Abstract
Background
Animal studies have demonstrated that timing of pubertal onset can be altered by prenatal exposure to dioxins or polychlorinated biphenyls (PCBs), but studies of human populations have been quite limited.
Methods
We assessed the association between maternal serum concentrations of dioxins and PCBs and the sons’ age of pubertal onset in a prospective cohort of 489 mother–son pairs from Chapaevsk, Russia, a town contaminated with these chemicals during past industrial activity. The boys were recruited at ages 8 to 9 years, and 4 years of annual follow-up data were included in the analysis. Serum samples were collected at enrollment from both mothers and sons for measurement of dioxin and PCB concentrations using high-resolution mass spectrometry. The sons’ pubertal onset—defined as pubertal stage 2 or higher for genitalia (G) or pubic hair (P), or testicular volume >3 mL—was assessed annually by the same physician.
Results
In multivariate Cox models, elevated maternal serum PCBs were associated with earlier pubertal onset defined by stage G2 or higher (4th quartile hazard ratio = 1.7 [95% confidence interval = 1.1– 2.5]), but not for stage P2 or higher or for testicular volume >3 mL. Maternal serum concentrations of dioxin toxic equivalents were not consistently associated with the sons’ pubertal onset, although a dose-related delay in pubertal onset (only for G2 or higher) was seen among boys who breast-fed for 6 months or more.
Conclusions
Maternal PCB serum concentrations measured 8 or 9 years after sons’ births—which may reflect sons’ prenatal and early-life exposures—were associated with acceleration in some, but not all, measures of pubertal onset.
Puberty is a time of dramatic physiologic and behavioral change, marked by sexual maturation and development of secondary sexual characteristics,1 growth in height and muscle mass,2 and important cognitive, emotional, and behavioral growth.3,4 Potential disruption of this critical developmental period by environmental chemicals is therefore of concern.
Numerous animal studies have shown that timing of pubertal onset can be altered by prenatal exposure to either 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most potent member of the dioxin family of chemical pollutants, or to other dioxin-like chemicals such as polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and coplanar polychlorinated biphenyls (C-PCBs).5–7 Polychlorinated biphenyls (PCBs) are mixtures of congeners with varying modes of action,8 and have complex associations with reproductive development in animal studies. Although one commercial PCB mixture (Aroclor 1254) delayed pubertal onset in male and female rats, another less chlorinated mixture (Aroclor 1221) advanced puberty in female rats.9 PCB exposure has furthermore been associated with increased adult testis weight in rats,10,11 and with altered regulation of gonadotropin-releasing hormone (GnRH),9 a hormone with a critical role in initiating puberty.
The limited number of epidemiologic studies conducted to date have reported no association between male pubertal stage and prenatal exposure to dioxins or PCBs,12–14 although one study found an association with reduced penile length.13 Recently, a large cross-sectional study found higher peripubertal serum PCB concentrations among adolescent boys in more mature pubertal stages, suggesting an association between PCBs and accelerated pubertal development.15 However, none of these epidemiologic studies specifically assessed the timing of pubertal onset, and none had detailed information on serum concentrations of both dioxins and PCBs. We recently reported an association between higher peripubertal serum dioxin concentrations (measured when the boys were 8 to 9 years old) with later male pubertal onset.16
In the present study, we assessed the association of maternal serum concentrations of dioxins and PCBs (measured 8 to 9 years after the pregnancy with the index son) with their son's age of pubertal onset. Because these chemicals have long half-lives in human adults (eg, 7.2 years17 for TCDD and 4.3 years18 for PCB15319), the mothers’ serum concentrations 8 to 9 years after pregnancy are correlated with their levels during pregnancy and serve as a surrogate measure of their son's in utero and lactational exposure. These data were collected as part of the Russian Children's Study, a prospective cohort study of boys (and their mothers) living in Chapaevsk, Russia. The primary objective of the study is to assess the association of environmental exposures with pubertal maturation and growth among the boys. Chapaevsk, approximately 950 kilometers southeast of Moscow, is an area with elevated environmental levels of dioxins and PCBs. Until 2003, it was the site of chlorinated chemical production at the Middle Volga Chemical Plant, that is, SVZH (also referred to as “Khimprom” in previous publications19,20). Dioxins were generated as unwanted by-products of chemical syntheses.21 PCBs were typically used in electrical capacitors and transformers, although their specific use at Middle Volga Chemical Plant is unknown. Environmental release of these chemicals most likely resulted from improper disposal of hazardous waste from the plant, including uncontrolled incineration.
METHODS
Study Population
The Russian Children's Study is an ongoing prospective cohort of 499 peripubertal boys (including 7 sibling pairs) and their mothers in Chapaevsk, Russia. A total of 572 eligible boys aged 8 or 9 years were identified using the town-wide health insurance information system and recruited between 2003 and 2005; 90% of the families agreed to participate.22 The study was approved by the human studies institutional review boards of the Chapaevsk Medical Association, Harvard School of Public Health, University of Massachusetts Medical School, US Centers for Disease Control and Prevention, and Brigham and Women's Hospital. Before participation, the parent or guardian signed an informed consent and the boy signed an assent form.
At study entry, a physical examination was conducted, and each boy and mother provided blood samples for analyses of dioxins and polychlorinated biphenyls. The boys’ blood samples were additionally analyzed for lead. Medical, lifestyle, and diet questionnaires, developed with Russian collaborators,20,23 were administered to each boy's mother or guardian by a nurse. Information was collected on birth and neonatal history; the child's medical history and physical activity; maternal and household smoking and alcohol use during the pregnancy with the child; family medical, occupational, and residential history; and socioeconomic measures such as household income and parental education. Birth weight and gestational age were also obtained from medical records. A validated Russian Institute of Nutrition semiquantitative food frequency questionnaire was modified to ascertain the child's typical dietary intake over the previous year24,25 and to estimate total daily energy intake and the proportion of energy obtained from fat, protein, and carbohydrate.
