Abstract
Rationale
Quetiapine has been shown to be a promising medication for the treatment of alcoholism. As an atypical antipsychotic medication with antagonist activity at D1 and D2, 5-HT1A and 5-HT2A, H1 and α1 and α2 receptors, quetiapine has been found to decrease impulsivity in other psychiatric disorders but its effects on impulsivity have not been studied in alcohol dependent patients.
Objective
This study seeks to test the effects of quetiapine on a specific dimension of impulsivity, namely response inhibition. This pilot study seeks to further elucidate the mechanisms of action of quetiapine for alcohol use disorders.
Method
A total of 20 non-treatment seeking alcohol dependent individuals were randomized to one of the following conditions in a double-blind, placebo-controlled design: (1) quetiapine (400 mg/day); or (2) matched placebo. Participants completed two counterbalanced intravenous placebo-alcohol administration sessions as well as behavioral measure of response inhibition (i.e. stop signal task) pre and post placebo-alcohol administration sessions.
Results
Analyses revealed a significant effect of quetiapine in improving response inhibition as measured by the stop signal task. These results provide preliminary evidence suggesting that quetiapine improves response inhibition in alcohol dependent patients, as compared to placebo.
Conclusion
This pilot study contributes a novel putative mechanism of action of quetiapine in alcoholism, namely an improvement in response inhibition.
Keywords: quetiapine, alcohol dependence, response inhibition, impulsivity, stop signal task
Introduction
Alcohol dependence is a complex and heterogeneous disorder, with available treatments that are only modestly effective. Quetiapine, a multiple receptor antagonist at 5-HT1A and 5-HT2A, dopamine D1 and D2, histamine H1, and adrenergic α1 and α2 receptors, represents a promising pharmacotherapy for alcohol dependence. In particular, 5-HT2A and D2 antagonism on the mesolimbic dopaminergic pathway into the nucleus accumbens is thought to decrease dopaminergic output in those areas, thereby putatively reducing the reinforcing effects of alcohol (Horacek et al., 2006). Nucleus accumbens dopamine is also thought to play a role in attentional and executive processes, including impulsivity, as it modulates cortico-limbic inputs from the prefrontal cortex (Besson et al., 2010; Pezze et al., 2007).
Due to its effects in the mesolimbic dopaminergic system, quetiapine may reduce the subjective effects and craving associated with alcohol consumption by blocking dopamine release in the brain’s reward system. Quetiapine was initially found to be an effective pharmacotherapy for alcoholism from a retrospective study that found that quetiapine-treated patients reported a significantly higher number of abstinent days and a significantly lower number of hospitalizations, as compared to non-quetiapine treated patients (Monnelly et al., 2004). In a clinical trial, quetiapine was found to reduce craving and decrease alcohol use among Type B alcohol dependent patients, whose clinical profile is marked by earlier onset of alcoholism, higher clinical co-morbidity, and impulsivity (Kampman et al., 2007). Recent research has found that quetiapine-treated patients reported reduced subjective intoxication in an alcohol administration paradigm, as well as lower alcohol craving during alcohol administration and on weekly craving ratings as compared to placebo-treated individuals (Ray et al., in press).
Medications such as quetiapine might also affect inhibitory control processes and reduce impulsive decision-making (Van den Eynde et al., 2008) as increases in behavioral inhibition have been reported for antagonists of dopamine D1 (Eagle et al., 2011) and alpha2 receptors (Bari et al., 2009). Impulsivity is defined as acting suddenly and without plan to satisfy an immediate desire and has been consistently implicated in addictive behaviors in both preclinical and clinical models (for a review see (Jentsch and Taylor, 1999; Kreek et al., 2005). Impulsivity, in turn, is considered to be heterogeneous and can be examined through various constructs, including impairment in response inhibition. Response inhibition concerns an individual’s ability to inhibit his/her thoughts or behaviors. In the context of addiction, self-control is critical to prevent initiation of substance use as well as throughout periods of abstinence in order to avoid relapse. In fact, studies of pharmacotherapies for alcoholism, such as topiramate, have found that this medication improved response inhibition, measured by the stop signal task, as compared to placebo (Rubio et al., 2009). Moreover, cue-exposure to detoxified alcohol dependent individuals was found to impair inhibitory performance on the stop signal task (Gauggel et al., 2010), further supporting the role of inhibitory control as a potential treatment target in alcoholism.
