Abstract
Rationale
A behavioral economic approach to understanding the relative value of alcohol may be useful for advancing medication development for alcoholism. Naltrexone is a heavily researched and moderately effective treatment for alcohol dependence making it a good candidate for a proof-of-concept study of behavioral economics and alcoholism pharmacotherapy.
Objectives
This study examines naltrexone efficacy and pharmacogenetics in terms of the relative value of alcohol, assessed via demand curve analysis.
Materials and Methods
Participants were 35 heavy drinking (AUDIT ≥ 8) Asian Americans. A within-subjects cross-over medication design was used along with an intravenous alcohol challenge completed after four days of both naltrexone and placebo. At baseline and BrAC = 0.06 g/dl, participants completed an Alcohol Purchase Task, which assessed estimated alcohol consumption along escalating prices. Behavioral economic demand curve analysis yielded measures of Intensity, Elasticity, maximum expenditure (Omax), proportionate price insensitivity (Pmax) and breakpoint.
Results
Compared to placebo, naltrexone significantly reduced Intensity, Omax and breakpoint. There were also a trend level medication effects on Pmax. BrAC was associated with increases in Pmax and breakpoint. A significant naltrexone × OPRM1 genotype interaction was observed for intensity of demand.
Conclusion
The present study extends the literature on naltrexone’s mechanisms through the application of a novel behavioral economic paradigm. These results indicate that naltrexone reduces several indices of demand for alcohol. This preliminary report provides further evidence for the effectiveness of naltrexone and supports the utility of a behavioral economic approach to alcoholism pharmacotherapy development.
Keywords: Alcohol, Naltrexone, Behavioral Economics, Asian Americans
Introduction
The field of behavioral economics integrates aspects of psychology and microeconomics to understand choice behavior and decision-making processes (Bickel et al., 2007). Behavioral economic paradigms have been applied to both normative behaviors as well as psychiatric disorders, particularly substance use disorders (MacKillop, Amlung, Murphy, Acker, & Ray, In Press; MacKillop, Miranda, et al., 2010; MacKillop, O’Hagen, et al., 2010; Vuchinich & Heather, 2003). From a behavioral economic perspective, substance use disorders reflect an acquired state in which the relative value of the drug remains persistently high and insensitive to escalating negative consequences, which is putatively due to dysregulated decision making and molar environmental contingencies that maintain the behavior (Vuchinich & Heather, 2003).
Using a behavioral economics framework, demand curve analysis provides a comprehensive and multidimensional approach in order to assess the relative value of alcohol. Demand curves are a quantitative representation of the relationship between price and alcohol consumption. Laboratory studies have employed various paradigms for generating demand curves (Johnson & Bickel, 2006), the most efficient of which is a hypothetical purchase task, such as an Alcohol Purchase Task (APT) in which participants estimate how many standard drinks they would purchase from very low to very high prices. APT responses are then used to generate demand curves, reflecting the overall relationship between consumption and cost. This curve can then be partitioned into a number of indices of the reinforcing value of alcohol. Specifically, these include Intensity (i.e., consumption at zero cost), Elasticity (i.e., overall proportionate slope of the demand curve), Pmax (i.e. proportionate price insensitivity), Omax (i.e., maximum expenditure across prices) and Breakpoint (i.e., the price at which consumption drops to zero). Although these indices are conceptually interrelated and generally reflect dimensions of volumetric consumption versus price sensitivity (MacKillop et al., 2009), they are considered theoretically distinct (Bickel et al., 2000). Importantly, these demand indices are highly sensitive to both individual differences in alcohol involvement (MacKillop et al., 2010a; Murphy & MacKillop, 2006; Murphy et al., 2009) and dynamic changes in motivation for alcohol (MacKillop et al., 2010b), permitting state-level assessments that may be sensitive to the effects of pharmacological and psychosocial interventions for alcohol use disorders. The general utility of this approach has been supported by research on demand for cigarettes (Hitsman et al., 2008; MacKillop et al., 2008; Murphy, MacKillop, Tidey, Brazil, & Colby, 2011).
Effective pharmacotherapies for alcoholism theoretically reduce the relative value of alcohol, albeit via potentially diverse mechanisms (e.g., altering subjective effects, attenuating craving, or inducing adverse reactions) (Hursh, Galuska, Winger, & Woods, 2005; Monti & Mackillop, 2007). Therefore, medication effects could be captured using demand curve analysis as indexed by lower intensity, Omax, and Pmax, as well as greater demand curve elasticity (Hursh, et al., 2005). In other words, behavioral economics provides potentially useful motivational indices for clarifying pharmacological mechanisms. Reductions in alcohol demand of this nature would (a) suggest that the medication effectively decreases the motivational value of alcohol, (b) specify which domains are affected, and (c) help elucidate medication effects in the context of real-world consumption. The opioid antagonist, naltrexone, represents one such pharmacotherapy.
Naltrexone is one of three currently FDA-approved pharmacotherapies for treating alcohol dependence in the United States. Clinical trials have shown naltrexone to be a moderately effective treatment for alcohol dependence. Studies have found that naltrexone reduces the number of heavy drinking days (Balldin et al., 2003; Monti et al., 2001; Rubio et al., 2002; Rubio, Ponce, & Manzanares, 2002), increases time to relapse (Anton et al., 1999; Guardia et al., 2002; Kiefer et al., 2003), yields lower relapse rates (Heinala et al., 2001; Latt, Jurd, Houseman, & Wutzke, 2002; Volpicelli, Alterman, Hayashida, & O’Brien, 1992), reduces the number of drinking days (O’Malley et al., 1992; Volpicelli, et al., 1992), and reduces the number of drinks per drinking episode (Chick et al., 2000; Guardia, et al., 2002; Morris, Hopwood, Whelan, Gardiner, & Drummond, 2001; O’Malley, et al., 1992). A large multi-site controlled trial found that naltrexone was an effective treatment for alcohol dependence when delivered in combination with a brief medically-oriented behavioral intervention (Anton et al., 2006). A few studies, however, have not found naltrexone to be superior to placebo (Killeen et al., 2004; Kranzler, Modesto-Lowe, & Van Kirk, 2000; Krystal, Cramer, Krol, Kirk, & Rosenheck, 2001).
