Abstract
The protein menin is encoded by the MEN1 gene, which is mutated in patients with multiple endocrine neoplasia type 1 (MEN1) syndrome. Although menin acts as a tumor suppressor in endocrine organs, it is required for leukemic transformation in mouse models. Menin possesses these dichotomous functions likely because it can both positively and negatively regulate gene expression as well as interact with a multitude of proteins with diverse functions. Here we review the recent progress in understanding the molecular mechanisms by which menin functions. The crystal structures of menin with different binding partners reveal that menin is a key scaffold protein that functionally crosstalks with various partners to regulate gene transcription and interplay with multiple signaling pathways.
Keywords: menin, scaffold protein, gene transcription, cell signaling
Menin : an orphan protein mutated in an inherited tumor syndrome
The menin protein is encoded by the MEN1 gene, which is mutated in patients with multiple endocrine neoplasia type 1 (MEN1) syndrome1, 2. MEN1 syndrome is a dominantly inherited disease that is characterized by tumor formation in endocrine organs including the pituitary gland, the parathyroid gland and pancreatic islets3–7. The majority of patients with the inherited form of this disease have one germline mutation in the MEN1 gene, with loss of heterozygosity (LOH) at the MEN1 alleles in the endocrine tumor, highlighting menin as a bona fide tumor suppressor in endocrine organs. These mutations spread throughout the coding region of the gene, with no obvious mutation hotspots5, 8. MEN1 is also frequently mutated in patients with sporadic parathyroid9 and pancreatic endocrine tumors8, 10. Consistently, mice with heterozygous Men1 mutation phenocopy the human MEN1 syndrome11–13. Complete Men1 ablation in the mouse is embryonic lethal at E11.5–13.5, with deficiencies in multiple organs11.
Menin consists of 610 residues and is conserved from Drosophila to humans14, but is not found in yeast or Caenorhabditis elegans, indicating that it is evolutionarily a relatively new gene. While menin is ubiquitously expressed in various organs during mouse embryonic development15, 16, its function is tissue-specific, sometimes displaying opposing roles between different organs. For instance, it suppresses tumorigenesis in endocrine organs, yet is essential for leukemogenesis17, 18.
It has also been reported that menin plays a role in suppressing hyperplasia or tumors in several other organs, such as the lung19, 20, prostate21, and breast22, and it exacerbates diabetes in mouse models23–25. Menin also influences the function of other organs such as bone26–31 and liver32, 33. Detailed mechanisms for how menin impacts these organs are less clear, but it likely functions via regulating various distinct signaling pathways. Menin itself is also regulated by multiple signaling proteins such prolactin23 and transforming growth factor beta (TGF-β, and by posttranslational modifications such as phosphorylation and SUMOylation. Additionally, MEN1 disease-associated single amino acid substitutions can lead to enhanced polyubiquitylation and degradation via proteasomal pathway.34–37.
Because menin lacks domains that are homologous to other proteins, it is challenging to elucidate its biochemical function. As such, intensive efforts have been made by many groups to identify menin-interacting proteins, in the hopes of finding clues about how menin biochemically suppresses tumorigenesis, as previously reviewed38, 39. These menin partners provide valuable information regarding the biochemical function of menin. However, the detailed underlying structural mechanism has been elusive. Recent progress in elucidating the crystal structure of menin, coupled with the revelation that menin crosstalks with various signaling pathways, provide new insights into how menin controls gene expression and cell signaling40, 41. This review focuses on the latest progress in understanding how menin, as a scaffold protein, controls gene expression and interplays with multiple signaling pathways to control cell behavior. As certain studies were performed in one cell line or one model system and in some situations only a limited number of studies were performed, caution is to be exercised regarding generalizing the role of menin and underlying mechanisms in these situations.
Menin interacts with proteins involved in regulating gene transcription and cell signaling
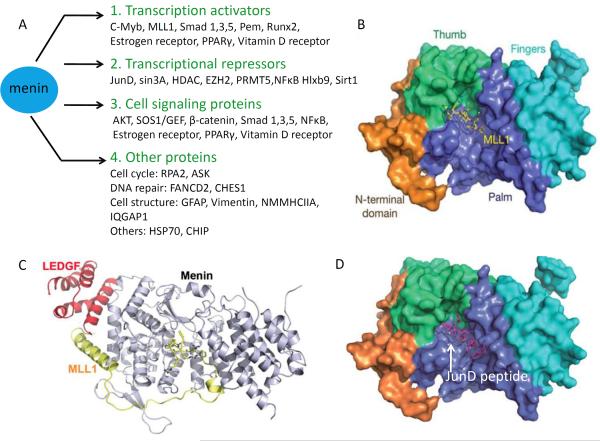
Although menin is ubiquitously expressed, its expression levels vary from tissue to tissue15, 23, 42. Menin is primarily a nuclear protein, but lesser amounts are also detectable in the cytoplasm and even the cell membrane. Its nuclear localization sequences (NLSs), located in its C-terminal region, can directly interact with DNA in a sequence-independent manner43, 44. Menin-interacting proteins can be classified into four main categories based on their cellular role in context to their association with menin: transcription activators, transcription repressors, and cell signaling proteins, and the other remaining interacting partners, which have diverse functions ranging from regulation of DNA repair to the structural integrity of the cell (Figure 1A). Menin-interacting transcriptional activators include transcription factors c-Myb, Protein-energy malnutrition (Pem) and runt-related transcription factor 2 (Runx2)45,46, 29, 47, and histone modifiers such as MLL1–2 (mixed lineage leukemia proteins) and histone H3 lysine 4 (H3K4) methyltransferases48, 49. The transcriptional repressors that directly or indirectly interact with menin include JunD, NFκB, homeobox HB9 Hlxb950, 51, 52, histone deacetylases 1/2 (HDAC1/2), the histone deacetylase Sirt1, the histone H3 lysine 27 methyltransferase EZH2, and protein arginine methyltransferase 5 (PRMT5)20, 53–55. Notably, menin also interacts with multiple proteins that mediate several signaling pathways, such as the SMAD proteins, which transduce TGFβ signaling29, 56; β-catenin, a member of the Wnt signaling pathway57–60; nuclear receptors such as estrogen receptor and PPARγ; and Ras-activating protein SOS119, 61, 62. The physiological and genetic impact of the interaction between menin and many of its interacting proteins in model organisms remains to be explored. It is noteworthy that these signaling proteins often can also act as either transcription activators or repressors, however for the purposes of this discussion we classify them based on the their activity in the context of their interaction with menin.
Figure 1. Menin-interacting proteins and structures of menin with selected interacting proteins.
