Catherine Weldon Donaghue Medical Research Foundation, and by the Betsy and Jonathan Blattmachr Family. Andrew Bauerschmidt was supported by the Doris Duke Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Plum F, Posner J. The diagnosis of stupor and coma. USA: Oxford University Press; 1982. [Google Scholar]
- 2.Ali F, Rickards H, Cavanna AE. The assessment of consciousness during partial seizures. Epilepsy Behav. 2012;23:98–102. doi: 10.1016/j.yebeh.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9:301–10. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld H. Epilepsy and the consciousness system: transient vegetative state? Neurol Clin. 2011;29:801–23. doi: 10.1016/j.ncl.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, Kelly JP, Rosenberg JH, Whyte J, Zafonte RD, Zasler ND. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 6.Provini F, Tinuper P, Bisulli F, Lugaresi E. Arousal disorders. Sleep Med. 2011;12 (Suppl 2):S22–6. doi: 10.1016/j.sleep.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Sanders RD, Tononi G, Laureys S, Sleigh JW. Unresponsiveness not equal unconsciousness. Anesthesiology. 2012;116:946–59. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiff N, Ribary U, Plum F, Llinas R. Words without mind. J Cogn Neurosci. 1999;11:650–6. doi: 10.1162/089892999563715. [DOI] [PubMed] [Google Scholar]
- 9.Johanson M, Valli K, Revonsuo A. How to assess ictal consciousness? Behav Neurol. 2011;24:11–20. doi: 10.3233/BEN-2011-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Shklyar I, Lee HW, Ezeani CC, Anaya J, Balakirsky S, Han X, Enamandram S, Men C, Cheng JY, Nunn A, Mayer T, Francois C, Albrecht M, Hutchison AL, Yap EL, Ing K, Didebulidze G, Xiao B, Hamid H, Farooque P, Detyniecki K, Giacino JT, Blumenfeld H. Impaired consciousness in epilepsy investigated by a prospective responsiveness in epilepsy scale (RES) Epilepsia. 2012;53:437–47. doi: 10.1111/j.1528-1167.2011.03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–9. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Cavanna AE, Mula M, Servo S, Strigaro G, Tota G, Barbagli D, Collimedaglia L, Viana M, Cantello R, Monaco F. Measuring the level and content of consciousness during epileptic seizures: the Ictal Consciousness Inventory. Epilepsy Behav. 2008;13:184–8. doi: 10.1016/j.yebeh.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Arthuis M, Valton L, Regis J, Chauvel P, Wendling F, Naccache L, Bernard C, Bartolomei F. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain. 2009;132:2091–101. doi: 10.1093/brain/awp086. [DOI] [PubMed] [Google Scholar]
- 14.Profitlich T, Hoppe C, Reuber M, Helmstaedter C, Bauer J. Ictal neuropsychological findings in focal nonconvulsive status epilepticus. Epilepsy Behav. 2008;12:269–75. doi: 10.1016/j.yebeh.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Bell WL, Park YD, Thompson EA, Radtke RA. Ictal cognitive assessment of partial seizures and pseudoseizures. Arch Neurol. 1998;55:1456–9. doi: 10.1001/archneur.55.11.1456. [DOI] [PubMed] [Google Scholar]
- 16.Inoue Y, Mihara T. Awareness and responsiveness during partial seizures. Epilepsia. 1998;39 (Suppl 5):7–10. doi: 10.1111/j.1528-1157.1998.tb05142.x. [DOI] [PubMed] [Google Scholar]
- 17.Wirrell EC. Epilepsy-related injuries. Epilepsia. 2006;47 (Suppl 1):79–86. doi: 10.1111/j.1528-1167.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith G, Ferguson PL, Saunders LL, Wagner JL, Wannamaker BB, Selassie AW. Psychosocial factors associated with stigma in adults with epilepsy. Epilepsy Behav. 2009;16:484–90. doi: 10.1016/j.yebeh.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Gloor P. Consciousness as a neurological concept in epileptology: a critical review. Epilepsia. 1986;27 (Suppl 2):S14–26. doi: 10.1111/j.1528-1157.1986.tb05737.x. [DOI] [PubMed] [Google Scholar]
- 20.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–26. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data S1. Responsiveness in Epilepsy Scale—Revised (RES-II)
Patients #1–24 tested with RES, patients #25–35 tested with RES-II. aBased on overall EEG of all seizures, not just seizure occurring during RES. Abbreviations: HC=hippocampus, R=right, L=left, O=occipital, T=temporal, P=parietal, F=frontal, Sup=superior, Lat=lateral, Inf=inferior, Bi=bilateral, H=hemisphere, GTC=secondarily generalized tonic-clonic, sz=seizure, Pt=patient.