Physical Examination and Pubertal Staging
At study entry and at annual follow-up visits, a standardized anthropometric examination and pubertal assessment were performed by a single study investigator (Dr. Sergeyev) according to a written protocol and without knowledge of the boys’ or mothers’ dioxin levels. Height was measured to the nearest mm with a stadiometer (Hite-Rite brand, Seca stadiometer, model 226, Hopkins Medical Products, Baltimore, MD). Weight was measured to the nearest 100 g with a metric scale. Body mass index (BMI) was calculated from the weight and height measurements (kg/m2). Pubertal status was staged from 1 to 5 on the basis of visual inspection and comparison with published photographs, according to internationally accepted criteria.26 Genitalia staging was assessed on the basis of the size and maturity of the genitalia. Pubic hair staging was based on the number and extent of terminal hair growth. Testicular volume was measured using Prader beads (orchidometer).
Analysis of Blood Samples for Dioxins, PCBs, and Lead
Blood samples were centrifuged and the serum was aliquoted and stored at –35°C until shipment on dry ice to the Centers for Disease Control and Prevention (CDC) for chemical analyses by the National Center for Environmental Health, CDC, Atlanta, GA. Serum samples were spiked with a mixture of 13C12-labeled PCDDs/PCDFs and C-PCBs as internal standards, and the analytes were isolated from serum by a C18 solid-phase extraction followed by a multicolumn automated cleanup and enrichment procedure.27 Samples were processed in batches of 10, which included a method blank and 2 quality control samples that were aliquots of pooled bovine sera spiked with PCDDs, PCDFs, and CPCBs. Quality control sample coefficients of variation (CVs), combining between- and within-run reproducibility, were <15% for all analytes, with the exception of PCB-77 and OCDF (CVs of 17% and 18%, respectively).
The analytes were separated on a DB-5 MS capillary column (Agilent JW Scientific DB-5 MS [p/n 122-5532]; Agilent Technologies, Santa Clara, CA) and quantified using selected ion monitoring, high-resolution (10,000 resolving power) mass spectrometry.28 Quantification was done by isotope dilution mass spectrometry using calibration standards containing 13C12- labeled and unlabeled analytes. For specific PCDDs, PCDFs, and C-PCBs that lack their own labeled standard, a labeled congener with the same degree of substitution and a similar retention time was used. Monoortho PCBs (M-PCBs) and nondioxin-like PCBs were extracted from an aliquot (1 g) of sample by SPE extraction.27 Total cholesterol and triglycerides were measured enzymatically, and the serum total lipid content was calculated as by Phillips and colleagues.29 All dioxin, furan, and PCB measurements are presented as lipid-adjusted. Blood lead levels were measured using atomic absorption spectrometry, as described previously.22
Statistical Analysis
The 2 serum concentrations of primary interest were the total dioxin toxic equivalents (TEQs) and the total concentration of PCBs. The total dioxin toxic equivalents (in pg/g lipid) were computed on a lipid-standardized basis using the 2005 WHO toxic equivalency factors (TEFs) to weight each congener's potency relative to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD).30 The total PCBs (ΣPCBs, ng/g lipid) are comprised of the summed concentrations of noncoplanar PCBs, including mono-ortho-substituted PCBs. We assessed concentrations of 7 PCDD congeners, 10 PCDFs, 4 C-PCBs, 6 M-PCBs, and 31 nondioxin-like PCBs.31 After log10 transformation of all variables to improve normality, Pearson coefficients were used to assess correlations between maternal dioxin and PCB concentrations, and between mother and son concentrations.
The analysis used longitudinal data on pubertal status from the initial visit as well as 4 years of annual follow-up visits. We evaluated associations using Cox proportional hazards models for time to pubertal onset. Onset was defined as testicular volume of >3 mL (either testis), genitalia stage 2 or higher, or pubic hair stage 2 or higher. Time of onset was defined as the midpoint between the first visit at which onset was observed and the previous visit. Pubertal onset before enrollment was assumed to occur 6 months before enrollment, and boys who were still prepubertal at their last study visit were censored at that visit.
Both ΣPCBs and total dioxin toxic equivalents (TEQs) were categorized into quartiles for analysis in regression models. Unadjusted associations for quartiles of maternal ΣPCBs or total TEQs with time to pubertal onset were assessed using Cox proportional hazards regression models (Model 1). Our model-building strategy focused on including potential confounders (such as household income), as well as covariates that were strong predictors of the outcome to improve the precision of the models and also to adjust for chance correlations of the covariates with exposures of interest. To avoid overadjustment,32 potential intermediates (such as the sons’ birth weight, gestational age, and peripubertal height and weight) were not included in the models despite being associated with age of pubertal onset in this population.16,22 Following these criteria, Model 2 was adjusted for household income, maternal age of menarche, son's blood lead level, proportions of dietary fat and protein, and total caloric intake. Household income and proportion of energy from dietary protein were only weakly associated with pubertal onset, but were included due to the former being of a priori interest, and the latter being necessary for interpretability of the other dietary coefficients.33 In Model 3, both the maternal total TEQs and ΣPCBs were included because the 2 measures were correlated and we sought to assess the independent association of each adjusted for the other.
Other covariates considered but not associated with pubertal onset included parity and maternal or household smoking during pregnancy. All covariates were analyzed as continuous measures except son's blood lead level at age 8 or 9 years (>5 μg/mL vs. less) and household income (<$75/month vs. higher).