(A) Four classes of menin interacting proteins based on their cellular functions in context of their interaction with menin: Transcription activators; transcription repressors; cell signaling proteins; and other proteins. Some menin interacting proteins can have dual roles as transcription factors and signaling molecules. (B) The surface representation of menin complexed with MLL1MBM (menin binding motif of MLL1). (C) Overall ternary structure of menin complexed with MLL1MBM-LBM (MLL1 menin binding motif- LEDGF binding motif) and LEDGFIBM.( lens epithelium-derived growth factor integrase binding motif) (D) Crystal structure of menin in complex with JunD peptide. Menin interacts directly with JunD. Menin binding motif of JunD consists of residues 27–47.JunDMBM is shown as purple stick model.(B–D) Reprinted by permission from [40].
Our group and others have proposed that menin might act as a scaffold or adaptor protein to interact with other proteins in controlling gene transcription, based on cellular and biochemical studies44, 63. This concept is consistent with the observation that menin distributes in a broad spectrum in gel filtration chromatography54, and that menin binds to thousands of gene loci in various cell lines64, 65. However, until recently the crystal structure of menin was not known, hindering the understanding of how menin interacts with its partners to exert its functions.
Crystal structure of menin and its interacting partners
Great efforts by many groups have been made to solve the crystal structure of menin. It has been challenging to obtain high quality menin protein crystals to decipher its structure. However, Huang et al. and Murai et al. have recently succeeded in solving the structure of menin alone40, 41, in complex with an MLL1 peptide (Fig. 1B), in a ternary complex with MLL1 and LEDGF (Lens Epithelium-Derived Growth Factor) (Fig. 1C), or in complex with a peptide from JunD (Fig. 1D)40.
The crystal structure of menin looks like a curved left hand, in which the N-terminal domain is represented by long β-hairpin, transglutaminase-like domain that resembles a thumb, the middle region adopts the shape of the palm, and the C-terminus resembles curved fingers (Fig. 1B). Notably, the `palm' forms a deep pocket that is occupied by the conserved MLL1 peptide (Fig. 1B). Mutagenesis studies show that the residues in either MLL1 or the menin pocket that mediate the menin--MLL1 interaction are crucial for binding each other as well as for the menin-mediated upregulation of Hox genes40.
Biochemical studies show that menin not only interacts with MLL1, but also interacts simultaneously with LEDGF, a protein important for MLL-AF9 induced leukemia63. The crystal structure of menin-MLL1-LEDGF clearly shows that the N-terminal region of MLL1 (yellow, Fig. 1C) binds the deep central pocket of menin, and the further downstream sequence of MLL1 loops around the N-terminal part of menin, forming an alpha helix that directly contacts the menin N terminus. On the top of the “V” shape structure that is co-formed by the alpha helix of MLL1 and the surface of the N-terminal part of menin, LEDGF (red, Fig. 1C) directly interacts with both MLL1 and menin. This is strong evidence demonstrating that menin acts as a scaffold protein to interact with multiple proteins. Consistent with this notion, a mutation in menin at the LEDGF-interaction site diminishes the ability of menin to promote expression of Hoxc8. Further work is needed to determine how menin structurally interacts with other partners.
Similar to its interaction with MLL1, Menin also binds JunD via its central pocket. The region of JunD that binds menin spans residues 27–47, and is known as the menin-binding motif (JunDMBM) (Fig. 1D)40. Comparison of the JunDMBM with the MLL1 peptide that binds menin (MLL1MBM) reveals a conserved sequence (FPXXP). Indeed, the co-crystal of menin and the JunD peptide shows that JunDMBM and MLL1MBM bind the menin pocket with the same orientation (Fig. 1B and D). MLL1MBM effectively competes out JunD binding to menin40, demonstrating that menin can use the same pocket to bind either MLL1 or JunD. MLL2 also binds to menin via a similar conserved sequence. It remains to be determined whether menin can also interact with other proteins containing similar sequences. Binding of menin to MLL1 serves to recruit these proteins to promoters of the genes, leading to an increase in gene transcription40.
Menin activates gene transcription
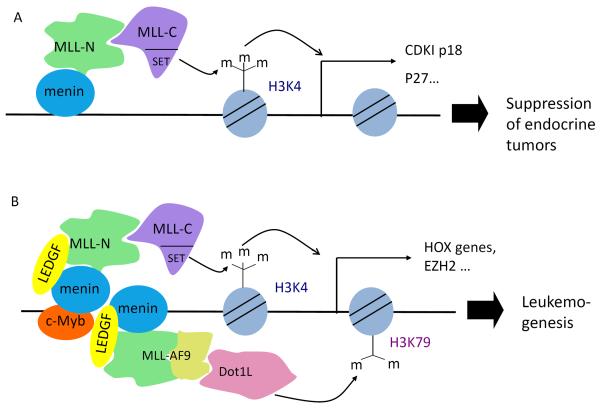
Menin upregulates the expression of cyclin-dependent kinase (CDK) inhibitors (CDKIs) p18 and p2742, 66, 67, thereby reducing beta cell proliferation. It activates transcription of the CDKIs at least partly via MLL1, which adds tri-methylation to histone H3 at lysine 4 (H3K4me3), a chromatin modification associated with transcriptional activation48, 66, 67 (Fig. 2A). Consistent with these findings, genetic ablation of RBP2, a histone H3 lysine 4 demethylase, reduced the development of insulinoma in a beta cell-specific Men1 knockout mouse model68. Whether a DNA sequence-specific factor recruits menin-MLL1 to the loci, and which factor that might be, remains unclear.
Figure 2. Menin acts as a scaffold protein to tissue-specifically activate gene transcription.
(A) In endocrine cells, menin recruits MLL1 to the promoters of cyclin dependent kinase (CDK) inhibitors p18 and p27. (B) In MLL-FP-induced leukemia cells c-Myb binds and recruits menin. Menin further recruits, via its central pocket, either wildtype MLL1 (green and violet) or MLL1-FPs, recruitment of MLL (wildtype containing MLL-N and MLL-C or MLL1-FP)can help recruit LEDGF and Dot1L. Menin can directly deposit H3K4me3, while Dot1L deposits H3K79 methylation, both leading to increased transcription of Hox genes, Meis1, and Ezh2, and blockade of myeloid differentiation. Depiction of c-Myb is not stoichiometric in this figure.