Quetiapine has been shown to affect impulsivity in psychiatric populations. Initially, a published case study found that quetiapine improved impulsivity and overall global functioning in patients diagnosed with borderline personality disorder (BPD) (Hilger et al., 2003). In an open-label study, Villeneuve and Lemlin (2005) found that quetiapine significantly decreased impulsivity, as measured by the Barratt Impulsivity Scale, in patients with BPD. Further, a 12-week clinical trial of quetiapine for BPD revealed a significant decrease in scores on the BIS (Van den Eynde et al., 2008). Other studies have found support for quetiapine-induced dampening of impulsivity in BPD patients (Bellino et al., 2006; Perrella et al., 2007). Although quetiapine has been shown to be a promising medication for alcoholism (Kampman et al., 2007), its effects on impulsivity have not been studied in this population.
This study seeks to test the effects of quetiapine on a specific dimension of impulsivity, namely response inhibition. These analyses are based on a pilot study of quetiapine for alcoholism (Ray et al., in press) and employ a behavioral task designed to measure response inhibition (i.e., stop-signal task) as opposed to relying on self-report measures. Based on the biological mechanisms of quetiapine and reports of quetiapine-induced dampening of impulsivity in other psychiatric samples, it is hypothesized that quetiapine will improve response inhibition in alcohol dependent patients, as compared to placebo. This pilot study seeks to further elucidate the mechanisms of action of quetiapine for alcohol use disorders.
Method
Participants
A total of 20 non-treatment seeking alcohol dependent individuals (mean age ± standard deviation: 32.8 ± 11.27) were randomized to one of the two medication conditions: quetiapine (400 mg/day) or matched placebo. The majority of the participants were male (80%). The average alcohol dependence symptom count, as determined by the Diagnostic Statistical Manual-IV (DSM-IV), was 4.5 (SD=1.19). The ethnic composition of the sample was as follows: 45% Caucasian, 25% African American, 15% Latino, 5% Asian, 10% did not specify ethnicity. A total of 15 out of the 20 randomized completed the study, 9 of whom were on placebo and 6 who were randomized to quetiapine.
All participants were between the ages of 21 and 65 and met DSM-IV diagnostic criteria for alcohol dependence. Exclusion criteria were: (1) current treatment for alcohol problems or are treatment seeking; (2) a current DSM-IV diagnosis of dependence on any psychoactive substances other than alcohol and nicotine; (3) a lifetime DSM-IV diagnosis of any psychotic disorder; (4) current use of a psychoactive drug, other than marijuana, as determined by a toxicology screen; (5) serious alcohol withdrawal symptoms; (6) clinically significant physical abnormalities as indicated by physical examination, hematological assessment, bilirubin concentration, or urinalysis; (7) history of epilepsy, seizures, or severe head trauma; (8) taking any medications that could interact adversely with quetiapine; (9) if female: pregnancy, nursing, or refusal to use reliable birth control.
Screening and Experimental Design
Following an initial phone screen assessing preliminary eligibility, participants were invited to the laboratory for an in-person screening session in which they read and signed the consent form and completed a series of individual difference measures including the Structured Diagnostic Interview for DSM-IV (SCID-IV) (First et al., 1995), used to verify current alcohol dependence. Eligible participants then completed a physical examination and laboratory exams at the UCLA General Clinical Research Center (GCRC). A total of 20 medically eligible participants were randomized to receive either quetiapine (400 mg/day) or placebo for a total of 6 weeks. The dosage schedule consisted of a dose escalation during week 1 (50 mg for days 1–2, 100 mg for day 3, 200 mg for days 4–5, and 300 mg for days 6–7), target dosage for weeks 2–5 (at 400 mg/day), and a dose decrease during week 6 (300 mg for days 36–37, 200 mg for days 38–39, 100 mg for days 40–41, and 0 mg for day 42). The titration schedule and target dose were consistent with a previous clinical trial of quetiapine for alcoholism (Kampman et al., 2007). On weeks 2 and 4 of the study, participants underwent a randomized, placebo-controlled, single blind, alcohol infusion session. During the alcohol challenge sessions, participants completed the stop signal task (SST) before and after alcohol (or saline) administration. The target Breath Alcohol Concentration (BrAC) for the alcohol infusion was 0.060 g/dl. Since the SST was administered before and after alcohol (or saline) administration, which was in turn randomized, practice effects are effectively accounted for in the design and cannot confound the results. For a complete description of the medication schedule and alcohol challenge see Ray et al. (in press).
