Abstract
Objectives
Brief episodes of ischemia and reperfusion after a lethal ischemic insult confer cardioprotection, a phenomenon termed “ischemic postconditioning.” However, all studies reported to date have been conducted in open-chest animal models. We sought to determine whether postconditioning occurs in conscious animals and whether it protects against severe myocardial injury.
Methods
Chronically instrumented rats were assigned to a 30- (Subset 1), 45- (Subset 2), or 60-min (Subset 3) coronary occlusion followed by 24 h of reperfusion. In each subset, rats received no further intervention (control), were preconditioned with 12 cycles of 2-min occlusion/2-min reperfusion immediately (early preconditioning; EPC) or 24 h (late preconditioning; LPC) before myocardial infarction, or were postconditioned with 20 cycles of 10-s occlusion/10-s reperfusion immediately after myocardial infarction (20-10 PostC).
Results
With a 30-min occlusion, infarct size (54.4 ± 2.3% of risk region in control-30) was significantly reduced in EPC-30, LPC-30, and 20-10 PostC-30 groups (by 72, 70, and 47%, respectively; all P < 0.05 vs. control-30). With a 45-min occlusion, infarct size (62.2 ± 2.4% in control-45) was reduced in EPC-45 and LPC-45 groups (by 47 and 41%, respectively; all P < 0.05 vs. control-45) but not in the 20-10 PostC-45 group [55.4 ± 2.3%, P = not significant (NS) vs. control-45]. With a 60-min occlusion, infarct size (72.7 ± 2.2% in control-60) was reduced in the EPC-60 (by 20%, P < 0.05) but not in the LPC-60 (63.6 ± 2.5%, P = NS) or in the 20-20 PostC group (71.5 ± 3.4%, P = NS).
Conclusions
Both early and late ischemic preconditioning as well as ischemic postconditioning confer protection in conscious rats; however, unlike early preconditioning, postconditioning protects only against coronary occlusions <45 min. In the conscious rat, the cardioprotection afforded by postconditioning is limited to mild to moderate myocardial injury.
Keywords: myocardium, ischemia, infarct size, preconditioning
Ischemic preconditioning, a powerful endogenous protective phenomenon (17), is manifest in all species tested and is associated with two distinct phases of cardioprotection (early and late) (3, 5). In addition, a recent study by Zhao et al. (32) in open-chest dogs has shown that brief episodes of ischemia and reperfusion after a lethal ischemic insult confer cardioprotection, a phenomenon termed ischemic postconditioning. This phenomenon has subsequently been confirmed in vivo in dogs (11), rabbits (1, 4, 13, 30), rats (14, 15, 31), and humans (22) and in vitro in rabbits (7, 29), rats (14, 26), and mice (12, 14), although a study in pigs (21) failed to detect myocardial protection with ischemic postconditioning. The cardioprotection conferred by postconditioning appears to vary greatly with species, experimental settings, and protocols and, unlike preconditioning, has not been observed consistently. For instance, in a study by Yang et al. (30) in anesthetized open-chest rabbits, postconditioning with four cycles of 30-s ischemia/ 30-s reperfusion reduced infarct size markedly. However, this same postconditioning protocol (4 cycles of 30-s ischemia/30-s reperfusion) had no significant infarct size-limiting effect in a study by a different group (13) using the same open-chest rabbit model and in a study by the same group (29) using isolated Langendorff perfused rabbit hearts.
To our knowledge, the infarct-sparing actions of postconditioning have not been explored in conscious animal models, which are more clinically relevant. Furthermore, most of the previous studies (1, 4, 7, 11, 13–15, 26, 29–32) that have obtained positive results with postconditioning were performed in animal models with coronary occlusion <45 min or relatively small infarct size (<55% of the risk region). Whether postconditioning is protective in animals with more severe myocardial injury (coronary occlusions >30 min or infarct size >60% of risk region) remains unclear. Accordingly, the present study was undertaken to investigate the phenomenon of ischemic postconditioning in a conscious animal model of myocardial infarction caused by various ischemic durations. For this purpose, we used a chronically instrumented rat model developed in our laboratory. We felt this would be appropriate because virtually all studies conducted in rats in vivo (14, 15, 31) have used open-chest preparations and a 30-min ischemic insult; data are lacking as to whether postconditioning is also protective in the conscious state and after longer (>30 min) durations of ischemia in this species. In addition, although the phenomenon of ischemic preconditioning has been extensively explored in various animal models, to our knowledge neither the early nor the late phase of ischemic preconditioning has been examined in conscious rats with different degrees of myocardial injury.
Thus the specific goals of the present study were to determine 1) whether the phenomenon of ischemic postconditioning exists in conscious animals; 2) whether postconditioning protects against severe injury induced by prolonged ischemic insults; and 3) whether, in conscious animals, the cardioprotection afforded by postconditioning is comparable with that afforded by ischemic preconditioning.
MATERIALS AND METHODS
The present study was performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85–23, Revised 1996) and was approved by the Institutional Animal Care and Use Committee of the University of Louisville School of Medicine, Louisville, KY.
Experimental preparation
Male Fisher 344 rats (Harlan Sprague-Dawley; 9–12 wk of age) were anesthetized with an intraperitoneal injection of ketamine (37.5 mg/kg) and xylazine (5 mg/kg), intubated with an endotracheal tube, and mechanically ventilated with 97% oxygen with a positive pressure rodent ventilator (Harvard Apparatus, model no. 683). Anesthesia was maintained with 1% isoflurane. Under sterile conditions, the heart was exposed through a left thoracotomy in the fourth intercostal space. After opening of the pericardium, a balloon occluder was placed around the left anterior descending coronary artery ~3 mm distal to the left atrial appendix. The balloon occluder was fashioned from Tygon tubing (inner diameter/outer diameter: 0.010/0.030 in.) and was secured to the left ventricle (LV) wall with one 6-0 prolene suture passing beneath the coronary artery. Proper function of the occluder was confirmed by noting cyanosis of the distal myocardium on inflation of the balloon and hyperemia after deflation. A bipolar lead was anchored to the chest wall to record the electrocardiogram (ECG). The wires and the occluder tubing were tunneled under the skin and exteriorized through a small incision between the scapulae. The chest wound was closed in layers. Gentamicin and ketoprofen were administered intramuscularly. Rats were allowed to recover for a minimum of 7 days after surgery.
Pilot studies
For studies of ischemic preconditioning, initially we used a protocol of six 4-min occlusion/4-min reperfusion cycles [the same protocol we have used in our previous studies in conscious rabbits (20, 23, 25) and in open-chest mice (9, 10)]. We tested this protocol in four conscious rats and found that all four animals developed ventricular fibrillation upon reperfusion after the first 4-min coronary occlusion. Thus we modified our preconditioning protocol to 12 cycles of 2-min occlusion/2-min reperfusion, so that the total ischemic burden remained the same. We found that this modified protocol induced a robust infarct size-limiting effect both in the early and in the late phase of preconditioning, and it avoided ventricular fibrillation on reperfusion.
Experimental protocol
Throughout the experiments, rats were kept in a cage in a quiet, dimly lit room. The ECG was continuously recorded on a thermal array chart recorder (Gould TA6000). Myocardial infarction was induced in conscious rats by performing a 30-, 45-, or 60-min coronary occlusion followed by 24 h of reperfusion. The performance of successful coronary occlusion/reperfusion was verified by observing the changes in the QRS complex on the ECG. Diazepam was administered 10 min before the onset of ischemia (4 mg/kg ip) to relieve the stress caused by the sustained coronary occlusion. No anti-arrhythmic agents were given at any time. Blood samples (0.3 ml each) were taken from a tail vein for creatine kinase (CK) activity assay at 1, 4, and 24 h after reperfusion.
