Abstract
Mating pheromone receptors of the yeast Saccharomyces cerevisiae are useful models for the study of G protein-coupled receptors. The mating pheromone receptors, Ste2 and Ste3, are not essential for viability so they can be readily targeted for analysis by a variety of genetic approaches. This chapter will describe methods for identification of two kinds of mutants that have been very informative about the mechanisms of receptor signaling: constitutively-active mutants and dominant-negative mutants. Interestingly, these distinct types of mutants have revealed complementary information. Constitutive signaling is caused by mutations that are thought to weaken interactions between the seven transmembrane domains, whereas the dominant-negative mutants apparently stabilize contacts between transmembrane domains and lock receptors in the off conformation. In support of these conclusions, certain combinations of constitutively-active and dominant-negative mutants restore nearly normal signaling properties.
1. Introduction
Saccharomyces cerevisiae mating pheromone receptors stimulate a G protein signal pathway that induces conjugation. The pheromone-induced responses include transcriptional induction, cell division arrest, and morphological changes. The ability of the yeast pheromone receptors to induce such robust and diverse responses has made them an interesting model for the analysis of G protein-coupled receptors (GPCRs). Some of the advantages of studying the pheromone receptors are that yeast cells grow rapidly and that the pheromone receptors can be easily manipulated since they are not essential for growth. However, the main advantage is the ease with which yeast cells can be manipulated by genetic approaches, ranging from classical genetic screens to targeted mutations. This has permitted detailed analysis of GPCR function by a wide range of genetic approaches, including the isolation of constitutive signaling and dominant-negative mutants that will be described in this chapter.
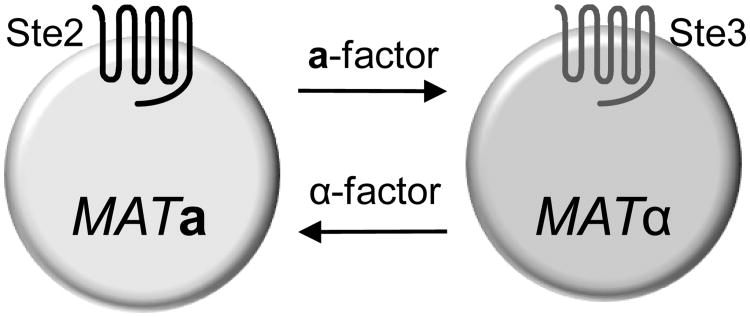
The pheromone pathway is activated when haploid cells of opposite mating type (MATa and MATα) stimulate each other with secreted mating pheromones to conjugate and form an a/α diploid cell (reviewed in (Dohlman and Thorner 2001; Bardwell 2005)). The MATa and MATα haploid cells differ primarily in the combination of receptor and pheromone they produce (Figure 1). MATa cells produce a-factor and the receptors for α-factor (STE2); MATα cells produce α-factor and the receptors for a-factor (STE3). Haploid cells are used for analysis of pheromone signaling because the a/α diploid cells do not express the pheromone receptor genes and are insensitive to pheromone. Most studies focus on the α-factor receptor (Ste2) in MATa cells since α-factor is a soluble peptide that is commercially available (e.g. Sigma-Aldrich Co. or Bachem Inc.). The a-factor receptor (Ste3) has been less studied because a-factor is a lipid-modified peptide that is difficult to use in conventional dose-response and ligand binding assays.
Figure 1.
Yeast cell identity.
The cell types are named for the type of pheromone they produce. MATa cells produce a-factor pheromone and the receptors for α-factor (Ste2). In contrast MATα cells produce α-factor pheromone and the receptors for a-factor Ste3.
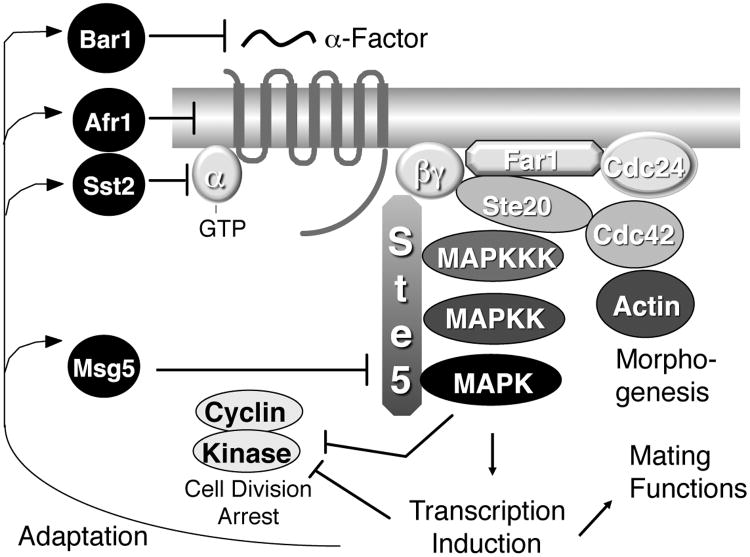
The key elements of the pheromone signal pathway are summarized in Figure 2. Ligand binding stimulates receptors to form an activated complex with a G protein that leads to the exchange of GDP for GTP on the α subunit (Gpa1p) and the subsequent release of the βγ complex (Ste4p, Ste18p) (Dohlman and Thorner 2001; Bardwell 2005). The free βγ subunits then recruit Ste5p to the membrane. Ste5p, a scaffolding protein, recruits the components of a MAP kinase cascade including a MAPKKK (Ste11p), a MAPKK (Ste7p) and a MAPK (Fus3p). This pathway ultimately activates the pheromone-responsive transcription factor Ste12p that induces genes such as FUS1.
Figure 2.
Yeast pheromone signal transduction pathway.
The major proteins involved in pheromone signaling are indicated.
