Abstract
Mounting evidence indicates that inflammatory cytokines contribute to the development of depression in both medically ill and medically healthy individuals. Cytokines are important for development and normal brain function, and have the ability to influence neurocircuitry and neurotransmitter systems to produce behavioral alterations. Acutely, inflammatory cytokine administration or activation of the innate immune system produces adaptive behavioral responses that promote conservation of energy to combat infection or recovery from injury. However, chronic exposure to elevated inflammatory cytokines and persistent alterations in neurotransmitter systems can lead to neuropsychiatric disorders and depression. Mechanisms of cytokine behavioral effects involve activation of inflammatory signaling pathways in the brain that results in changes in monoamine, glutamate, and neuropeptide systems, and decreases in growth factors, e.g. brain derived neurotrophic factor. Furthermore, inflammatory cytokines may serve as mediators of both environmental (e.g. childhood trauma, obesity, stress, and poor sleep) and genetic (functional gene polymorphisms) factors that contribute to depression’s development. This review explores the idea that specific gene polymorphisms and neurotransmitter systems can confer protection from or vulnerability to specific symptom dimensions of cytokine-related depression. Additionally, potential therapeutic strategies that target inflammatory cytokine signaling or the consequences of cytokines on neurotransmitter systems in the brain to prevent or reverse cytokine effects on behavior are discussed.
Keywords: inflammatory cytokines, depression, serotonin, dopamine, brain-derived neurotrophic factor, kynurenines
1. Introduction
There has been a great deal of interest in the effects of cytokines of the innate immune system on the brain and behavior. Cytokines are important in brain development, and can promote healthy brain function by supporting neuronal integrity, neurogenesis, and synaptic remodeling (Yirmiya and Goshen, 2011). Cytokines also have the capability of influencing neurocircuitry and neurotransmitter systems to produce behavioral alterations (Miller et al., 2009, Haroon et al., 2012). Acutely, administration of cytokines or activation of the innate immune system can induce a behavioral repertoire termed “sickness behavior” that includes anhedonia, anorexia, fever, sleep changes, and decreased social interaction (Dunn and Swiergiel, 1998, Dunn et al., 2005, Dantzer and Kelley, 2007). These potentially adaptive behavioral responses to cytokines can benefit an organism by promoting conservation of energy and allocation of resources to combat infection or recovery from injury, along with behaviors that may elicit care-giving from others (Lotrich, 2012). However, under conditions of chronic exposure to elevated inflammatory cytokines, persistent alterations in neurotransmitter function and behavior can lead to the development neuropsychiatric dysfunction, and especially depression. For instance, patients with increased inflammatory cytokines due to a variety of medical illnesses have increased rates of depression compared to the general population (Yirmiya et al., 1999, Yirmiya et al., 2000), and some patients with idiopathic major depression without co-morbid medical illness also exhibit increased circulating cytokines and inflammatory markers (Maes et al., 1992, Maes, 1999, Sluzewska, 1999, Dowlati et al., 2010). Furthermore, administration of cytokines to humans and laboratory animals produces neuropsychiatric symptoms and behavioral alterations consistent with depression (Miller et al., 2009).
To address the role of cytokines in depression, this review will provide an overview of the current literature from both human and animal studies regarding the effects of inflammatory cytokines on brain neurocircuitry and neurotransmitter systems that lead to behavioral change (mechanisms of cytokine actions in the brain summarized in Figure 1). We should note that not all types of depression necessarily involve cytokines, e.g. post-partum and peri-menopausal depression, hypothyroidism, depression secondary to cocaine withdrawal, and vascular depression to name just a few. Therefore, depression that is associated with inflammatory cytokines may be one subtype of depression. Nonetheless, it is biologically plausible that inflammatory cytokines serve as mediators of both environmental and genetic factors that may trigger the development of depressive disorders (Raison and Miller, 2011). Factors that may precipitate inflammation and influence the development of depression include medical illness, obesity, psychosocial stress, sleep disturbance, and gastrointestinal inflammation, and will be discussed herein. Additionally, there is growing interest in the iatrogenic depression that results from exogenous interferon-alpha therapy. This has facilitated mechanistic research interest into prospectively determining the pathways by which depression develops during inflammatory cytokine exposure. This set of various endogenous inflammatory cytokine and exogenous cytokine-associated depressions has been associated with specific risk factors that may allow for potential preventive interventions. That is, not all subjects exhibiting increased inflammatory cytokines develop depression, and there are numerous vulnerability and resilience factors for cytokine-induced depression (Kendler et al., 2001, Caspi et al., 2003, Duman and Monteggia, 2006, Heim et al., 2008, Lotrich, 2011) Moreover, the role of the immune system in depression suggests potential novel and targeted therapeutic strategies for reversing cytokine effects on the brain and behavior, which will be reviewed.
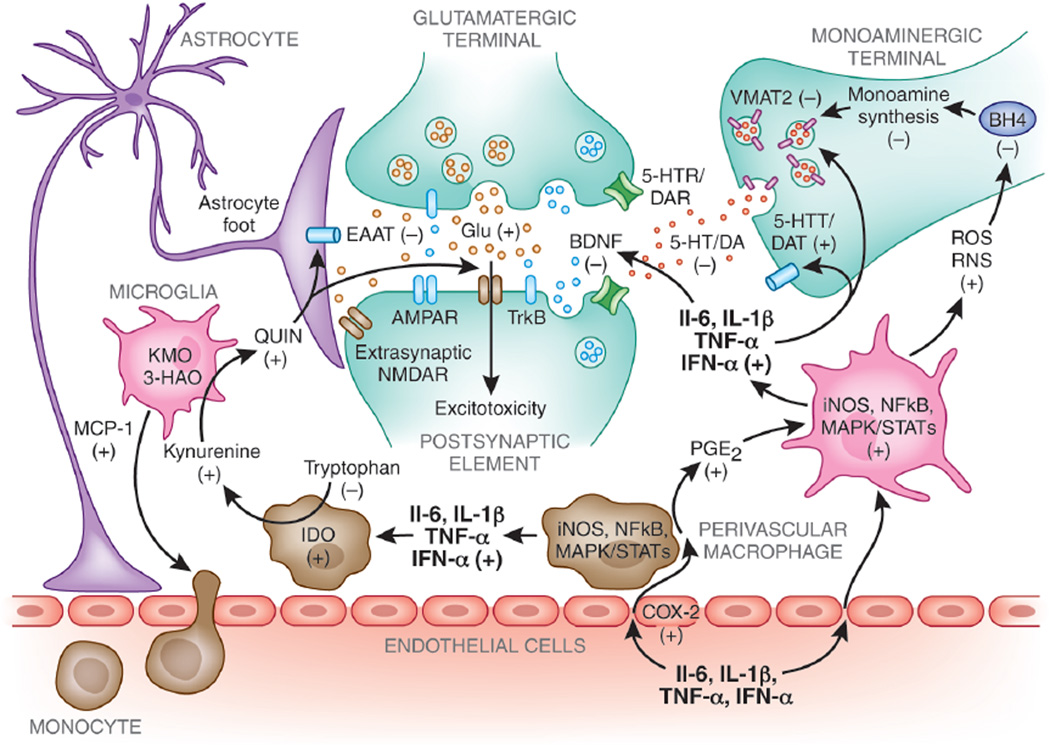
Figure 1. Potential mechanisms of inflammatory cytokine effects on brain monoamine, glutamate, and BDNF neurotransmitter systems.
Peripheral cytokines can access the central nervous system and increases production of local inflammatory mediators such as cyclooxegenase-2 (COX-2), prostaglandin E2 (PGE2), nitric oxide (NO), cytokines, and chemokines by endothelial cells, perivascular macrophages, and microglia. Production of monocyte chemotactic protein-1 (MCP-1) recruits peripheral immune cells into the brain that produce even more cytokines and inflammatory mediators. Inflammatory cytokines are associated with increased oxidative stress and generation of reactive oxygen and reactive nitrogen species (ROS and RNS). Increased ROS and RNS contribute to oxidation of tetrahydrobiopterin (BH4), a cofactor required for the synthesis of monoamines. Furthermore, evidence exists indicating that inflammatory cytokines and their signal transduction pathways, such as p38 mitogen-activated protein kinases (MAPK), may decrease expression or function of the vesicular monoamine transporter 2 (VMAT2) and/or increase expression or function of serotonin and dopamine transporters (5-HTT/DAT). Cytokines can also decrease brain-derived neurotrophic factor (BDNF) and interfere with TrkB receptor signaling, which may adversely influence neurogenesis and neuroplasticity. Finally, inflammatory cytokines can affect the glutamate (Glu) system by activation of the enzyme, indoleamine-2,3-dioxygenase (IDO), that catabolizes tryptophan, the primary amino-acid precursor of 5-HT, into kynurenine. Kynurenine is further broken down in the CNS into the neuroactive metabolites kynurenic acid and quinolinic acid (QUIN). Although not pictured, kynurenic acid can antagonize Glu receptors and decrease Glu release leading to decreased Glu neurotransmission. QUIN can directly activate the n-methyl-d-aspartate receptor (NMDAR), increase Glu release, and inhibit Glu uptake by astrocytes via the excitatory amino acid transporter (EAAT), thus allowing increased access of Glu to extrasynaptic NMDARs and contributing to excitotoxicity.
3-HAO, 3-hydroxyanthranilic acid oxygenase; 5-HT, serotonin; 5-HTT, serotonin transporter; AMPAR, 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl) propanoic acid receptor; BH4, tetrahydrobiopterin; BDNF, brain-derived neurotrophic factor; COX-2, cyclooxygenase-2; DAT, dopamine transporter; glu, glutamate; EAAT, excitatory amino acid transporter; IDO, indoleamine 2,3 dioxygenase; IFN, interferon; iNOS, inducible nitric oxide synthase; IL, Interleukin; KAT II, kynurenine aminotransferase II; KMO, kynurenine 3-monooxygenase; MAPK, mitogen-activated protein kinases; MCP-1, monocyte chemotactic protein-1; NMDAR, N-Methyl-D-aspartic acid receptor; NF-kB, nuclear factor-kappa B; PGE2, prostaglandin E2; QUIN, quinolinic acid; RNS, reactive nitrogen species; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; TH, tyrosine hydroxylase; TNF, tumor necrosis factor; TrkB, tyrosine kinase receptor B; VMAT2, vesicular monoamine transporter 2
2. Cytokines and depression
2.1 Elevated cytokines and inflammatory markers in idiopathic major depression
Numerous studies have reported increases in circulating proinflammatory cytokines, interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-alpha, their soluble receptors, and the acute phase protein, C-reactive protein (CRP), in patients with idiopathic major depression (Maes et al., 1992, Maes, 1999, Sluzewska, 1999). These findings have been corroborated by metaanalyses (Howren et al., 2009, Dowlati et al., 2010). A handful of studies have also measured cytokine concentrations in the cerebrospinal fluid (CSF) of depressed patients, and some have observed increased cytokines compared to controls or correlations between CSF cytokines and depression severity (Levine et al., 1999, Lindqvist et al., 2009, Martinez et al., 2012). For instance, one study observed higher CSF concentrations of IL-1beta, lower IL-6, and no change in TNF-alpha in depressed subjects compared to controls (Levine et al., 1999), and another reported increased CSF IL-6 concentrations that correlate with depressive scores in depressed subjects that had attempted suicide (Lindqvist et al., 2009). Another study reported no change in CSF cytokines between depressed and healthy control subjects as a group, but did find relationships between cytokines and depressive symptoms both before and after antidepressant treatment (Martinez et al., 2012). Furthermore, elevated levels of the inflammatory mediator, prostaglandin E2 (PGE2), have been observed in the saliva, plasma, and CSF of depressed subjects (Lieb et al., 1983, Linnoila et al., 1983, Calabrese et al., 1986, Ohishi et al., 1988, Nishino et al., 1989), and were found to correlate with depression severity (Ohishi et al., 1988, Nishino et al., 1989).
As well as exhibiting increased inflammatory markers at rest, depressed subjects have been reported to evince increased inflammatory responses to stress. For instance, subjects with major depression and a history of early life stress responded to psychological stress (the Trier Social Stress Test) with exaggerated circulating IL-6 production and increased DNA binding of nuclear factor (NF)-kB in peripheral blood mononuclear cells compared to non-depressed controls (Pace et al., 2006), Similarly, depressed subjects demonstrated a decreased ability for glucocorticoids to inhibit inflammatory cytokine production following exposure to a mock interview stressor compared to controls (Miller et al., 2005). Moreover, increased IL-6 production in adolescents with histories of childhood adversity has been shown to precede subsequent development of depression 6 months later (Miller and Cole, 2012), indicating a relationship between chronic stress, inflammation, and depression. Sleep disturbance may be another variable that is related to inflammation (Bryant et al., 2004, Motivala et al., 2005, Opp et al., 2007, Suarez, 2008) and thereby increased risk for depression. Sleep deprivation results in increased circulating levels of IL-6, TNF-α, and C-reactive protein when compared to periods of undisturbed sleep (Vgontzas et al., 1999, Meier-Ewert et al., 2004, Vgontzas et al., 2004).
Further solidifying the relationship between cytokines and immune activation in depression, several functional allelic variants and single-nucleotide polymorphisms (SNPs) of genes encoding immune and inflammatory molecules have been identified in associated with idiopathic major depression (Bosker et al., 2011, Bufalino et al., 2012, Raison and Miller, 2013). These findings have engendered speculation as to whether alleles that promote enhanced inflammatory cytokine secretion may have been evolutionary advantageous and thus conserved (Raison and Miller, 2013). Indeed, heightened inflammatory responses to environmental stimuli could have improved survival by conferring greater protection from bacterial and viral infection (Raison and Miller, 2013), and genetic priming to respond to stress and the environment with increased inflammatory and antiviral responses may contribute to the high prevalence of depression and comorbidity with chronic inflammatory disease.
2.2 Medical illness and depression
Patients that exhibit increased inflammation during chronic medical illness develop depression and fatigue at higher rates than the general population (Evans et al., 1999, Yirmiya et al., 1999, Yirmiya et al., 2000, Raison and Miller, 2003). For example, cancer patients exhibit elevated circulating proinflammatory cytokines and develop depression and fatigue at rates approaching 50% (Musselman et al., 2001b, Massie, 2004, Jehn et al., 2006). Circulating IL-1beta and IL-6 correlated with fatigue symptoms in cancer patients undergoing radiation or chemotherapy (Greenberg et al., 1993, Bower et al., 2002, Raison and Miller, 2003), indicating an association between proinflammatory cytokines and behavioral symptoms in these patients. Autoimmune disorders including multiple sclerosis, rheumatic disease, asthma, and allergies are also associated with high rates of neuropsychiatric symptoms and depression (Pollak and Yirmiya, 2002). Similarly, patients with human immunodeficiency virus (HIV), which is associated with increased inflammatory cytokine production, and particularly interferon (IFN)-alpha (Ries et al., 2012), manifest neuropsychiatric complications, such as cognitive decline and psychomotor disturbance, and often depression and fatigue (Treisman et al., 1998, Wojna et al., 2006, Payne et al., 2012). These data suggest that innate immune activation during chronic medical illnesses, as characterized by elevations in inflammatory cytokines, may contribute to the high rates of depression and other neuropsychiatric (e.g.cognitive or psychomotor) symptoms observed in medically ill populations.
Increased circulating inflammatory cytokines and CRP are risk factors for the development of illnesses associated with inflammation, such as heart disease and type 2 diabetes (Mendall et al., 1997, Spranger et al., 2003), and individuals with major depression have a several fold greater risk of comorbidity and mortality (Muskin, 2010, Shah et al., 2011). Furthermore, patients who are resistant to antidepressant treatment demonstrate higher concentrations of circulating cytokines and acute-phase proteins than patients who respond to antidepressant treatment (Sluzewska et al., 1997, Lanquillon et al., 2000, Fitzgerald et al., 2006), and are at even greater risk of morbidity and mortality in medical illness such as coronary heart disease (Carney and Freedland, 2009). Moreover, persons exposed to childhood trauma exhibit increased inflammatory markers as adults and increased risk of developing both medical illness and depression (Felitti et al., 1998, Dong et al., 2004, Danese et al., 2007).
2.3 Sources of cytokines and inflammation in medically healthy individuals
One of the major environmental factors that may interact with cytokines and genetic predisposition to major depression is stress (Haroon et al., 2012). Physical and psychological stressors can activate immune cells in both the periphery and CNS to release inflammatory cytokines that lead to neurotransmitter changes and behavioral alterations (Maier and Watkins, 1998, Koo and Duman, 2008). Recent interest has been paid to the mechanisms by which psychological stress can translate into immune system activation and release of proinflammatory cytokines (Fleshner, 2013). On a cellular level, the immune system can detect danger signals in the absence of a pathogen through the release of danger associated molecular patterns (DAMPs) such as heat shock protein-72, uric acid, and ATP, through a process termed “sterile inflammation” (Maslanik et al., 2012, Fleshner, 2013). These DAMPS are thought to be released during stress, and one key mechanism by which they may elicit an immune response is through the NLRP3 inflammasome, a multiprotein complex that is involved in the processing of IL-1beta. DAMPs are known to stimulate the inflammasome in the presence of LPS to activate caspase-1, which cleaves the immature precursor of interleukin IL-1beta and IL-18 into their mature releasable forms (Iwata et al., 2012, Maslanik et al., 2012). This increase in IL-1beta release can then induce the production of other inflammatory cytokines that are released during stress. Acutely, glucocorticoids provide a break on stress-induced inflammatory cytokine production, however the development of glucocorticoid resistance as the result of chronic stress or prolonged exposure to inflammatory cytokines can lead to increase predisposition for the release of IL-1beta and other cytokines (Maes et al., 1993, Pariante et al., 1999, Miller et al., 2002a, Pace et al., 2007, Cohen et al., 2012). Therefore, DAMPS and the NLRP3 inflammasome may serve as a primary link by which chronic psychological or physical stress is translated into damage signals that promote inflammatory activity, and may contribute to depression as well as associated co-morbid medical illnesses related to chronic stress (Iwata et al., 2012). Indeed, the ATP purinergic type 2X7 (P2X7) receptor, a primary activator of the NLRP3 inflammasome (Ferrari et al., 1997, Ferrari et al., 2006), may be involved in inflammatory processes in cardiovascular disease, obesity, neurologic disorders, pain, and pulmonary fibrosis (Skaper et al., 2009, Riteau et al., 2010, Sun et al., 2012), and has a polymorphic region (rs2230912) that is associated with major depression (Lucae et al., 2006).