Sensitivity Analyses
The sons’ peripubertal serum dioxin (TEQ) and PCB concentrations were categorized into quartiles and simultaneously added to Model 3, the final Cox model. This was done because the sons’ peripubertal serum dioxin concentrations were previously reported to be associated with pubertal onset16—as were the sons’ peripubertal PCB concentrations, in secondary analyses adjusting for both the sons’ TEQs and PCBs—and furthermore because of the correlation between the maternal and sons’ dioxin and PCB measures. This was considered a secondary analysis because the sons’ peripubertal serum dioxin and PCB concentrations are partially determined by the maternal body burden due to in utero and lactational transfer, and thus may be considered intermediate variables. Because adjusting for an intermediate variable has the potential to introduce bias, these results should be interpreted cautiously.32
As further sensitivity analyses, we conducted subgroup analyses in which multivariate Model 3 was applied to 2 subgroups in which stronger associations of maternal serum concentrations with pubertal onset might be expected. The first subgroup consisted of boys with duration of breast-feeding ≥6 months because they had the potential for substantial lactational exposure to dioxins and PCBs in addition to in utero exposure. The second subgroup consisted of boys whose mothers were living in Chapaevsk during the pregnancy. Their measured maternal serum concentrations might correlate more highly with their serum concentrations during pregnancy—because they were living in Chapaevsk at both times—as compared with women who recently arrived in Chapaevsk after having lived elsewhere during their pregnancy.
Because pubertal onset was assessed annually, approaches for interval-censored outcomes were also subsequently applied, under the assumption of a normal distribution for age at pubertal onset. In addition, repeated-measures models using generalized estimating equations (GEEs) were applied to pubertal onset at each annual visit, with adjustment for correlation among multiple visits using an autoregressive structure. GEE approaches were also used to evaluate the impact of clustering within household for twins and siblings included in the study.
RESULTS
Study Population
Our analysis included 444 mother–son pairs, or 89% of the 499 boys in the full cohort. Of the 55 pairs (11%) who were not included, 10 had chronic health conditions that might affect childhood growth and development. Forty-four were excluded because their mothers did not provide serum samples for analysis of dioxins, furans, and PCBs. The remaining son was excluded because he had been adopted, and his nonbiologic mother's serum sample would not be informative about his prenatal exposure. Seven sibling pairs (including 4 pairs of twins) were included. Birth, maternal, and household characteristics are presented in Table 1. The sons’ BMI, height, and other growth measures at study entry were consistent with World Health Organization child growth standards.34,35
TABLE 1.
Demographic, Maternal, and Son's Characteristics Among 444 Russian Boys
| Maternal characteristics (years); mean (SD) | |
| Maternal age at recruitment | 32 (5.1) |
| Maternal age at pregnancy | 24 (5.1) |
| Maternal age of menarche | 13 (1.4) |
| Son's characteristics at study entry | |
| Age (years); mean (SD) | 8.4 (0.5) |
| Height at study entry (cm); mean (SD) | 130 (6.3) |
| Weight at study entry (kg); mean (SD) | 27 (5.5) |
| BMI at study entry (kg/m2); mean (SD) | 16 (2.3) |
| Son's blood lead (≥5 μg/dL); no. (%) | 123 (28) |
| Proportion of dietary fat (%); mean (SD) | 34 (6.0) |
| Proportion of dietary protein (%); mean (SD) | 12 (1.6) |
| G2 pubertal onset at initial study visit | |
| Age 8a; no. (%) | 62 (22) |
| Age 9a; no. (%) | 65 (39) |
| TV pubertal onset at initial study visit | |
| Age 8a; no. (%) | 35 (13) |
| Age 9a; no. (%) | 29 (18) |
| P2 pubertal onset at initial study visit | |
| Age 8a, no. (%) | 10 (4) |
| Age 9a, no. (%) | 30 (18) |
| Birth and neonatal history | |
| Son's birthweight (kg); mean (SD) | 3.4 (0.5) |
| Preterm (gestational age <37 week); no. (%) | 35 (8) |
| Family living in Chapaevsk at son's birth; no. (%) | 367 (83) |
| Household characteristics; no. (%) | |
| Low maximum parental education (secondary school or less) | 33 (7) |
| Low household income (<$75/month) | 31 (7) |
Missing information: maternal age at menarche (n = 15), son's blood lead (n = 2), proportion of dietary fat (n = 1), proportion of dietary protein (n = 1), gestational age (n = 4). 279 boys were initially seen at age 8, 165 at age 9.
The median maternal ΣPCB concentration was 260 ng/g lipid, and total dioxin TEQs were 25 pg TEQ/g lipid. Detailed information on the serum concentrations of dioxins, furans, and PCBs among the mothers and sons has been published previously.19,31 The Pearson correlation between the maternal ΣPCBs and total TEQs (both log10-transformed) was 0.74. The Pearson correlations between the mothers’ and sons’ TEQs and ΣPCB serum concentrations (after log10 transformation) were modest: 0.32 and 0.24 for the maternal ΣPCBs and the sons’ ΣPCBs and TEQs, respectively, and 0.19 and 0.36 for the maternal TEQs and the sons’ ΣPCBs and TEQs, respectively.