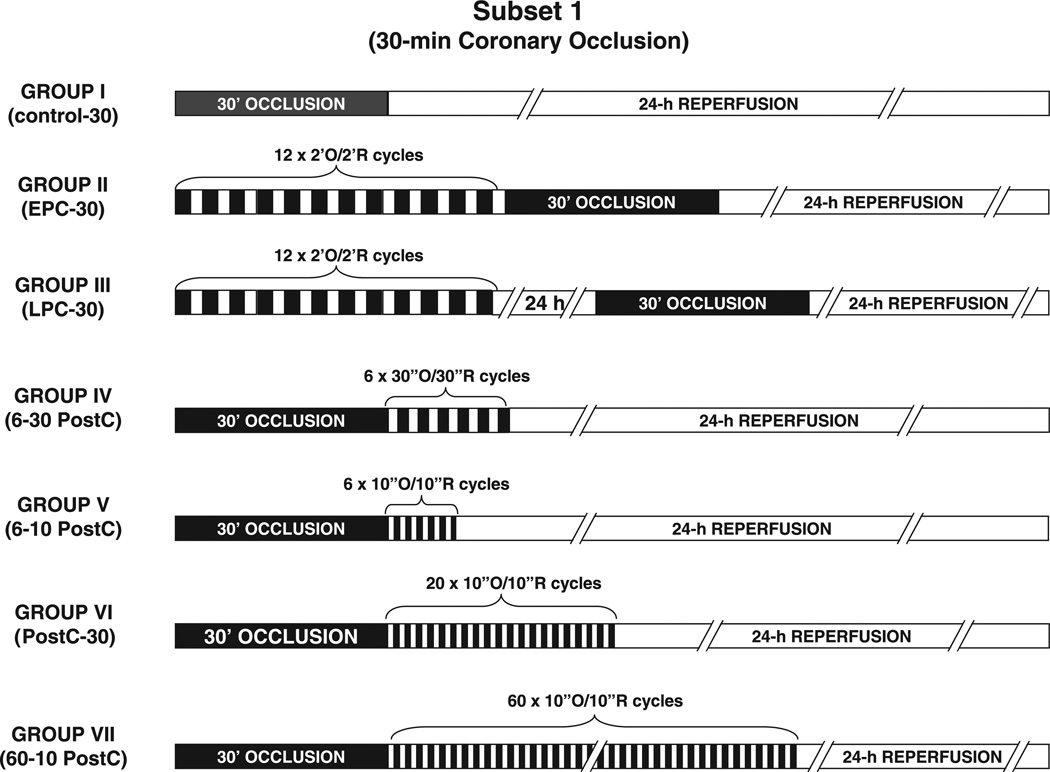
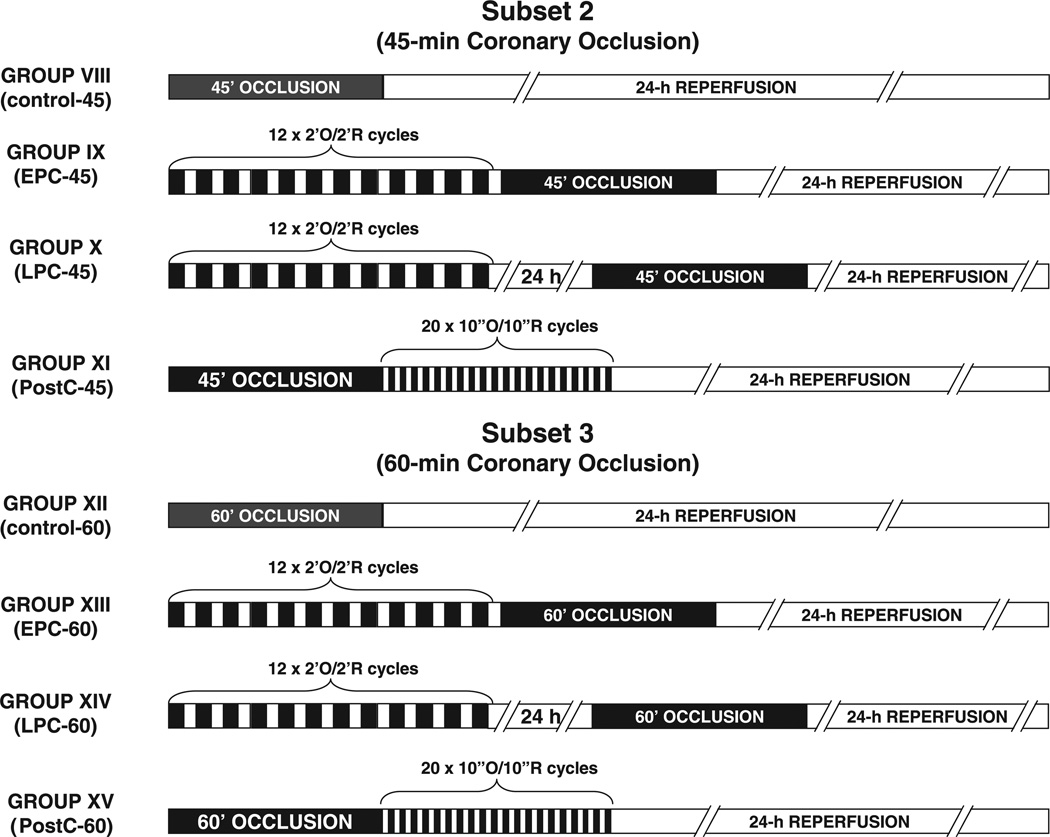
Three experimental subsets were studied. In Subset 1, all rats underwent a 30-min coronary occlusion and were assigned to seven groups (Fig. 1). Group I (control-30 group) received no further intervention. Groups II and III received an ischemic preconditioning protocol of 12 2-min coronary occlusion/2-min reperfusion cycles immediately (group II, EPC-30 group) or 24 h (group III, LPC-30 group) before the 30-min occlusion, respectively (EPC, early preconditioning; LPC, late preconditioning). Group IV (6–30 PostC group) received a postconditioning protocol of six 30-s occlusion/30-s reperfusion cycles at the onset of reperfusion after the 30-min occlusion. Groups V–VII were postconditioned with 6 (group V, 6–10 PostC group), 20 (group VI, 20–10 PostC-30 group), or 60 cycles (group VII, 60-10 PostC group) of 10-s occlusion/10-s reperfusion at the onset of reperfusion after the 30-min occlusion. In Subset 2, all rats underwent a 45-min coronary occlusion and were assigned to four groups (Fig. 2). Group VIII (control-45 group) received no further intervention. Groups IX and X were preconditioned with 12 cycles of 2-min occlusion/2-min reperfusion immediately (group IX, EPC-45 group) or 24 h (group X, LPC-45 group) before the 45-min occlusion, respectively. Group XI (20–10 PostC-45 group) was postconditioned with 20 cycles of 10-s occlusion/10-s reperfusion at the onset of reperfusion after the 45-min occlusion. In Subset 3, all rats underwent a 60-min coronary occlusion and were assigned to four groups (Fig. 2). Group XII (control-60 group) received no further intervention. Groups XIII and XIV were preconditioned with 12 cycles of 2-min occlusion/2-min reperfusion immediately (group XIII, EPC-60 group) or 24 h (group XIV, LPC-60 group) before the 60-min occlusion, respectively. Group XV (20-10 PostC-60 group) was postconditioned with 20 cycles of 10-s occlusion/10-s reperfusion at the onset of reperfusion after the 60-min occlusion.
Fig. 1.
Experimental protocols for studies of rats exposed to a 30-min coronary artery occlusion (Subset 1). O, occlusion; R, reperfusion; EPC, early preconditioning; LPC, late preconditioning; 6–30 PostC, six 30-s cycles of postconditioning; 6–10 PostC, six 10-s cycles of postconditioning; 60–10 PostC, sixty 10-s cycles of postconditioning.
Fig. 2.
Experimental protocols for studies of rats exposed to a 45-min (Subset 2) or a 60-min (Subset 3) coronary artery occlusion.
Measurement of region at risk and infarct size
At the conclusion of the study, the rats were given heparin (200 U iv), after which they were anesthetized with pentobarbital sodium (50 mg/kg iv) and euthanized with KCl. The heart was excised and perfused with Krebs-Henseleit solution through an aortic cannula by use of a Langendorff apparatus. To delineate infarcted from viable myocardium, the heart was then perfused with a 1% solution of 2,3,5-triphenyltetrazolium chloride in phosphate buffer (pH 7.4, 37°C) at a pressure of ~60 mmHg (10 ml over 5 min). To delineate the occluded-reperfused coronary vascular bed, the coronary artery was then tied at the site of the previous occlusion, and the aortic root was perfused with a 5% solution of phthalo blue dye (Heucotech, Fairless Hill, PA) in normal saline (3 ml over 3 min). As a result of this procedure, the portion of the LV supplied by the previously occluded coronary artery (region at risk) was identified by the absence of blue dye, whereas the rest of the LV was stained dark blue. The heart was then cut into six to seven transverse slices, and all atrial and right ventricular tissues were excised. The slices were weighed, fixed in a 10% neutral, buffered formaldehyde solution, and photographed (Nikon D100 digital camera with a Nikkor AF28–105-mm lens plus Promaster Spectrum 7 close-up lenses). Color pictures of heart slices were projected onto a paper screen at a 10-fold magnification, and the borders of the infarcted, ischemic-reperfused, and nonischemic regions were traced. The traced papers were then scanned with a computer and the corresponding areas measured by computerized planimetry (Adobe Photoshop, version 7.0); from these measurements, infarct size was calculated as a percentage of the risk region using methods analogous to those employed in previous studies in rabbits (20, 23).
Determination of plasma CK activity
The blood samples were centrifuged, and the plasma was analyzed with CK assay kits (Diagnostic Chemical Limited, Oxford, CT) spectrophotometrically at a 340-nm absorbance. CK activity was expressed as units per liter of plasma. Cumulative CK activity was calculated by integrating the area under the curve of CK release over the 24-h reperfusion period.
Statistical analysis
Data were analyzed by a one-way or a two-way repeated-measures (time and group) ANOVA, as appropriate, followed by Student’s t-tests with the Bonferroni correction using SigmaStat 2.0 for Windows. The relationship between infarct size and risk region size was compared among groups with an analysis of covariance (ANCOVA) using the size of the risk region as the covariate (20, 23) and was assessed by linear regression analysis using the least-squares method. ANCOVA was performed using SPSS 8.0 for Windows. P < 0.05 was considered significant. Results are reported as means ± SE.
RESULTS
Exclusions
A total of 160 conscious rats were used (4 for the pilot studies, 68 for the studies of Subset 1, and 44 for each of Subsets 2 and 3). Table 1 summarizes the initial assignments and exclusions. Ventricular fibrillation developed only during coronary occlusion. Rats that developed ventricular fibrillation but cardioverted spontaneously were included in the final analysis. The majority of exclusions for technical failure were due to balloon occluder malfunction except for two rats (1 in group IX and 1 in group XIII) that were excluded because of poor postmortem myocardial staining.
Table 1.