Behavioral Measure of Response Inhibition
In order to capture response inhibition participants completed the Stop Signal Task (SST) before and after each infusion (i.e., alcohol versus saline control). Before each infusion, participants were breathalyzed to ensure they had a BrAC of 0.00 g/dl. Participants completed the SST at baseline and then again upon reaching the target dose of a BrAC of 0.06 g/dl. The SST consisted of 64 total trials, including Go and Stop trials. Participants were shown a series of arrows pointing either left or right and were instructed to respond as quickly and as accurately as they could to the corresponding arrow, i.e. press the left button for the left arrow and vice versa. However, if they were presented with an audible beep, they were instructed to withhold their response to that particular arrow. The Stop trials were presented on 25% of the trials and the time intervals between the stop tone and the go stimulus [Stop Signal Delay (SSD)] began at 250 ms for ladder one and 350 ms for ladder two. The SSD varied from stop trial to trial based on the participant’s performance in a staircase fashion (i.e. ladder) such that if a participant was able to inhibit their response on the previous stop trial, the SSD would increase by 50 ms, thus increasing the difficulty of the task; if the participant failed to withhold their response, the SSD would decrease by 50 ms. From this, an average SSD was computed from each ladder of trials in order to determine the average time delay the participant would need in order to inhibit their response 50% of the time. Increased mSSD indicates the participant is better at inhibiting the prepotent response, thus improving their performance on the task. The Stop-Signal Reaction Time (SSRT), which is a sensitive measure of response inhibition, was calculated by subtracting mean SSD from median go reaction time (Aron et al., 2003).
Statistical Analysis
Repeated measures ANOVAs using the general linear model in SAS Statistical Software (PROC GLM), were used to test the effects of Medication, a two-level between subjects factor (Quetiapine vs. Placebo), Alcohol, a two-level within subjects factor (Alcohol vs. Saline), and Trial, a two-level within subjects factor (Pre-Infusion vs. Post-Infusion). The dependent variables were standard dimensions of response inhibition captured by the SST, namely: (a) median go reaction time (MGRT), (b) mean SSD (mSSD), (c) SSRT, and (d) percent of discrimination errors (Err).
Results
Baseline Comparisons
Baseline comparisons revealed no significant differences between the quetiapine and placebo groups on demographic and alcohol use variables. Specifically, there were no significant group differences on number of drinking days in the past 30 days (t (14) = 0.71, p = .49), total drinks in the past 30 days (t (14) = −0.54, p = .60), and DSM-IV Alcohol Dependence symptom count (t (14) = 0.33, p = .74) (Ray et al., in press).
Medication Effects on Response Inhibition
As can be seen in Table 1, there was a significant Trial × Medication interaction on mSSD (p < .05), suggesting that quetiapine-treated participants had increased mSSD post infusions, thereby improving performance on the task, as compared to placebo-treated participants (see Figure 1). Analyses of SSRT revealed a trend in the Trial × Medication interaction (p = .057), indicating that quetiapine-treated participants tended to perform better overall on the task after the infusions than placebo-treated participants. Analyses of percent of discrimination errors (Err) revealed a main effect of Alcohol (p < .05), such that overall, participants made more discrimination errors after alcohol administration, as compared to saline. There was no medication effect on MGRT or Err. Together, these results provide preliminary evidence suggesting that quetiapine improved response inhibition in alcohol dependent patients, as compared to placebo.
Table 1.
Results of general linear model analyses examining the effects of quetiapine on response inhibition measured by different dimensions of the stop signal task
| Variable and source | F | p-value |
|---|---|---|
| Median Go Reaction Time (MGRT) | ||
| Medication | 0.00 | 0.9851 |
| Trial | 0.31 | 0.5863 |
| Alcohol | 0.03 | 0.8745 |
| Trial × Medication | 0.25 | 0.6246 |
| Stop Signal Reaction Time (SSRT) | ||
| Medication | 0.44 | 0.5204 |
| Trial | 0.28 | 0.6048 |
| Alcohol | 0.83 | 0.3809 |
| Trial × Medication | 4.41 | 0.0574† |
| Mean Stop Signal Delay (mSSD) | ||
| Medication | 0.34 | 0.569 |
| Trial | 0.00 | 0.9969 |
| Alcohol | 0.24 | 0.6313 |
| Trial × Medication | 6.96 | 0.0216* |
| Percent Discrimination Error (Err) | ||
| Medication | 0.00 | 1.00 |
| Trial | 0.61 | 0.4482 |
| Alcohol | 4.86 | 0.0478* |
| Trial × Medication | 0.26 | 0.6211 |
Note:
p < .10;
p < .05
Figure 1.