Pubertal Onset at Entry and During Follow-up Period
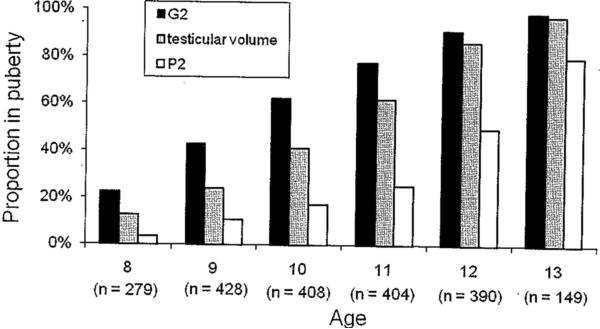
This analysis included follow-up of up to 4 annual visits after the baseline health examination (ie, until age 12 for the 279 boys recruited at age 8, and until age 13 for the 165 boys recruited at age 9). The proportion of boys with pubertal onset, by age, for the 444 boys included in this analysis is shown in Figure 1. The proportion with pubertal onset at the initial study visit is shown in Table 1. Pubertal onset, as defined by genitalia stage 2 or higher (G2) was observed at the initial clinical examination for 62/279 (22%) boys enrolled at age 8, 65/165 (39%) boys enrolled at age 9, and 355/389 (91%) by age 12. A similar increase was seen for pubertal onset defined by testicular volume >3 mL: 35/279 (13%) of those enrolled at age 8, 29/165 (18%) of those enrolled at age 9, and 336/389 (86%) by age 12. Pubertal onset as defined by pubic hair stage 2 (P2) occurred later in this population: 10/279 (4%) of those first seen at age 8, 30/165 (18%) of those first seen at age 9, and 193/389 (50%) by age 12. By the end of the 4-year follow-up, pubertal onset for G2, testicular volume, and P2 had occurred in 88%, 83%, and 55% of the boys, respectively.
FIGURE 1.
Proportion of the 444 Russian boys with pubertal onset, measured on the basis of genitalia staging (G2), testicular volume, and pubic hair staging (P2), by age at study visit. Sixty-three percent of boys were recruited at age 8 (n = 279), and the other 37% (n = 165) at age 9. A maximum of 4 years of follow-up occurred; therefore, only the boys recruited at age 9 were seen at age 13.
Association of Demographic, Body Size, and Socioeconomic Characteristics With Pubertal Onset
In multivariate models with adjustment for all other covariates, earlier pubertal onset was associated with higher caloric intake (testicular volume and P2), and larger proportions of dietary fat (G2 and testicular volume). Later pubertal onset was associated with older maternal age at menarche (G2, testicular volume, and P2), and the son's higher blood lead level (G2, testicular volume, and P2). For consistency, covariates associated with at least one pubertal-onset measure were included in the final adjusted models for every pubertal-onset measure.
Association of Maternal Serum ΣPCB and Total TEQ Concentrations With Sons’ Pubertal Onset in Cox Proportional Hazards Models
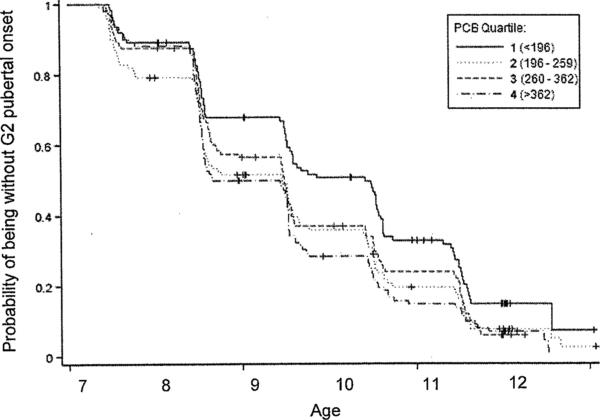
In unadjusted Cox models for time to pubertal onset (Table 2, Model 1), earlier onset of G2 was observed in the 3 upper quartiles of maternal ΣPCB serum concentrations (hazard ratio [HR] = 1.56 [95% confidence interval (CI) = 1.14–2.14] in the highest quartile). Similar results were seen in the Kaplan–Meier plot for G2 (Fig. 2), stratified by quartile of PCBs. No association with PCBs was seen for the other 2 pubertal onset measures.
TABLE 2.
Hazard Ratios for Pubertal Onset, Assessed by Genitalia Staging (G2), Testicular Volume, and Pubic Hair Staging (P2), by Quartilea of Maternal ΣPCBs
| Maternal ΣPCBs | Model 1 Unadjusted (n = 426) HR (95% CI) | Model 2 Adjustedb (n = 426) HR (95% CI) | Model 3 Additionally Adjusted for Maternal TEQsc (n = 424) HR (95% CI) |
|---|---|---|---|
| Genitalia stage 2 or higher | |||
| Q1d | 1.00 | 1.00 | 1.00 |
| Q2 | 1.45 (1.06–1.98) | 1.34 (1.00–1.80) | 1.33 (0.98–1.81) |
| Q3 | 1.37 (1.00–1.88) | 1.25 (0.93–1.68) | 1.25 (0.89–1.77) |
| Q4 | 1.56 (1.14–2.14) | 1.51 (1.13–2.03) | 1.65 (1.10–2.48) |
| Testicular volume >3mL | |||
| Q1d | 1.00 | 1.00 | 1.00 |
| Q2 | 1.04 (0.74–1.46) | 0.95 (0.70–1.30) | 0.95 (0.69–1.32) |
| Q3 | 1.17 (0.84–1.62) | 1.09 (0.81–1.47) | 1.07 (0.76–1.51) |
| Q4 | 1.16 (0.83–1.62) | 1.22 (0.90–1.64) | 1.21 (0.79–1.84) |
| Pubic hair stage 2 or higher | |||
| Q1d | 1.00 | 1.00 | 1.00 |
| Q2 | 1.13 (0.67–1.90) | 1.03 (0.70–1.51) | 1.07 (0.71–1.61) |
| Q3 | 1.12 (0.67–1.87) | 1.19 (0.82–1.71) | 1.21 (0.79–1.87) |
| Q4 | 1.03 (0.60–1.74) | 1.12 (0.77–1.63) | 1.06 (0.63–1.79) |
Q1: <196; Q2: 196 to 259; Q3: 260 to 362; Q4: >362 ng/g lipid.
The adjusted model includes maternal age of menarche, son blood lead, proportion of dietary fat, proportion of dietary protein, total caloric intake, and household income.
Adjusted for the variables listed in the previous footnote, as well as for maternal TEQs.