Exclusions from the study
| Group | Initial Assignment |
Exclusions |
Final Analysis |
|
|---|---|---|---|---|
| Died of VF during occlusion |
Technical failure |
|||
| Subset 1:30-min occlusion | ||||
| I (control-30) | 12 | 2 | 10 | |
| II (EPC-30) | 10 | 1 | 9 | |
| III (LPC-30) | 10 | 10 | ||
| IV (6–30 PostC) | 6 | 6 | ||
| V (6–10 PostC) | 10 | 10 | ||
| VI (PostC-30) | 12 | 2 | 10 | |
| VII (60-10 PostC) | 8 | 1 | 7 | |
| Subset 2: 45-min occlusion | ||||
| VIII (control-45) | 12 | 1 | 1 | 10 |
| IX (EPC-45) | 10 | 2 | 8 | |
| X (LPC-45) | 10 | 1 | 1 | 8 |
| XI (PostC-45) | 12 | 1 | 11 | |
| Subset 3: 60-min occlusion | ||||
| XII (control-60) | 12 | 2 | 10 | |
| XIII (EPC-60) | 10 | 1 | 9 | |
| XIV (LPC-60) | 10 | 1 | 1 | 8 |
| XV (PostC-60) | 12 | 1 | 1 | 10 |
| Total | 156 | 12 | 8 | 136 |
VF, ventricular fibrillation; EPC, early preconditioning (12 cycles of 2-min ischemia/2-min reperfusion applied immediately before occlusion); LPC, late preconditioning (12 cycles of 2-min ischemia/2-min reperfusion applied 24 h before occlusion); PostC, postconditioning with 20 cycles of 10-s ischemia/ 30-s reperfusion; 6–30 PostC, postconditioning with 6 cycles of 30-s ische-mia/30-s reperfusion; 6–10 PostC, postconditioning with 6 cycles of 10-s ischemia/30-s reperfusion; 60–10 PostC, postconditioning with 60 cycles of 10-s ischemia/30-s reperfusion.
Heart rate and basic parameters
As shown in Table 2, heart rate remained constant during coronary occlusion and reperfusion within each group and was not different among groups at any given time. Basic parameters including body weight, LV weight, and the size of the region at risk were also comparable among groups (Table 3).
Table 2.
Heart rate
| Group | n | Baseline | Occlusion |
Reperfusion |
||||
|---|---|---|---|---|---|---|---|---|
| 15 min | 30 min | 45 min | 60 min | 5 min | 30 min | |||
| Subset 1: 30-min occlusion | ||||||||
| I (control-30) | 10 | 475±12 | 456±8 | 450±10 | 462±14 | 472±14 | ||
| II (EPC-30) | 9 | 454±16 | 424±15 | 427±11 | 448±11 | 450±8 | ||
| III (LPC-30) | 10 | 482±10 | 458±10 | 436±11 | 456±12 | 450±12 | ||
| IV (6–30 PostC) | 6 | 473±12 | 460±7 | 453±10 | 473±8 | 460±10 | ||
| V (6–10 PostC) | 10 | 474±10 | 454±7 | 466±6 | 450±13 | 454±12 | ||
| VI (PostC-30) | 10 | 469±6 | 458±7 | 444±9 | 446±9 | 440±11 | ||
| VII (60-10 PostC) | 7 | 474±14 | 460±12 | 463±12 | 466±13 | 462±12 | ||
| Subset 2: 45-min occlusion | ||||||||
| VIII (control-45) | 10 | 468±12 | 434±12 | 440±14 | 439±18 | 464±11 | 466±11 | |
| IX (EPC-45) | 8 | 487±10 | 435±11 | 435±12 | 424±11 | 443±10 | 435±11 | |
| X (LPC-45) | 8 | 455±13 | 433±11 | 425±15 | 426±23 | 440±21 | 437±14 | |
| XI (PostC-45) | 11 | 476±7 | 445±7 | 445±8 | 440±9 | 476±7 | 445±7 | |
| Subset 3: 60-min occlusion | ||||||||
| XII (control-60) | 10 | 482±9 | 454±8 | 444±10 | 440±9 | 438±6 | 448±8 | 448±7 |
| XIII (EPC-60) | 9 | 467±8 | 464±9 | 462±8 | 456±9 | 456±12 | 467±12 | 460±10 |
| XIV (LPC-60) | 8 | 478±7 | 445±12 | 425±8 | 438±9 | 433±13 | 455±11 | 463±10 |
| XV (PostC-60) | 10 | 482±6 | 462±8 | 442±8 | 451±11 | 447±7 | 468±5 | 468±8 |
Values are means±SE (in beats/min). For abbreviations, see legend to Table 1.
Table 3.
Body weight, total LV weight, and area at risk
| Group | n | Body Weight, g |
Total LV Weight, g |
Region at Risk |
|
|---|---|---|---|---|---|
| Weight, g | %LV | ||||
| Subset 1: 30-min occlusion | |||||
| I (control-30) | 10 | 271 ±16 | 0.56±0.02 | 0.15±0.01 | 26.8±1.9 |
| II (EPC-30) | 9 | 253 ±13 | 0.60±0.02 | 0.16±0.01 | 27.1 ±1.9 |
| III (LPC-30) | 10 | 268 ±8 | 0.59 ±0.01 | 0.16±0.01 | 27.6±1.8 |
| IV (6–30 PostC) | 6 | 270±10 | 0.56±0.03 | 0.16±0.02 | 29.1 ±2.0 |
| V (6–10 PostC) | 10 | 234±4 | 0.54±0.02 | 0.16±0.01 | 29.6±2.4 |
| VI (PostC-30) | 10 | 266 ±9 | 0.57 ±0.03 | 0.15±0.01 | 26.5±2.1 |
| VII (60-10 PostC) | 7 | 268±17 | 0.55 ±0.02 | 0.14±0.01 | 24.7±2.3 |
| Subset 2: 45-min occlusion | |||||
| VIII (control-45) | 10 | 270±16 | 0.58 ±0.03 | 0.17±0.01 | 29.1 ±2.1 |
| IX (EPC-45) | 8 | 234±13 | 0.54±0.02 | 0.15±0.01 | 29.0±3.1 |
| X (LPC-45) | 8 | 249 ±3 | 0.57 ±0.04 | 0.16±0.01 | 29.0±1.8 |
| XI (PostC-45) | 11 | 270 ±6 | 0.65 ±0.02 | 0.19±0.02 | 30.8±2.3 |
| Subset 3: 60-min occlusion | |||||
| XII (control-60) | 10 | 242±10 | 0.64±0.04 | 0.18±0.01 | 28.7±1.4 |
| XIII (EPC-60) | 9 | 238±12 | 0.60±0.04 | 0.16±0.02 | 27.6±2.3 |
| XIV (LPC-60) | 8 | 237 ±6 | 0.64±0.02 | 0.19±0.02 | 28.9±2.3 |
| XV (PostC-60) | 10 | 251 ±8 | 0.62±0.03 | 0.18±0.01 | 28.5±1.1 |
Values are means ± SE. LV, left ventricle. For group abbreviations, see legend to Table 1.
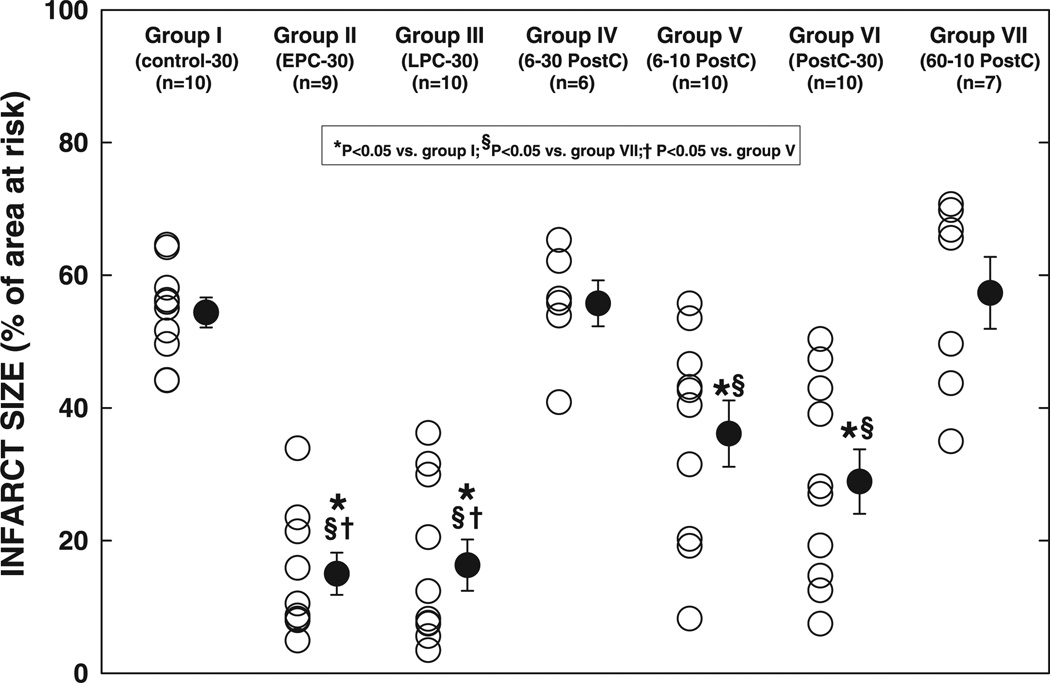
Myocardial infarction after a 30-min occlusion (Subset 1)
The average infarct size, expressed as a percentage of the region at risk, was 54.4 ± 2.3% in control animals (group I) and was significantly reduced in groups II (EPC-30 group, 15.0 ± 3.2%, P < 0.05) and III (LPC-30 group, 16.3 ± 3.9%, P < 0.05) (−72 and −70%, respectively, vs. group I) (Fig. 3, and see Fig. 12), indicating a robust infarct size-limiting effect of both EPC and LPC in conscious rats. Infarct size in group IV (6–30 PostC group, 55.8 ± 3.5% of the region at risk) was similar to that in group I (Fig. 3), suggesting that postconditioning with six 30-s cycles does not produce a protective effect in conscious rats. However, in group V (6–10 PostC group), infarct size (36.1 ± 5.0% of the region at risk) was 34% smaller than that in group I (P < 0.05), although it was larger than that in both groups II (P < 0.05) and III (P < 0.05) (Fig. 3), suggesting that postconditioning with six 10-s cycles is effective but affords relatively mild protection. In group VI (20-10 PostC-30 group), infarct size (28.9 ± 4.9% of region at risk) was reduced by 47% compared with group I (P < 0.05) and tended to be smaller than that in group V [P = not significant (NS)] (Fig. 3); it was larger than that in the two preconditioning groups (groups II and III, P = NS), although the differences were not significant (Fig. 3, and see Fig. 12), indicating that extending the postconditioning algorithm from 6 to 20 cycles of 10-s occlusions increases the cardioprotective potency of the protocol, although the potency is still less than that afforded by ischemic preconditioning. Interestingly, infarct size in group VII (60-10 PostC group, 57.3 ± 5.4% of the region at risk) was similar to that in group I and significantly larger than that in groups V and VI (Fig. 3), suggesting that further extension of the postconditioning algorithm to 60 cycles not only failed to produce additional reduction in infarct size, but also reversed the cardioprotection.