The MLL1 gene can undergo chromosomal translocations with one of various partner genes, resulting in the expression of MLL1 fusion proteins (MLL-FPs) that can induce leukemia. In these circumstances leukemogenesis is driven by c-Myb, a transcription factor that directly binds menin and likely recruits MLL1-FP, wild-type (WT) MLL1, and LEDGF to Hoxa9 and Meis1 gene loci to promote their expression (Fig. 2B)40, 45, 63, 69. Deletion of menin in these leukemia cells abolishes recruitment of WT MLL1 and MLL1-FPs and decreases H3K4me3 at these gene loci, demonstrating that menin is an essential cofactor for MLL1 function (Fig. 2B)70. Consistent with their role in promoting Hoxa9 and Meis1 expression, MLL1 fusion proteins are found in complexes associated with enhancing transcriptional activation, including the Dot1L complex, which methylates H3K79, and the pTEFb complex, which mediates transcriptional elongation71, 72.
As menin plays an important role in regulating gene expression through interaction with various partners such as MLL1 and MLL1 fusion protein, small molecule inhibitors that block menin-MLL1 interaction were developed. These inhibitors were shown to suppress menin--MLL1-dependent expression of Hox genes and inhibit proliferation of MLL1 fusion-transformed leukemia cells73. These findings support a new therapeutic strategy for aggressive leukemias with MLL1 rearrangements. Further optimization of these menin inhibitors yielded another compound (MI-2-2) that binds to menin with low nanomolar affinity (K(d) = 22nM) and very effectively disrupts the interaction between menin and MLL174. Moreover, co-crystallization of the human menin protein with MI-2-2 gave a high resolution (1.6Å) structure that displayed a close interaction between menin and MI-2-2. MI-2-2 has increased efficacy in blocking the menin--MLL1 interaction and expression of Hox genes compared to the earlier compounds74. These findings provide a structural basis to design better inhibitors to effectively inhibit the menin-MLL1 interaction.
Consistent with these findings, recently reported structure-based design of cyclic peptidomimetics, based on the co-crystal structure of menin--MLL1 peptide75, also generated a potent macrocyclic peptidomimetic compound that binds to menin with a Ki value of 4.7 nM, more than 600 times more potent than the corresponding acyclic MLL1 peptide75. Collectively, this fast progress in designing small molecule compounds to inhibit menin--MLL1 interaction, and expression of menin--MLL1 targets such as the Hox genes in leukemia, pave the way to develop better compounds with ideal pharmacokinetics, and might eventually lead to effective drugs to treat aggressive AML.
Menin suppresses gene transcription
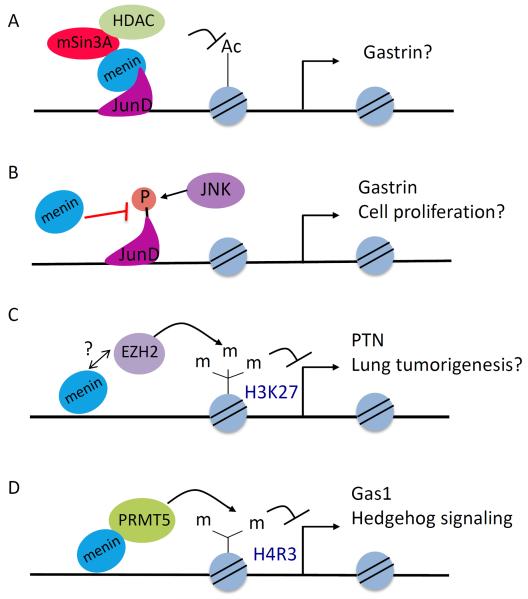
Menin has also been shown to repress gene transcription directly or indirectly. Recent studies have identified more factors that repress gene transcription through association with menin, such as various chromatin-modifying enzymes. Menin also regulates the expression of miRNAs and indirectly controls post-transcriptional expression. Moreover, menin directly interacts with JunD (as discussed previously), and represses JunD-induced transcription through its interaction with the co-repressor mSin3A and its associated HDAC (Fig. 3A)50, 55. It also represses the G2-M phase transition by repressing cyclin B2 expression, partly via recruiting HDAC3 to its promoter76.
Figure 3. Menin represses gene expression via multiple mechanisms.
(A) Menin interacts with JunD and recruits the Sin3A/histone deacetylase (HDAC) complex to reduce histone acetylation and suppress the expression of JunD targets such as Gastrin. The endogenous targets of JunD in this setting are unclear. (B) Menin also blocks JNK mediated phosphorylation of JunD, leading to repression of JunD transcriptional targets such as Gastrin. (C) In lung cancer cells, menin recruits EZH2 to the promoter of PTN to increase H3K27 trimethylation, a repressive mark, to downregulate the transcription of PTN. Whether menin directly interacts with EZH2 is yet to be determined (D) Menin can inhibit Hedgehog signaling by interacting with PRMT5 at the Gas1 promoter in pancreatic islets to increase PRMT5-mediated symmetric H4R3 dimethylation, which inhibits the expression of Gas1 and therefore Hedgehog signaling.
Menin inhibits JunD-mediated gene transcription through an additional mechanism involving competition for a binding partner40. c-Jun N-terminal kinase (JNK) normally phosphorylates JunD, activating JunD-induced gene expression. The consensus JNK-docking domain (D-domain) in JunD contains a cluster of basic amino acids preceding two leucine residues. The JunDMBM sequence partially overlaps with a putative D-domain of JunD (JunDD) (Fig. 3B)77. Both the basic residues and the leucine residues in JunDD are essential for JNK to dock on (or bind to) JunD and phosphorylate it, as well as for binding by menin (Fig. 3B). As such, menin binding to JunD blocks JNK-mediated phosphorylation and activation of JunD. Menin and JunD both bind to the promoter of the endogenous Gastrin gene and suppress its expression40. These findings unravel a new means for menin-mediated suppression of JunD activity and provide a structural basis for the mechanism of suppression (Fig. 3B). These findings also help explain why JunD activates proliferation of mouse embryonic fibroblasts (MEFs) in the absence of menin but suppresses proliferation in its presence; presumably, JunD binds promoters of pro-proliferative genes, and the presence or absence of menin controls whether JunD inhibits or activates expression of these genes, respectively78.
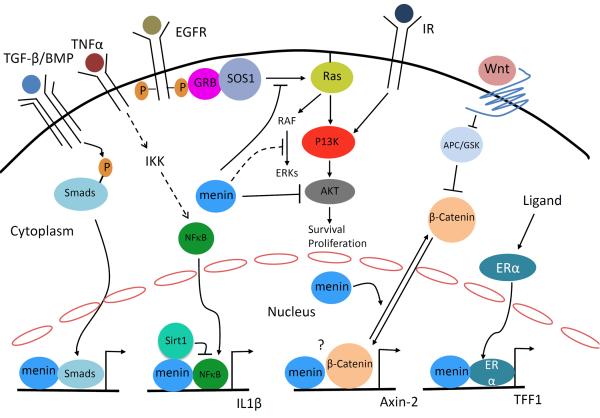
Menin also directly interacts with the p65 subunit of NF-κB and represses NF-κB-dependent transcription, as shown by luciferase reporter assays in cultured cells51. It has recently been reported that menin inhibits NF-κB-mediated transactivation via recruiting Sirt1, a histone deacetylase, to deacetylate lysine 310 (K310) of p65 in hepatocellular carcinoma cells (Fig. 4)53. It remains unclear whether menin-induced and Sirt1-mediated deacetylation of p65 represses expression of the endogenous targets of NF-κB.