Another source of inflammatory cytokines is the gut, and there has been attention paid to the role of intestinal microorganisms in influencing the immune responses of an organism (Lee and Mazmanian, 2010). The dense and complex community of gastrointestinal microbiota of an individual may be influenced by genetic and environmental factors, and the balance between protective and harmful bacteria species may be important for general health (Sekirov et al., 2010). The intestinal lining also harbors immune cells, including dendritic cells and T cells (Coombes and Powrie, 2008, Fagarasan et al., 2010), and may serve as a source of circulating cytokines. Increased exposure to toxins, food allergens, and stress may promote a loss of protective microorganisms and increase inflammation in the gut, thus contributing to behavioral symptoms associated with increased inflammatory cytokines (Arrieta et al., 2006, Buret, 2006). Furthermore, manipulation of the gut microbiota of mice altered behavior and hippocampal brain-derived neurotrophic factor (BDNF) content independent of changes in circulating inflammatory cytokines and neuroendocrine hormones, indicating a potentially direct gut to brain connection that can influence behavior (Bercik et al., 2011).
Finally, obesity is associated with increased circulating cytokines, and BMI significantly influences concentrations of L-6 and other inflammatory markers (Khaodhiar et al., 2004). A potential association between obesity, inflammatory cytokines, and behavioral alterations has been postulated (Lim et al., 2005). Adipose tissue, and especially visceral adiposity (Park et al., 2005), is thought to be a major source of inflammatory cytokines, and histologic examination has determined that macrophage accumulate among adipose cells (Weisberg et al., 2003, Suganami and Ogawa, 2010). Macrophage in visceral adiposity have been proposed to release cytokines into the portal vein, thus contributing to hepatic insulin resistance and type 2 diabetes (Rytka et al., 2011). Adiposity has been suggested as a link between depression, increased inflammatory markers, and increased risk of coronary heart disease (Miller et al., 2002b, Miller et al., 2003), and the cytokines released from adipose tissue may also affect the brain and behavior.
2.4 Access of peripheral cytokines to the brain and activation of local inflammatory networks
Cytokines released by peripheral immune cells or adipose tissue can access the CNS to initiate local immune activation by several mechanisms, including 1.) passage through leaky regions in the blood-brain-barrier at circumventricular organs (Katsuura et al., 1990, Pan and Kastin, 2003), 2.) active uptake mechanisms of cytokines across the blood-brain-barrier (Banks et al., 1995, Banks et al., 2002, Banks and Erickson, 2010), 3.) local actions at peripheral vagal nerve afferents that relay cytokine signals to relevant brain regions, including the nucleus of the solitary tract and hypothalamus (the so called ‘neural route’) (Bluthe et al., 1994, Ericsson et al., 1994, Watkins et al., 1994, Watkins et al., 1995), 4.) activation of endothelial cells and perivascular macrophages in the cerebral vasculature to produce local inflammatory mediators such as cytokines, chemokines, PGE2, and nitric oxide (NO) (Fabry et al., 1993, Cao et al., 1997, Miller, 1999, Matsumura and Kobayashi, 2004), and 5.) activated immune cells such as monocytes/macrophages and T cells can be recruited from the periphery to the brain parenchyma, and these cells can in turn produce cytokines in the brain (Shaftel et al., 2007, D'Mello et al., 2009).
Peripheral cytokine signals are amplified in the CNS by local inflammatory networks, including inflammatory signal transduction pathways, cytokine production, and PGE2 release (see Figure 1 for inflammatory pathways in the brain). In the brain, endothelial cells and perivascular macrophages respond to circulating cytokines to induce expression of COX-2 and release of PGE2 (Elmquist et al., 1997, Lacroix and Rivest, 1998, Konsman et al., 2002, Konsman et al., 2004). Cytokines in the brain are produced primarily by microglia, but can also be produced by astrocytes (Lieberman et al., 1989, Chung and Benveniste, 1990) and to some extent by neurons (Breder et al., 1988, Schobitz et al., 1992) and oligodendrocytes (Blasi et al., 1999, Palma et al., 2003). Following an acute inflammatory stimulus, increased CNS cytokines can confer protection to the brain (Kreutzberg, 1996, Kim and de Vellis, 2005, Chen et al., 2012), yet under conditions of chronic immune activation, microglia can become a source of inflammatory mediators that may influence brain neurotransmitter systems and neuronal integrity. Activated microglia can produce indoleamine-2,3-dioxygenase (IDO) and kynurenine-3-monooxygenase that catabolizes KYN (Guillemin et al., 2003), inducible NO synthase (iNOS) (Possel et al., 2000, Lu et al., 2012), reactive oxygen and nitrogen species (ROS/RNS) (Qin et al., 2004, Block et al., 2007), and MCP-1/CCL2 (Kim et al., 2011), a chemokine involved in attracting peripheral immune cells into the brain (see Figure 1)(D'Mello et al., 2009).
2.5 Exogenous cytokine administration and depression
Further evidence that inflammatory cytokines can cause behavioral alterations exists in the numerous reports of neuropsychiatric symptoms induced by chronic administration of cytokines, such as the antiviral and antiproliferative cytokine, interferon (IFN)-alpha, as treatment for certain cancers and viral infections (Capuron and Miller, 2004, Loftis and Hauser, 2004). IFN-alpha is notorious for producing behavioral symptoms including fatigue and neurovegetative symptoms, cognitive and sleep changes, anxiety, and anhedonia, and induces depression in 30–50% of treated-patients depending on the dose (Capuron et al., 2002, Maddock et al., 2005, Raison et al., 2005a, Lotrich, 2009). IFN-alpha-induced depression is very similar to major depression, and has been frequently used as a paradigm to investigate the pathophysiology and treatment of cytokine-induced behavioral changes (Capuron and Miller, 2004, Capuron et al., 2009).
In terms of immunologic mechanisms of IFN-alpha-induced depression, IFN-alpha may act directly on the central nervous system (CNS), but may also exert indirect effects via the activation of other peripheral and central inflammatory cytokines (Raison et al., 2009). Data from rodents and monkeys suggest that CNS penetration of peripherally administered IFN-alpha is low (Collins et al., 1985, Greig et al., 1988). Nevertheless, acute IFN-alpha administration to humans potently induces IL-6, while mildly stimulating IL-1 and TNF-alpha in the periphery (Sissolak et al., 1992, Shimizu et al., 1995, Cassidy et al., 2002, Capuron et al., 2003b), and increases IL-6 and monocyte chemotactic protein-1 (MCP-1/CCL2) in the CNS (Raison et al., 2009). Many pathways by which IFN-alpha and these other inflammatory cytokines induced by IFN-alpha may affect brain neurotransmitter systems to induce depressive behaviors have been investigated and will be discussed in section 3.
Because patients develop depression primarily during the first few months of IFN-alpha treatment, this clinical model has been useful in prospectively examining various risk factors for developing depression. As one example, evidence of mild pre-existing inflammatory activity may signal vulnerability to IFN-alpha-induced depression. For instance, mild stressors can increase IL-6 levels (Rohleder and Miller, 2008), and as noted above IL-6 can become further elevated during IFN-alpha therapy (Bonaccorso et al., 2001, Wichers et al., 2007). To examine, which comes first, time-lagged hierarchical regression has been employed – which provides evidence for a feed-forward relationship during IFN-alpha therapy -- in which slightly elevated IL-6 predicts increased depression one month later -- which then predicts subsequent increases in IL-6, and vice versa until major depression is manifest (Prather et al., 2009). In those resilient subjects who don’t develop depression, this feed-forward cycle does not exist and IL-6 does not increase (Prather et al., 2009). This type of finding highlights the advantages of studying depression developing prospectively.
2.6 Genetic vulnerability to exogenous cytokine-induced depression
Several functional gene polymorphisms have been variously associated with vulnerability to depression during IFN-alpha therapy or with certain symptom dimensions of this cytokine-induced depression (see Table 1 for details). A unique possibility has been raised by these findings that specific polymorphisms may be associated with risk for specific sets of symptoms. It has long been known in humans (Foley et al., 2003, Jang et al., 2004) and mice (Henderson et al., 2004) that distinct chromosomal regions are specifically associated with distinct mood and anxiety traits. Therefore, identifying genes that confer susceptibility to or protection from certain aspects of cytokine-induced depression may provide insight into the potential mechanisms of cytokine effects on behavior and novel treatment strategies.
Table 1.
Functional gene polymorphisms that have been examined in inflammatory cytokine-associated depression. All subjects received pegylated IFN-alfa-2a (PEGAYS) or IFN-alfa-2b (PEGINTRON) plus ribavirin for chronic hepatitis C virus (HCV) infection.
| Gene symbol |
Gene name | Study | Polymorphism/ SNP |
Subjects and design | Findings |
|---|---|---|---|---|---|
| Immune-related genes | |||||
| CCL2 | Chemokine (C- C motif) ligand 2/monocyte chemotactic protein-1 |
Smith et al., 2012 | rs1024611, rs2530797, rs4586 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| COX2 | Cyclooxygena se 2 |
Su et al., 2010 | rs4648308, rs2745557, rs689466 |
N=132, depression monitored by M.I.N.I., BDI and HDRS for 24 wk |
The rs4648308 A/G “at risk”genotype was associated with greater risk of depression and lower DHA levels during IFN-a treatment. |
| IDO1 | Indoleamine- 2,3- dioxygenase 1 |
Smith et al., 2012 | rs3739319, rs6991530, rs7820268. rs9657182 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
The rs9657182 (C/T) promoter polymorphism risk allele (CC) was associated with moderate or severe depression in Caucasians but not African Americans, who exhibited a low frequency of the risk allele. |
| Galvao-de Almeida et al., 2011 | rs3824259, rs10089084 |
N=277, depression monitored by M.I.N.I. 1 month after end of treatment |
No significant association with cytokine-induced depressive symptoms. |
||
| IFNAR1 | Interferon- alpha/beta receptor 1 |
Smith et al., 2012 | rs1012334, rs16997869, rs2843710 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| Yoshida et al., 2005 | −408C/T, −3C/T, and GT repeat dinucleotide microsatellite |
N=50, depression assessed by SDS at baseline and at 4, 8, 12 and 24 wk treatment |
The 5/14 genotype of the GT repeat dinucleotide microsatellite polymorphism was associated with a greater increase in neurovegetative/ somatic symptoms. |
||
| IFNG | Interferon- gamma |
Oxenkrug et al., 2011 | +874T/A promoter region |
N=204, retrospective examination of depression monitored by SCID |
Carriers of at least one T allele had higher rates of cytokine-induced depression. |
| IL1A | Interleukin-1 alpha |
Smith et al., 2012 | rs3783516, rs3783546 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| IL1B | Smith et al., 2012 | rs1143643, rs1143644, rs16944, rs4848306 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
|
| IL6 | Interleukin 6 | Bull et al., 2009 | rs1800795, rs1800796, rs1800797 |
N=98, depression monitored by BDI or SDS for 24 wk |
The ‘low IL-6’ synthesizing genotype (CC) was associated with significantly fewer symptoms of cytokine-induced depression. |
| Smith et al., 2012 | rs1800795 rs1800796 rs1800797 1 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
||
| Su et al., 2010 | rs1800795 (−174G/C) |
N=132, depression monitored by M.I.N.I., BDI, and HDRS for 24 wk |
There were only IL-6 G/G subjects in this Chinese sample and risk could not be assessed. |
||
| IL28B | Interleukin 28B | Lotrich et al., 2010 | rs1297860 | N=133, depressive symptoms monitored by BDI and SCID-1 for 48 wk |
The T/T genotype of the IL28B polymorphism was associated with less sustained viral response, and with less appetite, energy, and sleep complaints during IFN-a treatment. |
| TNF | Tumor necrosis factor-alpha |
Lotrich et al., 2010 | rs1800629 | N=105, depression and aggression were monitored by BDI and AIAG, respectively, for 16 wk treatment |
The A allele in A- 308G polymorphism (rs1800629), was associated with labile anger and fatigue, but not with depression. |
| Smith et al., 2012 | rs1799964, rs1800629, rs3093662, rs769178 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
||
| Neurotransmitter-related genes | |||||
| COMT | catechol-O- methyltransferase |
Smith et al., 2012 | rs165599, rs4680 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| DBH | Dopamine beta- hydroxylase |
Smith et al., 2012 | rs1611115, rs2519152, rs6271 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| HTR1A | Serotonin receptor 1A |
Kraus et al., 2007 | −1019A/G | N=139, depression monitored by HADS |
Homozygosity for the HTR1A-1019G variant significantly increased both incidence and severity of interferon- induced depression. Maximum increases in HADS depression scores during antiviral therapy correlated with HTR1A variation. |
| SLC6A3 | Dopamine transporter |
Smith et al., 2012 | rs2652511, rs2937639 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| SLC6A4 | Serotonin transporter |
Bull et al., 2009 | 5-HTTLPR (L/S) | N=98, depression and fatigue monitored at 4, 8, 12, 24 wk by BDI, SDS, CFQ |
The L/L genotype was associated with fewer depressive symptoms, and this ‘protective’ effect was evident only in the presence of the ‘low expression IL-6’ genotype. |
| Kraus et al., 2007 | 5-HTTLPR (L/S) | N=139, depression monitored by HADS |
No significant association with cytokine-induced depressive symptoms. |
||
| Lotrich et al., 2009 | 5-HTTLPR (LG, LA, and S) |
N=71, depression and related symptoms monitored by SCID, BDI, PSQI, NEO-Five Factor Inventory, CIRS-G at weeks 2, 4, 8, 12, and 16 wk treatment |
The LA allele was associated with a decreased rate of depression, with the LA/LA genotype being the most resilient. This genotype was also associated with better sleep quality. |
||
| Pierucci-Lagha et al., 2010 | 5-HTTLPR (L/S) | N=1,015, depression monitored by CIDI and BDI-II for 24 or 48 wk |
The L allele was associated with higher depression scores in non- Hispanic Caucasians and S allele associated with depression scores in Hispanic Caucasians. |
||
| Su et al., 2010 | 5-HTTLPR (L/S) | N=132, depression monitored by M.I.N.I., BDI and HDRS for 24 wk |
No significant association with cytokine-induced depressive symptoms, however there were only IL-6 G/G subjects in this Chinese sample and the effects of the “at risk” allele on the 5- HTTLPR could not be assessed. |
||
| TPH2 | Tryptophan hydoxylase-2 |
Kraus et al., 2007 | −703G/T transcriptional control region |
N=139, depression monitored by HADS |
No significant association with cytokine-induced depressive symptoms. |
| Neuropeptide and growth factor-related genes | |||||
| BDNF | Lotrich et al., 2013 | rs6265 (Val66Met) |
|||
| CRH1 | Corticotropin releasing hormone receptor 1 |
Smith et al., 2012 | rs110402, rs242924, rs7209436 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk D |
Significant association of rs7209436 with moderate to severe depression at 12 weeks that was N.S. after Bonferroni correction for multiple comparisons. |
| CRH | Corticotropin releasing hormone |
Smith et al., 2012 | rs11997416, rs12721510, rs1870390 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| Other genes | |||||
| APOE | apolipoprotein E | Gochee et al., 2004 | epsilon 4 allele | N=110, retrospective investigation of psychiatric referral and neuropsychiatric symptoms experienced during treatment |
Subjects with the epsilon4 allele were more likely to experience neuropsychiatric symptoms |
| FKBP5 | FK506 binding protein 5 |
Smith et al., 2012 | rs1360780, rs3800373 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
No significant association with cytokine-induced depressive symptoms. |
| PLA2G4A | Phospholipase A2 | Su et al., 2010 | rs10798059 (BanI), rs4651330, rs3736741 |
N=132, BDI and HDRS levels for 24 wk |
The BanI G/G or “at risk” genotype was associated with greater risk of depression and with lower EPA levels during IFN-a treatment. |
| POMC | Pro- opiomelanocortin |
Smith et al., 2012 | rs1009388, rs1042571, rs1866146 |
Caucasians (n=800) +African Americans (n=232), depression monitored by the 20 item CES-D rating scale for 48 wk |
Significant association of rs1866146 with moderate to severe depression at 12 weeks that was N.S. after Bonferroni correction for multiple comparisons. |
Abbreviations: AIAG= anger irritability and assault questionnaire, BDI= Beck Depression Inventory, CES-D= Center for Epidemiological Studies-Depression Scale, CFQ= Chalder fatigue questionnaire, CIDI= Composite International Diagnostic Interview, CIRS-G= Cumulative Illness Rating Scale Geriatric, HADS= Hospital Anxiety and Depression Scale, HDRS= Hamilton Depression Rating Scale, M.I.N.I= Mini International Psychiatric Interview for DSM-IV Axis I Disorders, PEG= pegylated, PSQI= Pittsburgh Sleep Quality Index, SCID= Structured Clinical Interview for DSM-IV Axis I Disorders, SDS= Zung self-rating depression scale.
Consistent with the relationship between circulating IL-6 and IFN-alpha-induced depression, a functional polymorphisms in the promoter region of the IL-6 gene (rs1800795), the genotype that produces more IL-6, was found to be associated with depression but not the fatigue that worsens during IFN-alpha therapy (Bull et al., 2008). We have similarly observed that a promoter TNF-alpha polymorphism (A-308G rs1800629) was associated with vulnerability for developing irritable anger but not depression (Lotrich et al., 2010a), and an IL-28B polymorphism (rs1297860) was associated with poor appetite, sleep, and fatigue, but not depression-specific symptoms (Lotrich et al., 2010b).
We and others also initially reported that a serotonin reuptake promoter polymorphism (5-HTTLPR) was associated with increased depression scores during IFN-alpha therapy (Bull et al., 2008, Lotrich et al., 2009), but another group did not find an association using the Hospital Anxiety Depression Scale (HADS) (Kraus et al., 2007). We have subsequently replicated the lack of association with the HADS, and observed that this was the result of the 5-HTTLPR being associated primarily with neurovegetative symptoms and not the more psychological symptoms that are the focus of the HADS (Lotrich et al., 2013). Similarly, a GT repeat dinucleotide microsatellite of the IFN-alpha receptor (IFN-AR) gene was found to be primarily related to neurovegetative and somatic symptoms (Yoshida et al., 2005). Conversely, a polymorphism for brain derived neurotrophic factor (a BNDF Val/Met allele) was not associated with vulnerability for the neurovegetative symptoms but was better associated with the more psychological symptoms of depression (Lotrich et al., 2013).