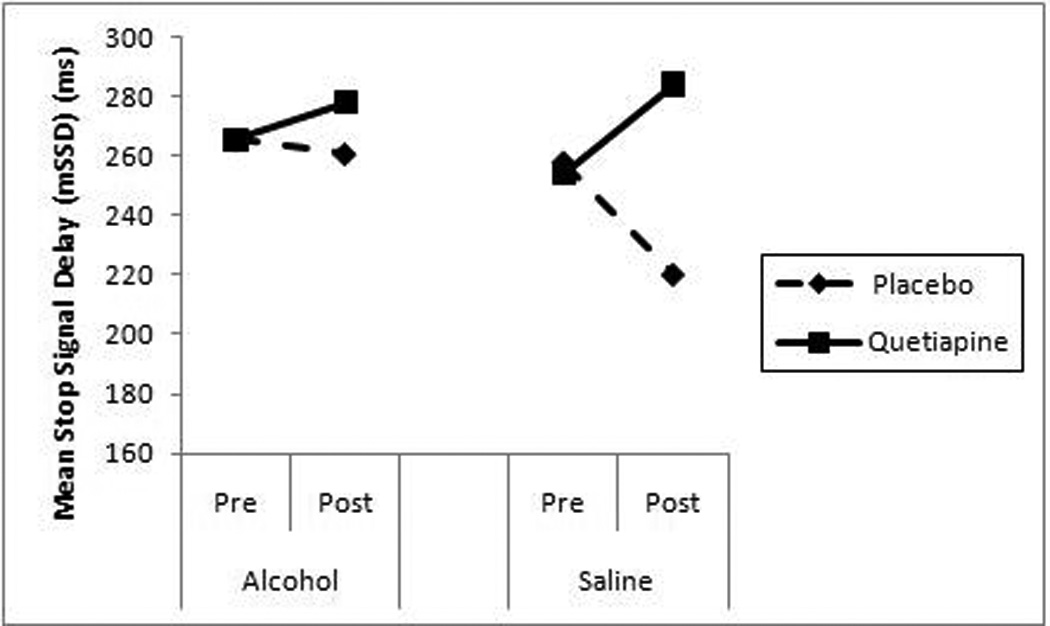
Mean SSD for placebo and quetiapine groups, pre and post alcohol and saline administrations.
Discussion
This pilot study provides initial evidence that quetiapine may improve response inhibition in alcohol dependent individuals. Specifically, analyses revealed that quetiapine-treated participants had increased mean stop signal delay (mSSD) across trials in comparison to placebo-treated participants. The increase in mSSD suggests that quetiapine-treated participants improved their performance on the task, thus improving their response inhibition. In contrast, the median go reaction time (MGRT) variable of the SST did not suggest medication effects indicating that quetiapine does not impact response time, but rather seems to affect the individual’s ability to withhold or inhibit their response. This is consistent with a study of topiramate for alcohol dependence in which the medication improved response inhibition, measured by the stop signal task, as compared to placebo (Rubio et al., 2009). Rubio and colleagues (2009), as well as the current study, highlight the potential utility of pharmacotherapies that improve response inhibition in treating alcohol dependence. To that end, while pharmacologically different, topiramate and quetiapine may share a common mechanism of action in treating alcohol dependence by improving response inhibition. Moreover, the statistical trend of the trial by medication interaction on the stop signal reaction time (SSRT) variable further supports the notion that quetiapine improves participants’ ability to inhibit responses, as compared to placebo. Given that quetiapine affects the “stop” reaction rather than the “go” response, this indicates that quetiapine did not simply contribute to improved performance on the task but rather increased participants’ ability to inhibit a prepontent response, thus improving their overall response inhibition.