Reference category.
FIGURE 2.
Kaplan–Meier curve for G2 onset, stratified by quartile of total PCBs (ng PCB/g lipid). Time of pubertal onset was defined on the basis of the midpoint between the first visit at which onset was observed and the previous visit. Visits occurred on or near the son's birthday.
The association of maternal ΣPCBs with G2 was slightly weakened in all quartiles after adjusting for multiple covariates (Model 2), although with slightly increased precision for the hazard ratios. After additionally adjusting for maternal TEQs (Model 3), the HR for G2 in the highest PCB quartile was slightly increased compared with Model 2 (HR = 1.65 [95% CI 1.10–2.48] in Model 3 vs. 1.51 [95% CI 1.13–2.03] in Model 2), although the 95% CI also widened. In Models 2 and 3, the associations of maternal ΣPCBs with pubertal onset assessed by testicular volume >3 mL or stage P2 or above remained weak and nonmonotonic.
No association of maternal total TEQ serum concentrations with the sons’ age of pubertal onset was found (Table 3). A weak association of higher maternal total TEQ with earlier G2 and testicular volume pubertal onset (Models 1 and 2) was no longer observed after adjustment for PCBs in Model 3.
TABLE 3.
Hazard Ratios for Pubertal Onset Assessed by Genitalia Staging (G2), Testicular Volume, and Pubic Hair Staging (P2), by Quartilea of Maternal Total TEQs
| Maternal Dioxin Toxic Equivalents | Model 1 Unadjusted n = 424 HR (95% CI) | Model 2 Adjustedb n = 424 HR (95% CI) | Model 3 Additionally Adjusteda for Maternal PCBsc n = 424 HR (95% CI) |
|---|---|---|---|
| Genitalia stage 2 or higher | |||
| Q1d | 1.00 | 1.00 | 1.00 |
| Q2 | 1.17 (0.86–1.59) | 1.23 (0.92–1.65) | 1.11 (0.82–1.51) |
| Q3 | 1.22 (0.89–1.67) | 1.24 (0.92–1.67) | 1.05 (0.74–1.50) |
| Q4 | 1.26 (0.93–1.71) | 1.21 (0.91–1.63) | 0.91 (0.60–1.37) |
| Testicular volume > 3mL | |||
| Q1d | 1.00 | 1.00 | 1.00 |
| Q2 | 1.10 (0.79–1.54) | 1.08 (0.80–1.46) | 1.05 (0.76–1.45) |
| Q3 | 1.09 (0.78–1.53) | 1.12 (0.83–1.52) | 1.05 (0.74–1.50) |
| Q4 | 1.13 (0.81–1.58) | 1.18 (0.87–1.59) | 1.01 (0.66–1.54) |
| Pubic hair stage 2 or higher | |||
| Q1d | 1.00 | 1.00 | 1.00 |
| Q2 | 1.15 (0.67–1.95) | 0.90 (0.62–1.30) | 0.85 (0.57–1.27) |
| Q3 | 1.32 (0.77–2.23) | 1.01 (0.70–1.47) | 0.94 (0.60–1.46) |
| Q4 | 1.14 (0.67–1.95) | 1.09 (0.76–1.57) | 1.04 (0.62–1.72) |
Q1: <17.2; Q2: 17.2–24.6; Q3: 24.6–36.0; Q4: >36.0 pg TEQ/g lipid.
The adjusted model includes maternal age of menarche, son blood lead, proportion of dietary fat, proportion of dietary protein, total caloric intake, and household income.
Adjusted for the variables listed in the previous footnote, as well as for maternal dioxin toxic equivalents.
Reference category.
HR indicates hazard ratio; CI, confidence interval.
Sensitivity Analyses for Multivariate Models
The associations between maternal PCBs and earlier G2 pubertal onset seen in Model 3 were unchanged when the sons’ peripubertal TEQs and ΣPCBs were simultaneously added, despite the association of the sons’ measured TEQs with pubertal onset,16 and the correlations (although modest) between the maternal and son's serum concentrations.
To supplement the Cox model analyses, the association of maternal PCBs and TEQs with the sons’ pubertal onset (using the covariates from Model 3) was also analyzed in both generalized estimating equation (GEE) models for repeated measures, and interval-censoring models (under the assumption of a normal distribution for age at pubertal onset). These alternate models were consistent with the results shown in Tables 2 and 3. On average, G2 pubertal onset occurred 7, 5, and 11 months earlier for those in maternal ΣPCB quartiles 2, 3, and 4 as compared with the lowest quartile (adjusting for covariates as in Model 3). Furthermore, results were similar after excluding all siblings, or accounting for clustering within families. In the interval-censored model, the mean age of pubertal onset for G2, TV, and P2 was 9.5 years, 10.3 years, and 12.0 years, respectively.
Subgroup Analyses
Because we hypothesized that maternal ΣPCB serum concentrations were a proxy for the in utero and potential lactational exposure of the son, we explored whether maternal ΣPCB serum concentrations were more strongly associated with pubertal onset among the 40% of boys who breast-fed for at least 6 months, which could lead to increased dioxin and PCB exposure through lactational transfer. As hypothesized, we found a stronger association of maternal serum PCBs with G2 pubertal onset among these boys: the hazard ratios (95% CIs) for quartiles 2, 3, and 4, respectively, were 1.18 (0.72 to 1.92), 1.44 (0.78 to 2.66), and 3.06 (1.32 to 7.12), adjusting for the same covariates as in Model 3. Among this subgroup of boys there was also a dose-related delay in G2 pubertal onset with increasing quartiles of maternal total TEQs, with hazard ratios of 0.85 (0.52 to 1.39), 0.81 (0.44 to 1.49), and 0.41 (0.17 to 0.99) for quartiles 2, 3, and 4, respectively. No association of maternal total TEQs with testicular volume or P2 was observed in any subgroup.