Fig. 3.
Infarct size after a 30-min coronary occlusion and 24 h of reperfusion. ○, Individual hearts; ●, group means ± SE.
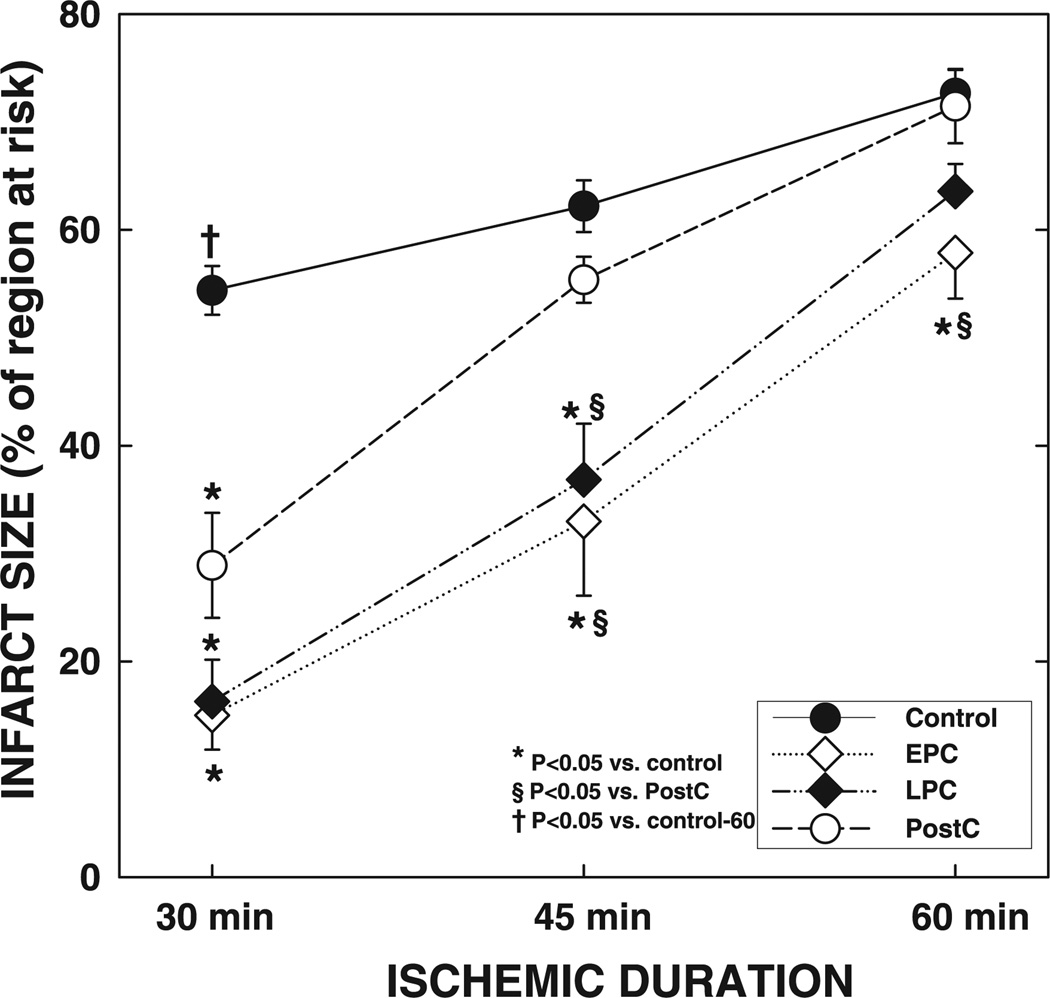
Fig. 12.
Myocardial infarct size after a 30-, 45-, or 60-min coronary occlusion and the effects of ischemic preconditioning and postconditioning. Data are means ± SE.
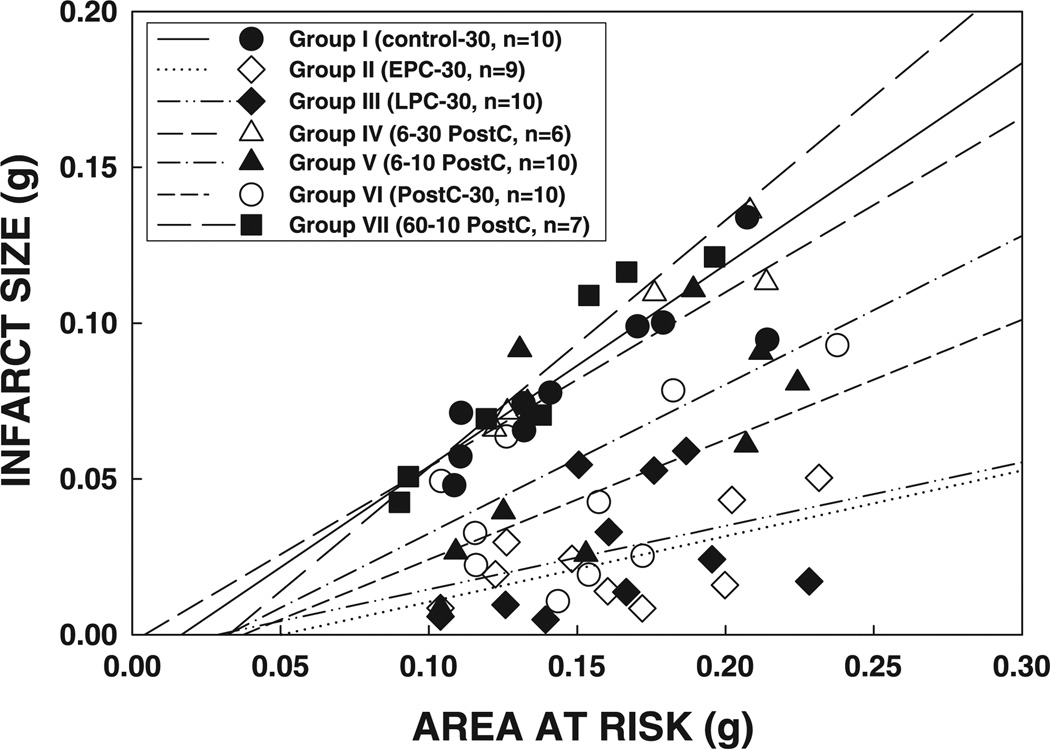
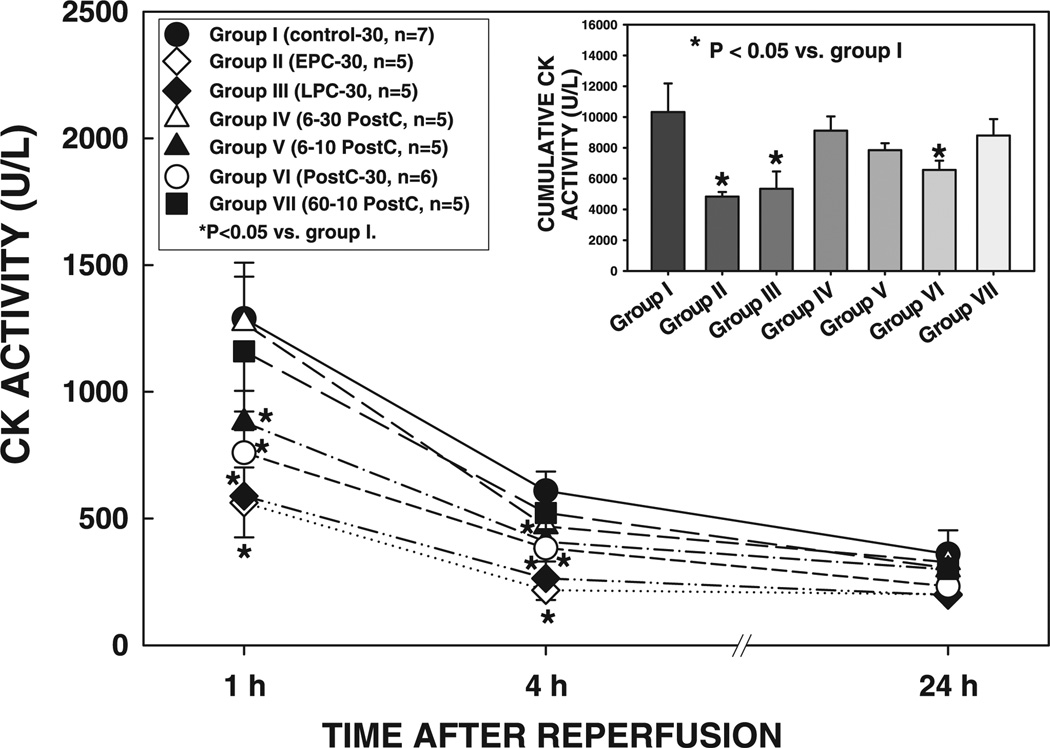
In all seven groups, the size of the infarction was positively and linearly related to the size of the region at risk (r = 0.883, 0.602, 0.348, 0.949, 0.634, 0.565, and 0.958, respectively, in groups I, II, III, IV, V, VI, and VII) (Fig. 4). As shown in Fig. 4, the regression lines were significantly shifted downward and to the right in groups II and III compared with group I (for both groups II and III, P < 0.05 by ANCOVA), indicating that for any given size of the region at risk, the resulting infarction was smaller in the hearts of the two preconditioned groups. The regression lines in groups V and VI were also significantly shifted to the right compared with group I (for both groups V and VI, P < 0.05 by ANCOVA) (Fig. 4), although to a lesser extent than in groups II and III, suggesting that the postconditioning algorithm of 6 or 20 10-s cycles affords infarct size-limiting effects (although the magnitude of this effect was slightly lower than ischemic preconditioning). In contrast, in groups IV and VII, the regression lines were indistinguishable from that in group I and significantly (P < 0.05 by ANCOVA) different from those in groups V and VI, indicating that for any given size of the region at risk, the resulting infarction was greater in rats subjected to the postconditioning algorithm of 6 30-s or 60 10-s cycles (Fig. 4). In agreement with the results obtained with tetrazolium staining, plasma CK activity at 1 and 4 h of reperfusion and cumulative CK activity over the 24-h reperfusion period were reduced in groups II, III, V, and VI compared with groups I, IV, and VII (Fig. 5), confirming the presence of an infarct size-limiting effect of the appropriate algorithm of postconditioning as well as of the two phases of ischemic preconditioning.