Genetic strategies have been used to identify a wide range of mutant phenotypes for STE2 that have contributed to models for ligand binding, signaling across the plasma membrane, G protein activation, receptor desensitization, and ligand-induced endocytosis (Konopka and Thorner 2004). Two strategies that have been very successful are the identification of constitutively active mutants and dominant-negative mutants. Constitutively-active mutants are primarily caused by mutations that are predicted to change the packing of the seven α-helical transmembrane domains (TMDs) (Konopka et al. 1996; Dube and Konopka 1998; Parrish et al. 2002). For example, the strongest constitutively active mutant identified thus far results from a substitution of Pro-258 for Leu in transmembrane domain six, which is expected to alter the structure of this domain in a way that impacts the way that the adjacent third intracellular loop interacts with the G protein (Konopka et al. 1996). In contrast, dominant-negative mutations tend to cluster near the extracellular ends of the TMDs, where they affect ligand binding or receptor activation (Dosil et al. 1998; Leavitt et al. 1999). These inactive receptors are dominant because they can sequester the G protein away from the wild-type receptors (Dosil et al. 2000). It should be possible to extend some of the genetic approaches described in this chapter to the study of GPCRs that can be heterologously expressed in yeast. We have previously described methods for expressing heterologous GPCRs in yeast (Mentesana et al. 2002).
2. Selecting a Yeast Strain and Expression Vector
2.1. Choosing a Yeast Strain
S. cerevisiae genes are designated by three letters, in italics, followed by a number corresponding to a specific gene (e.g. STE2). Dominant alleles are written in capital letters; recessive alleles are written in lower case letters. Mutant alleles are distinguished by the addition of a dash followed by a specific identifying allele number. (e.g. ste2-3). Deletion of a gene or disruption by insertion of another DNA sequence is indicated either with a Δ symbol (e.g. ste2Δ) or a double colon followed by the name of the marker gene used to create the deletion allele (e.g. ste2∷LEU2). Proteins are usually designated with a capital first letter and not italics (e.g. Ste2 or Ste2p).
Isolation of constitutively-active receptor mutants requires a specialized yeast strain to avoid the mating pheromone induced cell division arrest, which would kill off the desired mutant cells. To avoid this, the FAR1 gene should be mutated to prevent cell division arrest in response to activation of the pathway without affecting induction of transcriptional responses (Chang and Herskowitz 1990). The endogenous STE2 should be deleted to facilitate analysis of mutagenized versions to be introduced on plasmids. The sensitivity of cells for detection of the pheromone signal can also be increased by deleting genes that promote adaptation to α-factor (Figure 2). A major increase in sensitivity can be obtained by deleting the SST2 gene, which encodes an RGS protein that promotes GTP hydrolysis by the Gα subunit (Dohlman and Thorner 2001; Bardwell 2005), but in practice this does not work that well since the sst2Δ mutation causes a high basal activity that can interfere with detection of constitutive receptor mutants. For studies in which cells will be treated with α-factor pheromone, it will also be important to delete the BAR1 gene, which encodes a secreted protease that degrades α-factor in the medium. Finally, it is important to introduce a sensitive reporter gene to detect signaling. Most commonly used reporter genes use the promoter from the FUS1 gene, which is dramatically induced by pheromone signaling (Trueheart et al. 1987). The FUS1 promoter is fused to reporter genes such as lacZ or HIS3 to provide convenient assays for detection of receptor activation (Trueheart et al. 1987; Konopka et al. 1996).
Different considerations must be used for the isolation of dominant-negative STE2 mutants (Dosil et al. 1998). The cells must contain a wild-type FAR1 gene and a wild-type STE2 gene so that they can undergo cell division arrest in response to pheromone. As described above, the cells should carry a mutation of BAR1 to prevent recovery from cell division arrest by degradation of pheromone and they should also contain a FUS1-lacZ reporter gene.
2.2. Selecting an Expression Vector
Mutagenesis strategies in yeast are greatly facilitated by the availability of shuttle plasmids that can be propagated as episomal plasmids in both yeast and E. coli. A variety of plasmids are available, so care should be taken to choose an appropriate vector. Yeast episomal plasmids (YEp) replicate to high copy number. (YEp plasmids are derived from the 2μ circle, an autonomously replicating plasmid, and are thus also called 2μ plasmids.) YEp plasmids are the first choice for expression of STE2 in mutagenesis studies because they lead to overproduction of the Ste2 protein. This compensates for the fact that many mutant receptors are not stable at the cell surface and therefore increases the phenotypic effects of many constitutively-active and dominant-negative mutants. Overexpression of STE2 does not result in activation of the pathway, so it does not interfere with genetic screens for constituteively active mutants. Yeast centromeric plasmids (YCp) are present in low copy number (1 or 2 copies per cell) because they contain a centromeric element (CEN) that mediates even partitioning at mitosis. This type of vector can be advantageous for experiments where a consistent level of expression in each cell is desired. An HA (hemagglutinin) epitope tag should be introduced at the C terminal end of the receptor coding region to facilitate detection of the receptor protein.
Most currently used yeast plasmids are based on pBluescript or pUC19 plasmids (Gietz and Sugino 1988; Sikorski and Hieter 1989) to facilitate replication in E. coli. These shuttle plasmids typically carry one of several different selectable marker genes that can be used to select for plasmid maintenance in yeast by complementing a specific auxotrophy in the host strain. The most commonly used selectable markers are URA3, HIS3, LEU2, and TRP1, which are involved in the biosynthesis of uracil, histidine, leucine and tryptophan, respectively. We have had good success using vectors containing URA3, since there are media that can be used to select for or against the presence of URA3 plasmids in yeast (Sherman 2002).