Interestingly, selective serotonin (5-HT) reuptake inhibitors (SSRIs) have been shown to alleviate cytokine-induced depression (Musselman et al., 2001a, Raison et al., 2007), and depressive and anxiety symptoms appear to be most responsive, while fatigue and neurovegetative symptoms are less responsive (Capuron et al., 2002, Raison et al., 2005b, McNutt et al., 2012). These data are consistent with findings in patients with advanced cancer undergoing chemotherapy who also exhibit increased inflammation in association with fatigue that is not responsive to SSRIs (Bower et al., 2002, Morrow et al., 2003, Miller et al., 2008). In addition, fatigue is one of the primary residual symptoms in SSRI-treated medically healthy depressed patients, who, as noted above, have been shown to exhibit evidence of increased inflammation (Nierenberg et al., 2010, Targum and Fava, 2011). Therefore, differential responsiveness of symptom dimensions coupled with the association of specific gene loci with behavioral symptoms suggests that distinct mechanisms may mediate the various aspects of cytokine-induced depression, and understanding these mechanisms may improve treatment strategies.
In terms of immunologic mechanisms, an inflammatory pathway that may be involved in inflammatory cytokine-associated depression is the metabolism of polyunsaturated fatty acids involved in prostaglandin and resolvin synthesis. Omega-6 and omega-3 fatty acids have opposing effects on inflammatory signaling. The omega-6 fatty acid, arachidonic acid, is metabolized to form PGE2, while omega-3 fatty acids have anti-inflammatory properties and are metabolized to form ‘resolvins’ and ‘protectins’. These can influence cytokine synthesis and resolve inflammation (Hong et al., 2003, Serhan, 2010). As one example, docosahexaenoic acid (DHA) can be converted to ‘neuroprotectin’ which has potent inflammatory resolving activities in the brain (Lalancette-Hebert et al., 2011). Resolvin receptors are newly being discovered and potentially include formyl peptide receptor 2 (FPR2) (Dufton et al., 2010). Conversely, arachidonic acid is both an intracellular second messenger and is also a substrate for the synthesis of prostacyclins and thromboxanes, which can stimulate inflammatory activity (Ramwell et al., 1980). Interestingly, omega-3 fatty acids are reduced in major depression and also predict vulnerability to cytokine-induced depression (Su et al., 2010, Lotrich et al., 2012, McNamara and Lotrich, 2012). Su et al. (2012) examined functional polymorphisms of the phospholipase A2 (PLA2) and the cyclooxygenase 2 (COX2) genes, two key enzymes in the metabolism of polyunsaturated fatty acid and production of PGE2, and found that decreased omeg-3 fatty acids were dependent on the presence of the PLA2 BanI GG or COX2 (rs4648308) AG “risk” genotypes that conferred a higher risk of IFN-alpha-induced depression.
Another inflammatory pathway by which cytokines may mediate behavioral effects is by activation of IDO (Pemberton et al., 1997, Fujigaki et al., 2006), an enzyme expressed in multiple cell types, including macrophages, dendritic cells, microglia, astrocytes, and neurons (Guillemin et al., 2005c, Huang et al., 2010). IDO catabolizes tryptophan, the primary amino-acid precursor of serotonin (5-HT), into kynurenine (KYN). KYN is then further broken down in the CNS into the neuroactive metabolites kynurenic acid (KA), by KYN aminotransferase in astrocytes, or to quinolinic acid (QUIN), by kynurenine-3-monooxygenase, in microglia (Heyes et al., 1992) (see Figure 1). These metabolites can then interact with neurotransmitter systems in the brain, particularly glutamate (see Section 3.3), and are thought to be involved in the behavioral effects of cytokines (Dantzer et al., 2011, Haroon et al., 2012). Interestingly, a polymorphism (rs9657182) in the promoter region of the gene encoding IDO1 was found to be associated with IFN-alpha-induced depression in Caucasian subjects homozygous for the C risk allele, but not African Americans who exhibited a markedly lower frequency of the risk allele at this locus (Smith et al., 2012)(see Table 1 for details). No association between IDO SNPs (rs3824259; rs10089084 and rs35099072) and cytokine-induced depression was observed in a study enrolling HCV+ Brazilian subjects (Galvao-de Almeida et al., 2011).
Finally, higher rates of depression have also been observed in carriers of at least one T allele of the IFN-gamma +874T/A promoter polymorphic region in HCV+ Caucasian subjects receiving IFN-alpha treatment (Oxenkrug et al., 2011), and the epsilon4 allele of the apolipoprotein E gene has also been reported to confer increased susceptibility to neuropsychiatric symptoms reported during IFN-alpha treatment (Gochee et al., 2004). However, it is worthy to note that one study observed no association between the development of IFN-alpha-induced depression and polymorphic regions examined in the genes encoding TNF (rs1799964, rs1800629, rs3093662, rs769178), IL6 (rs1800795, rs1800796, rs1800797), IL1A (rs3783516, rs3783546), IL1B (rs1143643, rs1143644, rs16944, rs4848306), chemokine MCP-1/CCL2 (rs1024611, rs2530797,rs4586), or IFN-AR1 (rs1012334, rs16997869, rs2843710) (Smith et al., 2012).
3. Cytokine effects on neurotransmitter systems that may contribute to depression
Major depression likely involves complex interactions between genes and the environment that converge in the brain (Caspi et al., 2003, Kaufman et al., 2006, Lotrich, 2011). Numerous pathophysiologic mechanisms have been identified -- including dysfunction of monoaminergic systems and the hypothalamic-pituitary adrenal (HPA)-axis, changes in growth factors and neuropeptides, alterations in glutamate neurotransmission, and decreased neurogenesis (Heim et al., 1997, Charney, 1998, Berman et al., 2000, Pariante and Miller, 2001, Duman and Monteggia, 2006). Interestingly, all of these pathways have been demonstrated to be affected by inflammatory cytokines (Pace et al., 2007, Dantzer et al., 2008, Miller et al., 2009, Haroon et al., 2012) (Figure 1).
3.1 Cytokine effects on the monoamines
3.1.1 Serotonin (5-HT)
The 5-HT system is undoubtedly one of the most studied neurotransmitter systems in depression, and SSRIs are the most widely prescribed antidepressant medication (Masand and Gupta, 1999, Vaswani et al., 2003). Many aspects of the 5-HT system are altered in major depression, including changes in 5-HT turnover, and receptor and transporter binding (Perry et al., 1983, Arango et al., 1995, Malison et al., 1998, Mann et al., 2000, Willeit et al., 2000, Arango et al., 2001). In animal studies employing acute administration of inflammatory cytokines or immune activation with lipopolysaccharide (LPS), 5-HT and other monoamines are released in the hypothalamus to mediate fever and early behavioral changes associated with sickness behavior (Dunn et al., 2005, O'Connor et al., 2008, 2009b). Administration of inflammatory cytokines also acutely increase 5-HT turnover, as determined by increased 5 hydroxyindoleacetic acid (5-HIAA) or 5HIAA/5-HT ratios, in brain regions such as the cortex and nucleus accumbens (Song et al., 1999, De La Garza and Asnis, 2003), and these changes in 5-HT turnover occur in concert with the appearance of later, more persistent depressive-like behaviors (Frenois et al., 2007, O'Connor et al., 2009b). In patients treated chronically with IFN-alpha for hepatitis C virus (HCV), CSF concentrations of IL-6 negatively correlated with 5-HIAA concentrations, which in turn negatively correlated with IFN-alpha-induced depression severity (Raison et al., 2009). Lower plasma concentrations of 5-HT and higher circulating TNF-alpha at baseline have also been associated with somatic symptoms of depression during IFN-alpha treatment (Loftis et al., 2013).
Inflammatory cytokine administration and low-grade chronic inflammation have been shown to increase IDO activity and the metabolisms of tryptophan, the primary amino-acid precursor of serotonin, to KYN in the periphery (Capuron et al., 2003a, Raison et al., 2010b, Capuron et al., 2011a). In the brain, IDO activity is significantly increased at 24 hr and peaks at 48 hr in response to LPS administration, corresponding to the expression of some depressive-like behaviors, e.g. increased immobility in the forced-swim and tail suspension tests, in laboratory animals (Lestage et al., 2002, O'Connor et al., 2008). Therefore, IDO-induced decreases in 5-HT synthesis in the brain are thought to mediate cytokine-induced depressive symptoms. Indeed, correlations between IFN-alpha–induced depression, decreased plasma tryptophan, and increased KYN and/or the KYN/tryptophan ratio, have been reported (Bonaccorso et al., 2002, Capuron et al., 2003a). However, in a subsequent study examining KYN pathway metabolites and tryptophan in both the CSF and periphery of IFN-alpha-treated patients, increased CSF KYN and QUIN concentrations were observed that correlated with depressive symptoms and with CSF cytokines, yet tryptophan concentrations were not decreased in the CSF despite decreased blood tryptophan concentrations (Raison et al., 2010b). These findings are consistent with recent work in rodents indicating that KYN administration alone was sufficient to induce depressive-like behavior, and pharmacological inhibition of IDO with 1-methyl-tryptophan prevented LPS-induced depressive-like behavior but did not prevent changes in 5-HT turnover (O'Connor et al., 2008, O'Connor et al., 2009a, Salazar et al., 2012). These finding suggest that neuroactive KYN metabolites, KA and QUIN, are likely responsible for many behavioral changes associated with cytokine-induced IDO activity, and may involve effects on other neurotransmitter systems (see Section 3.3 for discussion).
Although alterations in 5-HT metabolism may not be the primary mediator of many behavioral symptoms resulting from cytokine-induced IDO activity (Dantzer et al., 2011, Maes et al., 2011), the 5-HT transporter (5-HTT) may serve as a biological substrate by which cytokines can affect 5-HT neurotransmission and subsequently behavior. The 5-HTT may be important in vulnerability to and treatment of depression, as the “short” (S) low-expression allele in the promoter region of the serotonin transporter (5-HTTLPR) has been shown to moderate the influence of stress on the development of depression (Caspi et al., 2003, Karg et al., 2011) and to predict responsiveness to SSRIs (Smeraldi et al., 1998, Lotrich et al., 2008, Porcelli et al., 2012). As discussed in section 2.6, the 5-HTTLPR and other functional polymorphisms related to 5-HT neurotransmission have been investigated in IFN-alpha-induced depression (Kraus et al., 2007, Bull et al., 2008, Lotrich et al., 2009) (see Table 1 for summary). Kraus et al. (2007) did not report a relationship between the 5-HTTLPR or functional variations in tryptophan hydroxylase 2 and cytokine-induced depression using the Hospital Anxiety and Depression Scale (HADS), but did observe a relationship with a 5-HT 1A receptor polymorphism. Relatedly, IFN-alpha administration has been shown to increase expression of 5-HT1A receptors in the brain (Abe et al., 1999). In contrast to the findings of Kraus et al. (2007), Lotrich et al. (2009) and Bull et al. (2009) did observe that the S allele of the 5-HTTLPR conferred vulnerability to cytokine-induced depression as defined by the Beck Depression Inventory and/or Zung Self-Rating Depression Rating Scale, respectively. Furthermore, the relationship between the 5-HTTLPR and IFN-alpha-induced depression was observed to depend on a functional polymorphism of the IL-6 promoter (Bull et al., 2008) (as mentioned in Section 2.2), indicating a potential relationship between increased production of other inflammatory cytokines, such as IL-6, and 5-HTT function in IFN-alpha-induced depression.
Interestingly, inflammatory cytokines, including IL-6, have been shown to increase 5-HTT expression and function (Morikawa et al., 1998, Mossner et al., 1998, Sakai et al., 2003, Tsao et al., 2008) (see Figure 1), an effect found to be mediated by induction of p38 mitogen-activated protein kinase (MAPK), both in vitro and in vivo (Zhu et al., 2005, Zhu et al., 2006, Zhu et al., 2010a). Increased p38 MAPK activation (phosphorylation) in lymphocytes following the initial injection of IFN-alpha has predicted IFN-alpha-induced depression and fatigue in HCV+ patients (Felger et al., 2011). Furthermore, p38 MAPK activation in peripheral blood monocytes have been shown to be related to decreased CSF concentrations of 5-HIAA in rhesus monkeys exposed to early maternal neglect and abuse (Sanchez et al., 2007). Interestingly, acute administration of cytokines, including IFN-gamma, IL-1beta, TNF-alpha (Clement et al., 1997) and IL-6 (Zhang et al., 2001), increase 5-HT release in several brain regions, effects that could be mediated by increased 5-HTT activity in addition to the previously mentioned cytokine-induced changes in 5-HT metabolism. Together these data indicate that inflammatory cytokines and their signal transduction pathways can increase expression and activity of the 5-HTT, and may interact with genetic vulnerability (S allele of the 5-HTTLPR, 5-HT receptors) to influence 5-HT neurotransmission and the development of depressive symptoms.
In terms of the role of 5-HT in the treatment of cytokine-induced depression, SSRIs have been very effective in treating anxiety, depressed mood, and cognitive aspects of cytokine-induce depression, but not as effective for fatigue and neurovegetative symptoms (Capuron et al., 2002, Raison et al., 2005b, McNutt et al., 2012). As noted in section 2.5, these symptoms are often residual symptoms in medically healthy patients that are treated with SSRIs for major depression (Nierenberg et al., 2010, Targum and Fava, 2011). Although genetic variability, such as with the 5-HTTLPR, may account for some differences in response to SSRIs (Smeraldi et al., 1998, Lotrich et al., 2008, Porcelli et al., 2012), it is interesting to consider that some symptom dimensions of depression, and particularly cytokine-induced depression, may correspond to alterations in different neurociruitry and neurotransmitter systems.
3.1.2 Dopamine (DA)
The fatigue of depression, which is often a residual symptom of SSRI therapy, is a prominent feature of cytokine-induced depression, and may represent cytokine effects on the basal ganglia and dopamine (DA) function (Capuron et al., 2001, Majer et al., 2008, Capuron et al., 2009). Alterations in basal ganglia activity have been observed in patients with both idiopathic major depression and IFN-induced depression (Epstein et al., 2006, Capuron et al., 2007, Furman et al., 2011, Capuron et al., 2012), and changes in DA synthesis, release and/or receptor signaling have been proposed as potential mechanisms that may contribute to anhedonic and psychomotor symptoms (Willner, 1983, Dunlop and Nemeroff, 2007, Stein, 2008, Felger and Miller, 2012).
Early evidence that IFN-alpha may affect DA neurotransmission comes from studies in rodents that reported both increases and decreases in brain dopamine and/or metabolites that either did or did not correspond to locomotor changes or depressive-like behavior following acute or sub-chronic IFN-alpha administration (Shuto et al., 1997, Kamata et al., 2000, Kumai et al., 2000, Kitagami et al., 2003, Sato et al., 2006). These mixed results are likely due to differences in dosing, length of cytokine exposure, and most importantly, the fact that species-specific cytokines were variably used and rodents do not respond to human IFN-alpha with activation of classic type I IFNR signaling (Loftis et al., 2006a, Loftis et al., 2006b, Wang et al., 2008) Rhesus monkeys that express functional IFNARs and activate relevant signal transduction pathways in response to human IFN-alpha (Felger et al., 2007), exhibit immune, neuroendocrine, and behavioral responses to IFN-alpha similar to humans, including decreases in psychomotor activity and increases in depressive-like huddling behavior (in ~50% of animals) (Felger et al., 2007, Felger and Miller, 2012). Of note, huddling behavior in non-human primates was first described following chronic administration of the monoamine depleting drug, reserpine (McKinney et al., 1971), and has been reported in monkeys treated with dopamine receptor antagonists and partial agonists (Rosenzweig-Lipson et al., 1994). Monkeys that display huddling behavior in response to IFN-alpha have been found to exhibit significantly lower CSF concentrations of the dopamine metabolites, homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) (Felger et al., 2007, Felger and Miller, 2012), which correlated with reduced locomotor activity (Felger and Miller, 2012). Similar correlations have been observed between reduced CSF HVA and physical fatigue in IFN-alpha-treated humans (Felger and Miller, 2012). These findings supported a role for DA function in fatigue and psychomotor slowing associated with inflammatory cytokines, and prompted further investigation into inflammatory cytokine effects on DA function.
The most substantial evidence that inflammatory cytokines affect DA and DA-relevant neurocircuitry comes from neuoroimaging studies in humans examining basal ganglia activity after administration of IFN-alpha or immune activating stimuli (e.g. LPS or typhoid vaccination) (Capuron et al., 2007, Brydon et al., 2008, Harrison et al., 2009b, Eisenberger et al., 2010, Capuron et al., 2012). Indeed, positron emission tomography (PET) in interferon (IFN)-alpha-treated patients revealed increased glucose metabolism in the basal ganglia, similar to Parkinson’ s disease (Eidelberg et al., 1994, Spetsieris et al., 1995, Mentis et al., 2002), where it is believed to reflect increased oscillatory burst activity in relevant basal ganglia nuclei secondary to the loss of inhibitory nigral DA input (Wichmann and DeLong, 1999, 2003). IFN-alpha-induced changes in basal ganglia glucose metabolism were in turn correlated with symptoms of fatigue (Capuron et al., 2007). Functional magnetic resonance imaging (fMRI) has also demonstrated decreased neural activation to a hedonic reward task during IFN-alpha administration that correlated with reduced motivation (Capuron et al., 2012). Similarly, lipopolysaccharide and typhoid vaccination have been shown to have effects on basal ganglia activity (Brydon et al., 2008, Harrison et al., 2009a, Eisenberger et al., 2010), including decreased ventral striatal activation to a reward task that was associated with increased depressive symptoms (Eisenberger et al., 2010), and increased nigral activity that correlated with psychomotor slowing (Brydon et al., 2008). Interestingly, a similar hypermetabolism of the basal ganglia has been observed in early HIV infection (Rottenberg et al., 1996), and decreased ventral striatal activation to reward has been observed in major depression (Epstein et al., 2006). Taken together, these data suggest that peripheral cytokine effects on basal ganglia function generalize to multiple inflammatory stimuli, and may be relevant to depression and specifically to symptoms of fatigue and psychomotor slowing.