Fig. 4.
Relationship between size of the region at risk and size of myocardial infarction in rats exposed to a 30-min coronary occlusion. Both individual values and the regression lines obtained by linear regression analysis for groups I, II, III, IV, V, VI, and VII are illustrated. In all groups, infarct size was positively and linearly related to the size of region at risk. The linear regression equations were as follows: group I, y = 0.56x − 0.002 (r = 0.88); group II, y = 0.21x − 0.011 (r = 0.60); group III, y = 0.20x − 0.001 (r = 0.35); group IV, y = 0.65x − 0.011 (r = 0.95); group V, y = 0.48x − 0.015 (r = 0.63); group VI, y = 0.38x − 0.014 (r = 0.57); group VII, y = 0.79x − 0.026 (r = 0.96). Analysis of covariance (ANCOVA) demonstrated that the regression lines for groups II, III, V, and VI were significantly different from that for group I (P < 0.05 for each comparison), indicating that for any given size of the region at risk, infarct size was smaller in rats that received either the 2 preconditioning protocols or the 2 appropriate postconditioning protocols compared with the controls; in contrast, the lines for groups IV and VII were similar to that for group I.
Fig. 5.
Plasma creatine kinase (CK) activity in rats exposed to a 30-min coronary occlusion at 1, 4, and 24 h of reperfusion. Inset: cumulative CK activity over the 24-h reperfusion period in groups I, II, III, IV, V, VI, and VII. Data are means ± SE.
Myocardial infarction after a 45-min occlusion (Subset 2)
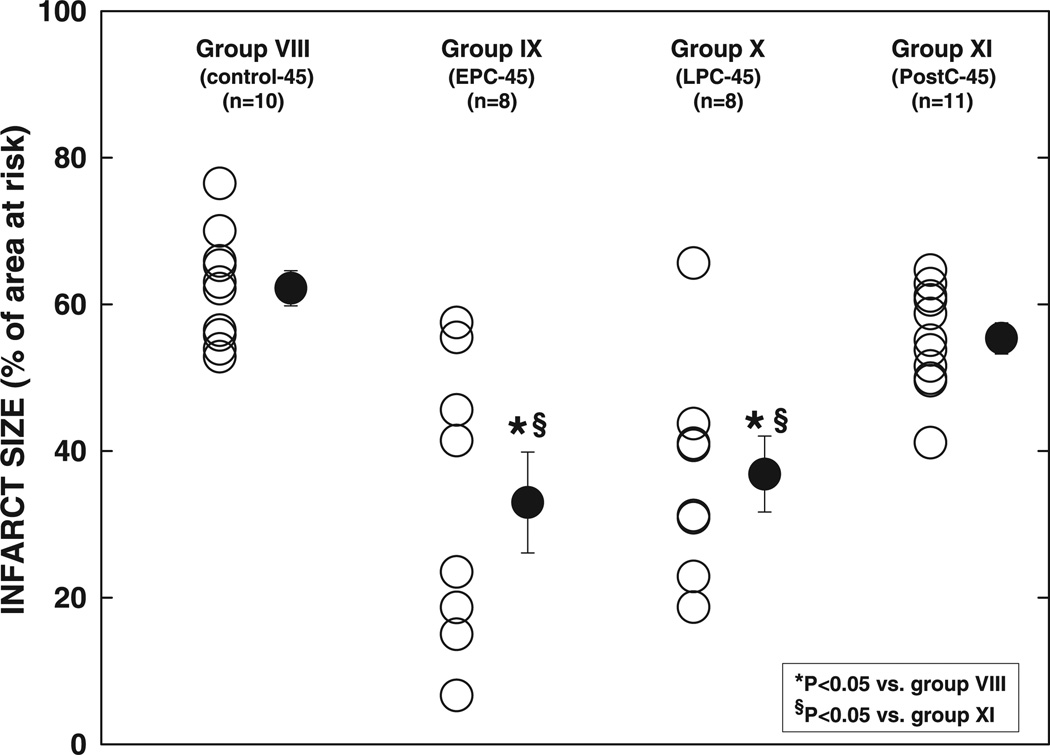
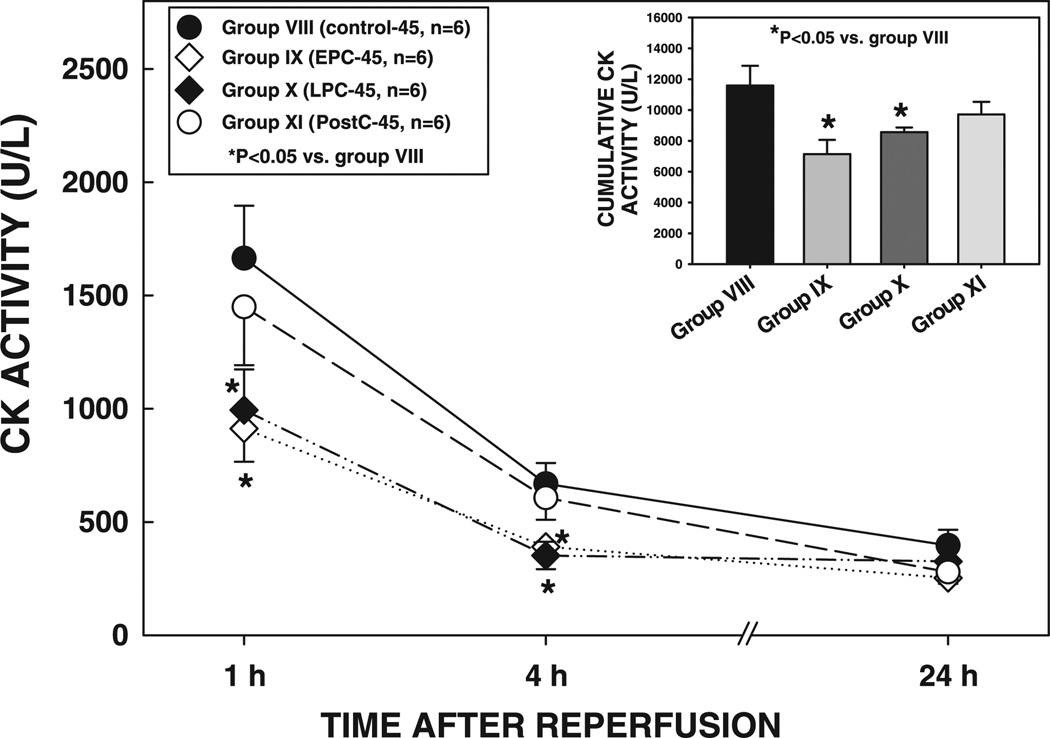
Infarct size in group VIII (control-45 group, 62.2 ± 2.4% of the region at risk) was slightly (+14%) larger compared with group I (control-30 group, P = NS) (Fig. 6, and see Fig. 12). It was markedly reduced by 47% in group IX (EPC-45 group, P < 0.01) and by 41% in group X (LPC-45 group, P < 0.01) (Fig. 6, and see Fig. 12), demonstrating that EPC and LPC protect against a 45-min occlusion. However, infarct size in group XI (20-10 PostC-45 group, 55.4 ± 2.4% of the region at risk) was not significantly different from that in group VIII (P = NS) (Fig. 6, and see Fig. 12), indicating that postconditioning does not alleviate the injury induced by a 45-min coronary occlusion.
Fig. 6.
Infarct size after a 45-min coronary occlusion and 24 h of reperfusion. ○, Individual hearts; ●, group means ± SE.