2.3. General Methods for Growth and Storage of Yeast
A rich medium (YPD) is used for general propagation of yeast strains under non-selective conditions (Sherman 2002). Plasmids are maintained by growth of cells on chemically defined synthetic medium. The starting point for synthetic media is “Yeast Nitrogen Base”, which consists of salts, vitamins, and ammonium sulfate as nitrogen source (Sherman 2002). The medium is supplemented with amino acids and other appropriate constituents except for the one that will provide the selective pressure. Dextrose is used as an energy source for optimal growth unless the experiment specifically involves the use of other sugars to regulate inducible promoters etc. Laboratories that do not routinely grow yeast can purchase commercially made media. In our experience, media purchased from Qbiogene (Carlsbad, CA) is comparable to our homemade media. Recipes for preparing commonly used media are given below.
2.3.1. Non-Selective YPD Medium
Add 10 g Yeast Extract, 20 g Bacto-Peptone and 0.120 g adenine1 to 900 ml distilled water. Mix with a magnetic stir bar in a 2 L flask. If making solid medium, add 20 g Bacto Agar.
Autoclave for 30 minutes.
Add 100 ml of sterile 20% dextrose.
2.3.2. Selective Media
10× Media Supplement
To 1 l of distilled water, add 0.2 g of histidine, arginine, and methionine, 0.3 g of tyrosine, isoleucine and lysine, 0.4 g each of adenine sulfate, uracil, and tryptophan, 0.5 g phenylalanine, 0.6 g leucine, and 1.0 g each of glutamic acid and aspartic acid, 1.5 g valine, 2.0 g threonine and 4.0 g serine.
Omit any of the above constituent(s) that will be used for selection of plasmids.
Aliquot into bottles and autoclave for 30 min.
Selective Media (dropout media)
Add 6.7 g Bacto Yeast Nitrogen Base (e.g. BD Diagnostics Systems catalog # 239210) to 800 ml distilled water. If making solid agar medium add 20 g bacteriological grade agar.
Autoclave 300 ml aliquots for 30 minutes.
Add 37 ml sterile 20% dextrose to cooled aliquots.
Add 37 ml of the appropriate 10× Media Supplemental solution.
2.3.3. Storage of Yeast Cultures
Yeast can typically be stored at 4°C for 1-3 months. Most strains will remain viable for even longer periods of time, but there is the potential danger of selecting for a mutant strain that has adapted for survival on old plates. Yeast go into a deep resting state so they should be restreaked onto a fresh plate to allow a period of logarithmic growth prior to starting a new experiment. Long-term stocks can be prepared by adjusting a fresh culture of yeast to 15% glycerol and then freezing at −70°c. Frozen cultures are viable for many years.
3. Transforming Plasmids into Yeast
The most common method for the introduction of plasmid DNA into yeast cells involves treating cells with lithium acetate (Gietz et al. 1995). This method yields up to 1×106 transformants/μg of DNA. The steps that are important for a successful transformation include the addition of properly prepared single-stranded carrier DNA, the use of the proper-sized polymer of polyethylene glycol, and the heat shock at 42°C. There is a very useful internet site that describes recent variations on these protocols: http://www.umanitoba.ca/medicine/biochem/gietz/.
3.1. Preparation of Single-Stranded Carrier DNA
Dissolve Salmon Testes DNA (Sigma-D1626) in TE (10 mM Tris-HCl pH 8.0, 1 mM EDTA) to 2 mg/ml by mixing on a magnetic stirrer overnight at 4°C.
Aliquot DNA into microcentrifuge tubes and store at −20°C.
Prior to use, boil an aliquot for 5 minutes and then cool in an ice water bath. Small aliquots should be used so that the sample is not thawed too many times. If kept frozen or on ice it is not necessary to boil before each use, but samples can be reheated to generate single-stranded DNA.
3.2. Transformation Protocol
Special reagent solutions needed
LiAc/TE: (10 mM Tris, pH 7.5; 1 mM EDTA; 100 mM Lithium acetate, pH 7.5)
PEG/LiAc/TE: (10 mM Tris, pH 7.5; 1 mM EDTA; 100 mM lithium acetate, pH 7.5; 40% PEG 3350 (e.g. Sigma cat.# P-3640; note that size of PEG is critical).
Procedure
Inoculate 50 ml YPD to a cell density of 2.5 × 106 cells/ml (OD660 ∼ 0.15) with cells from a fresh overnight culture.
Incubate with shaking for ∼4 hours at 30°C until cells reach 5-10 × 106 cells/ml (OD660 = 0.4-0.7).
Pellet cells by centrifugation at 3,000 × g for 5 min.
Wash cell pellet once with sterile water and then once with LiAc/TE solution.
Resuspend the final cell pellet in 0.5 ml LiAc/TE (1/100 volume).
Add 50 μl of the yeast cell suspension to a tube containing 10 μl (20 μg) carrier DNA and 2-4 μl transforming DNA (up to 5 μg) and vortex. Minimize the volume of added DNA to avoid diluting the other reagents.
Add 0.4 ml PEG/LiAc/TE and mix well.
Incubate at 30°C for 30 min.
Heat shock by incubating at 42°C for 15 min.
Add 1 ml selective media to dilute the PEG, mix and centrifuge for 10 sec to pellet cells.
Resuspend pellet in 0.2 ml selective medium and plate on selective agar plate.
Incubate at 30°C for 2-3 days then check for growth of transformed colonies. Be sure to include a control reaction lacking plasmid DNA to determine if the transformants are genuine and not due to a contamination in the yeast culture.