Figure 4. Menin regulates multiple signaling pathways.
Menin interacts with SMAD3 or SMAD1/5 to enhance TGFβ or BMP signaling, respectively, in various types of cells. Menin also interacts with NFκB and recruits Sirt1 to deacetylate p65 to suppress NFκB-induced gene expression. β-catenin is located in cell membrane, in presence of Wnt signaling it is translocated to the nucleus. In insulinoma cells, menin interacts with β-catenin to upregulate gene transcription, whether they associate with each other at the promoter of Axin 2 is not known. In contrast in MEFs, menin promotes nuclear export of β-catenin to suppress its transcriptional activity. Menin also interacts with nuclear receptors such as ERα to promote expression their target genes. In the cytoplasm, inhibits receptor tyrosine kinase signaling through multiple mechanisms: inhibition of AKT, inhibition of SOS1-dependent activation of Ras, and suppression of ERK activation.
Menin is also reported to repress expression of the PTN gene, which encodes a pro-proliferative receptor, pleotrophin, in lung cancer cells20. Both menin and a polycomb repressive complex 2 (PRC2) bind to the PTN gene promoter and increase the repressive chromatin mark H3K27me3 at the locus (Fig. 3C). A direct interaction between menin and EZH2, the PRC2 enzyme that catalyzes H3K27 trimethylation, has not been demonstrated.
Menin also directly interacts with protein arginine methyltransferase 5 (PRMT5), a negative regulator of gene transcription54, and recruits it to the promoter of the Gas1 gene, a crucial factor for binding of Sonic hedgehog (Shh) ligand to its receptor79. Thus, menin antagonizes Shh signaling, partly via increasing PRMT5-mediated repressive histone arginine dimethylation (H4R3me2) at the Gas1 promoter (Fig. 3D).
Menin was reported to repress gene expression posttranscriptionally via upregulation of microRNA expression, which can reduce protein translation or the stability of the target mRNA. Specifically, menin induces expression of microRNA-26a (miR-26a) by binding to the promoter of the miR-26a gene80. miR-26a targets and reduces the protein expression of the bone morphogenetic protein (BMP) signaling effector SMAD1, antagonizing the osteoblastic differentiation of human adipose tissue-derived stem cells by BMP. Interestingly, menin also promotes BMP signaling by binding SMADs at target loci and promoting expression of SMAD-target genes. Thus, induction of miR-26a by menin is likely a dampening or desensitizing mechanism for menin-mediated BMP signaling (Fig. 4). It remains unclear whether or how these various modes operate in different types of cells or for different target genes.
Menin regulates multiple signaling pathways
TGFß signaling pathway
Menin directly interacts with the TGFß downstream signaling molecule SMAD3 in COS cells56. Menin knockdown (KD) reduces SMAD3 binding to DNA in GH4C1 cells, a pituitary endocrine tumor cell line, suggesting a role for menin in recruiting SMAD3 to target genes to regulate their expression. Consistently, menin KD reduces the effect of TGFβ-induced inhibition of proliferation56.
BMP signaling
Menin also regulates BMP signaling, which is critical for bone development29. Consistent with this role, Men1-null embryos display defects in cranial and facial development29. Menin is required for BMP-dependent multipotent mesenchymal stem cell commitment to the osteoblast lineage, and it facilitates this commitment through its interaction with the BMP effectors SMAD1/5 and their binding partner Runx231. At later stages of differentiation menin inhibits differentiation, likely through inhibiting JunD function30. These results indicate that menin-mediated regulation of various signaling pathways may be not only tissue-specific, but also specific to a certain stage of differentiation.
Wnt Signaling
Wnt signaling stimulates pancreatic islet β cell proliferation, possibly by increasing expression of paired-like homeodomain 2 (Pitx2)81. At least part of the effects of Wnt on β cells is mediated by the canonical Wnt effector and transcription factor β-catenin. Overexpression of menin reduces nuclear accumulation of β-catenin and therefore its transcriptional activity in MEFs, in part by directly interacting with β-catenin and excluding it from the nucleus (Fig. 4)58. By contrast, menin was shown to be crucial for canonical Wnt/β-catenin signaling in cultured rodent islet tumor cells via interaction with β-catenin59. It is possible that menin may promote Wnt signaling in certain stages of islet tumor development or inhibit Wnt signaling to prevent β cells from tumorigenesis (Fig. 4). Further detailed biochemical and genetic mechanisms remain to be explored.
Nuclear receptor signaling
Menin interacts with several nuclear receptor transcription factors including estrogen receptor (ERα) and PPARγ61, 62. Menin directly interacts with ERα in a hormone-dependent manner and is recruited to the ERα target gene, trefoil factor 1 (TFF1). There, it increases the active histone mark H3K4me3, likely via recruitment of MLL, and activates target gene expression (Fig. 4). Menin also binds to ERα to enhance its activity in MCF7 breast cancer cells82, and menin expression correlates with a poorer prognosis in ER-positive breast cancer patients treated with tamoxifen. It is not yet clear whether menin can regulate endogenous ER targets to promote proliferation or survival of normal or transformed breast epithelial cells.
Ras signaling
Consistent with a role for menin in suppressing proliferation, menin overexpression slows the proliferation of Ras-transformed NIH-3T3 cells83. Menin inhibits ERK-dependent phosphorylation, a downstream target in Ras pathway, and activation of JunD84. Consistently, menin also inhibits ERK activation and lung cancer cell migration85. Upstream of extracellular signal-regulated kinase (ERK), menin antagonizes Ras/ERK signaling by reducing the level of active Ras-GTP. This is achieved at least partly by preventing the Ras guanine nucleotide exchange factor Son of Sevenless (SOS1), and its adaptor, growth factor receptor-bound protein 2 (GRB2), from binding to Ras in lung cancer cells (Fig. 4)19. It remains to be determined how menin blocks SOS1 from binding to Ras and precisely how menin inhibits ERKs.