Laboratory studies of naltrexone have elucidated the mechanism of action of naltrexone by suggesting that it reduces feelings of alcohol induced stimulation (Drobes, Anton, Thomas, & Voronin, 2004), decreases liking of alcohol (McCaul, Wand, Stauffer, Lee, & Rohde, 2001), increases fatigue and tension following alcohol exposure (King, Volpicelli, Frazer, & O’Brien, 1997) and slows the progression of drinking (Anton, Drobes, Voronin, Durazo-Avizu, & Moak, 2004). Using an in vivo behavioral choice paradigm that is behavioral economic in nature, naltrexone reduced the relative value of alcohol (Drobes, Anton, Thomas, & Voronin, 2003; O’Malley, Krishnan-Sarin, Farren, Sinha, & Kreek, 2002). Specifically, compared to placebo, naltrexone reduced alcohol self-administration of standardized ‘mini-drinks’ that cost $2 or $3 each (participants could keep any money they did not spend). However, the preceding studies used a task that only used a single price and only examined choice behavior following a priming dose. No studies to date have used behavioral economic demand curve analysis in order to more comprehensively and precisely delineate naltrexone’s effects on the relative value of alcohol.
Importantly, recent studies of naltrexone have also focused on pharmacogenetic predictors of treatment response, namely the μ-opioid receptor (OPRM1) gene. One of the most widely studied polymorphisms of the OPRM1 gene is the A118G SNP (rs1799971), which molecular studies have found affects binding affinity for β-endorphin, leading to a gain in function for the G allele variant (Bond et al., 1998). Pharmacogenetic studies of naltrexone have found that the G allele carriers exhibit greater naltrexone-induced blunting of alcohol high (Ray & Hutchison, 2007), and lower relapse rates in clinical trials of naltrexone for alcoholism (Anton et al., 2008; Oslin et al., 2003). Some studies, however, have failed to support this pharmacogenetic effect (Gelernter et al., 2007; Tidey et al., 2008). No studies to date have examined the efficacy of naltrexone, pharmacogenetic or otherwise, using behavioral economic demand curve analysis.
Genetic studies have found significant discrepancies in allele frequency between ethnicities for the A118G SNP. In Caucasian samples, the minor allele frequency is around 20% however, in East Asian populations the minor allele frequency is roughly 50% (Arias, Feinn, & Kranzler, 2006). Consequently, naltrexone’s pharmacogenetic effects observed in Caucasian samples may not replicate in Asian American samples. In fact, one study assessing cortisol response to opiate blockade by the opioid receptor antagonist naloxone found support for the moderating role of OPRM1 in Caucasians, but not in individuals of Asian descent (Hernandez-Avila et al., 2007). However, a study of Korean alcohol dependent patients found that among treatment adherent individuals, carriers of the G allele had longer time to relapse than A allele homozygotes (Kim et al., 2009).
In brief, naltrexone is a well-researched medication for alcoholism, which has been demonstrated to reduce alcohol craving and subjective intoxication relative to placebo. Recent studies have also suggested that carriers of the G allele of the OPRM1 gene may be associated with better clinical response to naltrexone, and given that this polymorphism is more prevalent in individuals of Asian descent, ethnicity considerations have become relevant to understanding naltrexone’s efficacy and pharmacogenetics. The present study focused on individuals of East Asian descent given the higher minor allele frequency and in order to extend the medication main effects and its pharmacogenetic findings from primarily Caucasian samples.
This study represents an extension of our recent work demonstrating that naltrexone alters subjective intoxication and craving in Asian American heavy drinkers and that these effects are moderated by OPRM1 genotype (Ray, Bujarski, Chin, & Miotto, 2011). Specifically, the objective of the present study is to extend the literature on both behavioral economics and naltrexone efficacy by assessing medication effects on the relative value of alcohol both before and after acute alcohol administration in a sample of Asian American heavy drinkers. Based on previous studies of naltrexone using de facto behavioral economic approaches, it is hypothesized that, compared to placebo, naltrexone will significantly reduce alcohol demand (including increasing alcohol price elasticity). Additionally, this study seeks to assess naltrexone pharmacogenetics in the context of demand curve analysis. Based on the available literature, it is hypothesized that naltrexone will produce greater reductions of the relative value of alcohol, indexed via behavioral economic markers, among OPRM1 A118G G-allele carriers as compared to A allele homozygotes.
Method
Participants
The present study was approved by the University of California, Los Angeles Institutional Review Board and all participants provided written informed consent after receiving a full explanation of the study. Inclusion criteria were the following: (1) a score of 8 or higher on the Alcohol Use Disorders Identification Test (AUDIT), indicating heavy alcohol use (Allen, Litten, Fertig, & Babor, 1997); (2) no history of adverse reactions to needle puncture; (3) self-reported East Asian ethnicity (i.e., Chinese, Korean, or Japanese); (4) no prior major psychiatric disorder and (5) not be currently taking any psychiatric medications including any opiates. In total, thirty-five (10 female) non-treatment seeking heavy drinkers were randomized in this trial. Mean AUDIT score of randomized participants was 13.21 (SD = 3.96). The average age was 22.3 (SD = 1.98; Range = 21 to 29) and of the 35 participants enrolled in this study 17 (48.6%) were Chinese, 15 (42.9%) were Korean, and 3 (8.5%) were Japanese. No randomized participants identified with more than one ethnic group, and all participants reported 100% East Asian heritage. All female subjects tested negative for pregnancy prior to each alcohol administration session and all subjects were required to have a breath alcohol concentration (BrAC) of 0.00 g/dl before each session. In order to ensure a BrAC of zero on visit days, participants were asked not to consume any alcohol the day before their visit.