4. “Gap-Repair” Approach for Targeting Mutagenesis to Genes on Plasmids
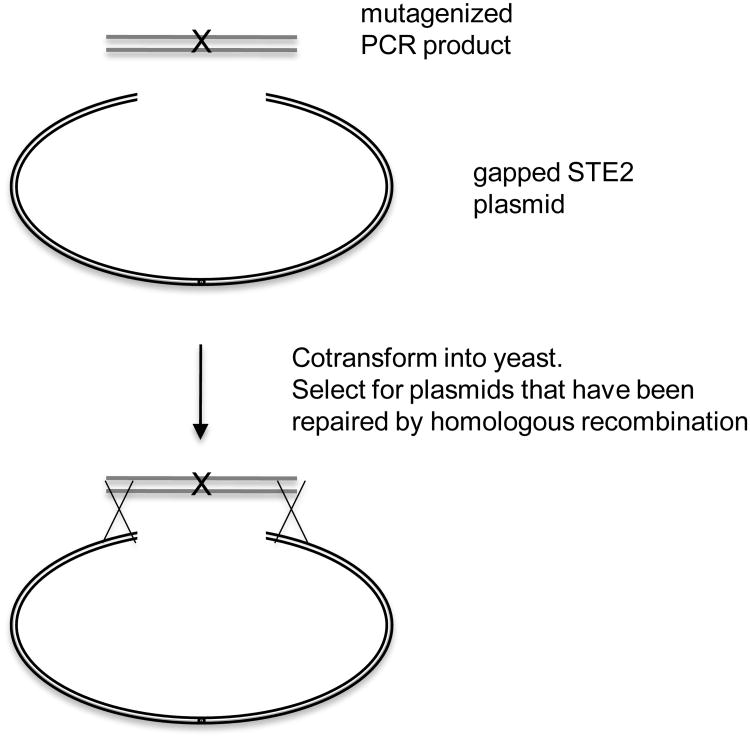
The starting point for isolating constitutively active and dominant-negative mutants in STE2 is to mutagenize a plasmid-borne copy of STE2 (Konopka et al. 1996; Dosil et al. 1998). This facilitates rapid recovery of the plasmid into E. coli so that the mutant phenotype can be confirmed by transforming the plasmid back into yeast. It also facilitates recovery of mutant genes for DNA sequence analysis to identify the specific mutation. To save time mapping the sites of the mutations within the STE2 gene, we target the mutagenesis to small regions of STE2 using a “Gap Repair” method (Kunes et al. 1987; Parrish et al. 2002). The basis of this technique is outlined in Figure 3. First, a gap is introduced by digesting a plasmid with two enzymes that remove a small 250 – 300 bp region of the STE2 coding region. Specially designed PCR primers are then used to amplify a DNA fragment that spans the gap and contains about 50 bp of homology to the ends of the digested plasmid. The PCR is performed under sub-optimal conditions using error-prone Taq polymerase to introduce a wide range of random mutations. (Note: Do not mutagenize to such a high level that multiple mutations are introduced or it will take a lot of effort later on to determine which mutations actually contribute to the phenotype.) This fragment is then co-transformed into yeast along with the gapped plasmid whose ends are homologous to those of the PCR DNA. The gap in the plasmid is then repaired by homologous recombination with the ends of the PCR product (Kunes et al. 1987). Other procedures have also been developed to use oligonucleotide-directed mutagenesis to introduce a broad range of mutations in STE2 (Martin et al. 2002).
Figure 3.
Gap-Repair method for mutagenizing STE2 on plasmids.
PCR amplification under suboptimal conditions for the Taq polymerase is used to introduce random mutations within the GPCR gene. The PCR products are then transformed into yeast along with a GPCR plasmid that has been linearized within the same region. Homologous recombination that occurs between the plasmid and the PCR product repairs the gap and introduces mutations into the targeted region. Yeast colonies carrying the mutagenized plasmids can then be replica-plated to appropriate media to assay for constitutive activity or dominant interference of the pheromone pathway.
Procedure
PCR amplify a region of STE2 using Taq polymerase lacking the proofreading function.
Adjust the standard conditions by using 1/5 the normal concentration of one nucleotide. This will cause a higher error rate in the polymerase and introduce mutations.
Amplify using a standard protocol, such as 94° 1 min, 55° 1 min, 72° 1 min per kb, 30 cycles, 72°C 10 min.
Digest the plasmid with restriction enzymes that will create a gap within the region of STE2 that is being mutagenized. Design the primers so that there is at least 50 bp of homology between the PCR product and the ends of the gap on the plasmid.
Co-transform the purified PCR fragment and the gapped plasmid (section 3.2). We typically use a molar ratio of about least 3:1, although good results are also obtained with a 1:1 ratio.
Plate transformations on selective medium and then incubate at 30°C for 2-3 days until colonies are visible.
A control transformation of gapped plasmid alone should be performed to ensure that the plasmid was digested efficiently. Linearized plasmids do not propagate in yeast so the gapped plasmid should yield only a few colonies
5. Isolation of Constitutively-Active Mutants
Constitutively-active mutants can be readily identified by performing the Gap-Repair mutagenesis strategy and into a yeast strain that is MATa ste2Δ far1Δ FUS1-lacZ. The yeast strain should also contain a mutation such as ura3Δ to select for the plasmid. We have had best results by first plating the transformation mixture on selective media to allow for colonies to grow, and then replica plating onto special medium containing X-gal to detect colonies in which the FUS1-lacZ reporter gene is constitutively stimulated resulting in higher levels of β-galactosidase. Although the transformation mixture can be plated directly onto the X-gal medium, the transformation efficiency and growth are not optimal on this type of medium so it is more difficult to identify the constitutive mutants. The exact amount of X-gal in the plates should be titrated so that control cells carrying a wild-type STE2 plasmid display a barely detectable blue color after 3 or 4 days. Constitutively-active mutants are readily apparent as darker blue colonies. The initial colonies should then be restreaked to purify them away from the non-transformed cells and retested to confirm that the phenotype is consistent. Plasmids can be recovered from the mutant cells and retransformed into a fresh yeast strain to confirm that the mutation is linked to the plasmid and not to a chromosomal mutation in another gene.