In a final subgroup analysis, the association of maternal ΣPCB concentration with G2 pubertal onset was not stronger in the subset of 367 mothers who were living in Chapaevsk when their son was born, compared with those who were not living in Chapaevsk at that time. Because all the mothers’ dioxin and PCB concentrations were measured while they were living in Chapaevsk (when the son was 8 or 9 years old), we had hypothesized that among women also living in Chapaevsk at the time of their son's birth, the measured ΣPCB serum concentrations might be more strongly correlated with their ΣPCB serum concentration during pregnancy, 8 or 9 years before, than among the women who were living in a different town during pregnancy.
DISCUSSION
Primary Results
Our principal study hypothesis was that boys’ exposure to dioxins and PCBs in utero and in early life— estimated by measuring their mothers’ serum TEQ and PCB concentrations when the boys were aged 8 or 9 years—would be associated with the boys’ timing of pubertal onset. In our primary analyses, higher maternal serum concentrations of ΣPCBs were associated with earlier onset of puberty, defined by genitalia stage G2 or above, but not with pubertal onset defined by testicular volume or pubic hair staging. This association was unaffected by adjusting for the son's serum concentrations of total dioxin TEQs and ΣPCBs. However, no association of maternal serum total TEQs was seen with any measure of sons’ pubertal onset among the full cohort.
Results From Subgroup Analyses
A stronger association of maternal ΣPCBs with G2 pubertal onset was seen in the subgroup of boys who breast-fed for at least 6 months, compared with those who breast-fed less. Furthermore, among this same subgroup, a strong dose-related delay in G2 pubertal onset was seen for increasing quartiles of maternal TEQs. This finding of stronger associations among boys who breastfed longer is consistent with greater early-life exposure due to lactational transfer of PCBs and dioxins. However, in the present study this subgroup analyses was not prespecified, and thus should be interpreted cautiously. A similarly stronger association of maternal dioxin exposure with male semen quality among breastfed boys than among non-breastfed boys has recently been reported.36
Comparison With the Previously Published Sons’ Peripubertal Serum Dioxin and PCB Associations With Pubertal Onset
The associations of the sons’ peripubertal serum dioxin and PCB concentrations with pubertal onset (G2 and testicular volume) are described in a separate publication.16 Briefly, in secondary analyses adjusting for both peripubertal serum TEQ and PCB concentrations, TEQs were associated with delayed testicular volume and G2 pubertal onset, and PCBs with accelerated G2 and testicular volume pubertal onset. Thus, the peripubertal PCB associations were in the same direction as those reported here for the maternal PCBs (although more consistent across multiple pubertal staging measures), whereas the peripubertal TEQ associations were stronger than those observed here for maternal TEQs. It was recently reported that the association between dioxin exposure and semen quality differed depending on whether exposure occurred during infancy and/or prepuberty versus during puberty or adulthood.37 Exposure to TCDD in infancy reduced sperm quality, whereas an opposite effect was seen with exposure during puberty. Exposure in either period was associated with a reduction in estradiol and increased follicle-stimulating hormone. It remains to be determined whether the findings from the present study indicate that the peripubertal period is a time of greater susceptibility than early life for the association of dioxins and PCBs with pubertal onset. Alternately, exposure misclassification in our measure of early-life exposure might be responsible for the weaker associations observed here.
Discordance Between the Different Measures of Pubertal Onset
Pubertal onset defined as genital stage G2 or above was the only pubertal measure that was associated with maternal ΣPCBs or total TEQs. Testicular development is regulated by the hypothalamic-pituitary-gonadal axis, and can be delayed or arrested by either a peripheral or central mechanism due to inadequate secretion of hypothalamic GnRH or pituitary gonadotropins (hypogonadotropic hypogonadism). Genital staging is based on growth of the external genitalia (phallic and scrotal development), whereas pubic hair growth typically reflects adrenarche (adrenal androgens) and has a strong familial and ethnic influence. Although genital staging and testicular volume are tightly correlated, the mechanisms underlying these 2 measures are not completely identical. Genital maturation is primarily dependent on androgens, whereas testicular growth, in addition, requires gonadotropins because the seminiferous tubular compartment constitutes an increasingly larger percentage of the testicular volume with pubertal maturation. In male rats, the fungicide vinclozolin, an antiandrogen, has been found to delay preputial separation—an androgen-dependent indicator of pubertal development38 similar to G2 pubertal onset in humans—but did not affect testis weight.39 In humans, testosterone therapy for hypogonadotropic hypogonadism restores normal genital maturation, but does not increase testicular volume. The latter requires administration of GnRH or gonadotropins such as follicle-stimulating hormone (FSH) or human chorionic gonadotropin (hCG).40 Thus, it is mechanistically plausible that a chemical exposure such as PCBs could differentially affect G2 and testicular volume pubertal onset. Alternatively, the lack of consistency may be due to differences in assessment, in that genital staging is based on visual inspection, whereas testicular volume requires palpation and is deemed a more precise measure of puberty.