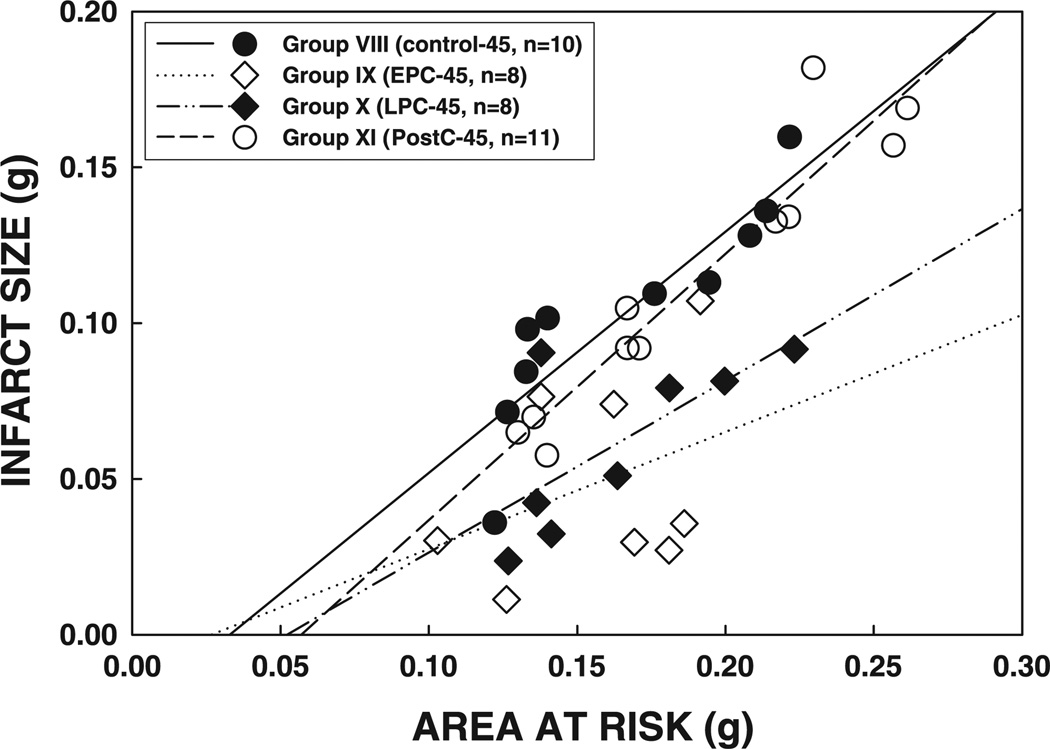
As in Subset 1, the size of the infarction was positively and linearly related to the size of the region at risk (r = 0.887, 0.362, 0.701, and 0.951, respectively, in groups VIII, IX, X, and XI) (Fig. 7). The regression lines in groups IX and X were shifted down compared with group VIII (P < 0.05 for both groups IX and X), indicating that for any given size of the region at risk, the resulting infarction was smaller in the preconditioned groups (Fig. 7). In contrast, the regression line in group XI was virtually superimposable to that in group VIII (P = NS by ANCOVA) (Fig. 7). Consistent with these data, plasma CK activity at 1 and 4 h after reperfusion and cumulative CK activity were significantly reduced in groups IX and X, but not in group XI, compared with groups VIII and XI (Fig. 8), confirming the infarct size-limiting effect of both the early and late phases of ischemic preconditioning but not postconditioning.
Fig. 7.
Relationship between size of the region at risk and size of myocardial infarction in rats exposed to a 45-min coronary occlusion. Both individual values and the regression lines obtained by linear regression analysis for groups VIII, IX, X, and XI are shown. In all groups, infarct size was positively and linearly related to the size of region at risk. The linear regression equations were as follows: group VIII, y = 0.77x− 0.025 (r = 0.89); group IX, y = 0.38x− 0.010 (r = 0.36); group X, y = 0.55x− 0.028 (r = 0.70); group XI, y = 0.85x− 0.049 (r = 0.95). ANCOVA demonstrated that the regression lines for groups IX and X were significantly shifted downward and to the right compared with that for group VIII (P < 0.05 for each comparison), indicating that, for any given size of the region at risk, infarct size was smaller in rats that received either the early or the late preconditioning protocol; in contrast, the line for group XI was similar to that for group VIII, indicating that no protective effect is exerted by postconditioning.
Fig. 8.
Plasma CK activity in rats exposed to a 45-min coronary occlusion at 1, 4, and 24 h of reperfusion. Inset: cumulative CK activity over the 24-h reperfusion period in groups VIII, IX, X, and XI. Data are means ± SE.
Myocardial infarction after a 60-min occlusion (Subset 3)
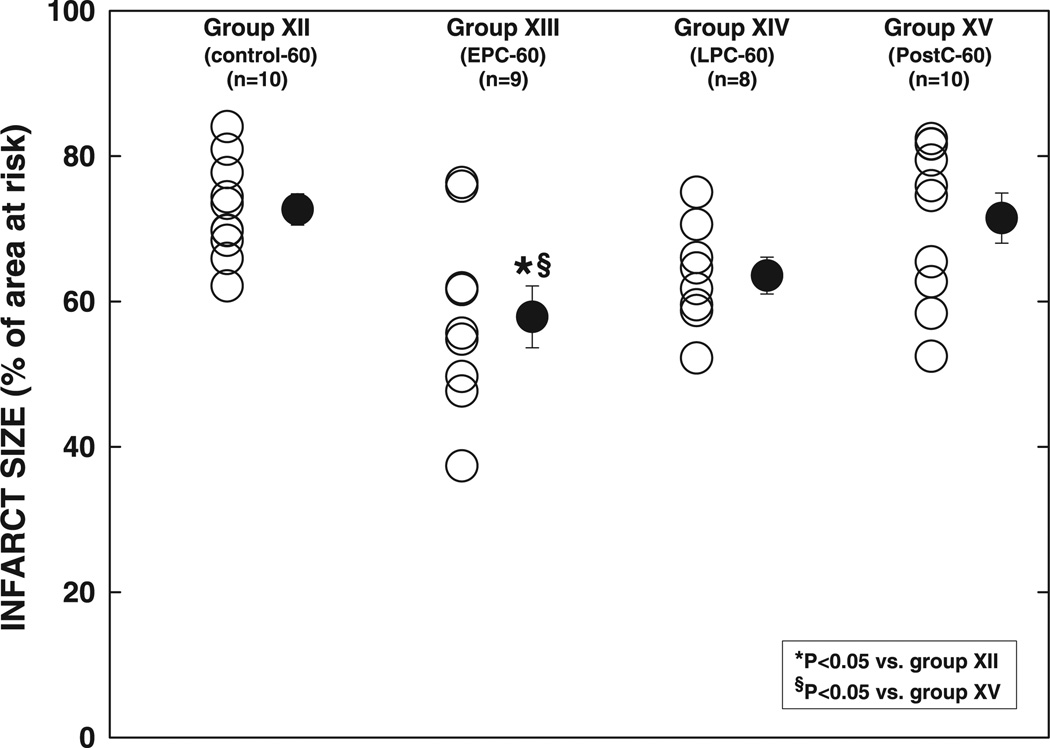
As shown in Fig. 9, infarct size in group XII (control-60 group) (72.7 ± 2.2% of the region at risk) was 34 and 17% larger than that in groups I (control-30 group, P < 0.05) and VIII (control-45 group, P = NS), respectively (Fig. 12). Infarct size was reduced significantly, albeit slightly (−20%), in group XIII (EPC-60 group, P < 0.05 vs. group XII) (Fig. 9, and see Fig. 12), indicating that, after a 60-min occlusion, the cardioprotection afforded by EPC is still present although not robust. In group XIV (LPC-60 group), infarct size tended to be smaller compared with group XII, but the difference was not significant (P = NS) (Fig. 9, and see Fig. 12), suggesting that LPC is not powerful enough to protect against the injury induced by a 60-min occlusion. Infarct size in group XV (20-10 PostC-60 group, 71.4 ± 3.4% of region at risk) was similar to that in group XII (P = NS) and was significantly larger than that in group XIII (P < 0.05) (Fig. 9, and see Fig. 12), suggesting that postconditioning does not confer cardioprotection after a 60-min occlusion.
Fig. 9.
Infarct size after a 60-min coronary occlusion and 24 h of reperfusion. ○, Individual hearts; ●, group means ± SE.