Akt and FOXO signaling
Menin has been reported to interact with the protein kinase Akt1 in cultured cells and mouse pancreata and reduce the level of active Akt1, at least in part by reducing its kinase activity86. Menin suppresses both Akt1-dependent proliferation and anti-apoptotic activity in nonendocrine and endocrine cells, partly by reducing the translocation of Akt1 from the cytoplasm to the plasma membrane (Fig. 4). It is unclear how menin prevents translocation of Akt1 to the cell membrane. Further work will be needed to determine the role of Akt1 in menin-mediated suppression of endocrine tumors, which can be done using tissue-specific double knockout of Men1 and Akt1. Menin is also reported to interact with transcription factor FOXO1 in the cytoplasm of hepatocytes87, but it is unclear whether menin directly or indirectly interacts with FOXO1 and what the biological consequence of this interaction is. As the Akt/mTOR pathway is upregulated in pancreatic neuroendocrine tumors, it would be important to investigate precisely how menin suppresses this pathway.
Hedgehog signaling
Menin directly interacts with PRMT5, a negative regulator of gene transcription54, and recruits PRMT5 to the promoter of the Gas1 gene, a crucial factor for binding of Sonic hedgehog (Shh) ligand, to activate the Hh signaling, a pro-proliferative pathway (Fig. 3D).
Overall, it has been proposed that menin plays an important and tissue specific role in various signaling pathways, ranging from Ras to Akt to Hedgehog signaling. However, more experimental evidence is needed to completely understand the function of menin with reference to these signaling pathways.
Regulation of menin by various proteins and signaling pathways
Prolactin pathway
It has been reported that menin influences islet growth in pregnant mice23. Prolactin, a hormonal regulator of pregnancy, represses islet menin expression via the Stat5/Bcl6 axis, leading to enhanced β cell proliferation during mouse pregnancy. Consistent with these findings, another recent report also shows that prolactin signaling and menin regulate pregnancy-induced β cell proliferation88.
TGFβ pathway
TGFβ stimulation leads to the upregulation of menin expression in rat GH4C1 cells, suggesting crosstalk between menin and TGFβ signaling29. Consistently, menin expression is also upregulated by TGFβ in MLL-AF9-transformed leukemia cells and mouse hepatocytes89. As menin also interacts with SMAD3, a TGFβ signal transducer, to increase gene expression (Fig. 4), these studies suggest a potential positive feedback loop. It is unclear how TGFβ upregulates menin expression and whether the crosstalk between menin and TGFβ signaling operates in animal models.
Somatostatin pathway
Gastrin is a peptide hormone that stimulates gastric acid secretion from parietal cells of the stomach, while Somatostatin is a peptide hormone and inhibitor of gastrin secretion as well as expression. It has been proposed that menin inhibits the expression of gastrin. Menin protein expression was reduced in the gastrointestinal system of somatostatin-null mice90. Mice treated with the somatostatin analog octreotide show an increased number of menin-expressing cells, menin mRNA, and menin protein expression predominantly in the duodenum. Menin induction appears to depend on suppression of protein kinase A (PKA). Whether and how the somastatin pathway regulates PKA to induce the expression of menin remains to be elucidated.
Glucose and the PI3K/Akt pathway
Menin acts as a negative regulator of β-cell proliferation, especially during pregnancy23. Also, acute ablation of menin prevents the development of streptozotocin-induced diabetes and reverses pre-existing diabetes in several mouse diabetes models24, 25. Short term glucose stimulation reduced menin levels and increased proliferation in INS1 insulinoma cells and primary rat islets in a PI3K/Akt signaling-dependent manner91. It has been reported that PI3K/Akt reduces expression of menin by phosphorylating and inhibiting the transcription factor FOXO1, which binds the promoter and stimulates transcription of the Men1 gene91. It remains to be explored whether this mechanism operates in primary islets and mouse diabetes models.
Silencing of the MEN1 expression by K-Ras-induced DNA methylation or microRNA
K-Ras-activation inhibits menin expression via DNMT1-dependent DNA de-methylation of the promoter of the MEN1 gene in human lung cancer cells19. Moreover, Luzi et al. discovered that miR-24-1 binds to the 3′UTR of MEN1 mRNA, and reduces MEN1 expression posttranscriptionally, unraveling another means of suppression of the MEN1 gene92.
Post-translational modifications of menin
Menin is post-translationally modified by SUMOylation, phosphorylation and ubiquitylation. Menin is SUMOylated by SUMO1 at Lysine 59135. Point mutations associated with the MEN1 disease lead to rapid degradation of menin by the ubiquitin-proteosome pathway in 293T cells34. Menin is specifically phosphorylated at Serine 394 on treatment with γIR and UV. Menin is also phosphorylated at Ser487 dynamically and at Ser543 constitutively, but the impact of phosphorylation on menin function remains unclear93.
Menin-mediated regulation of integrity of genome
Menin interacts with various proteins involving DNA replication and repair, such as checkpoint suppressor 1 (CHES1)94, and menin is localized to telomeres during meiosis95, suggesting a role for menin in telomere maintenance. Recently, mutations in chromatin modifier Daxx/ATRX genes as well as the MEN1 gene were identified in pancreatic neuroendocrine tumors (PNETs)10. Daxx is localized at the telomere in PNETs, and it not yet clear whether there is any functional crosstalk between menin and Daxx/ATRX in regulating the telomere. Whether and how these interactions of menin with DNA modifiers influence the biochemical function of menin in normal cells or disease states is not yet clear.
Concluding remarks
Recently rapid progress has been made in understanding the molecular and biochemical functions of menin. In particular, as the crystal structures of menin with several of its binding partners have been solved, and these new findings have established menin as a key scaffold protein that can functionally crosstalk with various partners in controlling a diverse range of biological functions. Menin positively or negatively controls gene transcription by interacting with distinct proteins. Menin may also exert its functions via either its central pocket or other parts of its surface. Moreover, menin not only regulates multiple signaling pathways, but itself is also regulated by multiple proteins, signaling pathways and numerous types of posttranslational modifications. It is noteworthy that these various mechanisms could be highly tissue-specific, or cell type or context-dependent, and more genetic and biochemical work remains to expand these observations. It is noteworthy that certain important findings were only observed in a limited number of studies or conditions; caution need to be exercised in generalizing such functions of menin and further detailed studies are expected to resolve these issues. Nevertheless, this progress has provided novel insights into how menin controls gene transcription and interplays with various signaling pathways. With the accumulation of this novel knowledge regarding the molecular mechanisms underlying the function of menin, many exciting questions (Box 1) can now be raised and addressed to improve the therapy of MEN1 disease and other related diseases such as leukemia. In particular, how does menin choose its partners in different contexts? For example, in MLL1 fusion protein (MFP)-induced leukemia, the interaction between menin and MLL1/MFP dimers is particularly important, as a menin/MLL1/MFP are required to maintain high level expression of HOX genes, which are crucial for MFP-induced leukemia. On the other hand, menin interaction with MLL1 may be important for upregulation of CDKI, p18 and p27 in endocrine cells, and menin interaction with JunD may be important to inhibit endocrine cells, but not necessary for MFP-induced leukemia. As such, further studies are necessary to explore these potential mechanisms.