Screening and Experimental Procedures
Initial assessment of the eligibility criteria (above) was conducted through a telephone interview. Eligible participants were invited to the laboratory for an additional screening session. Upon arrival at the lab, participants read and signed an informed consent form, provided a saliva sample for DNA analyses, and completed a series of individual differences measures. Given that the expected minor allele frequency for the study population was predicted to be approximately 50%, no prospective genotyping was conducted. Prior to participating in the alcohol challenge sessions, participants attended a physical examination session with the study physician at the UCLA General Clinical Research Center (GCRC) to determine medical eligibility to take the study medication and to participate in the ethanol infusion procedure. A total of 49 participants (12 women) were screened in the laboratory, 41 completed the physical exam, 3 of whom were ineligible for medical reasons and 3 of whom decided not to continue with the study, leaving 35 participants who enrolled in the study. Of the 35 individuals randomized, 32 completed the entire study and 3 dropped out after completing one alcohol administration session.
Participants completed two alcohol challenge sessions. One after taking naltrexone for four days and one after taking a matched placebo for four days. Active medication and placebo were delivered in a counterbalanced and double-blinded fashion. During the experimental sessions, participants were seated in a recliner chair and an IV was placed in their non-dominant arm. Participants were asked to complete a baseline assessment packet before receiving any alcohol. After completing the baseline assessment, participants received intravenous doses of alcohol, as described below. Participants then completed identical assessment measures at BrAC of 0.06 g/dl. After the infusion procedure was finished, participants were given a meal and asked to remain in the lab until their BrAC fell below 0.02 g/dl or to 0.00 g/dl if driving.
Alcohol Administration and Medication Procedures
Prior research with alcohol challenge paradigms has emphasized the importance of accurately controlling participants’ blood alcohol concentrations in order to reduce experimental variability (Li, Yin, Crabb, O’Connor, & Ramchandani, 2001; O’Connor, Morzorati, Christian, & Li, 1998; Ramchandani, Bolane, Li, & O’Connor, 1999). Therefore in the present study, alcohol was administered intravenously, consistent with procedures developed in our previous work (Ray, Meskew-Stacer, & Hutchison, 2007). The ethanol infusion sessions took place under medical supervision at the UCLA GCRC.
The infusion was performed using a 5% ethanol IV solution. An infusion algorithm was developed which took into account participant’s gender and weight. The formulas for determining target infusion rates were: 0.166-ml/minute × weight, in kilograms, for males, and 0.126-ml/minute × weight, for females. Participants started the intravenous administration at their target rate and breath alcohol concentration (BrAC) was monitored every 3 to 5 minutes. Upon reaching the target BrAC of 0.06 g/dl, participants’ infusion rates were reduced to half their target rate, in order to maintain stable BrAC levels during testing. The ethanol infusion yielded highly controlled BrACs, such that the observed Mean (SD) BrAC was 0.060 (.002) g/dl.
Medication was a double-blinded randomized and balanced within-subjects variable, such that each participant completed one infusion session after taking naltrexone for four days (25 mg for days 1–2 and 50 mg for days 3–4) and one session after taking a matched placebo for four days. Participants were required to take the first medication (naltrexone or placebo) once a day for three days prior to the first experimental session and on the morning of their appointment. After the first session, participants were given the second medication, which they took for three days prior to and on the morning of their second ethanol infusion session. Between sessions there was a week-long washout period, which studies have shown to be sufficient to avoid carry-over effects (Schuh, Walsh, & Stitzer, 1999). Medication procedures were consistent with previous laboratory research on acute dosing of naltrexone, (O’Malley, et al., 2002; Ray & Hutchison, 2007), which have been shown to produce a steady state of the medication (Schuh, et al., 1999).
Medication compliance was examined by packing the medication and placebo into capsules with 50 mg of riboflavin. Urine samples were collected prior to each ethanol infusion session and were analyzed for riboflavin content under an ultraviolet light for detection (Del Boca, Kranzler, Brown, & Korner, 1996). All samples tested positive for riboflavin content. More details on medication and alcohol administration procedures for this study can be found elsewhere (Ray, Bujarski, Chin, & Miotto, 2011).
Behavioral Assessments
The following measures were used in this study: (1) Alcohol Use Disorders Identification Test (AUDIT): The AUDIT was administered over the phone in order to establish a pattern of heavy drinking to determine eligibility. The AUDIT has demonstrated strong reliability and validity (Allen, et al., 1997). (2) Alcohol Purchase Task (APT): The APT is a hypothetical alcohol purchase task wherein participants report how many standard drinks they would consume in a typical drinking situation at 16 price points ranging from free ($0) to $1120 per drink. These prices were based on an initial validation study of purchase tasks for opiates and cigarettes (Jacobs & Bickel, 1999), and are based on typical units in an operant progressive-ratio schedule. Alcohol demand curves generated from the APT were used to calculate behavioral economic indices. The APT has been previously validated in several studies (MacKillop & Murphy, 2007; Murphy & MacKillop, 2006). Importantly, demand curve indices have been shown to be relatively stable over time (Murphy, MacKillop, Skidmore, & Pederson, 2009) while also allowing for repeated administration.