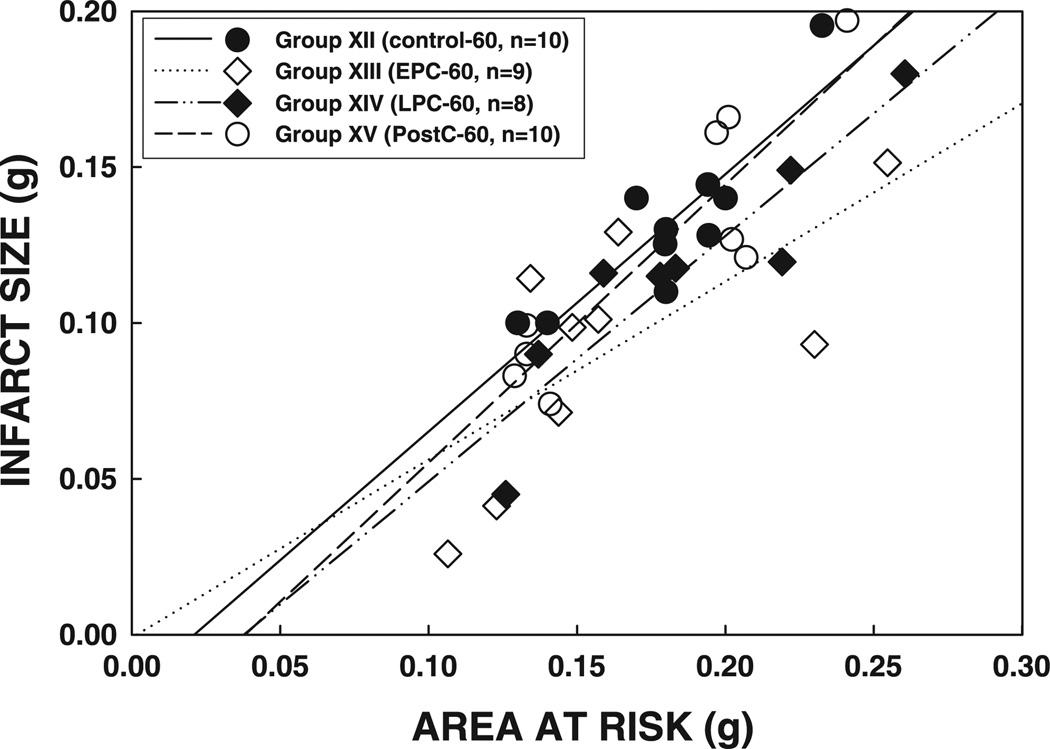
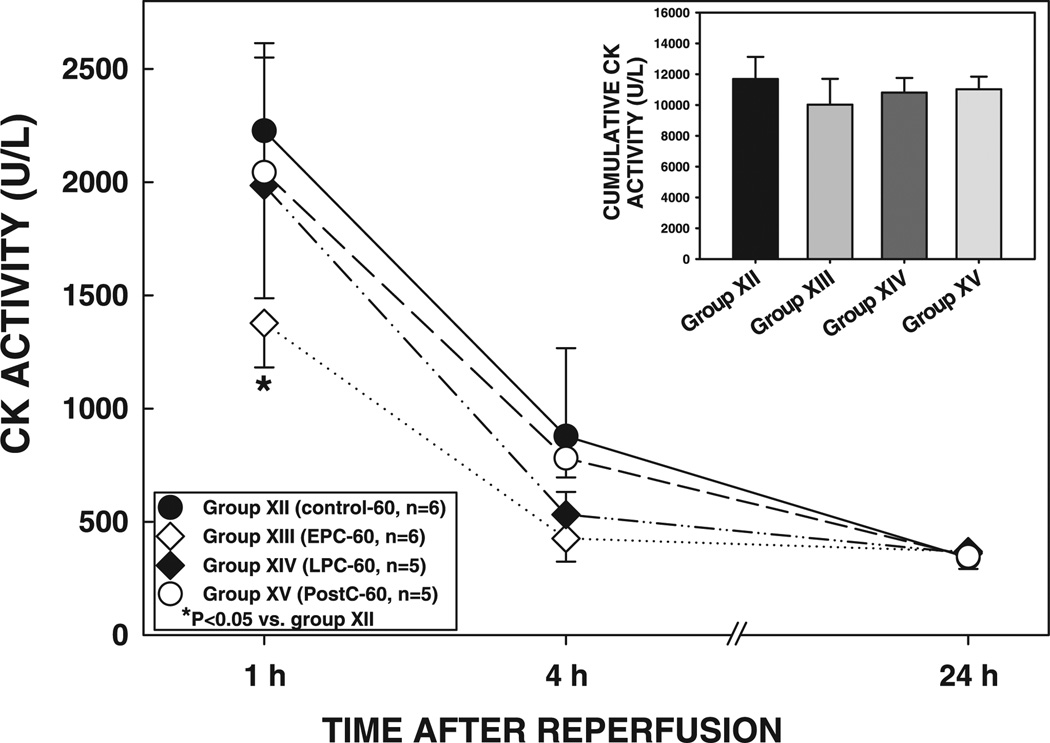
As in the previous subsets, the size of the infarction was positively and linearly related to the size of the region at risk (r = 0.876, 0.695, 0.916, and 0.890, respectively, in groups XII, XIII, XIV, and XV) (Fig. 10). The regression line in group XIII was significantly shifted down compared with group XII (P < 0.05), confirming the infarct-sparing effects of EPC (Fig. 10). In contrast, the regression lines in groups XIV and XV did not differ significantly from that in group XII (P = NS by ANCOVA) (Fig. 10). In group XIII, plasma CK activity was significantly reduced at 1 h of reperfusion compared with that in group XII (P < 0.05) (Fig. 11); however, CK activity at 4 h of reperfusion and cumulative CK activity were not significantly different (P = NS) (Fig. 11), suggesting that EPC affords mild protection against the myocardial infarction induced by a 60-min occlusion.
Fig. 10.
Relationship between size of the region at risk and size of myocardial infarction in rats exposed to a 60-min coronary occlusion. Both individual values and the regression lines obtained by linear regression analysis for groups XII, XIII, XIV, and XV are illustrated. In all groups, infarct size was positively and linearly related to the size of region at risk. The linear regression equations were as follows: group XII, y = 0.83x− 0.017 (r = 0.88); group XIII, y = 0.57x− 0.001 (r = 0.70); group XIV, y = 0.79x− 0.029 (r = 0.92); group XV, y = 0.89x− 0.034 (r = 0.89). ANCOVA demonstrated that the regression line for group XIII was significantly shifted downward and to the right compared with that for group XII (P < 0.05), indicating that, for any given size of region at risk, infarct size was smaller in rats that received the early preconditioning protocol; in contrast, the lines for groups XIV and XV were not significantly different from that for group XII.
Fig. 11.
Plasma CK activity in rats exposed to a 60-min coronary occlusion at 1, 4, and 24 h of reperfusion. Inset: cumulative CK activity over the 24-h reperfusion period in groups XII, XIII, XIV, and XV. Data are means ± SE.
DISCUSSION
The purpose of this study was to comprehensively assess the cardioprotection afforded by ischemic preconditioning and postconditioning in conscious rats. Our results can be summarized as follows: 1) a sequence of 12 cycles of 2-min coronary occlusion/2-min reperfusion applied immediately before a 30-,45-, or 60-min ischemic insult markedly reduces infarct size and CK release, demonstrating a robust early preconditioning effect against myocardial infarction; 2) the same sequence of 12 cycles of occlusion/reperfusion applied 24 h earlier also significantly reduces infarct size and CK release induced by a 30- or 45-min occlusion but not by a 60-min occlusion, indicating that late preconditioning affords protection only when the coronary occlusion is <60 min; 3) performing six cycles of 30-s occlusion and 30-s reperfusion at the onset of reperfusion after a 30-min occlusion does not limit infarct size, but shortening the postconditioning cycle length from 30 to 10 s significantly reduces infarct size and CK release, suggesting that cardioprotection can be conferred by postconditioning in conscious rats; 4) extending the number of postconditioning cycles from 6 to 20 results in a small gain in cardioprotection, and further extension of the postconditioning sequence to 60 cycles not only does not provide any further gain but also reverses the cardioprotection; and 5) the 20-cycle postconditioning protocol that reduces infarct size after a 30-min occlusion does not significantly reduce infarct size or CK release after a 45- or 60-min occlusion, suggesting that postconditioning protects only against the myocardial injury caused by coronary occlusions <45 min. To our knowledge, this is the first demonstration that the phenomenon of postconditioning protects against myocardial infarction in conscious animals and that this protective mechanism is limited to relatively short (<45 min) coronary occlusions.
In this investigation, we have developed a model of myocardial infarction in conscious rats. The Fisher 344 inbred rat was used because inbred strains are thought to yield less interanimal variation (2). Indeed, we noticed that the anatomy of the coronary circulation is highly consistent in this strain of rats and the variability in infarct size reasonably modest in controls (Figs. 3, 6, and 9). The rationale for using a conscious preparation was to avoid a number of factors that could interfere with the assessment of myocardial injury, of ischemic preconditioning, and of ischemic postconditioning, including anesthesia, fluctuations in body temperature, abnormal hemodynamic conditions, elevated catecholamine levels, and cytokine release. In this regard, many studies have shown that anesthetics can induce the cardioprotective effect of both preconditioning (6, 18, 19, 24, 27) and postconditioning (4, 8, 28). Because the primary end point of the present study was the assessment of cardioprotection, we felt it was important to avoid the confounding factors that are associated with anesthetics and open-chest animal models. An important aspect of this study was that the results obtained with tetrazolium staining of the myocardium were verified by measurements of CK release after reperfusion. The fact that, in all groups, the planimetry-based infarct size data by tetrazolium were in agreement with the data regarding CK release strengthens our conclusions and confirms the validity of using CK release as a surrogate measure of infarct size (31).
Using this model, we have demonstrated that myocardial infarct size increases progressively with the ischemic duration, from 54% of the region at risk after a 30-min occlusion to 72% after a 60-min coronary occlusion (Fig. 12).
The recently discovered phenomenon of postconditioning has broader clinical applications than preconditioning (16). However, although effective cardioprotection by postconditioning has been found in various species (1, 4, 7, 11, 13–15, 26, 29–32), most of these studies have used animal models with mild to moderate degrees of ischemic injury, i.e., relatively small infarct size (<55% of region at risk), except for one report in open-chest rabbits (30) that showed that postconditioning is still protective after a 45-min coronary occlusion causing infarct size >60% of the region at risk. In the present study, we found that postconditioning with the 10-s cycle protocol significantly reduced infarct size and CK release after a 30-min occlusion (when infarct size is <60%). These findings in conscious rats are analogous to those in open-chest rats (14, 15, 31). We also found that increasing the number of postconditioning cycles from 6 to 20 produced a further, although not significant, reduction of infarct size and CK release. Importantly, we found that the most effective postconditioning protocol (20 10-s cycles) did not limit infarct size after coronary occlusions lasting 45 min or longer (when infarct size was >60% of the region at risk), despite the presence of robust cardioprotection by ischemic preconditioning. Hence, our data demonstrate that, in conscious rats, the protection afforded by postconditioning is modest relative to that afforded by early and late preconditioning, being observed only when the index ischemic insult is <45 min.