We have also tried using a FUS1-HIS3 gene to directly select for constitutively active mutant receptors on medium lacking histidine (Mentesana et al. 2002). This approach can be optimized by adding aminotriazole to the plates, which is a competitive inhibitor of the HIS3-encoded enzyme and selects for cells with the highest level of pheromone pathway activation. However, we found that this strategy yielded a high degree of false positives that were apparently caused by mutations that somehow activated the FUS1-HIS3 reporter gene but not the pheromone pathway. These false positives take considerable effort to weed out, so we found it to be more efficient to screen for mutants on X-gal medium. In most experiments we were able to titrate the efficiency of mutagenesis so that we identified about 1 constitutive mutant per 1,000 colonies screened. In cases where multiple mutations were identified within the DNA fragment, we typically found it was more efficient to continue screening until the corresponding single mutant was identified rather than taking the time to recreate the single mutations.
In order to quantify the degree of constitutive activity, the mutant strains are then compared to the wild-type control in liquid assays for β-galactosidase activity. This is a relatively easy assay that gives a reliable comparison of the relative level of constitutive activity that will be described in detail below (see section 7).
5.1. Identification of Constitutively-Active Mutants
Replica-plate colonies obtained after Gap-Repair mutagenesis to indicator plates containing X-gal (See Section 4). Incubate at 30°C for 3-5 days. Constitutive mutants will be identified as blue colonies.
Restreak candidates to new X-gal plates to verify phenotype.
Recover the plasmid using a previously described procedure (Hoffman and Winston 1987). Reference not seen in Bibliography. Basically, the cell pellet from 1.5 ml of liquid culture is resuspended in 200 μl lysis buffer (2% Triton X-100, 1% SDS, 100 mM NaCl, 10 mM Tris pH 8.0, 1 mM EDTA) and then mixed with 200 μl phenol/chloroform. Add 200 μl glass beads and vortex for 2 min. Spin 5 min in a microcentrifuge. One μl of the supernatant can be used to transform E. coli. The efficiency is low because of an inhibitor in the yeast extract.
Re-transform yeast to confirm that the observed phenotype is plasmid-dependent.
5.2. X-gal Plates for β-galactosidase Reporter Gene Detection
To detect activation of the FUS1-lacZ (β-galactosidase) reporter gene, add X-gal (5-bromo-4-chloro-3-indoyl β D-galactoside) to the agar medium at a final concentration of 40 μg/ml. The X-gal should be added when the agar is cooled to about 50°C, just prior to pouring to prevent inactivation. The X-gal plates differ from standard plates in that they are buffered to pH 7.0 for optimal β-galactosidase activity.
Special reagent solutions needed
20 mg/ml stock of X-gal in dimethylformamide
KPI solution: 1 M KH2PO4, 0.15 M (NH4)2SO4, 0.75 M KOH, adjusted to pH 7.0
Prepare solid synthetic medium lacking the appropriate amino acid as described above in section 2.3.2.
Add 100 ml of the appropriate 10× Media Supplemental solution (minus the appropriate additive), 100 ml 20% dextrose, 100 ml KPI
Allow medium to cool, and then just prior to pouring plates add 2 ml of a 20 mg/ml stock of X-gal.
5.3. Quantitative Assay for FUS1-lacZ Reporter Gene Activity
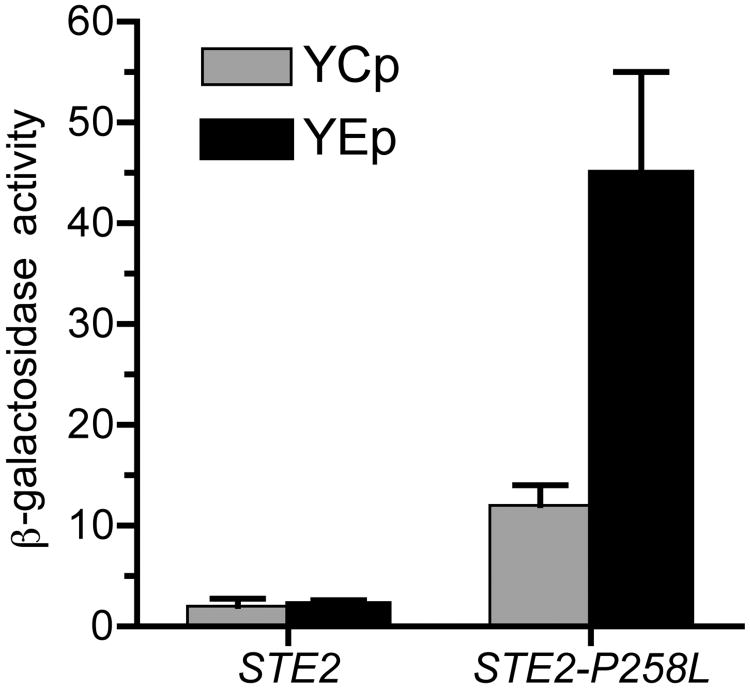
The colorimetric substrate ONPG can be conveniently used to assay β-galactosidase activity (Miller 1972) if the yeast cells are permeabilized to allow access of the substrate to the enzyme. Since wild type S. cerevisiae cells do not produce an endogenous β-galactosidase, reporter genes that make use of the E. coli β-galactosidase gene (lacZ) provide a sensitive and convenient indicator of gene expression. Sample data for a constitutive mutant is shown in Figure 4. Note that increased constitutive activity is detected when the STE2-P258L mutant is carried on a high copy YEp plasmid. Overproduction of this mutant receptor compensates for the fact that it is not stably produced and is present at lower levels at the cell surface (Konopka et al. 1996).
Figure 4.
Elevated FUS1-lacZ reporter gene activity in constitutively signaling mutant strains. β-galactosidase activity resulting from the FUS1-lacZ pheromone-responsive reporter gene is shown for cells carrying the indicated STE2 gene on either a low copy YCp plasmid or a high copy YEp plasmid. Overproduction of the STE2-P258L mutant results in higher β-galactosidase activity because it compensates for the reduced stability of this mutant receptor at the cell surface.
Special Reagents
Z buffer (10 mM KCl, 1 mM MgSO4, 60 mM Na2HPO4.7H2O, 40 mM NaH2PO4.H2O, 50 mM β-mercaptoethanol). (Note: add β-mercaptoethanol just prior to use.)