Box 1: Outstanding questions.
How does menin choose its partner among a diverse group of interacting proteins? Does menin choose certain partners in distinct types of cells or in distinct settings of biological conditions?
How does menin functionally interact with its partners in vivo in model organisms such as mice?
How is menin regulated and how do various posttranslational modifications impact its function?
How does menin exert its crucial functions in various organ systems in development, homeostasis, and disease?
Can MEN1 mutation-induced dysregulation of tumorigenic pathways, such as the Hedgehog signaling pathway, be targeted to improve therapy of MEN1 syndrome?
Can the structure of menin be used to develop better inhibitors to treat relevant diseases such as MLL-FP-induced leukemia, perhaps by blocking menin-MLL/MLL-FP interactions?
Highlights
Molecular and structural basis for menin-mediated activation of gene transcription
Multiple modes and molecular basis of menin-mediated suppression of gene transcription
Menin regulates multiple cell signaling pathways
Menin is regulated by multiple factors and signaling pathways
Acknowledgements
We thank the close collaboration on studying the structural and functional relationship with Dr. Ming Lei's group, our colleagues for critically reading the manuscript, Ms. Erin Quinn for editing the manuscript. We also thank Dr. Chang-Xian Zhang for valuable discussion and inputs. This work was supported in part by grants from the NIH (R01-DK085121) and Caring for Carcinoid Foundation-AACR Grant Care for Carcinoid Foundation (11-60-33), and a TRP grant from Leukemia and Lymphoma Society. We apologize for being unable to cite all the relevant literatures due to limit of the space.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest No conflicts of interests are declared by any of the authors.
References
- 1.Chandrasekharappa SC, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 2.Lemmens I, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet. 1997;6:1177–1183. doi: 10.1093/hmg/6.7.1177. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal SK, et al. Menin molecular interactions: insights into normal functions and tumorigenesis. Horm Metab Res. 2005;37:369–374. doi: 10.1055/s-2005-870139. [DOI] [PubMed] [Google Scholar]
- 4.Boikos SA, Stratakis CA. Molecular genetics of the cAMP-dependent protein kinase pathway and of sporadic pituitary tumorigenesis. Hum Mol Genet. 2007;16(Spec No 1):R80–87. doi: 10.1093/hmg/ddm019. [DOI] [PubMed] [Google Scholar]
- 5.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 6.Libe R, Bertherat J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol. 2005;153:477–487. doi: 10.1530/eje.1.02004. [DOI] [PubMed] [Google Scholar]
- 7.Thakker RV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal SK, et al. Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet. 1997;6:1169–1175. doi: 10.1093/hmg/6.7.1169. [DOI] [PubMed] [Google Scholar]
- 9.Carling T, et al. Parathyroid MEN1 gene mutations in relation to clinical characteristics of nonfamilial primary hyperparathyroidism. J Clin Endocrinol Metab. 1998;83:2960–2963. doi: 10.1210/jcem.83.8.4977. [DOI] [PubMed] [Google Scholar]
- 10.Jiao Y, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertolino P, et al. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol. 2003;17:1880–1892. doi: 10.1210/me.2003-0154. [DOI] [PubMed] [Google Scholar]
- 12.Crabtree JS, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding B, et al. Multiple endocrine neoplasia type 1 knockout mice develop parathyroid, pancreatic, pituitary and adrenal tumours with hypercalcaemia, hypophosphataemia and hypercorticosteronaemia. Endocr Relat Cancer. 2009;16:1313–1327. doi: 10.1677/ERC-09-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guru SC, et al. Characterization of a MEN1 ortholog from Drosophila melanogaster. Gene. 2001;263:31–38. doi: 10.1016/s0378-1119(00)00562-x. [DOI] [PubMed] [Google Scholar]
- 15.Guru SC, et al. Isolation, genomic organization, and expression analysis of Men1, the murine homolog of the MEN1 gene. Mamm Genome. 1999;10:592–596. doi: 10.1007/s003359901051. [DOI] [PubMed] [Google Scholar]
- 16.Stewart C, et al. Characterization of the mouse Men1 gene and its expression during development. Oncogene. 1998;17:2485–2493. doi: 10.1038/sj.onc.1202164. [DOI] [PubMed] [Google Scholar]
- 17.Chen YX, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A. 2006;103:1018–1023. doi: 10.1073/pnas.0510347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokoyama A, et al. The Menin Tumor Suppressor Protein Is an Essential Oncogenic Cofactor for MLL-Associated Leukemogenesis. Cell. 2005;123:207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, et al. Interplay between menin and K-Ras in regulating lung adenocarcinoma. J Biol Chem. 2012;287:40003–40011. doi: 10.1074/jbc.M112.382416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao SB, et al. Suppression of lung adenocarcinoma through menin and polycomb gene-mediated repression of growth factor pleiotrophin. Oncogene. 2009;28:4095–4104. doi: 10.1038/onc.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seigne C, et al. Characterisation of prostate cancer lesions in heterozygous Men1 mutant mice. BMC Cancer. 2010;10:395. doi: 10.1186/1471-2407-10-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seigne C, et al. High incidence of mammary intraepithelial neoplasia development in Men1-disrupted murine mammary glands. J Pathol. 229:546–558. doi: 10.1002/path.4146. [DOI] [PubMed] [Google Scholar]
- 23.Karnik SK, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318:806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, et al. Reversal of preexisting hyperglycemia in diabetic mice by acute deletion of the Men1 gene. Proc Natl Acad Sci U S A. 2010;107:20358–20363. doi: 10.1073/pnas.1012257107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, et al. Deletion of the Men1 gene prevents streptozotocin-induced hyperglycemia in mice. Exp Diabetes Res. 2010;2010:876701. doi: 10.1155/2010/876701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aziz A, et al. Menin expression modulates mesenchymal cell commitment to the myogenic and osteogenic lineages. Dev Biol. 2009;332:116–130. doi: 10.1016/j.ydbio.2009.05.555. [DOI] [PubMed] [Google Scholar]
- 27.Hendy GN, et al. Menin and TGF-beta superfamily member signaling via the Smad pathway in pituitary, parathyroid and osteoblast. Horm Metab Res. 2005;37:375–379. doi: 10.1055/s-2005-870152. [DOI] [PubMed] [Google Scholar]
- 28.Inoue Y, et al. Menin interacts with beta-catenin in osteoblast differentiation. Horm Metab Res. 2011;43:183–187. doi: 10.1055/s-0030-1270527. [DOI] [PubMed] [Google Scholar]