During the laboratory screening session, participants completed a battery of individual difference measures that included demographics and drinking behavior. During the alcohol infusion, the APT was administered at baseline and at the target BrAC of 0.060 g/dl.
Demand Curve Analysis
Demand curve analysis was conducted using Hursh and Silberberg’s (2008) exponential model:
where Q = consumption at a given price; Q0 = maximum consumption (consumption at zero or minimal price); k = a constant across conditions that denotes the range of consumption values in log powers of ten; C = the cost of the commodity (price), and α = the derived demand parameter reflecting the rate of decline of consumption in standardizd price, also referred to as essential value (Hursh and Silberberg 2008). In addition to α, three other demand indices were examined: Intensity was defined as the Y intercept of the derived demand curve (Q0); normalized Pmax was determined from the extrapolated demand curve, (Q0 × C)/100 (hereafter simply referred to as Pmax); and Omax was generated based on Pmax (i.e., expenditure at Pmax). Of note, observed values for several of the variables can be generated from purchase task data using raw or arithmetically-calculated values, however, the observed and derived indices are very highly correlated (MacKillop et al., 2008; Murphy and MacKillop, 2006), as was the case in this study, and thus only derived values are reported to avoid alpha error inflation. Parallel analyses of observed values revealed no substantial differences in findings. Finally, the demand index Breakpoint was also used, defined as the price that first suppresses consumption to zero (Breakpoint does not have a derived counterpart). Prior to the primary analyses, outliers were assessed (z-score cutoff of 4.0) and identified outliers were subsequently removed from analysis.
Genotyping
A complete description of the genotyping methods in the present sample is reported in Ray et al (2011). DNA samples were collected via Oragene saliva collection kits. Polymerase chain reaction (PCR) was used to genotype participants according to the OPRM1 SNP of interest. Participants were also genotyped with respect to both aldehyde dehydrogenase (ALDH2, rs671) and alcohol dehydrogenase (ADH1B, rs1229984) to serve as control variables. Genotypes were automatically scored by Sequence Detection Systems (SDS) software version 2.3 and verified by visual inspection. Observed genotype call rates in this sample was 98.6%.
Data Analytic Plan
Analyses were conducted using a multilevel model framework (Singer, 1998), where medication and BrAC were Level 1 variables (nested within subjects), while subject and genotype were Level 2 variables. All analyses initially controlled for total AUDIT score gender, and self-reported disposable income and ALDH2 and ADH1B genotypes. Hypothesis testing was conducted using PROC MIXED in SAS Statistical Software version 9.2 and utilizing data from all 35 participants who completed at least one experimental session. Mixed models examined the effects of Medication, a two-level within subjects factor (Naltrexone vs. Placebo), Genotype, a two-level between subjects factor (A allele homozygotes vs. G allele carriers), assessment BrAC, a two-level within subjects factor (BrAC = 0.00, 0.06 g/dl), and their interactions. The dependent variables were Elasticity (α), Intensity, Pmax, Omax, and Breakpoint.
Results
Pre-Test Comparisons
Of the 49 participants screened, 21 (42.9%) were homozygous for the A allele of OPRM1, 22 (44.9%) had one copy of the G allele, and 6 (12.2%) were homozygous for the G allele. Of the 35 participants enrolled in the study, 13 were homozygous for the A-allele of the OPRM1 SNP of interest, 17 were heterozygous and 5 were G-allele homozygotes. Consistent with previous research of naltrexone pharmacogenetics, OPRM1 G-allele carriers and G-allele homozygotes were grouped together for all analyses (Ray et al. 2007c; Anton et al. 2008; Oslin et al. 2003). With respect to ALDH2 genotype, 26 participants were *1 homozygotes and 9 were *2 carriers. In terms of ADH1B genotype, 4 participants were *1 homozygotes, 12 were heterozygous and 19 were *2 homozygotes. All allelic frequencies were in conformity with Hardy Weinberg equilibrium, no allele frequency imbalance was observed, and there was no differential drop out by genotype (for complete description see Ray, Bujarski, Chin, & Miotto, 2011).
There were no significant genotype group differences with regard to baseline alcohol use or demographic variables, including gender, ethnicity, AUDIT score, and past month drinking frequency (ps > .10). Gender and self-reported disposable income were not significant predictors of any behavioral economic indices presented herein (ps > .10) and were therefore excluded from the final models. AUDIT score, in turn, was a significant predictor of Intensity only (p < 0.01), and was thus retained only in this model. Additionally, ALDH2 and ADH1B genotypes were not significantly associated with any behavioral economic indices (ps > .10) and were thus excluded from final models.
Behavioral Economic Indices
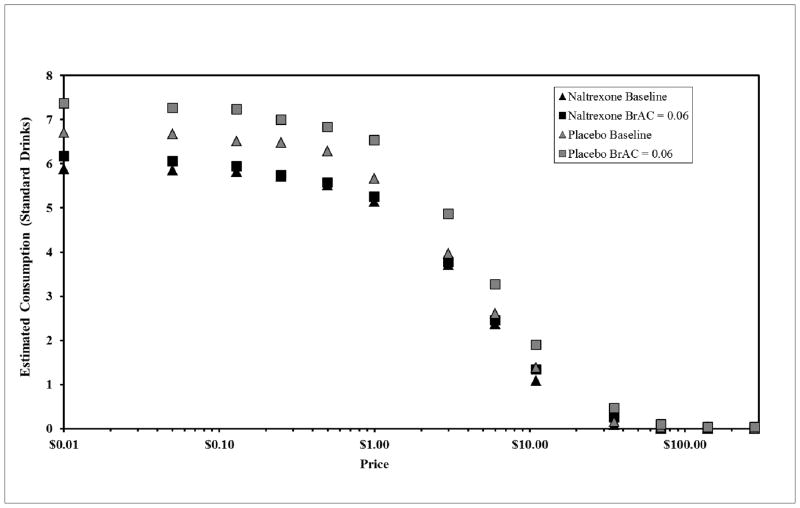
Demand curves for the two medication conditions (Naltrexone and Placebo) and two BrAC conditions (BrAC = 0.00, 0.06) are shown in Figure 1.