ONPG (4 mg/ml o-nitrophenyl-β-D-galactopyranoside in 0.1 M phosphate buffer pH 7.0)
β-galactosidase assay procedure
Grow cells overnight at 30°C so that they remain in log phase (i.e. OD660 < .7; less than 107 cells/ml). To ensure that the basal level remains at a consistently low level, cells should be kept growing for two days before performing the assay.
Adjust 2 ml of culture to 2.5 × 106 cells/ml and then add α factor (1 × 10−7M) and incubate for two hours. A longer incubation can be used for greater sensitivity.
Place the cells on ice. For careful measurements add cycloheximide to 10 μg/ml.
Measure the OD600 of the cells.
Add 0.1 ml of the cell culture to a 1.5 ml tube containing 0.7 ml Z buffer with 2-mercaptoethanol added.
Add 50 μl chloroform and 50 μl 0.1% SDS. Vortex for 30 seconds.
Add 0.16 ml ONPG and mix by vortexing.
Incubate at 37°C for one hour or until the reactions turn visibly yellow.
Quench the reactions by adding 0.4 ml 1 M Na2CO3.
Spin tubes for 10 minutes to remove debris and then read OD420.
Calculate the β-galactosidase units using the formula: Units = (1000 × OD420)/(t × V × OD600) (t = time of incubation in minutes and V = volume of cell culture added to Z buffer in ml.)
6. Isolation of Dominant-Negative Mutants
Identification of dominant-negative mutants begins with the Gap-Repair mutagenesis procedure described above (Section 4). However, in this case the yeast strain used is MATa STE2 FAR1 FUS1-lacZ and ura3Δ. The colonies carrying the mutagenized plasmids are then replica-plated to agar plates containing different concentrations of α-factor in the medium. We have found it useful to replica plate the colonies to two sets of agar medium plates. One set contains the lowest dose of α-factor that causes cell division arrest and the second set of plates contain a 10-fold higher dose. The low dose helps to identify even weakly dominant mutants, but may also give some false positives. The higher dose helps to more quickly identify the strongest mutants (see Figure 5).
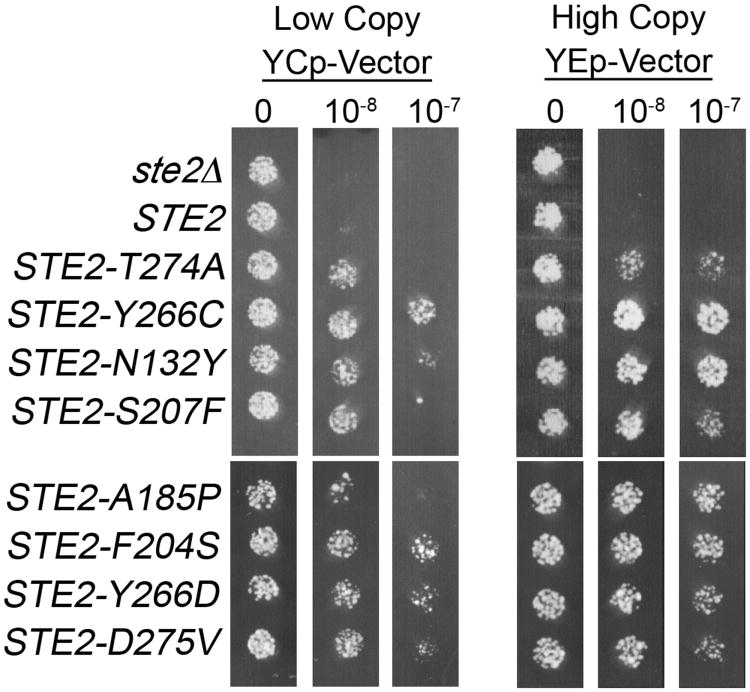
Figure 5.
Dominant-negative STE2 mutants are resistant to α-factor-induced cell division arrest. Yeast strain JKY25 carrying a wild-type copy of STE2 in the genome were transformed with either low copy or high copy plasmids carrying the STE2 genes indicated on the left. For simplicity, the empty vector control was labeled as ste2Δ. Equal amounts of cells were then spotted on medium lacking α-factor, or containing 10−8 or 10−7 M α-factor as indicated above. Growth of cells was photographed after incubation for 2 days at 30°C. [Reproduced from (Dosil et al. 1998) with permission from the American Society for Microbiology (DOI = 0270-7306/98/$04.00+0)].
One special caution about this approach is that mutation of genes that are part of the downstream signal pathway can also cause resistance to α-factor induced cell division arrest. To avoid wasting time studying such false-positives, only pick candidates for further analysis where it looks like many cells in the colony are resistant to α-factor, and not just that there is a rare spontaneous mutant in one part of the colony. Furthermore, cells picked for retesting should be taken off of a control plate that lacks α-factor so there has not been any selective pressure to become resistant to α-factor. A second caution is that an unmodified STE2 gene should be used for this approach. The C-terminal tail of Ste2 is critical for dominant-negative function (Dosil et al. 2000). Thus, tagging the C terminus of Ste2 with an epitope tag or GFP prior to the mutagenesis might limit the range of mutations that are identified.
The dominant-negative STE2 mutant plasmids that are recovered should be examined in a wild-type STE2 strain and also in a ste2Δ strain. The STE2 strain will be used to determine the properties of the dominant-negative mutant, such as the strength of the interference with normal receptor function. In contrast, the use of the ste2Δ strain will permit analysis of the new STE2 mutants in the absence of wild-type receptors. Our previous studies identified at least two types of receptor mutants (Dosil et al. 1998). One type fails to bind ligand and the other class binds ligand but cannot transduce a signal to the G protein effectively. An advantage of isolating mutants in this manner is that they are very stably produced at the plasma membrane, since they have been selected to compete with the wild-type receptors. In contrast, many previously identified loss of function mutants have been difficult to study, since they are present at low levels at the cell surface.