- 29.Kaji H. Menin and bone metabolism. J Bone Miner Metab. 2012;30:381–387. doi: 10.1007/s00774-012-0355-3. [DOI] [PubMed] [Google Scholar]
- 30.Naito J, et al. Menin suppresses osteoblast differentiation by antagonizing the AP-1 factor, JunD. J Biol Chem. 2005;280:4785–4791. doi: 10.1074/jbc.M408143200. [DOI] [PubMed] [Google Scholar]
- 31.Sowa H, et al. Menin is required for bone morphogenetic protein 2- and transforming growth factor beta-regulated osteoblastic differentiation through interaction with Smads and Runx2. J Biol Chem. 2004;279:40267–40275. doi: 10.1074/jbc.M401312200. [DOI] [PubMed] [Google Scholar]
- 32.Cheng P, et al. Menin prevents liver steatosis through co-activation of peroxisome proliferator-activated receptor alpha. FEBS Lett. 2011;585:3403–3408. doi: 10.1016/j.febslet.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Zindy PJ, et al. Upregulation of the tumor suppressor gene menin in hepatocellular carcinomas and its significance in fibrogenesis. Hepatology. 2006;44:1296–1307. doi: 10.1002/hep.21367. [DOI] [PubMed] [Google Scholar]
- 34.Canaff L, et al. Menin missense mutants encoded by the MEN1 gene that are targeted to the proteasome: restoration of expression and activity by CHIP siRNA. J Clin Endocrinol Metab. 2012;97:E282–291. doi: 10.1210/jc.2011-0241. [DOI] [PubMed] [Google Scholar]
- 35.Feng ZJ, et al. SUMO modification of menin. Am J Cancer Res. 2013;3:96–106. [PMC free article] [PubMed] [Google Scholar]
- 36.MacConaill LE, et al. Phosphorylation of the menin tumor suppressor protein on serine 543 and serine 583. Mol Cancer Res. 2006;4:793–801. doi: 10.1158/1541-7786.MCR-06-0123. [DOI] [PubMed] [Google Scholar]
- 37.Yaguchi H, et al. Menin Missense Mutants Associated with Multiple Endocrine Neoplasia Type 1 Are Rapidly Degraded via the Ubiquitin-Proteasome Pathway. Mol Cell Biol. 2004;24:6569–6580. doi: 10.1128/MCB.24.15.6569-6580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balogh K, et al. Menin and its interacting proteins: elucidation of menin function. Trends Endocrinol Metab. 2006;17:357–364. doi: 10.1016/j.tem.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Poisson A, et al. Menin interacting proteins as clues toward the understanding of multiple endocrine neoplasia type 1. Cancer Letters. 2003;189:1–10. doi: 10.1016/s0304-3835(02)00509-8. [DOI] [PubMed] [Google Scholar]
- 40.Huang J, Gurung B, Wan B, Matkar S, Veniaminova NA, Wan K, Merchant JL, Hua X, Lei M. The same pocketin menin binds both MLL and JUND to produce opposite effects on transcription. Nature. 2012;482:542–546. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murai MJ, et al. Crystal structure of menin reveals binding site for mixed lineage leukemia (MLL) protein. J Biol Chem. 2011;286:31742–31748. doi: 10.1074/jbc.M111.258186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnepp RW, et al. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res. 2006;66:5707–5715. doi: 10.1158/0008-5472.CAN-05-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guru SC, et al. Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci U S A. 1998;95:1630–1634. doi: 10.1073/pnas.95.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La P, et al. Tumor suppressor menin: the essential role of nuclear localization signal domains in coordinating gene expression. Oncogene. 2006;25:3537–3546. doi: 10.1038/sj.onc.1209400. [DOI] [PubMed] [Google Scholar]
- 45.Jin S, et al. c-Myb binds MLL through menin in human leukemia cells and is an important driver of MLL-associated leukemogenesis. J Clin Invest. 120:593–606. doi: 10.1172/JCI38030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemmens IH, et al. Menin interacts directly with the homeobox-containing protein Pem. Biochem Biophys Res Commun. 2001;286:426–431. doi: 10.1006/bbrc.2001.5405. [DOI] [PubMed] [Google Scholar]
- 47.Sowa H, et al. Menin is required for BMP-2-and TGF-beta -regulated osteoblastic differentiation through interaction with Smads and Runx2. J Biol Chem. 2004 doi: 10.1074/jbc.M401312200. [DOI] [PubMed] [Google Scholar]
- 48.Hughes CM, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–597. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama A, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal SK, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–152. doi: 10.1016/s0092-8674(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 51.Heppner C, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene. 2001;20:4917–4925. doi: 10.1038/sj.onc.1204529. [DOI] [PubMed] [Google Scholar]
- 52.Shi K, et al. The embryonic transcription factor Hlxb9 is a menin interacting partner that controls pancreatic beta-cell proliferation and the expression of insulin regulators. Endocr Relat Cancer. 2013;20:111–122. doi: 10.1530/ERC-12-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gang D, et al. The tumor suppressor protein menin inhibits NF-kappaB-mediated transactivation through recruitment of Sirt1 in hepatocellular carcinoma. Mol Biol Rep. 2012 doi: 10.1007/s11033-012-2326-0. [DOI] [PubMed] [Google Scholar]
- 54.Gurung B, et al. Menin Epigenetically Represses Hedgehog signaling in MEN1 Tumor Syndrome. Cancer Research. 2013 doi: 10.1158/0008-5472.CAN-12-3158. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim H, et al. Menin, a tumor suppressor, represses JunD-mediated transcriptional activity by association with an mSin3A-histone deacetylase complex. Cancer Res. 2003;63:6135–6139. [PubMed] [Google Scholar]
- 56.Kaji H, et al. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A. 2001;98:3837–3842. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bertolino P, et al. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63:4836–4841. [PubMed] [Google Scholar]
- 58.Cao Y, et al. Nuclear-cytoplasmic shuttling of menin regulates nuclear translocation of {beta}-catenin. Mol Cell Biol. 2009;29:5477–5487. doi: 10.1128/MCB.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen G, et al. Menin promotes the Wnt signaling pathway in pancreatic endocrine cells. Mol Cancer Res. 2008;6:1894–1907. doi: 10.1158/1541-7786.MCR-07-2206. [DOI] [PubMed] [Google Scholar]
- 60.Veschi S, et al. Alterations of MEN1 and E-cadherin/beta-catenin complex in sporadic pulmonary carcinoids. Int J Oncol. 2012 doi: 10.3892/ijo.2012.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dreijerink KM, et al. Menin links estrogen receptor activation to histone H3K4 trimethylation. Cancer Res. 2006;66:4929–4935. doi: 10.1158/0008-5472.CAN-05-4461. [DOI] [PubMed] [Google Scholar]