Comparison With Serum ΣPCB and Dioxin Concentrations in Other Populations
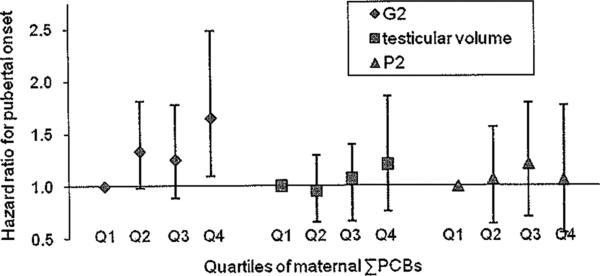
The serum PCDD/F/PCB concentrations measured in this cohort are elevated compared with the current U.S. general population.31,41 Compared with the 20- to 39-year-old U.S. population in 2003–2004, serum concentrations among these Russian women were elevated for both median ΣPCBs (75 vs. 260 ng/g lipid, respectively), and 90th percentile total TEQs (16 vs. 51 pg TEQ/g lipid respectively). In particular, the cutpoint between the first and second ΣPCB quartiles in our analysis was 196 ng/g lipid which is substantially higher than the aforementioned median U.S. concentration. Finer categorization of low-level exposures in our data is not possible because the 5th percentile ΣPCB concentration among these women is 134 ng/g lipid, and only 2 women have ΣPCB serum concentrations <75 ng/g lipid. Although the hazard ratio for G2 pubertal onset in the second quartile of maternal ΣPCBs is elevated relative to the first, the greatest increase in the hazard ratios is between ΣPCB quartiles 3 and 4 (Table 2, Model 3; Fig. 3), which corresponds to serum ΣPCB concentrations substantially higher than the current U.S. median.
FIGURE 3.
Adjusted hazard ratios from Cox proportional hazards model (Model 3) for pubertal onset among Russian boys, measured on the basis of genitalia staging (G2), testicular volume, and pubic hair staging (P2), by quartile of maternal ΣPCBs (as defined in key to Figure 2). Adjusted for maternal age of menarche, son's blood lead level, proportion of dietary fat, proportion of dietary protein, total caloric intake, household income, and maternal TEQs.
Strengths and Limitations
Strengths of this study include its use of a single physician to assess pubertal stage for all participants, as well as its large sample size and prospective design. Serum concentrations of many dioxin and PCB congeners (7 PCDDs, 10 PCDFs, 4 C-PCBs, 6 M-PCBs, and 31 nondioxin-like PCBs) were measured for each mother and son, and important covariates were assessed, including the son's blood lead levels,22 and family socioeconomic status.
The primary limitation is the 8- to 9-year interval between pregnancy and the collection of maternal serum for analysis of PCBs and TEQs. Despite the long biologic half-lives of dioxins and PCBs,17 this interval is likely to introduce exposure misclassification, which could bias the results either toward the null hypothesis or away from it.
CONCLUSIONS
These findings suggest that maternal ΣPCB serum concentrations collected 8 or 9 years after their sons’ births, which may reflect their sons’ prenatal and early-life exposures, are associated with acceleration in some measures of pubertal onset, specifically genital staging. This association was not attenuated by adjusting for multiple covariates including the son's peripubertal serum concentration of ΣPCBs and TEQs. However, there were no consistent associations of maternal total TEQs with measures of pubertal onset in their sons. Follow-up of the present cohort continues, providing the opportunity to explore associations of maternal serum concentrations of organochlorines with further measures of timing of pubertal progression (ie, G2 to G5).
ACKNOWLEDGMENTS
We thank the study participants and the staff of the Chapaevsk Medical Association. We also thank L. Earl Gray for his insights regarding mechanisms of pubertal onset in animals and humans.
The research described in this article has been reviewed by the National Institute of Environmental Health Sciences, and approved for publication. Approval does not signify that the contents necessarily reflect the views of the Agency, nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
The opinions expressed in this manuscript are those of the authors and do not necessarily reflect the official opinion of the Centers for Disease Control and Prevention.
This work was funded by U.S. EPA grant R82943701 and NIEHS grants ES014370, ES00002, and 5T32-ES07069-28. OH was supported by 5T32ES016645-02 from NIEHS/NHGRI. MML is a member of the UMass DERC (DK32520). CE is an International Consultant and the President of BioSimulation Consulting Inc. LA is a consultant for Environmental Health and Engineering, Inc. DGP is a consultant for Axys Analytical Solutions, Fluid Management Systems, Inc., and Exponent Inc.
Footnotes
The other authors reported no financial interests related to this research.
REFERENCES
- 1.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlberg J. Secular trends in pubertal development. Horm Res. 2002;57(suppl 2):19–30. doi: 10.1159/000058096. [DOI] [PubMed] [Google Scholar]
- 3.Giedd JN, Clasen LS, Lenroot R, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006:254–255. 154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Pettitt LM. Gender intensification of peer socialization during puberty. New Dir Child Adolesc Dev. 2004;106:23–34. doi: 10.1002/cd.114. [DOI] [PubMed] [Google Scholar]
- 5.Bell DR, Clode S, Fan MQ, et al. Toxicity of 2,3,7,8-tetrachlorodibenzop-dioxin in the developing male Wistar(Han) rat. II: chronic dosing causes developmental delay. Toxicol Sci. 2007;99:224–233. doi: 10.1093/toxsci/kfm141. [DOI] [PubMed] [Google Scholar]
- 6.Gray LE, Jr, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol Appl Pharmacol. 1995;131:108–118. doi: 10.1006/taap.1995.1052. [DOI] [PubMed] [Google Scholar]
- 7.Hamm JT, Chen CY, Birnbaum LS. A mixture of dioxins, furans, and non-ortho PCBs based upon consensus toxic equivalency factors produces dioxin-like reproductive effects. Toxicol Sci. 2003;74:182–191. doi: 10.1093/toxsci/kfg107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolff MS, Camann D, Gammon M, Stellman SD. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105:13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dys-function across the life cycle. Rev Endocr Metab Disord. 2007;8:143–159. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 10.Cooke PS, Zhao YD, Hansen LG. Neonatal polychlorinated biphenyl treatment increases adult testis size and sperm production in the rat. Toxicol Appl Pharmacol. 1996;136:112–117. doi: 10.1006/taap.1996.0013. [DOI] [PubMed] [Google Scholar]