To probe the DAergic mechanisms of IFN-alpha effects on neural activity in the basal ganglia, a PET study was conducted using [18F]fluorodopa (FDOPA) in HCV+, IFN-alpha-treated subjects (Capuron et al., 2012). FDOPA, like L-3,4-dihydroxyphenylalanine (L-DOPA), is taken up by DAergic neurons and converted by DOPA decarboxylase to DA, whereupon it is stored in vesicles for release. Interestingly, both increased uptake and decreased turnover of FDOPA in the caudate, putamen and ventral striatum of IFN-alpha-treated patients was found (Capuron et al., 2012). Baseline and percent change in FDOPA uptake and turnover were, in turn, correlated with IFN-alpha-induced depression and fatigue scores. Increased uptake of FDOPA in the basal ganglia following IFN-alpha administration is in stark contrast to that observed in Parkinson’s disease where decreased FDOPA uptake is representative of a loss of DAergic neurons and/or their projections throughout the basal ganglia (Leenders et al., 1986, Kaasinen et al., 2001, Kumakura and Cumming, 2009). Increased uptake and decreased turnover of FDOPA suggests a potential depletion of DA in DAergic terminals and increased synthetic capacity following IFN-alpha exposure. These findings support the idea that peripheral inflammatory cytokines produce alterations in basal ganglia function, possibly reflective of decreased DA availability, that in turn contribute to the development of depressive symptoms.
Numerous mechanisms exist by which cytokines and inflammatory signaling pathways may affect DA neurotransmission (see Felger and Miller, 2012 for review), and particularly DA synthesis. Dopamine synthesis relies on the conversion of tyrosine (Tyr) to L-DOPA by Tyr hydroxylase, the rate-limiting enzyme for dopamine synthesis. A major source of Tyr is phenylalanine (Phen), which is converted to Tyr by Phen hydroxylase (PAH). Both of these enzymes, Tyr hydroxylase and PAH, require tetrahydrobiopterin (BH4) as an essential enzyme co-factor. BH4 is also a co-factor for NO synthases (NOSs), which convert arginine to NO (Cunnington and Channon, 2010). Additionally, BH4 is highly redox-sensitive and is reversibly oxidized to dihydrobiopterin (BH2), or irreversibly to dihydroxanthopterin (Dumitrescu et al., 2007, Cunnington and Channon, 2010, Haroon et al., 2012). Although inflammatory cytokines have been shown to induce GTP-cyclohydrolase I, the enzyme necessary for BH4 synthesis, inflammation-mediated. increases in ROS/RNS can oxidize BH4, whereas increased inducible NOS (iNOS) activity can usurp BH4 (see Figure 1), thus reducing it’s availability for DA synthesis (Cunnington and Channon, 2010). Decreased PAH activity due to low BH4 availability results in increased Phen concentrations with respect to Tyr, and these amino acids, as well as BH4 and BH2, can be measured in the blood and CSF as indirect biomarkers of dopamine synthetic capacity (Candito et al., 1994, Hashimoto et al., 2004, Neurauter et al., 2008a, Neurauter et al., 2008b, Capuron et al., 2011b, Felger et al., 2012b).
Peripheral blood Phen concentrations and Phen/Tyr ratio have been shown to correlate with inflammatory mediators (e.g. IL-6, IL-2 receptor, and soluble TNF-alpha receptor-2) and markers of oxidative stress in patient populations with medical illnesses such as sepsis, cancer, and HIV that exhibit increased inflammation (Neurauter et al., 2008a, Neurauter et al., 2008b). Furthermore, in a recent study of healthy elderly persons with low-grade inflammation, peripheral blood concentrations of Phen, Tyr, and an increased Phen/Tyr ratio were associated with neuropsychiatric symptoms including anhedonia and altered sleep (Capuron et al., 2011b). Plasma Phen/Tyr ratios were increased in IFN-alpha-treated subjects compared to controls (Felger et al., 2012b, Zoller et al., 2012), and negatively correlated with CSF concentrations of DA and HVA, and positively correlated with fatigue, in IFN-alpha-treated patients (Felger et al., 2012b). Furthermore, CSF concentrations of BH2 were significantly increased in IFN-alpha-treated patients compared to controls, and decreased CSF BH4 concentrations correlated with increased CSF IL-6 (Felger et al., 2012b). These results indicate that inflammation is associated with decreased peripheral conversion of Phen to Tyr, which may in turn be associated with reduced DA in the brain as well as behavioral symptoms such as anhedonia and fatigue. These alterations may be related to oxidation of BH4 secondary cytokine-induced activation of CNS inflammatory responses.
Although much of the interest in cytokine effects on monoamine transporters has been focused on the 5-HTT (Zhu et al., 2005, Zhu et al., 2006, Zhu et al., 2010a) (see Section 3.1.1 and Figure 1), where both in vitro and in vivo data have established that stimulation of p38 MAPK, a major signaling pathway activated by IFNAR1 and other cytokines, can increase the expression and function of the 5-HTT. However, MAPK pathways have also been shown to influence the dopamine transporter in vitro (Moron et al., 2003). Furthermore, increased expression of the DAT has been observed in subjects with neuropsychiatric disturbances as a result of HIV infection (Gelman et al., 2006, Ferris et al., 2008). It is also worthy to note that there is some evidence that inflammatory cytokines may negatively affect the expression and function of the vesicular monoamine transporter 2 (VMAT2) that packages monoamines into vesicles for release (see Figure 1) (Kazumori et al., 2004, Felger and Miller, 2012). Normal vesicular sequestration and release is particularly important in DAergic cells due to the risk of auto-oxidation of DA and the formation of free radicals and neurotoxic quinones (Cadet and Brannock, 1998, LaVoie and Hastings, 1999, Guillot and Miller, 2009). Decreased VMAT2 function and increased cytosolic DA, such as with methamphetamine exposure, can lead to DA auto-oxidation and increased ROS formation (Riddle et al., 2006, Guillot and Miller, 2009). Therefore, decreased expression or function of the VMAT2 by inflammatory cytokines could not only affect the amount of synaptic DA released, but may also contribute to ROS formation and reduced availability of BH4, which is needed for DA synthesis.
In terms of genetic vulnerability, functional polymorphisms in genes involved in DA neurotransmission, e.g. the DA receptor 2 (DR2), DR4, DAT, and catechol-O-methyltransferase (COMT) polymorphisms, have been associated with neuropsychiatric disorders including depression (Manki et al., 1996, Haeffel et al., 2008, Wang et al., 2012). Of these gene polymorphisms, only one study has examined SNPs in the COMT and DAT genes in cytokine-induced depression (Smith et al., 2012), and no association with IFN-alpha-induced depressive symptoms were observed (see Table 1). Nonetheless, the growing evidence that cytokines affect the basal ganglia and DA function warrants further investigation into genetic vulnerability of the DA system that may contribute to cytokine-induced depression. However, environmental factors may be particularly relevant to effects of inflammatory cytokines on brain DA. The predisposition of DAergic neurons to oxidative stress make the DA system increasingly sensitive to agents such as pesticides and drugs of abuse, which are thought to decrease DA packaging and increase intraneuronal DA content (Guillot and Miller, 2009). Moreover, microglial activation and inflammatory cytokines are associated with worse outcome following exposure to these compounds (Gao et al., 2002, Sriram et al., 2006).
3.1.3 Norepinephrine (NE)
Increased concentrations of NE and the NE metabolite, 3-Methoxy-4-hydroxyphenylglycol (MHPG), have been reported in the CSF of patients with major depression (Roy et al., 1986, Gudmundsson et al., 2007). In addition to 5-HT, NE is targeted by tricyclic antidepressant drugs and 5-HT-NE reuptake inhibitors (SNRIs) (Williams et al., 2000). Most information regarding the inflammatory cytokine effects on NE come from studies administering cytokines or LPS acutely to laboratory animals (Dunn et al., 1999, Dunn et al., 2005). Acutely, cytokines and immune activation increase locus coeruleus activity and NE release in the hippocampus and hypothalamus. This increase in NE activity is relevant to HPA-axis activation, fever, and metabolic change associated with immune activation (Dunn et al., 1999, Dunn et al., 2005). Whereas 5-HT and DA metabolites in the CSF have been found to correlate with IFN-alpha-induced depressive and fatigue symptoms, respectively (Raison et al., 2009, Felger and Miller, 2012), MHPG has been examined in the CSF of IFN-alpha-treated patients but was not related to behavioral symptoms (Raison et al., 2009). SNPs of the gene for DA beta-hydroxylase, the enzyme that converts DA to NE, was examined in IFN-alpha-treated HCV+ subjects but was not associated with the development of depression (Smith et al., 2012). More work is necessary to understand the effects of cytokines on the NE system in relation to cytokine-induced depression.
3.2 Cytokine effects on neuropeptides and growth factors
3.2.1 Brain-derived neurotrophic factor
Inflammatory cytokines have repeatedly been observed to influence both neuronal development and apoptosis (Chawla-Sarkar et al., 2003, Hayley et al., 2005) (Mehler and Kessler, 1997, Zhao and Schwartz, 1998), (Peng et al., 2008, Anisman, 2009, Koo et al., 2010b). In fact, both stress and subsequent inflammatory cytokine activity may adversely influence neurogenesis and neuroplasticity (Patel et al., 2003, Goshen and Yirmiya, 2007, Peng et al., 2008, Chen et al., 2009, Goshen and Yirmiya, 2009, Koo et al., 2010b). IFN-alpa can likewise decrease cell proliferation in the hippocampus, an effect that is potentially mediated by IL-1 (Kaneko et al., 2006). IFN-alpha administration has also been found to decrease systemic BDNF levels in humans (Kenis et al., 2010, Lotrich et al., 2013) (see Figure 1), as can other inflammatory cytokines and LPS injections (Tong et al., 2008, Cortese et al., 2011) (Guan and Fang, 2006). Inflammatory cytokines also influence BDNF receptor (TrkB) phosphorylation, thereby further interfering with BDNF signaling (Cortese et al., 2011). Consistent with these findings, the reversal of LPS-induced apoptosis by antidepressants requires intact BDNF signaling (Peng et al., 2008).
However, BDNF decreases during IFN-alpha administration in most people whether they develop depression or not, consistent with findings in rodents in which IFN-alpha affected behavior without affecting BDNF levels (Fahey et al., 2007). However, as described in section 2.6, the met allele (which is associated with decreased BDNF release) has been associated with symptoms of depression during IFN-alpha treatment (Lotrich et al., 2013). Therefore, it is likely that there is an interaction between pre-existing low BDNF concentrations and subsequent risk forcytokine-induced depression, which is congruent with studies of association between depression risk and the met allele in other contexts (Sarchiapone et al., 2008, Kunugi et al., 2010, Kanellopoulos et al., 2011, Pregelj et al., 2011), (Drachmann et al., Aguilera et al., 2009, Cirulli et al., 2011), (Su et al., 2011), (Zhang et al., 2011). It is noteworthy that a direct comparison of the 5-HTTLPR and the met/val allele of BDNF found that the 5-HTTLPR Short allele was specifically associated with risk for neurovegetative symptoms of depression, while the BDNF Met allele was primarily associated with the cognitive symptoms (Lotrich et al., 2013). This finding reinforces the idea that specific gene polymorphisms and neurotransmitter systems may confer protection from or susceptibility to specific symptom dimensions of depression.
3.2.2 Corticotropin releasing hormone (CRH)
The role of corticotropin releasing hormone (CRH) and the HPA-axis in major depression and in cytokine-induced depression has been investigated extensively (Arborelius et al., 1999, Capuron et al., 2003b, Pariante and Lightman, 2008, Raison et al., 2010a) . Acutely, cytokine administration or immune activation in humans and laboratory animals stimulates the HPA-axis (Dunn, 2000), and some features of HPA-axis alterations that have been described in major depression are observed in cytokine-induced depression. Indeed, IFN-alpha-induced depression has been associated with flattening of the diurnal cortisol rhythm and elevated evening cortisol (Raison et al., 2010a). Interestingly, an exaggerated response in adrenocorticotropic hormone (ACTH) and cortisol to the initial injection of IFN-alpha predicted the subsequent development of IFN-alpha-induced depression in malignant melanoma patients (Capuron et al., 2003b). Furthermore, a similar relationship between an exaggerated ACTH response and depressive-like huddling behavior was observed in IFN-alpha-treated monkeys (Felger et al., 2007). This exaggerated HPA response to an inflammatory stimulus that conferred vulnerability to cytokine-induced depression was believed to be due to sensitization of CRH pathways in the brain, and cytokines had been shown to stimulate CRH release (Besedovsky and del Rey, 1996). Despite the many reports of elevated CSF concentrations of CRH in major depression (Arborelius et al., 1999), no changes in CSF CRH have been observed following IFN-alpha administration or in relation to IFN-alpha-induced depression (Felger et al., 2007, Raison et al., 2009). Therefore, it is reasonable that HPA-mediated vulnerability to cytokine-induced depression represents a more general hyper-responsiveness and pre-existing vulnerability to stress or inflammatory challenge, which may be mediated by changes in glucocorticoid receptor (GR) expression or function (Pace et al., 2007).
Functional gene polymorphisms for both the GR and CRH receptor 1 (CRHR1) gene have been associated with major depression (van Rossum et al., 2006, Ressler and Mayberg, 2007) and with the response to SSRI antidepressants (van Rossum et al., 2006, Liu et al., 2007). Three SNPs for the CRHR1 gene were examined in patients treated with IFN-alpha (Smith et al., 2012) (see Table 1). An association between the CRHR1 (rs7209436) SNP with IFN-alpha-induced depression was observed, but was not significant after adjusting for multiple SNP comparisons. In this same study, SNPs for the genes of CRH and proopiomelanocortin (POMC), the precursor for ACTH, were examined. An association between the POMC (rs1866146) SNP with IFN-alpha-induced depression was observed, but was not significant after adjusting for multiple SNP comparisons. The CRH SNPs were not associated with cytokine-induced depression. Although GR polymorphisms have not been examined in cytokine-induced depression, FK506 binding protein 5, a known regulator of the GR that has been associated with mood disorders, was examined in IFN-alpha-treated HCV+ subjects and also did not associate with cytokine-induced depression (Smith et al., 2012).
3.3 Cytokine effects on glutamate neurotransmission
The efficacy of N-Methyl-D-aspartate (NMDA) receptor antagonist drugs, such as ketamine, in rapid antidepressant responses has prompted much attention to the role of glutamate in the pathophsyiology of depression (Sanacora et al., 2012). Inflammatory cytokines activate IDO and KYN pathways in the brain that effect glutamate neurotransmission and may contribute to glutamate dysfunction in depression (McNally et al., 2008, Dantzer et al., 2011, Haroon et al., 2012). KYN, which is formed from the IDO catabolization of tryptophan, can be produced locally in the CNS or transported across the blood-brain barrier by large neutral amino acid transporters (Smith et al., 1987, Fukui et al., 1991). KYN is then further catabolized into the neuroactive metabolites kynurenic acid (KA) and QUIN (see section 2.2 and Figure 1). Whereas KA is considered neuroprotective, QUIN is thought to mediate neurotoxic effects of IDO, and both metabolites have been shown to affect glutamate neurotransmission (Stone, 2000a, Schwarcz and Pellicciari, 2002, Tavares et al., 2002, Tavares et al., 2005). Both KA and QUIN have been observed to be increased in the CSF of IFN-alpha-treated patients (Schwarcz and Pellicciari, 2002, Raison et al., 2010b), and CSF QUIN correlated with depressive symptoms during IFN-alpha administration (Raison et al., 2010b). Evidence of increased glutamate (Matute et al., 2006, Hashimoto et al., 2007, Matute, 2011), as well as microglial activation and increased expression of QUIN (Steiner et al., 2008), have been found in the frontal cortex of patients with mood disorders.
In terms of KYN metabolite effects on glutamate, the protective metabolite, KA, reduces glutamate release, and has been shown to be an antagonist of NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Stone, 2000b, a). However, DA release is in part under glutamatergic control (Schwarcz and Pellicciari, 2002), and intra-striatal infusions of KA to rodents leads to marked reductions DA release, as determined by in vivo microdialysis, an effect that can be reversed by activating alpha 7 nicotinic acetylcholine receptors on glutamatergic afferents (Wu et al., 2007). Therefore, KA may exert behavioral effects through interactions between glutamate and DA neurotransmitter systems, even in the absence of increase glutamate activity. On the other hand, QUIN contributes to oxidative stress (Rios and Santamaria, 1991, Behan et al., 1999, Schwarcz and Pellicciari, 2002), and can directly activate the NMDA receptor (Schwarcz and Pellicciari, 2002, Tavares et al., 2002, Tavares et al., 2005) (See Figure 1). This combined effect of increased oxidative stress and NMDA activity contributes to QUIN-mediated excitotoxicity (Perez-De La Cruz et al., 2012), and QUIN has been implicated in a number of neurodegenerative diseases, including Huntington’s disease, Amyotrophic Lateral Sclerosis, Alzheimer’s disease, and dementia secondary to infection with HIV (Schwarcz and Pellicciari, 2002, Guidetti and Schwarcz, 2003, Guillemin et al., 2005a, Guillemin et al., 2005b, Guillemin et al., 2005d). QUIN and inflammatory cytokines have also been shown to increase glutamate release and decrease the astrocytic uptake of glutamate by the excitatory amino acid transporter (Tavares et al., 2002, Tilleux and Hermans, 2007, Ida et al., 2008), thus making glutamate more available to extrasynaptic NMDARs and contributing to glutamate toxicity (Hardingham et al., 2002). Additionally, IL-1b is also an inhibitor of NMDAR-mediated long-term potentiation (LTP), likely through a MAP kinase mechanism (Coogan et al., 1999), as is IL-18 (Curran and O'Connor, 2001). Interestingly, the IL-18 receptor recruits the IL-1 receptor associated kinase (IRAK) and effects subsequent MAPK signaling, potentially sharing similar intracellular pathways. Additionally, the mRNA specific adenosine deaminase 1 (ADAR1), which edits the AMPA glutamate receptor, is strongly induced by IFN-alpha (Liu and Samuel, 1999). Thus there are a variety of routes by which cytokines may influence the glutamatergic system and conceivably increase risk for depression.