Special reagent needed
α-factor stock solution: α-factor is dissolved at 0.5 mM final concentration in a buffer with 10 mM HCl, 0.2 mM EDTA, and 1 mM β-mercaptoethanol.
Synthetic medium plates containing α-factor: these plates are made as described in Section 2 and then the appropriate amount of α-factor is added just before pouring when the agar is cooled to about 50°C.
Procedure
The colonies carrying the mutagenized STE2 plasmids are created as described in section 4.
The resulting colonies are replica-plated to agar plates containing different concentrations of α-factor in the medium. One plate contains the lowest dose of α-factor that causes cell division arrest (1×10−8 M) and another plate contains a 10-fold higher dose (10−7 M). Be sure to include a third plate that lacks α-factor that can be used to for future studies. Since these colonies have not been exposed to α-factor there is less chance to select for a rare spontaneous mutation in a chromosomal gene that will cause resistance to α-factor.
The plates are incubated at 30°C and inspected after 48 and 74 h for signs of growth. The low dose helps to identify even weakly dominant mutants, but may also give some false positives. The higher dose helps to more quickly identify the strongest mutants.
All mutants should be retested by purifying the plasmids into E. coli and retesting following transformation into fresh yeast cells as described above for the constitutive mutants.
7. Further Methods for Analysis of Mutant Receptors
Mutant receptors identified by the above strategies can be further characterized by the strategies presented in this section. Protocols for assaying the ligand binding properties of α-factor receptors have been described previously (Jenness et al. 1983; Dosil et al. 2000; Ding et al. 2001).
7.1. Analysis of Receptor Protein Production by GFP-tagging
A very convenient way to detect mutant receptors is to fuse them to the Aequorea victoria green fluorescent protein (GFP). Fusion of GFP to the C terminus of the α-factor receptor results in a receptor protein that displays wild-type functional properties yet can be detected by fluorescence microscopy. Fluorescence is seen as a prominent ring around the cell demonstrating that the protein is stable and properly transported to the plasma membrane. Mutants that do not localize properly or receptors that are down-regulated by endocytosis are generally found in punctate-looking intracellular compartments (Li et al. 1999; Choi and Konopka 2006). Best results are obtained using a version of GFP that is enhanced for brightness and whose codons are optimized for expression in yeast codons (Longtine et al. 1998).
7.2. Western Blot Analysis of Receptor Protein Production
Western blot analysis is another very convenient method to detect Ste2 proteins produced in yeast. We use a triple HA tag, which provides a very sensitive level of detection (Dosil et al. 2000). The preparation of yeast cell extracts requires disruption of the cell wall. Mechanical disruption by vortexing cells with glass beads is the simplest and will be described here. Whole cell extracts can be analyzed, but sensitivity can be increased by analyzing a crude membrane fraction. Methods to separate the protein extract by polyacrylamide gel electrophoresis and transfer to nitrocellulose are common practices that are described in detail elsewhere (Sambrook et al. 1989).
Special Reagents
Acid treated 450 micron glass beads (e.g. Sigma Cat.# G8772)
TE/PP buffer: 100 mM NaCl, 50mM Tris-HCl pH 7.5, 1mM EDTA, 100 μg/ml PMSF, 2 μg/ml pepstatin A
PAGE sample buffer (8 M urea, .3% SDS, 25 mM Tris pH 6.8, 0.01% Bromophenol Blue, 0.01% Xylene Cyanol)
Procedure
Grow a 50 ml culture of yeast to a density of about 1 × 107 cells/ml (OD660 = 0.5-1). Either YPD or synthetic medium can be used.
Cool centrifuge tubes and buffers on ice.
Transfer about 2.5 × 108 cells to a 50 ml conical tube and harvest cell pellet by centrifugation at 1,000 × g for 5 min.
Wash cell pellet with 10 ml sterile water and then pellet cells again by centrifugation for 5 min.
Resuspend cell pellet in 1ml sterile water and transfer to 1.5 ml microfuge tube.
Centrifuge at 14,000 × g for 30 seconds in a microfuge and then remove the supernatant. (Cells may be stored at –70°C at this point or placed on ice until needed.)
Resuspend 2.5 × 108 cells in 250 μl TE/PP buffer and add 250 μl glass beads.
Vortex cells at very high speed for 1 min. Tilt tube so that there is maximum smashing action of the glass beads to break the yeast cell walls. Incubate on ice for 1 min to keep the sample cool and prevent proteolysis. Repeat 3 times.
Transfer cell extract to fresh tube (leaving behind the glass beads)
Centrifuge at low speed (330 × g) for 5 min at 4°C to remove unbroken cells.
Transfer supernatant to a new tube. Centrifuge at top speed for 15 min at 4°C in a microfuge to pellet membranes.
The crude membrane pellet can be used immediately or stored at −70°C.
Dissolve the crude membrane pellet in 100 μl 2X PAGE loading buffer. Warm tubes at 37°C for 10 minutes prior to loading gel. Do not boil GPCR samples or they will aggregate.
Centrifuge at top speed in a microfuge for 3 min prior to loading and then perform gel electrophoresis and Western blot according to standard procedures.
7.3. Halo Assay for Cell Division Arrest
The halo assay is a useful way to assay α-factor-induced cell division arrest (Dosil et al. 1998). In brief, cells are spread on the surface of an agar plate and then filter disks containing α-factor are placed on the surface. The α-factor diffuses out into the medium and causes a zone of growth inhibition that is proportional to the dose of α-factor in the disk.
Special Reagents
α-factor stock solution: α-factor is dissolved at 0.5 mM final concentration in a buffer with 10 mM HCl, 0.2 mM EDTA, and 1 mM β-mercaptoethanol.