Figure 1.
Empirical alcohol demand curves for the two medication conditions and two BrAC conditions showing average self-reported consumption along rising price. Note that Intensity is not presented because zero price cannot be depicted on a logarithmic axis and the last two prices are not depicted because all responses were zeros.
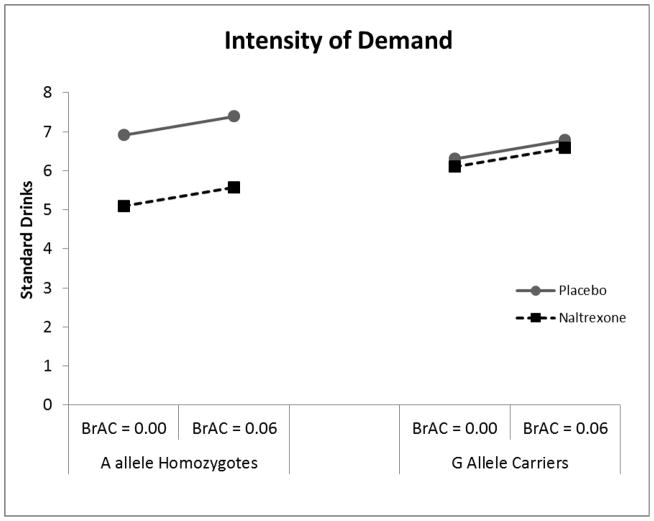
Analysis of intensity of alcohol demand revealed a significant main effect of medication (β = 2.15, SE = 0.83, t = 2.58, p < 0.05) such that naltrexone was associated with lower intensity than placebo. There was also a trend towards a significant medication × genotype interaction, (β = −1.94, SE = 1.04, t = −1.86, p = 0.07) however this interaction was not in the hypothesized direction. Among A-allele homozygotes naltrexone reduced intensity relative to placebo, however this relationship was not seen in G-allele carriers (Figure 2). There was also a trend towards a significant main effect of genotype (β = 3.34, SE = 1.91, t = 1.75, p = 0.085), such that G allele carriers reported greater level of Intensity. There was no significant main effect of alcohol or any significant alcohol interactions (ps > 0.10).
Figure 2.
Intensity of alcohol demand (i.e. consumption at zero cost) as a function of medication condition (Naltrexone vs. Placebo) and BrAC (0.00 vs. 0.06).
Analysis of Omax indicated a significant main effect of medication (β =5.97, SE = 2.99, t = 2.00, p = 0.05) such that naltrexone significantly reduced maximal expenditure compared to placebo. There was no significant main effect of alcohol, or genotype and no significant interactions were found (ps > 0.10).
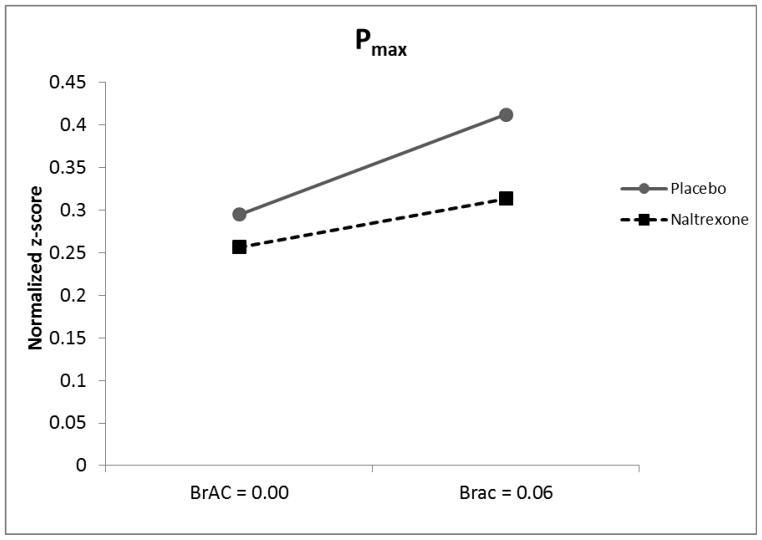
Analysis of Pmax revealed a significant main effect of alcohol (β = 0.085, SE = 0.040, t = 2.14, p < 0.05) such that alcohol exposure resulted in greater Pmax (Figure 3). There was also a trend level main effect of naltrexone (β = 0.67, SE = 0.040, t = 1.66, p = 0.10) such that naltrexone was associated with decreased Pmax. No significant genotype effects were seen nor were any interactions significant (ps > 0.10).
Figure 3.
Pmax (i.e. proportionate price insensitivity) as a function of medication condition (Naltrexone vs. Placebo) and BrAC (0.00 vs. 0.06).
Analysis of Breakpoint revealed a significant main effect of medication (β = 6.01, SE = 2.89, t = 2.08, p < 0.05) such that naltrexone was associated with lower Breakpoint as compared to placebo (Figure 4). A significant main effect of alcohol was also found (β = 9.33, SE = 3.19, t = 2.92, p < 0.01) such that alcohol exposure resulted in greater Breakpoint relative to baseline. No significant genetic main effects or interactions were observed with respect to breakpoint. No significant main effects or any significant interactions were found with respect to elasticity of demand for alcohol (ps > 0.10).