4. Translational and therapeutic implications
4.1 Clinical significance and limitations
Depression is a significant health concern, with lifetime prevalence of major depression in up to 20% individuals (Blazer et al., 1994, Kessler et al., 2005, Kruijshaar et al., 2005). However, medically ill patients can experience depression at rates up to 50%, which can have devastating consequences on treatment adherence, quality of life, and morbidity and mortality (Musselman et al., 1998, Evans et al., 1999, Loberiza et al., 2002, Raison and Miller, 2003, Lotrich, 2010). As described in this review, inflammatory cytokines released during medical illness can affect neurotransmitter systems that are involved in the pathophysiology of depression, and administration of cytokines produces an iatrogenic depression that is very similar to idiopathic major depression. It follows that the inflammatory markers associated with major depression and the vulnerabilities that have been described for IFN-alpha-induced depression, including circulating IL-6 concentrations, increased p38 MAPK responses to IFN-alpha, and gene polymorphisms for cytokines (IL-6, IL28B), cytokine receptors (IFNRA), and inflammatory mediators (IDO, COX2), may serve as reliable markers to identify patients at risk for the development of depression during medical illness and/or medical interventions associated with inflammation (e.g. cytokine therapies, chemotherapy, radiation, surgery). Another potential vulnerability for the development of depression during IFN-alpha-therapy are baseline depression scores (Capuron et al., 2004), and could also be considered when assessing risk for depression prior to medical interventions such as cytokine treatment or chemotherapy.
Medically healthy depressed patients who are resistant to antidepressant therapy demonstrate higher concentrations of circulating inflammatory cytokines and CRP than patients who respond to antidepressants (Sluzewska et al., 1997, Lanquillon et al., 2000, Fitzgerald et al., 2006). Therefore, baseline concentrations of circulating cytokines, such as IL-6, and markers of inflammation, like CRP, may be useful for indentifying subjects that will fail to respond to current antidepressant therapies and for determining potential novel treatment strategies. In fact, a recent study determined that TNF antagonism with infliximab alleviated depressive symptoms in patients with treatment resistant depression compared to placebo, but only in those patients with high CRP (hsCRP >5 mg/L). These findings indicating that treatment resistant patients with increased inflammation may be candidates for novel antidepressant therapies that target cytokines and inflammatory signaling pathways, “as discussed below in section 4.2.1.
In addition to measuring circulating biomarkers or genetic polymorphisms, leukocyte mRNA expression has been examined prospectively to identify predictors of cytokine-induced depression or antidepressant response and potential therapeutic targets. For example, one study that examined early (initial injection) changes in the response to IFN-alpha administration reported differential expression of IFN stimulated genes and genes that have been related to depression or neural development in patients that subsequently developed depression (Schlaak et al., 2012). Some of these genes were also found to be up-regulated following in vitro stimulation with IFN-alpha (GCH1, TOR1B, DYNLT1, DISC1) in leukocytes isolated from subjects with depression compared to controls in this same study, and numerous genes encoding IFNs, IFN-regulated genes and toll-like receptors were also up-regulated at baseline in depressed subjects compared to controls. A second study examining transcriptional differences later in treatment (12 weeks) identified up-regulation of IFN-alpha-regulated genes that are common to chronic fatigue syndrome, such as OAS2 (which correlated with depression and fatigue scores), and decreased expression of genes regulated by cAMP response element-binding protein, in patients that developed depression during IFN-alpha therapy compared to ,those that did not (Felger et al., 2012a). These IFN-related genes or a genome-wide analysis of gene expression have yet to be examined for prediction of treatment response in depression. However, a recent study took an interesting approach to examining the relationship between inflammation and antidepressant response in depressed patients by examining expression of a targeted subset of genes related to inflammation, GR signaling, and neuroplasticity, both before and after 8 weeks of treatment with escitalopram or nortriptyline (Cattaneo et al., 2013). Non-responders had higher baseline mRNA levels of IL-1beta, macrophage inhibitory factor (MIF), and TNF-alpha, and these inflammatory genes that predicted treatment-resistance were not the genes that were affected by antidepressants in responders. Rather, successful antidepressant response was associated with changes in the expression of a different set of genes, decreased IL-6 and the GR-related FKBP5, and increased BDNF and VGF nerve growth factor. Interestingly, increases in circulating BDNF in response to successful antidepressant therapy have been reported (Molendijk et al., 2011, Rojas et al., 2011), as have decreased circulating cytokines, such as IL-6 (Frommberger et al., 1997, Lanquillon et al., 2000), Therefore, prospective studies examining transcriptomic changes over time, such as this one, may be useful in identifying not only genes that predict treatment response, but also those that are involved in the mechanisms of antidepressant action.
In terms of the identification of depressed patients that may exhibit increased inflammation and that may be less responsive to standard antidepressant therapy, persons with a history of childhood trauma tend to have higher inflammatory biomarkers as adults and also exhibit higher rates of depression (Danese et al., 2007, Danese et al., 2008). In fact, persons exposed to early life stress have been found to be less responsive to antidepressant interventions (Nemeroff et al., 2003, Nanni et al., 2011). Interestingly, a “biological embedding” or imprinting of stress through inflammatory processes in childhood has been proposed (Danese et al., 2011). Given the recent evidence that DAMP activation of the NLRP3 inflammasome may be a key mechanism of converting psychological stress into inflammatory signals and its importance in IL-1 production (see section 2.3), the inflammasome may be a target for novel antidepressant therapies that block inflammation associated with early life trauma or chronic stress (discussed below in section 4.2.1).
Prior to discussing novel therapeutic strategies that target inflammation or the effects of inflammatory cytokines on neurotransmitter systems to potentially reverse depressive symptoms, it is worthy to discuss several limitations of the research discussed in this review and important considerations for future research. One limitation is the fact that much of the literature examining vulnerability factors for cytokine-induced depression or treatment response to antidepressant therapy has relied on correlative measures between SNPs, biomarkers of inflammation or neurotransmitter function, and behavioral symptoms. Therefore, mechanistic studies using basic and translational animal models are necessary to integrate the genetic, genomic and biomarker findings identified in clinical studies, and to examine novel therapeutic strategies that target inflammatory pathways or the neural substrates that they interact with to reverse cytokine-related behavior change (as will be discussed below in section 4.2). It is also important that these findings then be translated back and tested in clinical trials. Furthermore, additional neuroimaging studies in humans are necessary to continue to understand the mechanisms of cytokine effects on the brain and to identify imaging biomarkers of treatment response. Moreover, studies that integrate transcriptomic changes in the periphery with neuroimaging findings in the brain may advance our understanding of how peripheral inflammatory processes affect specific neural substrates and neurocircuits. Another limitation in our analysis of the literature reviewed herein is that not all studies have examined the relationship between gene polymorphisms or biomarkers and specific symptoms of depression. Therefore, the use of appropriate rating scales and analyses to capture the full spectrum of depressive-symptom dimensions may be useful in identifying important relationships that could improve the future understanding and treatment of cytokine-related depression.
4.2 Potential therapeutic strategies for treatment of cytokine-induced depression
Although SRRIs and other classical antidepressants that target the 5-HT system are efficacious in treating many aspects of both major depression and cytokine-induced depression, drugs that target cytokine and inflammatory signaling pathways, the glutamate system, or that improve monoamine synthesis may confer increased benefit for the treatment of residual symptoms. Moreover, approximately 30% of depressed patients fail to respond to standard antidepressant treatments (Rush et al., 2006), and these patients have been shown to exhibit increased inflammatory cytokines (Sluzewska et al., 1997, Lanquillon et al., 2000, Fitzgerald et al., 2006) and may benefit from novel treatment strategies. The following section will discuss briefly therapeutic targets relevant to the pathways presented in this review that may be efficacious in the treatment of depression, either alone or as adjuvant to current antidepressant therapies, in both medically ill and medially healthy individuals. Because treatments for depression that may target inflammation or the effects of inflammatory cytokines on the brain are still being investigated, and some of the therapies are under development, some of the data regarding potential antidepressant efficacy have come from preclinical studies. Therefore, this section will discuss both clinical and translational findings.
4.2.1 Immune activation
Cytokine antagonists
Pharmacological strategies to inhibit cytokine activity has included the use of monoclonal cytokine antibodies, such as etanercept and infliximab that inhibit TNF-alpha activity, as well as small molecule inhibitors that have increased bioavailability and may permeate the CNS (McCoy and Tansey, 2008). Administration of the TNF-alpha antagonist, etanercept, has been shown to inhibit fatigue in patients with advanced cancer (Monk et al., 2006). As discussed above, a similar TNF-alpha antagonist, infliximab, was successful in a placebo-controlled trial in improving depressive symptoms in patients that were moderately resistant to antidepressant treatment, but only in those subjects that exhibited high baseline inflammatory biomarkers (Raison et al., 2012). These findings not only suggest that cytokine antagonism may improve depressive symptoms, but also reinforce the idea that identifying inflammatory risk factors in treatment refractory patients may be beneficial in targeting future treatment strategies. Finally, the inflammasome may serve as a novel target for inhibiting cytokine production to reduce the inflammatory consequences of stress that lead to depressive symptoms. Indeed, knockout mice for the ATP P2X7 receptor that stimulates inflammasome-mediated IL-1beta release exhibit an antidepressant-like profile, including increased mobility in the tail suspension and forced swim tests, and blockade with a high affinity P2X7 receptor antagonist, A-804598 (Donnelly-Roberts et al., 2009), may inhibit the effects of stress on neurogenesis and depressive-like behavior in laboratory animals (Iwata et al., 2012).
Inflammatory signal transduction
The most commonly used agents to block inflammatory signaling are COX inhibitors, which have been shown to inhibit depressive-like behaviors, including decreased sucrose preference, increased immobility in tail suspension and forced swim, and decreased activity in open field tests, in laboratory animals induced by LPS and chronic unpredictable stress (Guo et al., 2009, de Paiva et al., 2010, Teeling et al., 2010). In humans, COX inhibitors have been administered as adjuvant to antidepressant therapy. The selective COX-2 inhibitor, celecoxib, improved the efficacy of the NE reuptake inhibitor, reboxetine, in depressed subjects (Muller et al., 2006). Addition of both celecoxib (Akhondzadeh et al., 2009) and acetylsalicylic acid (which blocks COX-1 and COX-2) (Mendlewicz et al., 2006) augmented treatment responses to fluoxetine. Omega-3 fatty acids have increased the antidepressant efficacy of fluoxetine (Jazayeri et al., 2010) and citalopram (Gertsik et al., 2012), and a meta-analysis reported direct antidepressant effects when combining all sources of omega-3 fatty acids (e.g. docosahexaenoic acid, fish oils) (Lin and Su, 2007). Inhibition of p38 MAPK has been shown to reverse the development of LPS-induced behavioral changes in laboratory animals (Zhu et al., 2010b), and p38 MAPK inhibitors are currently being development for the treatment of autoimmune and inflammatory disorders (Kumar et al., 2003) and may have potential in treating cytokine-induced depression. NF-kB is another inflammatory signal transducer that is of great interest to cytokine-induced depressive behavior, and has been shown to mediate cytokine and stress effects on neurogenesis (Nadjar et al., 2005, Koo et al., 2010a). Although many specific NF-kB inhibitors are being developed for treatment of diseases such as cancer, naturally occurring compounds that inhibit NF-kB, as well as other inflammatory mediators such as NO, have all been demonstrated to have antidepressant-like effects in animal models. Examples include curcumin (a derivative of the curry spice, turmeric) (Lopresti et al., 2012), a tocopherol (a form of Vitamin E) (Godbout et al., 2005), and resveratrol (found in the skin of red grapes) (Lu et al., 2010).
Other potential modulators of inflammation that have been suggested (please see (McNamara and Lotrich, 2012) for review) include the anti-inflammatory cytokine IL-10 (Asadullah et al., 2000), the soluble IL-4 receptor (Borish et al., 1999, Borish et al., 2001), cannabinoids (Hill and Gorzalka, 2009), altering the intestinal microflora (Ma et al., 2004, Karimi et al., 2009) (Rhee et al., 2009, Bravo et al., 2011), minocycline (Plane et al., 2010) (Molina-Hernandez et al., 2008, Pae et al., 2008) which potentially reduces inflammatory cytokines by inhibiting caspase synthesis (Chen et al., 2000), MK2/3 (MAP kinase associated protein-kinase 2 and 3) inhibitors (Duman et al., 2007) (Anderson et al., 2007), IRAK inhibitors, and correction of zinc deficiencies (Dybala et al., 2006, Prasad, 2009, Roy et al., 2010) .
4.2.2 Monoamines
DA agonists
Stimulants such as amphetamines and DAT blockers have exhibited limited therapeutic efficacy in treating fatigue in medically ill and depressed populations (Sugawara et al., 2002, Stankoff et al., 2005, Butler et al., 2007, Pucci et al., 2007, Mar Fan et al., 2008, Moraska et al., 2010). Stimulant drugs that work through alternative mechanisms, such as modafinil, may have greater success in treating fatigue in medical illnesses, e.g. cancer and multiple sclerosis (Krupp et al., 1995, Rammohan et al., 2002, Zifko et al., 2002), yet their benefit remains to be determined (Stankoff et al., 2005, Pucci et al., 2007). Classic stimulants act to increase DA release and block DAT function, and these drugs may not provide long term efficacy if cytokine effects on DA function are primarily mediated through inhibitory effects on synthesis. For instance, Parkinson’s-like symptoms have been observed in some patients during IFN-alpha administration, and these symptoms have been successfully treated with levodopa, suggesting that DA replacement may be beneficial in some patients (Mizoi et al., 1997, Sarasombath et al., 2002, Bersano et al., 2008). Therefore compounds that improve DA synthesis, or stimulate DA receptor signaling, such as ropinirole, could be beneficial in improving DA-mediated symptoms. Interestingly, ropinirole augmentation of antidepressant treatment in a small sample of refractory patients reduced depression scores by half in 40% of patients (Cassano et al., 2005).
Monoamine synthesis
Cytokines may affect monoamine synthesis through degradation of BH4 (a co-factor necessary for both DA and 5-HT synthesis) (Cunnington and Channon, 2010). Therefore, compounds that increase BH4 activity may boost monoamine synthesis and improve depressive symptoms in patients exposed to inflammatory cytokines. BH4 can be restored from its oxidized form, BH2, through the salvage pathway, and following administration of synthetic pterin, by conversion via sepiapterin reductase (Nichol et al., 1983, Cunnington and Channon, 2010). Sapropterin (Kuvan) is the first non-dietary, FDA-approved, synthetic form of BH4 for patients with phenylketonuria that is effective in lowering blood Phen levels by stabilizing and increasing PAH activity (Trefz et al., 2009, Burton et al., 2010, Utz et al., 2012). Other strategies are available to improve BH4 activity, including the use of folic acid, L-methylfolate, and S-adenosyl-methionine (SAMe) (Stahl, 2007, Miller, 2008). Interestingly, low serum folate has been associated with depression and with resistance to antidepressant treatment (Fava et al., 1997, Papakostas et al., 2004a, Papakostas et al., 2004b, Gilbody et al., 2007a, Gilbody et al., 2007b), and administration of L-methylfolate and SAMe have demonstrated efficacy as adjuvant to antidepressant therapy (Godfrey et al., 1990, Papakostas et al., 2010, Ginsberg et al., 2011).
4.2.3 Glutamate
IDO/KYN pathway
Inhibition of the IDO and KYN pathway may be important in preventing cytokine effects on the glutamate system and preventing cytokine-induced behavioral alterations. Indeed, IDO-deficient mice are resistant to behavioral changes following infection with bacille Calmette–Guerin (BCG), while showing a normal inflammatory cytokine response to BCG administration (O'Connor et al., 2009a). The IDO antagonist, 1-methyl tryptophan (1-MT), has been shown inhibit depressive-like behavior in response to immune activation with LPS and Mycobacterium bovis (O'Connor et al., 2008, O'Connor et al., 2009a, O'Connor et al., 2009b, Salazar et al., 2012). IDO inhibitors are being developed and tested for efficacy in cancer and other diseases (Di Pucchio et al., 2010) and may be applicable for treating cytokine-mediated depression.
NMDA antagonists
Interactions between inflammatory cytokines and glutamate may explain some of the effectiveness of NMDA receptor antagonists in depression, and changes in glutamate may moderate many of the changes in other neurotransmitter systems that contribute to cytokine-induced depression. Administration of glutamate receptor antagonists, such as the NMDA antagonist, ketamine, have potent antidepressant effects especially in treatment resistant depressed patients (Price et al., 2009, aan het Rot et al., 2010), who, as mentioned previously (section 2.1), have been shown to exhibit increased inflammation. Given that the neurotoxic effects of QUIN may be mediated by excessive glutamate excitotoxicity (Schwarcz and Pellicciari, 2002, Tavares et al., 2002, Tavares et al., 2005), glutamate antagonists may be useful in preventing excitotoxic effects on other neurotransmitter systems. For instance, metabotropic glutamate receptor antagonists that modulate glutamate transmission in the basal ganglia have been successful in reducing dopamine cell loss in an animal model of PD (Masilamoni et al., 2011). Furthermore, inhibition of the NMDA receptor with memantine prevented the onset of DA deficits in the brains of SIV-infected macaques, and effect mediated by increased BDNF (Meisner et al., 2008). Finally, a recent study administering ketamine prior to LPS found that blockade of the NMDA receptor abrogated LPS-induced depressive-like behavior in mice (decreased sucrose preference and increased immobility in the forced swim test) without affecting inflammatory signaling in the periphery or in the brain (Walker et al., 2013).
5. Summary and conclusions
Mounting evidence exists that inflammatory cytokines are involved in the development of neuropsychiatric symptoms and depression. Inflammatory cytokines are elevated in medical illness, and are also produced in the gut, in adipose tissue, and both centrally and systemically following stress. Peripheral cytokines can access the brain and activate local CNS inflammatory networks to produce alterations in neurotransmitter function. Furthermore, exogenous administration of cytokines or innate immune activation with LPS, vaccines, or infectious agents promote behavioral alterations including cytokine-induced depression, and have be used to understand the mechanisms of cytokine effects on the brain. As described herein, neurotransmitter systems that are affected by inflammatory cytokines to produce behavioral alterations and depression include monoamines, glutamate, neuropeptide, and growth factors. Moreover, numerous biomarkers and gene polymorphisms that confer vulnerability to cytokine-induced depression have been indentified, and may be useful in identifying at-risk patients.