Our present findings in rats are apparently at odds with those of Yang et al. (30), who reported that in open-chest rabbits, postconditioning was still effective in reducing infarct size after a 45-min occlusion. The reason(s) for the apparent discrepancy is unknown. The divergent results may be secondary to differences in species (rabbits vs. rats), experimental preparations (open-chest vs. conscious animals), reperfusion duration (3 vs. 24 h), or sample size (6 rabbits vs. 11 rats). However, the results of Yang et al. (30) differ also from a recent study (13) in which the same postconditioning protocol (4 30-s cycles) in the same animal model (open-chest rabbits subjected to a 30-min occlusion) produced no effect on infarct size [although a significant infarct size-limiting effect could be induced with a different postconditioning protocol (6 10-s cycles)]. Interestingly, in this study (13), hypercholesterolemic rabbits with larger infarct size (−60% of the risk region) were not protected by either of the two postconditioning protocols, despite robust cardioprotection by ischemic preconditioning. The findings in hypercholesterolemic rabbits are similar to our findings in healthy rats. Although the authors concluded that postconditioning is ineffective in hypercholesterolemic and atherosclerotic rabbits (13), an alternative explanation may simply be that postconditioning is not powerful enough to alleviate myocardial injury when infarct size is as large as 60% of the risk region.
We also found that the six 30-s cycle protocol of postconditioning that has been shown to be effective in reducing infarct size in dogs (11, 32) and rabbits (30) is ineffective in conscious rats. This finding confirms that an appropriate postconditioning algorithm is crucial for cardioprotection to occur (13, 29). Interestingly, we also found that a further increase in postconditioning cycles from 20 to 60 not only failed to increase the potency of cardioprotection but also reversed the beneficial effect of postconditioning. The reason for this is unclear but may relate to spasm of the coronary artery resulting from excessive manipulations or to the ischemic injury inflicted by the cumulative additional 10 min of coronary occlusion associated with the postconditioning algorithm.
Fig. 12 shows that, in conscious rats, the relationship between the extent of damage (infarct size) and the duration of ischemia is not linear; that is, doubling the duration of ischemia from 30 to 60 min results only in a 34% relative increase in infarct size (from 54.4 ± 2.3% of the region at risk to 72.7 ± 2.2% after 60 min). It is also apparent that the protective efficacy of early preconditioning, late preconditioning, and postconditioning depends more on the duration of ischemia than on the extent of damage (measured as infarct size under control conditions). That is, early and late preconditioning and postconditioning are very protective after 30 min of coronary occlusion, when infarct size in control rats averages 54.4 ± 2.3% of the region at risk. They are, however, either ineffective or only mildly effective when the duration of coronary occlusion is doubled to 60 min, despite the fact that the extent of damage observed in control conditions at this time (72.7 ± 2.2% of the region at risk) is only 34% larger. Our data imply that increasing durations of ischemia increase tissue injury in a manner that cannot be measured simply by infarct size assessment; as the ischemia is prolonged, protective mechanisms are lost, even though the final extent of damage in the unprotected (control) state changes only modestly.
In conclusion, our data demonstrate that, in conscious rats, cardioprotection is conferred by both the early and the late phases of ischemic preconditioning as well as by postconditioning. Among these three manipulations, early preconditioning is the most powerful, followed (in descending order) by late preconditioning and by postconditioning. The infarct-sparing effects of postconditioning are limited, being evident only in settings in which the duration of ischemia is <45 min. As previously pointed out (16), postconditioning is clinically more feasible than preconditioning and could have broad practical applications. Our data, however, appear to suggest that the clinical potential of this intervention may be restricted by its limited protective efficacy.
Acknowledgments
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grants R01-HL-74351, -HL-55757, -HL-68088, -HL-70897, -HL-76794, and -HL-78825 and by American Heart Association Grant 0355391B.
REFERENCES
- 1.Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- 2.Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ. Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am J Physiol Heart Circ Physiol. 2000;278:H1395–H1400. doi: 10.1152/ajpheart.2000.278.4.H1395. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 4.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–109. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MV, Downey JM. Myocardial preconditioning promises to be a novel approach to the treatment of ischemic heart disease. Annu Rev Med. 1996;47:21–29. doi: 10.1146/annurev.med.47.1.21. [DOI] [PubMed] [Google Scholar]
- 6.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Darling CE, Jiang R, Maynard M, Whittaker P, Vinten-Johansen J, Przyklenk K. Postconditioning via stuttering reperfusion limits myocardial infarct size in rabbit hearts: role of ERK1/2. Am J Physiol Heart Circ Physiol. 2005;289:H1618–H1626. doi: 10.1152/ajpheart.00055.2005. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, Sun HY, Guyton RA, Vinten-Johansen J, Zhao ZQ. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78:961–969. doi: 10.1016/j.athoracsur.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Heusch G, Buchert A, Feldhaus S, Schulz R. No loss of cardioprotection by postconditioning in connexin 43-deficient mice. Basic Res Cardiol. 2006;101:354–356. doi: 10.1007/s00395-006-0589-0. [DOI] [PubMed] [Google Scholar]
- 13.Iliodromitis EK, Zoga A, Vrettou A, Andreadou I, Paraskevaidis IA, Kaklamanis L, Kremastinos DT. The effectiveness of postconditioning and preconditioning on infarct size in hypercholesterolemic and normal anesthetized rabbits. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2005.11.023. In press. [DOI] [PubMed] [Google Scholar]
- 14.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–133. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Kloner RA, Rezkalla SH. Preconditioning, postconditioning, and their application to clinical cardiology. Cardiovasc Res. 2006;70:297–307. doi: 10.1016/j.cardiores.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 18.Novalija E, Varadarajan SG, Camara AK, An J, Chen Q, Riess ML, Hogg N, Stowe DF. Anesthetic preconditioning: triggering role of reactive oxygen and nitrogen species in isolated hearts. Am J Physiol Heart Circ Physiol. 2002;283:H44–H52. doi: 10.1152/ajpheart.01056.2001. [DOI] [PubMed] [Google Scholar]
- 19.Piriou V, Chiari P, Lhuillier F, Bastien O, Loufoua J, Raisky O, David JS, Ovize M, Lehot JJ. Pharmacological preconditioning: comparison of desflurane, sevoflurane, isoflurane and halothane in rabbit myocardium. Br J Anaesth. 2002;89:486–491. [PubMed] [Google Scholar]
- 20.Qiu Y, Rizvi A, Tang XL, Manchikalapudi S, Takano H, Jadoon AK, Wu WJ, Bolli R. Nitric oxide triggers late preconditioning against myocardial infarction in conscious rabbits. Am J Physiol Heart Circ Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz LM, Lagranha CJ. Ischemic postconditioning during reperfusion activates Akt and ERK without protecting against lethal myocardial ischemia-reperfusion injury in pigs. Am J Physiol Heart Circ Physiol. 2006;290:H1011–H1018. doi: 10.1152/ajpheart.00864.2005. [DOI] [PubMed] [Google Scholar]
- 22.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 23.Takano H, Manchikalapudi S, Tang XL, Qiu Y, Rizvi A, Jadoon AK, Zhang Q, Bolli R. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation. 1998;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Ludwig LM, Krolikowski JG, Alcindor D, Pratt PF, Kersten JR, Pagel PS, Warltier DC. Isoflurane produces delayed preconditioning against myocardial ischemia and reperfusion injury: role of cyclooxygenase-2. Anesthesiology. 2004;100:525–531. doi: 10.1097/00000542-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Tang XL, Takano H, Xuan YT, Sato H, Kodani E, Dawn B, Zhu Y, Shirk G, Wu WJ, Bolli R. Hypercholesterolemia abrogates late preconditioning via a tetrahydrobiopterin-dependent mechanism in conscious rabbits. Circulation. 2005;112:2149–2156. doi: 10.1161/CIRCULATIONAHA.105.566190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 27.Wakeno-Takahashi M, Otani H, Nakao S, Imamura H, Shingu K. Isoflurane induces second window of preconditioning through upregulation of inducible nitric oxide synthase in rat heart. Am J Physiol Heart Circ Physiol. 2005;289:H2585–H2591. doi: 10.1152/ajpheart.00400.2005. [DOI] [PubMed] [Google Scholar]
- 28.Weber NC, Preckel B, Schlack W. The effect of anaesthetics on the myocardium-new insights into myocardial protection. Eur J Anaesthesiol. 2005;22:647–657. doi: 10.1017/s0265021505001080. [DOI] [PubMed] [Google Scholar]
- 29.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning’s protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100:57–63. doi: 10.1007/s00395-004-0498-4. [DOI] [PubMed] [Google Scholar]
- 30.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 31.Zatta AJ, Kin H, Lee G, Wang N, Jiang R, Lust R, Reeves JG, Mykytenko J, Guyton RA, Zhao ZQ, Vinten-Johansen J. Infarctsparing effect of myocardial postconditioning is dependent on protein kinase C signalling. Cardiovasc Res. 2006;70:315–324. doi: 10.1016/j.cardiores.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]