Figure 4.
Breakpoint for alcohol (i.e. price at which estimated consumption equals zero) as a function of medication (Naltrexone vs. Placebo) and BrAC (0.00, vs. 0.06).
Discussion
The aims of this study were to apply a behavioral economic approach to understanding the effects of naltrexone in Asian American heavy drinkers. This is a noteworthy effort as behavioral economic approaches have demonstrated significant utility in assessing the etiology and maintenance of substance misuse and addictive disorders (MacKillop, O’Hagen, et al., 2010; Vuchinich & Heather, 2003). This study is the first to have tested the effects of naltrexone, or any other pharmacotherapy for alcoholism, using a behavioral economic paradigm. In addition, the study examined naltrexone’s effects in individuals of Asian ancestry thereby extending the initial report of naltrexone’s pharmacogenetic effects on mechanisms of craving and subjective intoxication (Ray, et al., 2011)
Behavioral economic principles assert that alcohol use disorders develop as a result of persistent and escalating overvaluation of alcohol’s reinforcing properties compared to alternative sources of reinforcement. Consequently, decision making about alcohol consumption becomes increasingly impaired (Vuchinich & Heather, 2003). The present proof-of-concept study tested the effects of a well-established medication for alcoholism, naltrexone, according to behavioral economic principles. Specifically, this study tested whether naltrexone significantly reduced the relative value of alcohol compared to placebo and whether such effects could be detected using a hypothetical alcohol purchase task. The study design allowed us to test the effects of acute alcohol exposure via an intravenous alcohol administration and how alcohol exposure interacts with medication. This interaction is relevant given that some of the human laboratory and clinical effects of naltrexone may be dependent upon alcohol exposure (Ray, Chin, & Miotto, 2010; Ray, Krull, & Leggio, 2010).
Results revealed that naltrexone significantly reduced Intensity, Omax, and breakpoint relative to placebo, and a statistical trend-level effect in the same direction was present for Pmax. In other words, naltrexone significantly reduced unconstrained alcohol consumption, the amount of money spent on alcohol, and how sensitive the individual was to the increasing price of alcohol as measured by the APT. Additionally, intravenous administration of alcohol to a BrAC of 0.06 g/dl was associated with increases in Pmax and breakpoint suggesting that acute alcohol exposure may increase demand for alcohol. In summary, these results are largely consistent with previous studies indicating that naltrexone dampens the relative value of alcohol (Drobes, et al., 2003; O’Malley, et al., 2002), but extend those findings by including a baseline assessment and revealing the specific elements of relative value that are affected by naltrexone (Anton, et al., 2004; Ray & Hutchison, 2007; Swift, Whelihan, Kuznetsov, Buongiorno, & Hsuing, 1994).
Analysis of the A118G SNP of the OPRM1 gene revealed a trend level main effect of genotype on intensity of alcohol demand, such that the G allele carriers exhibited significantly greater estimated unconstrained alcohol use than A allele homozygotes. This finding is consistent with previous studies which show G allele carriers are more sensitive to the hedonic properties of alcohol (Ray & Hutchison, 2004, 2007). Moreover, this is the first instance, to our knowledge, in which individual differences in alcohol demand have been shown to systematically differ by genotype, suggesting these behavioral economic indices may be useful intermediate phenotypes for alcoholism, although not endophenotypes (for a discussion of the distinction between intermediate phenotypes and endophenotypes see Goldman & Ducci, 2007).
In addition, a trend level pharmacogenetic effect on intensity was observed; however, this interaction was not in the hypothesized direction. Specifically, naltrexone reduced intensity among A allele homozygotes, however no significant difference was seen among G allele carriers. In interpreting these results it is important to note that the effect of genotype was found in only one of the behavioral economic indices assessed. All other measures of the relative value of alcohol found no significant pharmacogenetic effect. Additionally, at least one previous pharmacogenetic study of naltrexone and the OPRM1 gene on alcohol craving has reported similar findings in the contrary direction to standard hypotheses (McGeary et al., 2006). On balance, these findings suggest that different pharmacogenetic results may be obtained through a behavioral economic approach, which in turn requires validation in both future lab studies and treatment studies.
While we were unable to examine the impact of naturalistic drinking on naltrexone versus placebo, there is little reason to suspect that potential differences in this regard explain the obtained results. First, participants were only permitted to drink on medication days one and two, which were both titration days (25 mg/day). In order to ensure a BrAC of zero on the day of the experimental visit participants were asked not to drink on day 3 and alcohol administrations were conducted on medication day 4 (upon verified BrAC of zero). Therefore it is unlikely that the influence of naltrexone on naturalistic drinking had a substantial effect on the results reported herein.
The present study represents the first application of behavioral economic demand curve analysis to the study of naltrexone in heavy drinkers. These results suggest that hypothetical consumption tasks and behavioral economic paradigms generally could be useful in medication development for alcoholism. These results should be interpreted in the context of this study’s strengths and limitations. This study utilized a novel approach to assessing pharmacological effects using a well validated measure of alcohol demand (i.e., APT) with strong psychometric properties (MacKillop & Murphy, 2007; Murphy & MacKillop, 2006). Additionally, the study sample draws from a well characterized population, namely Asian American young adults. Several genetic factors (i.e., ADH1B and ALDH2), which are largely unique to Asian Americans, were assessed as confound to these results. No significant effects of alcohol metabolizing genes were observed. This null finding may be partially explained by the significant homogeneity of alcohol metabolizing genotypes in this sample, resulting in limited statistical power. Furthermore, participants were selected on the basis of their heavy-drinking status (AUDIT ≥ 8) further narrowing the phenotype and limiting power to detect the protective effects of metabolic genes. In addition, medication compliance, as verified by riboflavin detection was high. The within-subjects cross-over design of the present study represents a significant strength as participants served as their own controls, increasing statistical power to capture both medication and alcohol administration effects. Another strength of the study was the intravenous alcohol administration procedure as it controls for learned alcohol cue effects in addition to providing considerable experimental control over BrAC (Ray, et al., 2007).