Although there is a wide-range of immunologic and neurobiological mechanisms that may explain cytokine-induced depression, many of the proposed therapeutic interventions are in conceptual or pre-clinical stages. Future directions in the field exist in the potential for targeted manipulation of the inflammatory response to prevent and reverse neuropsychiatric illness. Future research will aim to determine whether drugs that target inflammatory molecules will be as efficacious as current antidepressant therapies, while remaining safe and well tolerated. Furthermore, establishing the mechanisms of these compounds on brain function are necessary, as well as defining a reliable set of biomarkers to identify at-risk patients that may benefit from immune therapies. Finally, compounds that increase monoamine synthesis, promote growth factors, or modulate glutamate neurotransmission may be effective in combating the behavioral effects of heightened inflammatory cytokine activity in the brain.
-
□
Symptoms of cytokine-related depression may involve specific genes, neurotransmitters
-
□
Environmental factors (e.g. childhood trauma, stress, obesity) increase cytokines
-
□
Novel treatments for depression may target cytokines and their signaling mechanisms
Acknowledgements
This work was funded in part by NIMH R090250, the Glenn Family Breast Fund and the Winship Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors certify that there are no conflicts of interest and that they have nothing to declare.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Abe S, Hori T, Suzuki T, Baba A, Shiraishi H, Yamamoto T. Effects of chronic administration of interferon alpha A/D on serotonergic receptors in rat brain. Neurochemical Research. 1999;24:359–363. doi: 10.1023/a:1020929415443. [DOI] [PubMed] [Google Scholar]
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, van Os J, Ibanez M, Ruiperez M, Ortet G, Fananas L. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39:1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M, Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26:607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Meyers MJ, Vernier WF, Mahoney MW, Kurumbail RG, Caspers N, Poda GI, Schindler JF, Reitz DB, Mourey RJ. Pyrrolopyridine inhibitors of mitogen-activated protein kinase-activated protein kinase 2 (MK-2) Journal of Medical Chemistry. 2007;50:2647–2654. doi: 10.1021/jm0611004. [DOI] [PubMed] [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. Journal of Psychiatry & Neuroscience. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55:1512–1520. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadullah K, Docke WD, Sabat R, Volk HD, Sterry W. The treatment of psoriasis with IL-10: rationale and review of the first clinical trials. Expert Opinion on Investigational Drugs. 2000;9:95–102. doi: 10.1517/13543784.9.1.95. [DOI] [PubMed] [Google Scholar]
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Behan WM, McDonald M, Darlington LG, Stone TW. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. British journal of pharmacology. 1999;128:1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. 609, e591–e593. doi: 10.1053/j.gastro.2011.04.052. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bersano A, Aghemo A, Rumi MG, Ballabio E, Candelise L, Colombo M. Recovery after L-DOPA treatment in peginterferon and ribavirin induced parkinsonism. Eur J Intern Med. 2008;19:370–371. doi: 10.1016/j.ejim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- Blasi F, Riccio M, Brogi A, Strazza M, Taddei ML, Romagnoli S, Luddi A, D'Angelo R, Santi S, Costantino-Ceccarini E, Melli M. Constitutive expression of interleukin-1beta (IL-1beta) in rat oligodendrocytes. Biol Chem. 1999;380:259–264. doi: 10.1515/BC.1999.034. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature reviews Neuroscience. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M, Almerighi C, Levrero M, Egyed B, Bosmans E, Meltzer HY, Maes M. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105:45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, Agosti JM. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. Journal of Allergy and Clinical Immunology. 2001;107:963–970. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- Borish LC, Nelson HS, Lanz MJ, Claussen L, Whitmore JB, Agosti JM, Garrison L. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. American Journal of Respiratory & Critical Care Medicine. 1999;160:1816–1823. doi: 10.1164/ajrccm.160.6.9808146. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Hartman CA, Nolte IM, Prins BP, Terpstra P, Posthuma D, van Veen T, Willemsen G, DeRijk RH, de Geus EJ, Hoogendijk WJ, Sullivan PF, Penninx BW, Boomsma DI, Snieder H, Nolen WA. Poor replication of candidate genes for major depressive disorder using genome-wide association data. Mol Psychiatry. 2011;16:516–532. doi: 10.1038/mp.2010.38. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. PNAS early edition pnas. 2011:1102999108. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nature reviews Immunology. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buret AG. How stress induces intestinal hypersensitivity. Am J Pathol. 2006;168:3–5. doi: 10.2353/ajpath.2006.050958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton BK, Bausell H, Katz R, Laduca H, Sullivan C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU) Mol Genet Metab. 2010;101:110–114. doi: 10.1016/j.ymgme.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Butler JM, Jr, Case LD, Atkins J, Frizzell B, Sanders G, Griffin P, Lesser G, McMullen K, McQuellon R, Naughton M, Rapp S, Stieber V, Shaw EG. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69:1496–1501. doi: 10.1016/j.ijrobp.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochemistry international. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Skwerer RG, Barna B, Gulledge AD, Valenzuela R, Butkus A, Subichin S, Krupp NE. Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res. 1986;17:41–47. doi: 10.1016/0165-1781(86)90040-5. [DOI] [PubMed] [Google Scholar]
- Candito M, Nagatsu T, Chambon P, Chatel M. High-performance liquid chromatographic measurement of cerebrospinal fluid tetrahydrobiopterin, neopterin, homovanillic acid and 5-hydroxindoleacetic acid in neurological diseases. Journal of chromatography B, Biomedical applications. 1994;657:61–66. doi: 10.1016/0378-4347(94)80070-7. [DOI] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol. 1997;272:R1712–R1725. doi: 10.1152/ajpregu.1997.272.6.R1712. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003a;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003b;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63:376–386. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly person is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biological Psychiatry. 2011a doi: 10.1016/j.biopsych.2010.12.006. xx:xx. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011b;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. Treatment-resistant depression and mortality after acute coronary syndrome. Am J Psychiatry. 2009;166:410–417. doi: 10.1176/appi.ajp.2008.08081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polyorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cassano P, Lattanzi L, Fava M, Navari S, Battistini G, Abelli M, Cassano GB. Ropinirole in treatment-resistant depression: a 16-week pilot study. Can J Psychiatry. 2005;50:357–360. doi: 10.1177/070674370505000612. [DOI] [PubMed] [Google Scholar]
- Cassidy EM, Manning D, Byrne S, Bolger E, Murray F, Sharifi N, Wallace E, Keogan M, O'Keane V. Acute effects of low-dose interferon-alpha on serum cortisol and plasma interleukin-6. J Psychopharmacol. 2002;16:230–234. doi: 10.1177/026988110201600307. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59(Suppl 14):11–14. [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature medicine. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- Chen P, Jiang T, Ouyang J, Cui Y, Chen Y. Epigenetic programming of diverse glucocorticoid response and inflammatory/immune-mediated disease. Medical Hypotheses. 2009;73:657–658. doi: 10.1016/j.mehy.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-Induced Microglial Activation and Neuroprotection against Experimental Brain Injury Is Independent of Hematogenous TLR4. J Neurosci. 2012;32:11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- Cirulli F, Reif A, Herterich S, Lesch KP, Berry A, Francia N, Aloe L, Barr CS, Suom iSJ, Alleva E. A novel BDNF polymorphism affects plasma protein levels in interaction with early adversity in rhesus macaques. Psychoneuroendocrinology. 2011;36:382–379. doi: 10.1016/j.psyneuen.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement HW, Buschmann J, Rex S, Grote C, Opper C, Gemsa D, Wesemann W. Effects of interferon-gamma, interleukin-1 beta, and tumor necrosis factor-alpha on the serotonin metabolism in the nucleus raphe dorsalis of the rat. Journal of Neural Transmission. 1997;104:981–991. doi: 10.1007/BF01273312. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JM, Riccardi R, Trown P, O'Neill D, Poplack DG. Plasma and cerebrospinal fluid pharmacokinetics of recombinant interferon alpha A in monkeys: comparison of intravenous, intramuscular, and intraventricular delivery. Cancer Drug Deliv. 1985;2:247–253. doi: 10.1089/cdd.1985.2.247. [DOI] [PubMed] [Google Scholar]
- Coogan A, O'Neill LAJ, CO'Connor JJ. The p38 MAP kinase inhibitor SB203580 antagonises the inhibitory effect of interleukin-1b on long-term potentiation in the rat dentate gyrus in vitro . Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nature reviews Immunology. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. Journal of Neuroscience. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington C, Channon KM. Tetrahydrobiopterin: pleiotropic roles in cardiovascular pathophysiology. Heart (British Cardiac Society) 2010;96:1872–1877. doi: 10.1136/hrt.2009.180430. [DOI] [PubMed] [Google Scholar]
- Curran B, O'Connor JJ. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience. 2001;108:83–90. doi: 10.1016/s0306-4522(01)00405-5. [DOI] [PubMed] [Google Scholar]
- D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Werts H, Freeman J, Pariante CM, Moffitt TE, Arseneault L. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry. 2011;16:244–246. doi: 10.1038/mp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza RI, Asnis GM. The non-steroidal anti-inflammatory drug diclofenac sodium attenuates IFN-alpha induced alterations to monoamine turnover in prefrontal cortex and hippocampus. Brain Research. 2003;977:70–79. doi: 10.1016/s0006-8993(03)02757-4. [DOI] [PubMed] [Google Scholar]
- de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A. Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res. 2010;215:146–151. doi: 10.1016/j.bbr.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Di Pucchio T, Danese S, De Cristofaro R, Rutella S. Inhibitors of indoleamine 2,3-dioxygenase: a review of novel patented lead compounds. Expert Opin Ther Pat. 2010;20:229–250. doi: 10.1517/13543770903512974. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, Namovic MT, Surber B, Vaidyanathan SX, Perez-Medrano A, Wang Y, Carroll WA, Jarvis MF. [3H]A-804598 ([3H]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology. 2009;56:223–229. doi: 10.1016/j.neuropharm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A metaanalysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Drachmann BJ, Bock C, Vinberg M, Werg eT, Gether U. Vedel Kessing L Interaction between genetic polymorphisms and stressful life events in first episode depression. Journal of Affective Disorders. 119:107–115. doi: 10.1016/j.jad.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Dufton N, Hannon R, Brancaleone V, Dalli J, Patel HB, Gray M, D'Acquisto F, Buckingham JC, Perretti M, Flower RJ. Anti-inflammatory role of the murine formyl-peptide receptor 2: ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russel DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biological Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Cytokine activation of the HPA axis. Ann N Y Acad Sci. 2000;917:608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of cytokines in infection-related behavior. Ann N Y Acad Sci. 1998;840:577–585. doi: 10.1111/j.1749-6632.1998.tb09596.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- Dybala M, Maciag D, Cichy A, Pomierny-Chamiolo L, Partyka A, Librowski T, Nowak G. Medium supplementation with zinc enables detection of imipramine-induced adaptation in glycine/NMDA receptors labeled with [3H]L-689,560. Source Pharmacological Reports. 2006;58:753–757. [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, Saper CB. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. The Journal of comparative neurology. 1997;381:119–129. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Staab JP, Petitto JM, Morrison MF, Szuba MP, Ward HE, Wingate B, Luber MP, O'Reardon JP. Depression in the medical setting: biopsychological interactions and treatment considerations. J Clin Psychiatry. 1999;60(Suppl 4):40–55. discussion 56. [PubMed] [Google Scholar]
- Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Fahey B, Hickey B, Kelleher D, O'Dwyer AM, O'Mara SM. The widely-used anti-viral drug interferon-alpha induces depressive- and anxiogenic-like effects in healthy rats. Behavioural Brain Research. 2007;182:80–87. doi: 10.1016/j.bbr.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–428. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Pace TW, Woolwine BJ, Hu F, Raison CL, Miller AH. Early activation of p38 mitogen activated protein kinase is associated with interferon-alpha-induced depression and fatigue. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Cole SW, Pace TW, Hu F, Woolwine BJ, Doho GH, Raison CL, Miller AH. Molecular signatures of peripheral blood mononuclear cells during chronic interferon-alpha treatment: relationship with depression and fatigue. Psychol Med. 2012a;42:1591–1603. doi: 10.1017/S0033291711002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, Miller AH. Tyrosine metabolism during interferon-alpha administration: Association with fatigue and CSF dopamine concentrations. Brain Behav Immun. 2012b doi: 10.1016/j.bbi.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33:315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American journal of preventive medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. The Journal of cell biology. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neuroscience and biobehavioral reviews. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P, O'Brien SM, Scully P, Rijkers K, Scott LV, Dinan TG. Cutaneous glucocorticoid receptor sensitivity and pro-inflammatory cytokine levels in antidepressant-resistant depression. Psychol Med. 2006;36:37–43. doi: 10.1017/S003329170500632X. [DOI] [PubMed] [Google Scholar]
- Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun. 2013;27:1–7. doi: 10.1016/j.bbi.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Foley DL, Neale MC, Gardner CO, Pickles A, Prescott CA, Kendler KS. Major depression and associated impairment: same or different genetic and environmental risk factors? Am J Psychiatry. 2003;160:2128–2133. doi: 10.1176/appi.ajp.160.12.2128. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommberger UH, Bauer J, Haselbauer P, Fraulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247:228–233. doi: 10.1007/BF02900219. [DOI] [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biology of mood & anxiety disorders. 2011;1:11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao-de Almeida A, Quarantini LC, Sampaio AS, Lyra AC, Parise CL, Parana R, de Oliveira IR, Koenen KC, Miranda-Scippa A, Guindalini C. Lack of association of indoleamine 2,3-dioxygenase polymorphisms with interferon-alpha-related depression in hepatitis C. Brain Behav Immun. 2011;25:1491–1497. doi: 10.1016/j.bbi.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Spencer JA, Holzer CE, 3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2006;1:410–420. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- Gertsik L, Poland RE, Bresee C, Rapaport MH. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012;32:61–64. doi: 10.1097/JCP.0b013e31823f3b5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007a;165:1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007b;61:631–637. doi: 10.1136/jech.2006.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg LD, Oubre AY, Daoud YA. L-methylfolate Plus SSRI or SNRI from Treatment Initiation Compared to SSRI or SNRI Monotherapy in a Major Depressive Episode. Innov Clin Neurosci. 2011;8:19–28. [PMC free article] [PubMed] [Google Scholar]
- Gochee PA, Powell EE, Purdie DM, Pandeya N, Kelemen L, Shorthouse C, Jonsson JR, Kelly B. Association between apolipoprotein E epsilon4 and neuropsychiatric symptoms during interferon alpha treatment for chronic hepatitis C. Psychosomatics. 2004;45:49–57. doi: 10.1176/appi.psy.45.1.49. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005;169:97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. The role of pro-inflammatory cytokines in memory processes and neural plasticity. In: Ader R, et al., editors. Psychoneuroimmunology, 4E. Elsevier, Inc.; 2007. [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): A central regulator of stress responses. Frontiers in Neuroendocrinology. 2009;30:30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Greenberg DB, Gray JL, Mannix CM, Eisenthal S, Carey M. Treatment-related fatigue and serum interleukin-1 levels in patients during external beam irradiation for prostate cancer. J Pain Symptom Manage. 1993;8:196–200. doi: 10.1016/0885-3924(93)90127-h. [DOI] [PubMed] [Google Scholar]
- Greig NH, Soncrant TT, Wozniak KM, Rapoport SI. Plasma and tissue pharmacokinetics of human interferon-alpha in the rat after its intravenous administration. J Pharmacol Exp Ther. 1988;245:574–580. [PubMed] [Google Scholar]
- Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behavior and Immunity. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gudmundsson P, Skoog I, Waern M, Blennow K, Palsson S, Rosengren L, Gustafson D. The relationship between cerebrospinal fluid biomarkers and depression in elderly women. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2007;15:832–838. doi: 10.1097/JGP.0b013e3180547091. [DOI] [PubMed] [Google Scholar]
- Guidetti P, Schwarcz R. 3-Hydroxykynurenine and quinolinate: pathogenic synergism in early grade Huntington's disease? Adv Exp Med Biol. 2003;527:137–145. doi: 10.1007/978-1-4615-0135-0_16. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer's disease hippocampus. Neuropathol Appl Neurobiol. 2005a;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Meininger V, Brew BJ. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis. 2005b;2:166–176. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005c;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005d;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Molecular neurobiology. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, Luo F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur J Pharmacol. 2009;612:54–60. doi: 10.1016/j.ejphar.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Haeffel GJ, Getchell M, Koposov RA, Yrigollen CM, Deyoung CG, Klinteberg BA, Oreland L, Ruchkin VV, Grigorenko EL. Association between polymorphisms in the dopamine transporter gene and depression: evidence for a gene-environment interaction in a sample of juvenile detainees. Psychological science. 2008;19:62–69. doi: 10.1111/j.1467-9280.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nature neuroscience. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009a;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009b;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Nagatsu T, Ohta T, Mizutani M, Omura I. Changes in the concentrations of tetrahydrobiopterin, the cofactor of tyrosine hydroxylase, in blood under physical stress and in depression. Annals of the New York Academy of Sciences. 2004;1018:378–386. doi: 10.1196/annals.1296.047. [DOI] [PubMed] [Google Scholar]