- 62.Dreijerink KM, et al. The multiple endocrine neoplasia type 1 (MEN1) tumor suppressor regulates peroxisome proliferator-activated receptor gamma-dependent adipocyte differentiation. Mol Cell Biol. 2009;29:5060–5069. doi: 10.1128/MCB.01001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46. doi: 10.1016/j.ccr.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scacheri PC, et al. Genome-wide analysis of menin binding provides insights into MEN1 tumorigenesis. PLoS Genet. 2006;2:406–419. doi: 10.1371/journal.pgen.0020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang P, et al. Global analysis of H3K4 methylation defines MLL family member targets and points to a role for MLL1-mediated H3K4 methylation in the regulation of transcriptional initiation by RNA polymerase II. Mol Cell Biol. 2009;29:6074–6085. doi: 10.1128/MCB.00924-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karnik SK, et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A. 2005;102:14659–14664. doi: 10.1073/pnas.0503484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Milne TA, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin W, et al. Loss of the retinoblastoma binding protein 2 (RBP2) histone demethylase suppresses tumorigenesis in mice lacking Rb1 or Men1. Proc Natl Acad Sci U S A. 2011;108:13379–13386. doi: 10.1073/pnas.1110104108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thiel AT, et al. MLL-AF9-induced leukemogenesis requires coexpression of the wild-type Mll allele. Cancer Cell. 17:148–159. doi: 10.1016/j.ccr.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thiel AT, et al. The trithorax protein partner menin acts in tandem with EZH2 to suppress C/EBPalpha and differentiation in MLL-AF9 leukemia. Haematologica. 2013 doi: 10.3324/haematol.2012.074195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin C, et al. AFF4, a Component of the ELL/P-TEFb Elongation Complex and a Shared Subunit of MLL Chimeras, Can Link Transcription Elongation to Leukemia. Mol Cell. 37:429–437. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okada Y, et al. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121:167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 73.Grembecka J, et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol. 8:277–284. doi: 10.1038/nchembio.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi A, et al. Structural insights into inhibition of the bivalent menin-MLL interaction by small molecules in leukemia. Blood. 120:4461–4469. doi: 10.1182/blood-2012-05-429274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou H, et al. Structure-based design of high-affinity macrocyclic peptidomimetics to block the menin-mixed lineage leukemia 1 (MLL1) protein-protein interaction. J Med Chem. 56:1113–1123. doi: 10.1021/jm3015298. [DOI] [PubMed] [Google Scholar]
- 76.Wu T, et al. Regulation of cyclin B2 expression and cell cycle G2/m transition by menin. J Biol Chem. 2010;285:18291–18300. doi: 10.1074/jbc.M110.106575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hernandez JM, et al. Multiple facets of junD gene expression are atypical among AP-1 family members. Oncogene. 2008;27:4757–4767. doi: 10.1038/onc.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Agarwal SK, et al. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci U S A. 2003;100:10770–10775. doi: 10.1073/pnas.1834524100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007;21:1231–1243. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luzi E, et al. The regulatory network menin-microRNA 26a as a possible target for RNA-based therapy of bone diseases. Nucleic Acid Ther. 2012;22:103–108. doi: 10.1089/nat.2012.0344. [DOI] [PubMed] [Google Scholar]
- 81.Rulifson IC, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci U S A. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Imachi H, et al. Menin, a product of the MENI gene, binds to estrogen receptor to enhance its activity in breast cancer cells: possibility of a novel predictive factor for tamoxifen resistance. Breast Cancer Res Treat. 2010;122:395–407. doi: 10.1007/s10549-009-0581-0. [DOI] [PubMed] [Google Scholar]
- 83.Kim YS, et al. Stable overexpression of MEN1 suppresses tumorigenicity of RAS. Oncogene. 1999;18:5936–5942. doi: 10.1038/sj.onc.1203005. [DOI] [PubMed] [Google Scholar]
- 84.Gallo A, et al. Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation. Oncogene. 2002;21:6434–6445. doi: 10.1038/sj.onc.1205822. [DOI] [PubMed] [Google Scholar]
- 85.Feng ZJ, et al. Lung cancer cell migration is regulated via repressing growth factor PTN/RPTP beta/zeta signaling by menin. Oncogene. 2010;29:5416–5426. doi: 10.1038/onc.2010.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, et al. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Res. 2011;71:371–382. doi: 10.1158/0008-5472.CAN-10-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wuescher L, et al. Insulin regulates menin expression, cytoplasmic localization, and interaction with FOXO1. Am J Physiol Endocrinol Metab. 2011;301:E474–483. doi: 10.1152/ajpendo.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hughes E, Huang C. Participation of Akt, menin, and p21 in pregnancy-induced beta-cell proliferation. Endocrinology. 2011;152:847–855. doi: 10.1210/en.2010-1250. [DOI] [PubMed] [Google Scholar]
- 89.Zhang H, et al. Menin expression is regulated by transforming growth factor beta signaling in leukemia cells. Chin Med J (Engl) 2011;124:1556–1562. [PubMed] [Google Scholar]
- 90.Mensah-Osman E, et al. Somatostatin stimulates menin gene expression by inhibiting protein kinase A. Am J Physiol Gastrointest Liver Physiol. 2008;295:G843–854. doi: 10.1152/ajpgi.00607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H, et al. Glucose-mediated repression of menin promotes pancreatic beta-cell proliferation. Endocrinology. 2012;153:602–611. doi: 10.1210/en.2011-1460. [DOI] [PubMed] [Google Scholar]
- 92.Luzi E, et al. The negative feedback-loop between the oncomir Mir-24-1 and menin modulates the Men1 tumorigenesis by mimicking the “Knudson's second hit”. PLoS ONE. 2012;7:e39767. doi: 10.1371/journal.pone.0039767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Francis J, et al. The menin tumor suppressor protein is phosphorylated in response to DNA damage. PLoS One. 2011;6:e16119. doi: 10.1371/journal.pone.0016119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Busygina V, et al. Hypermutability in a Drosophila model for multiple endocrine neoplasia type 1. Hum Mol Genet. 2004;13:2399–2408. doi: 10.1093/hmg/ddh271. [DOI] [PubMed] [Google Scholar]
- 95.Suphapeetiporn K, et al. MEN1 tumor-suppressor protein localizes to telomeres during meiosis. Genes, Chromosomes & Cancer. 2002;35:81–85. doi: 10.1002/gcc.10113. [DOI] [PubMed] [Google Scholar]