The effect of quetiapine on response inhibition, a dimension of impulsivity, is consistent with previous literature on borderline personality disorder (BPD) patients (Hilger et al., 2003; Perrella et al., 2007; Van den Eynde et al., 2009). In previous research on individuals with BPD revealed that quetiapine decreased impulsivity, as captured through the BIS, a self-report measure. This study is the first randomized controlled trial of quetiapine monotherapy examining its effects on impulsivity in an alcohol dependent population. However, the present study is inconsistent with previous research showing that combined with naltrexone, quetiapine did not demonstrate any benefits on drinking outcomes above combined naltrexone and placebo (Guardia et al., 2011). However, these null results may be due to the lower target dose of quetiapine (25–200 mg/day). Additionally, the current study utilized quetiapine monotherapy rather than in combination with naltrexone. Another study found that quetiapine was associated with significant decreases in depressive symptoms but not with reduced alcohol use in patients with bipolar disorder and alcohol dependence (Brown et al., 2008). These divergent findings may be due to the sample characteristics regarding the bipolar disorder comorbidity and lighter baseline drinking levels in the Brown et al. (2008) study. Thus despite the null findings reviewed herein, methodological differences between these studies could help account for these inconsistencies and future studies of quetiapine for alcoholism appear warranted.
While quetiapine has been shown to be potentially effective for the treatment of alcohol dependence (Kampman et al., 2007), perhaps due in part to its reduction of craving, subjective intoxication, and subjective alcohol-induced sedation (Ray et al., in press), the present findings suggest that improvements in inhibitory control may be a mechanism of action of quetiapine for alcoholism. Given that increased impulsivity has been linked with alcohol dependence (Aragues et al., 2011; Rubio et al., 2008), including alcohol cue-induced disruptions in inhibitory control during early recovery (Gauggel et al., 2010), medications that can effectively target deficits in inhibitory control may be promising for the treatment of alcoholism. While quetiapine is a multiple receptor antagonist at 5-HT1A and 5-HT2A, dopamine D1 and D2, histamine H1, and adrenergic α1 and α2 receptors, based on the preclinical literature, it is likely that D1 and alpha2 antagonism are the neural pathways subserving quetiapine’s effect on response inhibition. D1 antagonists have been found to improve response inhibition, while D2 antagonism decreases performance on the SST (Eagle et al., 2011). Additionally, antagonism of alpha2 receptors by quetiapine increases NE output. Increases in NE activity, in turn has been found to improve response inhibition (Bari et al., 2009). Conversely, there is ample evidence, preclinical and clinical, to suggest that serotonin plays little role in response inhibition (Clark et al., 2005; Eagle et al., 2009). Thus, dopamine D1 and alpha2 blockade represent the most likely mechanisms underlyying quetiapine’s effect on impulsivity. Future studies should examine the neural basis of medication-induced improvements in inhibitory control as recent neuroimaging findings have suggested altered neural processing during the stop signal task in alcohol dependent individuals (Li et al., 2009).
These results should be interpreted in the context of the study’s strengths and limitations. This study utilized a behavioral measure of inhibitory control, allowing for an objective measure of a specific dimension of impulsivity, namely response inhibition. The study design was a double-blind placebo-controlled randomized trial of quetiapine, which strengthens causal inferences about medication effects. In addition, the within subjects nature of the alcohol and saline administration improved statistical power to detect medication effects and effectively controlled for practice effects on the task. Study limitations included the small sample size as well as the differential dropout between the two medication groups. On balance, this pilot study provides preliminary evidence suggesting that quetiapine improves response inhibition in alcohol dependent patients. These results offer a novel putative mechanism of action for this medication that is consistent with the literature on the use of quetiapine for other psychiatric disorders and with its pharmacological effects. If supported by larger trials, these results suggest that quetiapine may be effective for the treatment of alcoholism by improving response inhibition, thereby enhancing patients’ ability to inhibit pathological responses (i.e., alcohol use) hence promoting recovery. Future research should expand on the current study on the effects of quetiapine among individuals with alcohol dependence by including additional constructs of impulsivity, such as risky decision-making. Additional studies are also needed to effectively establish the utility of response inhibition as a mechanism of action in alcoholism treatment and recovery by testing its association with clinical outcomes in treatment-seeking samples.
Acknowledgements
This study was supported by seed funds from the Department of Psychology at the University of California Los Angeles (UCLA) and by NIH/NCRR grant number M01-RR00865.
The authors wish to thank Karen Miotto, M.D., Pauline Chin, Andia Heydari, Ryan Arellano, Ellen Chang, Belinda De La Torre, Ana Heydari, and Spencer Bujarski for their contribution to data collection and data management for this project.
Footnotes
None of the authors have any conflicts of interest to report.