- Hayley S, Poulter M, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135:659–678. doi: 10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull. 1997;33:185–192. [PubMed] [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behavior genetics. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, Dilling LA, Elia J, Kruesi MJ, Lackner A, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS & Neurological Disorders Drug Targets. 2009;8:451–458. doi: 10.2174/187152709789824624. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Huang L, Baban B, Johnson BA, 3rd, Mellor AL. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int Rev Immunol. 2010;29:133–155. doi: 10.3109/08830180903349669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett. 2008;432:232–236. doi: 10.1016/j.neulet.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: Pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Taylor S, Stein MB, Moon EC. Heritability of individual depressive symptoms. J Affect Disord. 2004;80:125–133. doi: 10.1016/S0165-0327(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Jazayeri S, Keshavarz SA, Tehrani-Doost M, Djalali M, Hosseini M, Amini H, Chamari M, Djazayery A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010;178:112–115. doi: 10.1016/j.psychres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Jehn CF, Kuehnhardt D, Bartholomae A, Pfeiffer S, Krebs M, Regierer AC, Schmid P, Possinger K, Flath BC. Biomarkers of depression in cancer patients. Cancer. 2006;107:2723–2729. doi: 10.1002/cncr.22294. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, Rinne JO. Increased frontal [(18)F]fluorodopa uptake in early Parkinson's disease: sex differences in the prefrontal cortex. Brain. 2001;124:1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- Kamata M, Higuchi H, Yoshimoto M, Yoshida K, Shimizu T. Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2000;10:129–132. doi: 10.1016/s0924-977x(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Kudo K, Mabuchi T, Takemoto K, Fujimaki K, Wati H, Iguch iH, Tezuka H, Kanba S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31:2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- Kanellopoulos D, Gunning FM, Morimoto SS, Hoptman MJ, Murphy CF, Kelly RE, Glatt C, Lim KO, Alexopoulos GS. Hippocampal volumes and the brain-derived neurotrophic factor val66met polymorphism in geriatric major depression. American Journal of Geriatric Psychiatry. 2011;19:13–22. doi: 10.1097/jgp.0b013e3181f61d62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi K, Inman MD, Bienenstock J, Forsythe P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. American Journal of Respiratory and Critical Care Medicine. 2009;179:186–193. doi: 10.1164/rccm.200806-951OC. [DOI] [PubMed] [Google Scholar]
- Katsuura G, Arimura A, Koves K, Gottschall PE. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1 beta-induced ACTH release. Am J Physiol. 1990;258:E163–E171. doi: 10.1152/ajpendo.1990.258.1.E163. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kazumori H, Ishihara S, Rumi MA, Ortega-Cava CF, Kadowaki Y, Kinoshita Y. Transforming growth factor-alpha directly augments histidine decarboxylase and vesicular monoamine transporter 2 production in rat enterochromaffin-like cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G508–G514. doi: 10.1152/ajpgi.00269.2003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Genetic risk, number of previous depressive episodes, and stressful life events in predicting onset of major depression. Am J Psychiatry. 2001;158:582–586. doi: 10.1176/appi.ajp.158.4.582. [DOI] [PubMed] [Google Scholar]
- Kenis G, Prickaerts J, van Os J, Koek GH, Robaeys G, Steinbusch HWM, Wichers M. Depressive symptoms following interferon-α therapy: mediated by immune-induced reductions in brain-derived neurotrophic factor? International Journal of Neuropsychopharmacology. 2010;14:247–253. doi: 10.1017/S1461145710000830. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaodhiar L, Ling PR, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and C-reactive protein correlate with body mass index across the broad range of obesity. JPEN J Parenter Enteral Nutr. 2004;28:410–415. doi: 10.1177/0148607104028006410. [DOI] [PubMed] [Google Scholar]
- Kim B, Jeong HK, Kim JH, Lee SY, Jou I, Joe EH. Uridine 5'-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J Immunol. 2011;186:3701–3709. doi: 10.4049/jimmunol.1000212. [DOI] [PubMed] [Google Scholar]
- Kim SU, de Vellis J. Microglia in health and disease. Journal of neuroscience research. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978:104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. The Journal of comparative neurology. 2004;472:113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010a;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler NJ, Duman RS. Nuclear factor-kb is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MR, Al-Taie O, Schafer A, Pfersdorff M, Lesch KP, Scheurlen M. Serotonin-1A receptor gene HTR1A variation predicts interferon-induced depression in chronic hepatitis C. Gastroenterology. 2007;132:1279–1286. doi: 10.1053/j.gastro.2007.02.053. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kruijshaar ME, Barendregt J, Vos T, de Graaf R, Spijker J, Andrews G. Lifetime prevalence estimates of major depression: an indirect estimation method and a quantification of recall bias. European journal of epidemiology. 2005;20:103–111. doi: 10.1007/s10654-004-1009-0. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Coyle PK, Doscher C, Miller A, Cross AH, Jandorf L, Halper J, Johnson B, Morgante L, Grimson R. Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology. 1995;45:1956–1961. doi: 10.1212/wnl.45.11.1956. [DOI] [PubMed] [Google Scholar]
- Kumai T, Tateishi T, Tanaka M, Watanabe M, Shimizu H, Kobayashi S. Effect of interferon-alpha on tyrosine hydroxylase and catecholamine levels in the brain of rats. Life Sci. 2000;67:663–669. doi: 10.1016/s0024-3205(00)00660-3. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Hor iH, Adachi N, Numakawa T. Interface between hypothalamic-pituitary-adrenal axis and brain-derived neurotrophic factor in depression. Psychiatry & Clinical Neurosciences. 2010;64:477–459. doi: 10.1111/j.1440-1819.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Julien C, Cordeau P, Bohacek I, Weng YC, Calon F, Kriz J. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke; a journal of cerebral circulation. 2011;42:2903–2909. doi: 10.1161/STROKEAHA.111.620856. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders KL, Palmer AJ, Quinn N, Clark JC, Firnau G, Garnett ES, Nahmias C, Jones T, Marsden CD. Brain dopamine metabolism in patients with Parkinson's disease measured with positron emission tomography. Journal of neurology, neurosurgery, and psychiatry. 1986;49:853–860. doi: 10.1136/jnnp.49.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Lieb J, Karmali R, Horrobin D. Elevated levels of prostaglandin E2 and thromboxane B2 in depression. Prostaglandins Leukot Med. 1983;10:361–367. doi: 10.1016/0262-1746(83)90048-3. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W, Hong S, Nelesen R, Dimsdale JE. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch Intern Med. 2005;165:910–915. doi: 10.1001/archinte.165.8.910. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Whorton AR, Rubinow DR, Cowdry RW, Ninan PT, Waters RN. CSF prostaglandin levels in depressed and schizophrenic patients. Arch Gen Psychiatry. 1983;40:405–406. doi: 10.1001/archpsyc.1983.01790040059008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Samuel CE. Editing of glutamate receptor subunit B pre-mRNA by splice-site variants of interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J Biol Chem. 1999;274:5070–5077. doi: 10.1074/jbc.274.8.5070. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu F, Wang G, Xiao Z, Tang J, Liu W, Wang H, Liu H, Wang X, Wu Y, Cao Z, Li W. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007;414:155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Loberiza FR, Jr, Rizzo JD, Bredeson CN, Antin JH, Horowitz MM, Weeks JC, Lee SJ. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J Clin Oncol. 2002;20:2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175–190. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Hauser P, Macey TA, Lowe JD. Can rodents be used to model interferon-alpha-induced depressive symptoms? Prog Neuropsychopharmacol Biol Psychiatry. 2006a;30:1364–1365. doi: 10.1016/j.pnpbp.2006.04.004. author reply 1366. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Patterson AL, Wilhelm CJ, McNett H, Morasco BJ, Huckans M, Morgan T, Saperstein S, Asghar A, Hauser P. Vulnerability to somatic symptoms of depression during interferon-alpha therapy for hepatitis C: A 16-week prospective study. J Psychosom Res. 2013;74:57–63. doi: 10.1016/j.jpsychores.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Wall JM, Pagel RL, Hauser P. Administration of pegylated interferon-alpha-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006b;31:1289–1294. doi: 10.1016/j.psyneuen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Lopresti AL, Hood SD, Drummond PD. Multiple antidepressant potential modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J Psychopharmacol. 2012;26:1512–1524. doi: 10.1177/0269881112458732. [DOI] [PubMed] [Google Scholar]
- Lotrich F. Management of Psychiatric Disease in Hepatitis C Treatment Candidates. Curr Hepat Rep. 2010;9:113–118. doi: 10.1007/s11901-010-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich F. Inflammatory cytokines, growth factors, and depression. Curr Pharm Des. 2012;18:5920–5935. doi: 10.2174/138161212803523680. [DOI] [PubMed] [Google Scholar]
- Lotrich FE. Major depression during interferon-alpha treatment: vulnerability and prevention. Dialogues Clin Neurosci. 2009;11:417–425. doi: 10.31887/DCNS.2009.11.4/felotrich. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE. Gene-environment interactions in geriatric depression. Psychiatr Clin North Am. 2011;34:357–376. viii. doi: 10.1016/j.psc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Albusaysi S, Ferrell RE. Brain-derived neurotrophic factor serum levels and genotype: association with depression during interferon-alpha treatment. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2012.263. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Labile anger during interferon alfa treatment is associated with a polymorphism in tumor necrosis factor alpha. Clin Neuropharmacol. 2010a;33:191–197. doi: 10.1097/WNF.0b013e3181de8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Loftis JM, Ferrell RE, Rabinovitz M, Hauser P. IL28B Polymorphism Is Associated with Both Side Effects and Clearance of Hepatitis C During Interferon-Alpha Therapy. J Interferon Cytokine Res. 2010b doi: 10.1089/jir.2010.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG, Kirshner M, Ferrell RF, Reynolds CF., Iii Serotonin transporter genotype interacts with paroxetine plasma levels to influence depression treatment response in geriatric patients. J Psychiatry Neurosci. 2008;33:123–130. [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Sears B, McNamara RK. Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: Relationship with interleukin-6. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY, Leung YM, Su KP. Interferon-alpha induces nitric oxide synthase expression and haem oxygenase-1 down-regulation in microglia: implications of cellular mechanism of IFN-alpha-induced depression. Int J Neuropsychopharmacol. 2012:1–12. doi: 10.1017/S1461145712000338. [DOI] [PubMed] [Google Scholar]
- Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang Z, Wang Z, Wang JM, Le Y. Resveratrol differentially modulates inflammatory responses of microglia and astrocytes. J Neuroinflammation. 2010;7:46. doi: 10.1186/1742-2094-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucae S, Salyakina D, Barden N, Harvey M, Gagne B, Labbe M, Binder EB, Uhr M, Paez-Pereda M, Sillaber I, Ising M, Bruckl T, Lieb R, Holsboer F, Muller-Myhsok B. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Human molecular genetics. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- Ma D, Forsythe P, Bienenstock J. Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alph-induced interleukin-8 expression. Infection and/Immunity. 2004;72:5308–5314. doi: 10.1128/IAI.72.9.5308-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock C, Landau S, Barry K, Maulayah P, Hotopf M, Cleare AJ, Norris S, Pariante CM. Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry. 2005;10:332–333. doi: 10.1038/sj.mp.4001634. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry. 1993;150:1189–1193. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Verkerk R, Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuro Endocrinol Lett. 2011;32:264–273. [PubMed] [Google Scholar]
- Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C, de Jonckheere C, Minner B, Raus J. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med. 1992;22:45–53. doi: 10.1017/s0033291700032712. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, Sanacora G, Owens MJ, Nemeroff CB, Rajeevan N, Baldwin RM, Seibyl JP, Innis RB, Charney DS. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry. 1998;44:1090–1098. doi: 10.1016/s0006-3223(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Manki H, Kanba S, Muramatsu T, Higuchi S, Suzuki E, Matsushita S, Ono Y, Chiba H, Shintani F, Nakamura M, Yagi G, Asai M. Dopamine D2, D3 and D4 receptor and transporter gene polymorphisms and mood disorders. J Affect Disord. 1996;40:7–13. doi: 10.1016/0165-0327(96)00035-3. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, Dwork AJ, Arango V. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, Braganza S, Tannock IF. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29:32–38. doi: 10.1002/da.20876. [DOI] [PubMed] [Google Scholar]
- Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. 1999;7:69–84. [PubMed] [Google Scholar]
- Masilamoni GJ, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T, Smith Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134:2057–2073. doi: 10.1093/brain/awr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci. 2004;9:2819–2826. doi: 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011 doi: 10.1111/j.1469-7580.2010.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney WT, Jr, Eising RG, Moran EC, Suomi SJ, Harlow HF. Effects of reserpine on the social behavior of rhesus monkeys. Dis Nerv Syst. 1971;32:735–741. [PubMed] [Google Scholar]
- McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Lotrich FE. Elevated immune-inflammatory signaling in mood disorders: a new therapeutic target? Expert Rev Neurother. 2012;12:1143–1161. doi: 10.1586/ern.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt MD, Liu S, Manatunga A, Royster EB, Raison CL, Woolwine BJ, Demetrashvili MF, Miller AH, Musselman DL. Neurobehavioral effects of interferon-alpha in patients with hepatitis-C: symptom dimensions and responsiveness to paroxetine. Neuropsychopharmacology. 2012;37:1444–1454. doi: 10.1038/npp.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Kessler JA. Hematolymphopoietic and inflammatory cytokines in neural development. Trends in Neurosciences. 1997;20:357–365. doi: 10.1016/s0166-2236(96)01045-4. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Meisner F, Scheller C, Kneitz S, Sopper S, Neuen-Jacob E, Riederer P, ter Meulen V, Koutsilieri E. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: a novel pharmacological action of memantine. Neuropsychopharmacology. 2008;33:2228–2236. doi: 10.1038/sj.npp.1301615. [DOI] [PubMed] [Google Scholar]
- Mendall MA, Patel P, Asante M, Ballam L, Morris J, Strachan DP, Camm AJ, Northfield TC. Relation of serum cytokine concentrations to cardiovascular risk factors and coronary heart disease. Heart. 1997;78:273–277. doi: 10.1136/hrt.78.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. International clinical psychopharmacology. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, Edwards C, Mattis P, Eidelberg D. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson's disease. Am J Psychiatry. 2002;159:746–754. doi: 10.1176/appi.ajp.159.5.746. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13:216–226. [PubMed] [Google Scholar]
- Miller DW. Immunobiology of the blood-brain barrier. J Neurovirol. 1999;5:570–578. doi: 10.3109/13550289909021286. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of proinflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002a;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Cole SW. Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry. 2012;72:34–40. doi: 10.1016/j.biopsych.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–285. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002b;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Mizoi Y, Kaneko H, Oharazawa A, Kuroiwa H. [Parkinsonism in a patient receiving interferon alpha therapy for chronic hepatitis C] Rinsho Shinkeigaku. 1997;37:54–56. [PubMed] [Google Scholar]
- Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RC, Elzinga BM. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:380–386. doi: 10.1016/j.pnpbp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T, Caligiuri MA, Villalona-Calero MA. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- Moraska AR, Sood A, Dakhil SR, Sloan JA, Barton D, Atherton PJ, Suh JJ, Griffin PC, Johnson DB, Ali A, Silberstein PT, Duane SF, Loprinzi CL. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28:3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa O, Sakai N, Obara H, Saito N. Effects of interferon-alpha, interferon-gamma and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol. 1998;349:317–324. doi: 10.1016/s0014-2999(98)00187-3. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PL, Flynn PJ, Hynes HE, Banerjee TK, Kirshner JJ, King DK. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21:4635–4641. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- Mossner R, Heils A, Stober G, Okladnova O, Daniel S, Lesch KP. Enhancement of serotonin transporter function by tumor necrosis factor alpha but not by interleukin-6. Neurochem Int. 1998;33:251–254. doi: 10.1016/s0197-0186(98)00026-6. [DOI] [PubMed] [Google Scholar]
- Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Muskin PR. Major depressive disorder and other medical illness: a two-way street. Ann Clin Psychiatry. 2010;22:S15–S20. [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001a;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001b;158:1252–1257. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Bluthe RM, May MJ, Dantzer R, Parnet P. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropsychopharmacology. 2005;30:1492–1499. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A. Childhood Maltreatment Predicts Unfavorable Course of Illness and Treatment Outcome in Depression: A Meta-Analysis. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci U S A. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurauter G, Grahmann AV, Klieber M, Zeimet A, Ledochowski M, Sperner-Unterweger B, Fuchs D. Serum phenylalanine concentrations in patients with ovarian carcinoma correlate with concentrations of immune activation markers and of isoprostane-8. Cancer Lett. 2008a;272:141–147. doi: 10.1016/j.canlet.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Neurauter G, Schrocksnadel K, Scholl-Burgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, Fuchs D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008b;9:622–627. doi: 10.2174/138920008785821738. [DOI] [PubMed] [Google Scholar]
- Nichol CA, Lee CL, Edelstein MP, Chao JY, Duch DS. Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1546–1550. doi: 10.1073/pnas.80.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, Miyahara S, Rush AJ. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40:41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S, Ueno R, Ohishi K, Sakai T, Hayaishi O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am J Psychiatry. 1989;146:365–368. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009a;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009b;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi K, Ueno R, Nishino S, Sakai T, Hayaishi O. Increased level of salivary prostaglandins in patients with major depression. Biol Psychiatry. 1988;23:326–334. doi: 10.1016/0006-3223(88)90283-1. [DOI] [PubMed] [Google Scholar]
- Opp MR, Born J, Irwin MR. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. New York, NY: Academic Press; 2007. pp. 570–618. [Google Scholar]