A limitation in this study is the small sample size, which given the inherently large variability in the outcome variables may limit power to detect substantive effects. In particular, this applies most obviously to the genetic analyses, where the sample was further divided into even smaller subsamples. Here, it is important to note that the effect of genotype was found for only one of the behavioral economic indices assessed, but only relatively large magnitude effects could be detected as a result of the small sample size. Additionally the sample was intentionally comprised entirely of Asian Americans and, as such, these findings may not generalize to other ethnic groups. Another methodological consideration is that the study used an APT for hypothetical alcohol and, although there is evidence that decision making for hypothetical rewards and actual rewards correspond closely for purchase tasks and other behavioral economic measures (Lagorio & Madden, 2005; MacKillop, M.T., Acker, & M., 2010; Madden et al., 2004), it will be important to further verify these findings using an APT paradigm utilizing actual alcohol and money. Finally, the present study assessed medication response in a subclinical sample of at risk-drinkers rather than a sample of alcohol dependent individuals for whom naltrexone would typically be prescribed.
On balance, this study extends the literature on the application of behavioral economics to pharmacotherapy research and naltrexone pharmacotherapy mechanisms. These findings support the utility of applying behavioral economic paradigms to medication development for alcohol use disorders. Through the use of an APT and the resulting demand curve analysis the results revealed that naltrexone significantly reduced several elements of the relative value of alcohol compared to placebo. Together, these findings are consistent with accumulating evidence that naltrexone’s functional mechanism of action is a blunting of alcohol’s hedonic properties, both prior to drinking and once drinking has commenced (Drobes, et al., 2004; McCaul, et al., 2001; Swift, et al., 1994). These findings extend our initial analyses of this sample demonstrating that naltrexone dampens the reinforcing subjective effects of alcohol and reduces alcohol craving relative to placebo (Ray et al., 2011). Most importantly, these results provide a much-needed demonstration of the utility of behavioral economic indices to behavioral pharmacology of alcoholism by leveraging a well-validated and FDA-approved pharmacotherapy for alcohol dependence. Future studies employing a similar behavioral economic approach to pharmacotherapy development for alcoholism appear to be highly feasible and warranted.
Acknowledgments
The authors would like to thank Pauline Chin, Eliza Hart, James Ashenhurst, and Kelly Courtney for their contributions to data collection and management. We wish to thank Dr. Karen Miotto, the study physician, for her role in patient care and for overseeing all medical aspects of the project, including physical exams, medication management, and alcohol infusion sessions.
This research was supported by a grant from the UCLA Academic Senate to L.R. and by a grant from the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health (M01-RR00865). Dr. MacKillop was supported by NIH grants K23 AA016936 and R21 AA017696-01A1.
Footnotes
Disclosure/Conflict of Interest
None of the authors have any conflicts of interest to disclose.
References
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21(4):613–619. [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004;173(1–2):32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83(3):262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berglund M, Borg S, Mansson M, Bendtsen P, Franck J, Willander A. A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res. 2003;27(7):1142–1149. doi: 10.1097/01.ALC.0000075548.83053.A9. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35(6):587–593. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Kranzler HR, Brown J, Korner PF. Assessment of medication compliance in alcoholics through UV light detection of a riboflavin tracer. Alcohol Clin Exp Res. 1996;20(8):1412–1417. doi: 10.1111/j.1530-0277.1996.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology. 2003;28(4):755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28(9):1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, Rosenheck R, Krystal JH. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31(4):555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Goldman D, Ducci F. Deconstruction of vulnerability to complex diseases: enhanced effect sizes and power of intermediate phenotypes. Scientific World Journal. 2007;7:124–130. doi: 10.1100/tsw.2007.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia J, Caso C, Arias F, Gual A, Sanahuja J, Ramirez M, Casas M. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26(9):1381–1387. doi: 10.1097/01.ALC.0000030561.15921.A9. [DOI] [PubMed] [Google Scholar]
- Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21(3):287–292. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Covault J, Wand G, Zhang H, Gelernter J, Kranzler HR. Population-specific effects of the Asn40Asp polymorphism at the mu-opioid receptor gene (OPRM1) on HPA-axis activation. [Controlled Clinical Trial Research Support, N.I.H., Extramural] Pharmacogenetics and genomics. 2007;17(12):1031–1038. doi: 10.1097/FPC.0b013e3282f0b99c. [DOI] [PubMed] [Google Scholar]
- Hitsman B, MacKillop J, Lingford-Hughes A, Williams TM, Ahmad F, Adams S, Munafo MR. Effects of acute tyrosine/phenylalanine depletion on the selective processing of smoking-related cues and the relative value of cigarettes in smokers. Psychopharmacology (Berl) 2008;196(4):611–621. doi: 10.1007/s00213-007-0995-5. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Galuska CM, Winger G, Woods JH. Addictive drugs, effective therapies: It’s all about the economy! Molecular Interventions. 2005;5(1):20–28. doi: 10.1124/mi.5.1.6. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav. 2006;85(1):73–93. doi: 10.1901/jeab.2006.102-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60(1):92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- Killeen TK, Brady KT, Gold PB, Simpson KN, Faldowski RA, Tyson C, Anton RF. Effectiveness of naltrexone in a community treatment program. Alcohol Clin Exp Res. 2004;28(11):1710–1717. doi: 10.1097/01.alc.0000145688.30448.2c. [DOI] [PubMed] [Google Scholar]
- Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, Son BK, Oslin DW. A micro opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 2009;201(4):611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129(1):15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology. 2000;22(5):493–503. doi: 10.1016/S0893-133X(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Lagorio CH, Madden GJ. Delay discounting of real and hypothetical rewards III: Steady-state assessments, forced-choice trials, and all real rewards. Behavioural Processes. 2005;69(2):173–187. doi: 10.1016/j.beproc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176(11):530–534. doi: 10.5694/j.1326-5377.2002.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Li TK, Yin SJ, Crabb DW, O’Connor S, Ramchandani VA. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res. 2001;25(1):136–144. [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Murphy CM, Acker J, Ray LA, editors. A Behavioral Economic Approach to Health Behavior. Jones and Bartlett Publishers, LLC; (In Press) [Google Scholar]
- MacKillop J, MTA, Acker J, MS Further validation of an alcohol purchase task: Equivalence of versions for hypothetical and actual rewards. Alcoholism: Experimental and Clinical Research. 2010;34(Suppl):48A. doi: 10.1111/j.1530-0277.2011.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Miranda R, Jr, Monti PM, Ray LA, Murphy JG, Rohsenow DJ, Gwaltney CJ. Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. J Abnorm Psychol. 2010;119(1):106–114. doi: 10.1037/a0017513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG. A behavioral economic measure of demand for alcohol predicts brief intervention outcomes. Drug Alcohol Depend. 2007;89(2–3):227–233. doi: 10.1016/j.drugalcdep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG, Ray LA, Eisenberg DT, Lisman SA, Lum JK, Wilson DS. Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol. 2008;16(1):57–65. doi: 10.1037/1064-1297.16.1.57. [DOI] [PubMed] [Google Scholar]
- MacKillop J, O’Hagen S, Lisman SA, Murphy JG, Ray LA, Tidey JW, Monti PM. Behavioral economic analysis of cue-elicited craving for alcohol. Addiction. 2010;105(9):1599–1607. doi: 10.1111/j.1360-0443.2010.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Lagorio CH, Begotka AM, Mueller AM, Hehli DJ, Wegener AA. Delay discounting of potentially real and hypothetical rewards: II. Between- and within-subject comparisons. Exp Clin Psychopharmacol. 2004;12(4):251–261. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic- pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25(4):537–547. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- McGeary JE, Monti PM, Rohsenow DJ, Tidey J, Swift R, Miranda R., Jr Genetic moderators of naltrexone’s effects on alcohol cue reactivity. Alcohol Clin Exp Res. 2006;30(8):1288–1296. doi: 10.1111/j.1530-0277.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- Monti PM, Mackillop J, editors. Advances in the treatment of craving for alcohol and tobacco. Amsterdam: Elsevier Press; 2007. [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25(11):1634–1647. [PubMed] [Google Scholar]
- Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96(11):1565–1573. doi: 10.1046/j.1360-0443.2001.961115654.x. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J. Relative reinforcing efficacy of alcohol among college student drinkers. Exp Clin Psychopharmacol. 2006;14(2):219–227. doi: 10.1037/1064-1297.14.2.219. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA. Reliability and validity of a demand curve measure of alcohol reinforcement. Exp Clin Psychopharmacol. 2009;17(6):396–404. doi: 10.1037/a0017684. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 2011;113(2–3):207–214. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22(1):202–210. [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49(11):881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160(1):19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28(8):1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23(4):617–623. [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Chin PF, Miotto K. Pharmacogenetics of Naltrexone in Asian Americans: A Randomized Placebo-Controlled Laboratory Study. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Miotto K. Naltrexone for the treatment of alcoholism: clinical findings, mechanisms of action, and pharmacogenetics. CNS Neurol Disord Drug Targets. 2010;9(1):13–22. doi: 10.2174/187152710790966704. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28(12):1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64(9):1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ray LA, Krull JL, Leggio L. The effects of Naltrexone among alcohol non-abstainers: results from the COMBINE Study. [Original Research] Frontiers in Psychiatry. 2010:1. doi: 10.3389/fpsyt.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Meskew-Stacer S, Hutchison KE. The relationship between prospective self-rating of alcohol sensitivity and craving and experimental results from two alcohol challenge studies. J Stud Alcohol Drugs. 2007;68(3):379–384. doi: 10.15288/jsad.2007.68.379. [DOI] [PubMed] [Google Scholar]
- Rubio G, Manzanares J, Lopez-Munoz F, Alamo C, Ponce G, Jimenez-Arriero MA, Palomo T. Naltrexone improves outcome of a controlled drinking program. J Subst Abuse Treat. 2002;23(4):361–366. doi: 10.1016/s0740-5472(02)00296-9. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Manzanares J. Naltrexone for alcohol dependence. N Engl J Med. 2002;346(17):1329–1331. doi: 10.1056/NEJM200204253461716. author reply 1329-1331. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. [Clinical Trial Research Support, U.S. Gov’t, P.H.S.] Psychopharmacology. 1999;145(2):162–174. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24(4):323–355. [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry. 1994;151(10):1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr, McGeary JE, Paty JA. Moderators of naltrexone’s effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin Exp Res. 2008;32(1):58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49(11):876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Heather N. Choice, behavioral economics, and addiction. Amsterdam; Boston: Pergamon; 2003. [Google Scholar]