- 11.Kim IS. Effects of exposure of lactating female rats to polychlorinated biphenyls (PCBs) on testis weight, sperm production and sertoli cell numbers in the adult male offspring. J Vet Med Sci. 2001;63:5–9. doi: 10.1292/jvms.63.5. [DOI] [PubMed] [Google Scholar]
- 12.Gladen BC, Ragan NB, Rogan WJ. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr. 2000;136:490–496. doi: 10.1016/s0022-3476(00)90012-x. [DOI] [PubMed] [Google Scholar]
- 13.Guo YL, Lai TJ, Ju SH, Chen YC, Hsu CC. Sexual developments and biological findings in Yucheng children. Organohalogen Compounds. 1993;14:235–238. [Google Scholar]
- 14.Mol NM, Sørensen N, Weihe P, et al. Spermaturia and serum hormone concentrations at the age of puberty in boys prenatally exposed to polychlorinated biphenyls. Eur J Endocrinol. 2002;146:357–363. doi: 10.1530/eje.0.1460357. [DOI] [PubMed] [Google Scholar]
- 15.Den Hond E, Dhooge W, Bruckers L, et al. Internal exposure to pollutants and sexual maturation in Flemish adolescents. J Expo Sci Environ Epidemiol. 2011;21:224–233. doi: 10.1038/jes.2010.2. [DOI] [PubMed] [Google Scholar]
- 16.Korrick SA, Lee MM, Williams PL, et al. Dioxin exposure and age of pubertal onset among Russian boys. Environ Health Perspect. doi: 10.1289/ehp.1003102. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milbrath MO, Wenger Y, Chang CW, et al. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryan JJ, Levesque D, Panopio LG, Sun WF, Masuda Y, Kuroki H. Elimination of polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs) from human blood in the Yusho and Yu-Cheng rice oil poisonings. Arch Environ Contam Toxicol. 1993;24:504–512. doi: 10.1007/BF01146170. [DOI] [PubMed] [Google Scholar]
- 19.Burns JS, Williams PL, Sergeyev O, et al. Predictors of serum dioxins and PCBs among peripubertal Russian boys. Environ Health Perspect. 2009;117:1593–1599. doi: 10.1289/ehp.0800223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauser R, Williams P, Altshul L, et al. Predictors of serum dioxin levels among adolescent boys in Chapaevsk, Russia: a cross-sectional pilot study. Environ Health. 2005;4:8. doi: 10.1186/1476-069X-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revich B, Aksel E, Ushakova T, et al. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere. 2001;43:951–966. doi: 10.1016/s0045-6535(00)00456-2. [DOI] [PubMed] [Google Scholar]
- 22.Williams PL, Sergeyev O, Lee MM, et al. Blood lead levels and delayed onset of puberty in a longitudinal study of Russian boys. Pediatrics. 2010;125:e1088–e1096. doi: 10.1542/peds.2009-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MM, Sergeyev O, Williams P, et al. Physical growth and sexual maturation of boys in Chapaevsk, Russia. J Pediatr Endocrinol Metab. 2003;16:169–178. doi: 10.1515/jpem.2003.16.2.169. [DOI] [PubMed] [Google Scholar]
- 24.Martinchik AN, Baturin AK, Baeva VS, et al. [Development of a method of studying actual nutrition according to analysis of the frequency of consumption of food products: creation of a questionnaire and general evaluation of the reliability of the method]. Voprosy pitaniia. 1998:8–13. [PubMed] [Google Scholar]
- 25.Rockett HR, Breitenbach M, Frazier AL, et al. Validation of a youth/adolescent food frequency questionnaire. Preven med. 1997;26:808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 26.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner W, DiPietro E, Lapeza C, Green V, Gill J, Patterson DG. A fast universal automated cleanup system for the isotope-dilution high-resolution mass spectrometric analysis of PCDDs, PCDFs, coplanar PCBs, PCB congeners, and persistent pesticides from the same serum sample. Organohalogen Compounds. 1997;31:26–31. [Google Scholar]
- 28.Patterson DG, Jr, Hampton L, Lapeza CR, Jr, et al. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzop-dioxin. Anal Chem. 1987;59:2000–2005. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- 29.Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr, Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 30.Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humblet O, Williams PL, Korrick SA, et al. Predictors of serum dioxin, furan, and PCB concentrations among women from Chapaevsk, Russia. Environ Sci Technol. 2010;44:5633–5640. doi: 10.1021/es100976j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 33.Willett W, Stampfer M. In: Nutritional Epidemiology. Willet W, editor. United Kingdom: Oxford University Press; Oxford: 1998. pp. 273–301. [Google Scholar]
- 34.Burns JS, Williams PL, Sergeyev O, et al. Serum dioxins and polychlorinated biphenyls are associated with growth among Russian boys. Pediatrics. 2011;127:e59–e68. doi: 10.1542/peds.2009-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mocarelli P, Gerthoux PM, Needham LL, et al. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119:713–718. doi: 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mocarelli P, Gerthoux PM, Patterson DG, Jr, et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116:70–77. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 39.Monosson E, Kelce WR, Lambright C, Ostby J, Gray LE., Jr Peripubertal exposure to the antiandrogenic fungicide, vinclozolin, delays puberty, inhibits the development of androgen-dependent tissues, and alters androgen receptor function in the male rat. Toxicol Ind Health. 1999;15:65–79. doi: 10.1177/074823379901500107. [DOI] [PubMed] [Google Scholar]
- 40.Han TS, Bouloux PM. What is the optimal therapy for young males with hypogonadotropic hypogonadism? Clin Endocrinol (Oxf) 2010;72:731–737. doi: 10.1111/j.1365-2265.2009.03746.x. [DOI] [PubMed] [Google Scholar]
- 41.Patterson DG, Jr, Wong LY, Turner WE, et al. Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ Sci Technol. 2009;43:1211–1218. doi: 10.1021/es801966w. [DOI] [PubMed] [Google Scholar]