- Oxenkrug G, Perianayagam M, Mikolich D, Requintina P, Shick L, Ruthazer R, Zucker D, Summergrad P. Interferon-gamma (+874) T/A genotypes and risk of IFN-alpha-induced depression. J Neural Transm. 2011;118:271–274. doi: 10.1007/s00702-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have an antidepressant effect? Biomedical Pharmacotherapy. 2008;62:308–311. doi: 10.1016/j.biopha.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Palma JP, Kwon D, Clipstone NA, Kim BS. Infection with Theiler's murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-kappaB activation: potential role for the initiation of demyelinating disease. J Virol. 2003;77:6322–6331. doi: 10.1128/JVI.77.11.6322-6331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Interactions of cytokines with the blood-brain barrier: implications for feeding. Curr Pharm Des. 2003;9:827–831. doi: 10.2174/1381612033455332. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mischoulon D, Green CH, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 2: predictors of relapse during the continuation phase of pharmacotherapy. J Clin Psychiatry. 2004a;65:1096–1098. doi: 10.4088/jcp.v65n0811. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004b;65:1090–1095. doi: 10.4088/jcp.v65n0810. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Pearce BD, Pisell TL, Sanchez CI, Po C, Su C, Miller AH. The proinflammatory cytokine, interleukin-1alpha, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Patel HC, Boutin H, Allan SM. Interleukin-1 in the brain: mechanisms of action in acute neurodegeneration. Annals of the New York Academy of Science. 2003;992:39–47. doi: 10.1111/j.1749-6632.2003.tb03136.x. [DOI] [PubMed] [Google Scholar]
- Payne B, Hateley C, Ong E, Premchand N, Schmid M, Schwab U, Newton J, Price D. HIV-associated fatigue in the era of highly active antiretroviral therapy: novel biological mechanisms? HIV Med. 2012 doi: 10.1111/j.1468-1293.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- Pemberton LA, Kerr SJ, Smythe G, Brew BJ. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interferon Cytokine Res. 1997;17:589–595. doi: 10.1089/jir.1997.17.589. [DOI] [PubMed] [Google Scholar]
- Peng CH, Chiou SH, Chen SJ, Chou YC, Ky HH, Cheng CK. Neuroprotection by imipramine against lipopolysaccharide-induced apoptosis in hippocampus-dreived neural stem cellsmediated by activation of BDNF and the MAPK pathway. European Neuropsychopharmacology. 2008;18:128–140. doi: 10.1016/j.euroneuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Perez-De La Cruz V, Carrillo-Mora P, Santamaria A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int J Tryptophan Res. 2012;5:1–8. doi: 10.4137/IJTR.S8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Marshall EF, Blessed G, Tomlinson BE, Perry RH. Decreased imipramine binding in the brains of patients with depressive illness. Br J Psychiatry. 1983;142:188–192. doi: 10.1192/bjp.142.2.188. [DOI] [PubMed] [Google Scholar]
- Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Archives of neurology. 2010;67:1442–1448. doi: 10.1001/archneurol.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for 'depression due to a general medical condition', immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Fabbri C, Serretti A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22:239–258. doi: 10.1016/j.euroneuro.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Possel H, Noack H, Putzke J, Wolf G, Sies H. Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia. 2000;32:51–59. doi: 10.1002/1098-1136(200010)32:1<51::aid-glia50>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Prasad AS. Impact of the discovery of human zinc deficiency on health. Journal of the American College of Nutrition. 2009;28:257–265. doi: 10.1080/07315724.2009.10719780. [DOI] [PubMed] [Google Scholar]
- Prather AA, Rabinovitz M, Pollock BG, Lotrich FE. Cytokine-induced depression during IFN-alpha treatment: the role of IL-6 and sleep quality. Brain Behav Immun. 2009;23:1109–1116. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pregelj P, Nedic G, Paska AV, Zupanc T, Nikolac M, Balazic J, Tomori M, Komel R, Seler DM, Pivac N. The association between brain-derived neurotrophic factor polymorphism (BDNF Val66Met) and suicide. Journal of Affective Disorders. 2011;128:287–290. doi: 10.1016/j.jad.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci E, Branas P, D'Amico R, Giuliani G, Solari A, Taus C. Amantadine for fatigue in multiple sclerosis. Cochrane database of systematic reviews (Online) 2007:CD002818. doi: 10.1002/14651858.CD002818.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005a;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2010a;15:535–547. doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010b;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005b;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry. 2003;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. Arch Gen Psychiatry. 2012:1–11. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP, Zajecka JM, Bruno CJ, Henderson MA, Reinus JF, Evans DL, Asnis GM, Miller AH. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25:1163–1174. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- Rammohan KW, Rosenberg JH, Lynn DJ, Blumenfeld AM, Pollak CP, Nagaraja HN. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. Journal of neurology, neurosurgery, and psychiatry. 2002;72:179–183. doi: 10.1136/jnnp.72.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramwell PW, Foegh M, Loeb R, Leovey EM. Synthesis and metabolism of prostaglandins, prostacyclin, and thromboxanes: the arachidonic acid cascade. Seminars in perinatology. 1980;4:3–13. [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature Reviews Gastroenterology and Hepatology. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. The AAPS journal. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M, Pritschet K, Schmidt B. Blocking type I interferon production: a new therapeutic option to reduce the HIV-1-induced immune activation. Clin Dev Immunol. 2012:534929. doi: 10.1155/2012/534929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, Ryffel B, Couillin I. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Miller GE. Acute deviations from long-term trait depressive symptoms predict systemic inflammatory activity. Brain Behav Immun. 2008;22:709–716. doi: 10.1016/j.bbi.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Rojas PS, Fritsch R, Rojas RA, Jara P, Fiedler JL. Serum brain-derived neurotrophic factor and glucocorticoid receptor levels in lymphocytes as markers of antidepressant response in major depressive patients: a pilot study. Psychiatry Res. 2011;189:239–245. doi: 10.1016/j.psychres.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Rosenzweig-Lipson S, Hesterberg P, Bergman J. Observational studies of dopamine D1 and D2 agonists in squirrel monkeys. Psychopharmacology (Berl) 1994;116:9–18. doi: 10.1007/BF02244865. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, Price RW. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1996;37:1133–1141. [PubMed] [Google Scholar]
- Roy A, Evers SE, Avison WR, Campbell MK. Higher zinc intake buffers the impact of stress on depressive symptoms in pregnancy. Nutrition Research. 2010;30:695–704. doi: 10.1016/j.nutres.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Roy A, Linnoila M, Karoum F, Pickar D. Relative activity of metabolic pathways for norepinephrine in endogenous depression. Acta psychiatrica Scandinavica. 1986;73:624–628. doi: 10.1111/j.1600-0447.1986.tb02734.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60:56–63. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Hasegawa C, Okura M, Morikawa O, Ueyama T, Shirai Y, Sakai N, Saito N. Novel variants of murine serotonin transporter mRNA and the promoter activity of its upstream site. Neurosci Lett. 2003;342:175–178. doi: 10.1016/s0304-3940(03)00292-1. [DOI] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O'Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Hormones and behavior. 2012;62:202–209. doi: 10.1016/j.yhbeh.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, Maestripieri D, Miller AH. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12:895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- Sarasombath P, Sumida K, Kaku DA. Parkinsonism associated with interferon alpha therapy for chronic myelogenous leukemia. Hawaii Med J. 2002;61:48–57. [PubMed] [Google Scholar]
- Sarchiapone M, Carli V, Roy A, Iacoviello L, Cuomo C, Latella M, di Giannantonio M, Janiri L, de Gaetano M, Janal M. Association of polymorphism (Val66Met) of brain-derived neurotrophic factor with suicide attempts in depressed patients. Neuropsychobiology. 2008;57:139–145. doi: 10.1159/000142361. [DOI] [PubMed] [Google Scholar]
- Sato T, Suzuki E, Yokoyama M, Semba J, Watanabe S, Miyaoka H. Chronic intraperitoneal injection of interferon-alpha reduces serotonin levels in various regions of rat brain, but does not change levels of serotonin transporter mRNA, nitrite or nitrate. Psychiatry Clin Neurosci. 2006;60:499–506. doi: 10.1111/j.1440-1819.2006.01538.x. [DOI] [PubMed] [Google Scholar]
- Schlaak JF, Trippler M, Hoyo-Becerra C, Erim Y, Kis B, Wang B, Scherbaum N, Gerken G. Selective hyper-responsiveness of the interferon system in major depressive disorders and depression induced by interferon therapy. PLoS One. 2012;7:e38668. doi: 10.1371/journal.pone.0038668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobitz B, Voorhuis DA, De Kloet ER. Localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Neurosci Lett. 1992;136:189–192. doi: 10.1016/0304-3940(92)90046-a. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010;177:1576–1591. doi: 10.2353/ajpath.2010.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AJ, Veledar E, Hong Y, Bremner JD, Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch Gen Psychiatry. 2011;68:1135–1142. doi: 10.1001/archgenpsychiatry.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Ohtani K, Sato N, Nagamine T, Mori M. Increase in serum interleukin-6, plasma ACTH and serum cortisol levels after systemic interferon-alpha administration. Endocr J. 1995;42:551–556. doi: 10.1507/endocrj.42.551. [DOI] [PubMed] [Google Scholar]
- Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747:348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- Sissolak G, Hoffbrand AV, Mehta AB, Ganeshaguru K. Effects of interferon-alpha (IFN) on the expression of interleukin 1-beta (IL-1), interleukin 6 (IL-6), granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor-alpha (TNF) in acute myeloid leukemia (AML) blasts. Vol. 6. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK; 1992. pp. 1155–1160. [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P. P2X(7) Receptors in Neurological and Cardiovascular Disorders. Cardiovascular psychiatry and neurology. 2009:861324. doi: 10.1155/2009/861324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluzewska A. Indicators of immune activation in depressed patients. Adv Exp Med Biol. 1999;461:59–73. doi: 10.1007/978-0-585-37970-8_4. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Sobieska M, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35:123–127. doi: 10.1159/000119332. [DOI] [PubMed] [Google Scholar]
- Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry. 1998;3:508–511. doi: 10.1038/sj.mp.4000425. [DOI] [PubMed] [Google Scholar]
- Smith AK, Simon JS, Gustafson EL, Noviello S, Cubells JF, Epstein MP, Devlin DJ, Qiu P, Albrecht JK, Brass CA, Sulkowski MS, McHutchinson JG, Miller AH. Association of a polymorphism in the indoleamine- 2,3-dioxygenase gene and interferon-alpha-induced depression in patients with chronic hepatitis C. Mol Psychiatry. 2012;17:781–789. doi: 10.1038/mp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Song C, Merali Z, Anisman H. Variations of nucleus accumbens dopamine and serotonin following systemic interfleukin-1, interleukin-2, or interleukin-6 treatment. Neuroscience. 1999;88:823–836. doi: 10.1016/s0306-4522(98)00271-1. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Moeller JR, Dhawan V, Ishikawa T, Eidelberg D. Visualizing the evolution of abnormal metabolic networks in the brain using PET. Comput Med Imaging Graph. 1995;19:295–306. doi: 10.1016/0895-6111(95)00011-e. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O'Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12:739–744. doi: 10.1017/s1092852900015418. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Waubant E, Confavreux C, Edan G, Debouverie M, Rumbach L, Moreau T, Pelletier J, Lubetzki C, Clanet M. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 2005;64:1139–1143. doi: 10.1212/01.WNL.0000158272.27070.6A. [DOI] [PubMed] [Google Scholar]
- Stein DJ. Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr. 2008;13:561–565. doi: 10.1017/s1092852900016837. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of psychiatric research. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends in pharmacological sciences. 2000a;21:149–154. doi: 10.1016/s0165-6147(00)01451-6. [DOI] [PubMed] [Google Scholar]
- Stone TW. Inhibitors of the kynurenine pathway. Eur J Med Chem. 2000b;35:179–186. doi: 10.1016/s0223-5234(00)00121-5. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC, Aitchison KJ, Pariante CM. Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry. 2010;67:550–557. doi: 10.1016/j.biopsych.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N, Zhang L, Fei F, Hu H, Wang K, Hui H, Jiang XF, Li X, Zhen HN, Li J, Cao BP, Dang W, Qu Y, Zhou F. The brain-derived neurotrophic factor is associated with alcohol dependence-related depression and antidepressant response. Brain Research. 2011:1415. doi: 10.1016/j.brainres.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun. 2008;22:960–968. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. Journal of leukocyte biology. 2010;88:33–39. doi: 10.1189/jlb.0210072. [DOI] [PubMed] [Google Scholar]
- Sugawara Y, Akechi T, Shima Y, Okuyama T, Akizuki N, Nakano T, Uchitomi Y. Efficacy of methylphenidate for fatigue in advanced cancer patients: a preliminary study. Palliat Med. 2002;16:261–263. doi: 10.1191/0269216302pm547xx. [DOI] [PubMed] [Google Scholar]
- Sun S, Xia S, Ji Y, Kersten S, Qi L. The ATP-P2X7 signaling axis is dispensable for obesity-associated inflammasome activation in adipose tissue. Diabetes. 2012;61:1471–1478. doi: 10.2337/db11-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targum SD, Fava M. Fatigue as a residual symptom of depression. Innov Clin Neurosci. 2011;8:40–43. [PMC free article] [PubMed] [Google Scholar]
- Tavares RG, Schmidt AP, Abud J, Tasca CI, Souza DO. In vivo quinolinic acid increases synaptosomal glutamate release in rats: reversal by guanosine. Neurochemical research. 2005;30:439–444. doi: 10.1007/s11064-005-2678-0. [DOI] [PubMed] [Google Scholar]
- Tavares RG, Tasca CI, Santos CES, Alves LB, Porciuncula LO, Emanuelli T, Souza DO. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochemistry international. 2002;40:621–627. doi: 10.1016/s0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- Teeling JL, Cunningham C, Newman TA, Perry VH. The effect of non-steroidal antiinflammatory agents on behavioural changes and cytokine production following systemic inflammation: Implications for a role of COX-1. Brain Behav Immun. 2010;24:409–419. doi: 10.1016/j.bbi.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. Journal of neuroscience research. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiology of Aging. 2008;29:1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trefz FK, Burton BK, Longo N, Casanova MM, Gruskin DJ, Dorenbaum A, Kakkis ED, Crombez EA, Grange DK, Harmatz P, Lipson MH, Milanowski A, Randolph LM, Vockley J, Whitley CB, Wolff JA, Bebchuk J, Christ-Schmidt H, Hennermann JB. Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. J Pediatr. 2009;154:700–707. doi: 10.1016/j.jpeds.2008.11.040. [DOI] [PubMed] [Google Scholar]
- Treisman G, Fishman M, Schwartz J, Hutton H, Lyketsos C. Mood disorders in HIV infection. Depress Anxiety. 1998;7:178–187. doi: 10.1002/(sici)1520-6394(1998)7:4<178::aid-da6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT, Lin CF, Wu HT, Wu SR, Tsai WH. Interferon-alpha-induced serotonin uptake in Jurkat T cells via mitogen-activated protein kinase and transcriptional regulation of the serotonin transporter. J Psychopharmacol. 2008;22:753–760. doi: 10.1177/0269881107082951. [DOI] [PubMed] [Google Scholar]
- Utz JR, Lorentz CP, Markowitz D, Rudser KD, Diethelm-Okita B, Erickson D, Whitley CB. START, a double blind, placebo-controlled pharmacogenetic test of responsiveness to sapropterin dihydrochloride in phenylketonuria patients. Mol Genet Metab. 2012;105:193–197. doi: 10.1016/j.ymgme.2011.10.014. [DOI] [PubMed] [Google Scholar]
- van Rossum EF, Binder EB, Majer M, Koper JW, Ising M, Modell S, Salyakina D, Lamberts SW, Holsboer F. Polymorphisms of the glucocorticoid receptor gene and major depression. Biol Psychiatry. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML, Licinio J, Gold PW, Hermida RC, Mastorakos G, Chrousos GP. Circadian interleukin-6 secretion and quantity and depth of sleep. The Journal of clinical endocrinology and metabolism. 1999;84:2603–2607. doi: 10.1210/jcem.84.8.5894. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. The Journal of clinical endocrinology and metabolism. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R. NMDA Receptor Blockade By Ketamine Abrogates Lipopolysaccharide-Induced Depressive-Like Behavior IN C57BL/6J MICE. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Campbell IL, Zhang H. Systemic interferon-alpha regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry. 2008;13:293–301. doi: 10.1038/sj.mp.4002013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu X, Yu Y, Han Y, Wei J, Collier D, Li T, Ma X. The role of single nucleotide polymorphism of D2 dopamine receptor gene on major depressive disorder and response to antidepressant treatment. Psychiatry Res. 2012;200:1047–1050. doi: 10.1016/j.psychres.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Mooney-Heiberger K, Martinez J, Furness L, Smith KP, Maier SF. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Oscillations in the basal ganglia. Nature. 1999;400:621–622. doi: 10.1038/23148. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O, Tauscher J, Hilger E, Stastny J, Brucke T, Kasper S. [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry. 2000;47:482–489. doi: 10.1016/s0006-3223(99)00293-0. [DOI] [PubMed] [Google Scholar]
- Williams JW, Jr, Mulrow CD, Chiquette E, Noel PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med. 2000;132:743–756. doi: 10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- Willner P. Dopamine and depression: a review of recent evidence. I. Empirical studies Brain Res. 1983;287:211–224. doi: 10.1016/0165-0173(83)90005-x. [DOI] [PubMed] [Google Scholar]
- Wojna V, Skolasky RL, Hechavarria R, Mayo R, Selnes O, McArthur JC, Melendez LM, Maldonado E, Zorrilla CD, Garcia H, Kraiselburd E, Nath A. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12:356–364. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmacher T. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Barak O, Reichenberg A, Cohen E, Shavit Y, Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Alagbe O, Wang X, Woolwine B, Thornbury M, Raison CL, Miller AH. Promoter polymorphisms of the interferon-alpha receptor gene and development of Interferon-induced depressive symptoms in patients with chronic hepatitis C: preliminary findings. Neuropsychobiology. 2005;52:55–61. doi: 10.1159/000086605. [DOI] [PubMed] [Google Scholar]
- Zhang J-j, Terreni L, De Simoni MG, Dunn AJ. Peripheral interleukin-6 administration increases extracellular concentrations of serotonin and the evoked release of serotonin in the rat striatum. Neurochemistry International. 2001;38:303–308. doi: 10.1016/s0197-0186(00)00099-1. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fang Y, Zeng Z, Lian Y, Wei J, Zhu H, Jia Y, Zhao X, Xu Y. BDNF gene polymorphisms are associated with Alzheimer's disease-related depression and antidepressant response. Journal of Alzheimer's Disease. 2011;26:523–530. doi: 10.3233/JAD-2011-110113. [DOI] [PubMed] [Google Scholar]
- Zhao B, Schwartz JP. Involvement of cytokines in normal CNS development and neurological diseases: recent progress and perspectives. Journal of neuroscience research. 1998;52:7–16. doi: 10.1002/(SICI)1097-4547(19980401)52:1<7::AID-JNR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010a;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology. 2010b doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifko UA, Rupp M, Schwarz S, Zipko HT, Maida EM. Modafinil in treatment of fatigue in multiple sclerosis. Results of an open-label study. Journal of neurology. 2002;249:983–987. doi: 10.1007/s00415-002-0765-6. [DOI] [PubMed] [Google Scholar]
- Zoller H, Schloegl A, Schroecksnadel S, Vogel W, Fuchs D. Interferon-alpha therapy in patients with hepatitis C virus infection increases plasma phenylalanine and the phenylalanine to tyrosine ratio. J Interferon Cytokine Res. 2012;32:216–220. doi: 10.1089/jir.2011.0093. [DOI] [PubMed] [Google Scholar]