References
- Aragues M, Jurado R, Quinto R, Rubio G. Laboratory paradigms of impulsivity and alcohol dependence: a review. Eur Addict Res. 2011;17:64–71. doi: 10.1159/000321345. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of quetiapine in the treatment of borderline personality disorder: A pilot study. J Clin Psychiatry. 2006;67:1042–1046. doi: 10.4088/jcp.v67n0705. [DOI] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Garza M, Carmody TJ. A randomized, double-blind, placebo-controlled add-on trial of quetiapine in outpatients with bipolar disorder and alcohol use disorders. J Clin Psychiatry. 2008;69:701–705. doi: 10.4088/jcp.v69n0502. [DOI] [PubMed] [Google Scholar]
- Clark L, Roiser JP, Cools R, Rubinsztein DC, Sahakian BJ, Robbins TW. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology (Berl) 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Lehmann O, Theobald DE, Pena Y, Zakaria R, Ghosh R, Dalley JW, Robbins TW. Serotonin depletion impairs waiting but not stop-signal reaction time in rats: implications for theories of the role of 5-HT in behavioral inhibition. Neuropsychopharmacology. 2009;34:1311–1321. doi: 10.1038/npp.2008.202. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neurosci. 2011;31:7349–7356. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient edition (SCID-I/P, version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Gauggel S, Heusinger A, Forkmann T, Boecker M, Lindenmeyer J, Cox WM, Staedtgen M. Effects of alcohol cue exposure on response inhibition in detoxified alcohol-dependent patients. Alcohol Clin Exp Res. 2010;34:1584–1589. doi: 10.1111/j.1530-0277.2010.01243.x. [DOI] [PubMed] [Google Scholar]
- Guardia J, Roncero C, Galan J, Gonzalvo B, Burguete T, Casas M. A double-blind, placebo-controlled, randomized pilot study comparing quetiapine with placebo, associated to naltrexone, in the treatment of alcohol-dependent patients. Addict Behav. 2011;36:265–269. doi: 10.1016/j.addbeh.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Hilger E, Barnas C, Kasper S. Quetiapine in the treatment of borderline personality disorder. World J Biol Psychiatry. 2003;4:42–44. doi: 10.3109/15622970309167910. [DOI] [PubMed] [Google Scholar]
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Hoschl C. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati HM, Lynch KG, Whittingham T, Macfadden W, Dackis C, Tirado C, Oslin DW, Sparkman T, O'Brien CP. A double-blind, placebo-controlled pilot trial of quetiapine for the treatment of Type A and Type B alcoholism. J Clin Psychopharmacol. 2007;27:344–351. doi: 10.1097/JCP.0b013e3180ca86e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: neural measures of stop signal performance. Alcohol Clin Exp Res. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnelly EP, Ciraulo DA, Knapp C, LoCastro J, Sepulveda I. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24:532–535. doi: 10.1097/01.jcp.0000138763.23482.2a. [DOI] [PubMed] [Google Scholar]
- Perrella C, Carrus D, Costa E, Schifano F. Quetiapine for the treatment of borderline personality disorder; an open-label study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:158–163. doi: 10.1016/j.pnpbp.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology. 2007;32:273–283. doi: 10.1038/sj.npp.1301073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Heydari A, Miotto K. A human laboratory study of the effects of quetiapine on subjective intoxication and alcohol craving. Psychopharmacology (Berl) doi: 10.1007/s00213-011-2287-3. in press. [DOI] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, Jimenez-Arriero MA, Ponce G, Palomo T. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res. 2008;32:1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Rubio G, Martinez-Gras I, Manzanares J. Modulation of impulsivity by topiramate: implications for the treatment of alcohol dependence. J Clin Psychopharmacol. 2009;29:584–589. doi: 10.1097/JCP.0b013e3181bfdb79. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, De Saedeleer S, Naudts K, Day J, Vogels C, van Heeringen C, Audenaert K. Quetiapine treatment and improved cognitive functioning in borderline personality disorder. Hum Psychopharmacol. 2009;24:646–649. doi: 10.1002/hup.1075. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Senturk V, Naudts K, Vogels C, Bernagie K, Thas O, van Heeringen C, Audenaert K. Efficacy of quetiapine for impulsivity and affective symptoms in borderline personality disorder. J Clin Psychopharmacol. 2008;28:147–155. doi: 10.1097/JCP.0b013e318166c4bf. [DOI] [PubMed] [Google Scholar]