Blank filter discs: 6 mm diameter discs from BD Diagnostic Systems (cat# 231039)
Procedure
1. Dilute an overnight culture of yeast cells to 106 cells/ml.
2. Spread 150 μl of cells on the surface of an agar petri plate. If plates are dry, add extra sterile water so that the cells will spread evenly. (Note: some labs mix the yeast cells with relatively cool molten agar and then pour a thin layer across the top an agar plate. This gives nice-looking results, but takes more time to set up.)
3. Allow the plate to dry so that the excess liquid has soaked into the plate. It should take about 20-30 min for the plates to dry
4. While the plates are drying, prepare appropriate dilutions of α-factor. For a bar1 mutant strain, prepare a series of 2-fold dilutions between 10−5 M and 10−6 M α-factor. α-factor sticks to plastic, so it should be diluted into culture medium to help reduce non-specific sticking.
5. Place 4 sterile filter discs on a clean sheet of Saran Wrap and then add 10 ul of the appropriate α-factor solution. When the liquid has been absorbed by the filter, transfer the filter to the agar plate. Arrange the discs so that they are well separated and the halos will be distinct.
7. Incubate until the edge of the halo is well-defined and then measure the diameter of the halo.
Acknowledgments
We thank our colleagues for contributing suggestions for protocols. J. B. K. gratefully acknowledges research grant support from the American Heart Association and the National Institutes of Health (GM087368).
Footnotes
Adenine should be added to the medium for strains that carry the ade2 mutation to suppress the formation of a toxic red pigment that accumulates due to disruption of the adenine biosynthetic pathway.
References
- Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides. 2005;26(9):339–350. doi: 10.1016/j.peptides.2004.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell. 1990;63:999–1011. doi: 10.1016/0092-8674(90)90503-7. [DOI] [PubMed] [Google Scholar]
- Choi Y, Konopka JB. Accessibility of Cys residues substituted into the cytoplasmic regions of the a-factor receptor identifies the intracellular residues that are available for G protein interaction. Biochemistry. 2006;(45):15310–15317. doi: 10.1021/bi0614939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding FX, Lee BK, Hauser M, Davenport L, Becker JM, Naider F. Probing the binding domain of the Saccharomyces cerevisiae α-mating factor receptor with fluorescent ligands. Biochemistry. 2001;40(4):1102–1108. doi: 10.1021/bi0021535. [DOI] [PubMed] [Google Scholar]
- Dohlman HG, Thorner JW. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu Rev Biochem. 2001;70:703–754. doi: 10.1146/annurev.biochem.70.1.703. [DOI] [PubMed] [Google Scholar]
- Dosil M, Giot L, Davis C, Konopka JB. Dominant-negative mutations in the G protein-coupled a-factor receptor map to the extracellular ends of the transmembrane segments. Mol Cell Biol. 1998;18(10):5981–5991. doi: 10.1128/mcb.18.10.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosil M, Schandel K, Gupta E, Jenness DD, Konopka JB. The C-terminus of the Saccharomyces cerevisiae α-factor receptor contributes to the formation of preactivation complexes with its cognate G protein. Mol Cell Biol. 2000;20:5321–5329. doi: 10.1128/mcb.20.14.5321-5329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube P, Konopka JB. Identification of a polar region in transmembrane domain 6 that regulates the function of the G protein-coupled α-factor receptor. Mol Cell Biol. 1998;18:7205–7215. doi: 10.1128/mcb.18.12.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA. Studies on the transformation of intact yeast cells by the LiAc/SS- DNA/PEG procedure. Yeast. 1995;11(4):355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- Gietz RD, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Jenness DD, Burkholder AC, Hartwell LH. Binding of α-factor pheromone to yeast a cells: chemical and genetic evidence for an α-factor receptor. Cell. 1983;35:521–529. doi: 10.1016/0092-8674(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Konopka JB, Margarit M, Dube P. Mutation of pro-258 in transmembrane domain 6 constitutively activates the G protein-coupled α-factor receptor. Proc Natl Acad Sci USA. 1996;93:6764–6769. doi: 10.1073/pnas.93.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka JB, Thorner J. Pheromone Receptors (Yeast) Encyclopedia Biochem. 2004;3:256–261. [Google Scholar]
- Kunes S, Ma H, Overbye K, Fox MS, Botstein D. Fine structure recombinational analysis of cloned genes using yeast transformation. Genetics. 1987;115(1):73–81. doi: 10.1093/genetics/115.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt LM, Macaluso CR, Kim KS, Martin NP, Dumont ME. Dominant negative mutations in the α-factor receptor, a G protein-coupled receptor encoded by the STE2 gene of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1999;261(6):917–932. doi: 10.1007/s004380051039. [DOI] [PubMed] [Google Scholar]
- Li Y, Kane T, Tipper C, Spatrick P, Jenness DD. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol Cell Biol. 1999;19(5):3588–3599. doi: 10.1128/mcb.19.5.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14(10):953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Martin NP, Celic A, Dumont ME. Mutagenic mapping of helical structures in the transmembrane segments of the yeast α-factor receptor. J Mol Biol. 2002;317(5):765–788. doi: 10.1006/jmbi.2002.5444. [DOI] [PubMed] [Google Scholar]
- Mentesana PE, Dosil M, Konopka JB. Functional assays for mammalian G-protein-coupled receptors in yeast. Methods Enzymol. 2002;344:92–111. doi: 10.1016/s0076-6879(02)44708-8. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor, Cold Spring Harbor Laboratory Press; 1972. pp. 325–355. [Google Scholar]
- Parrish W, Eilers M, Ying W, Konopka JB. The cytoplasmic end of transmembrane domain 3 regulates the activity of the Saccharomyces cerevisiae G-protein-coupled α-factor receptor. Genetics. 2002;160(2):429–443. doi: 10.1093/genetics/160.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor; 1989. [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueheart J, Boeke JD, Fink GR. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol Cell Biol. 1987;7:2316–2328. doi: 10.1128/mcb.7.7.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]