Abstract
Background and Objective
Conflicting results were found between the I-gel™ and the LMA-Supreme™ during anesthesia, so we conducted a meta-analysis of randomized controlled trials (RCTs) to compare the effectiveness and safety of the I-gel™ vs. the LMA-Supreme™during anesthesia.
Methods
A comprehensive search was conducted using Pubmed, EMbase, ISI Web of Knowledge, the Cochrane Library, China Journal Full-text Database, Chinese Biomedical Database, Chinese Scientific Journals Full-text Database, CMA Digital Periodicals, and Google scholar to find RCTs that compare the LMA-S™ with the i-gel™during anesthesia. Two reviewers independently selected trials, extracted data, and assessed the methodological qualities and evidence levels. Data were analyzed by RevMan 5.0 and comprehensive meta-analysis software.
Results
Ten RCTs were included. There were no significant differences in oropharyngeal leak pressures (mean difference [MD] 0.72, 95% confidence interval [CI] –1.10 2.53), device placement time (MD –1.3, 95%CI –4.07 1.44), first attempt insertion success (risk ratio [RR] 1.01, 95% CI 0.9 1.14), grade 3 and 4 fiberoptic view (RR 0.89, 95%CI 0.65 1.21), and blood on removal (RR 0.62, 95%CI 0.32 1.22) between the i-gel™ and the LMA-Supreme™, respectively. However, the LMA-Supreme™was associated with easier gastric tube insertion (RR 1.17, 95%CI 1.07 1.29), and more sore throat (RR 2.56, 95%CI 1.60 4.12) than the i-gel™ group.
Conclusions
The LMA-Supreme™ and i-gel™ were similarly successful and rapidly inserted. However, the LMA-Supreme™ was shown to be easier for gastric tube insertion and associated with more sore throat compared with the i-gel™.
Introduction
Since the introduction of the classic laryngeal mask airway, the field of supraglottic airway devices (SGA) has experienced a remarkable evolution and SGA are now routinely used in clinical anaesthesia [1]. The i-gel™ (Intersurgical Ltd, Wokingham, Berkshire, United Kingdom) and the Laryngeal Mask Supreme™ (LMA-S™; Laryngeal Mask Company, Henley-on-Thames, United Kingdom) are two single-use supraglottic airway devices. The i-gel™comprises a soft, gel-like, non-inflatable cuff made of thermoplastic elastomer, a widened, flattened stem with a rigid bite-block that acts as a buccal stabilizer to reduce axial rotation and malpositioning, and an oesophageal vent through which a gastric tube can be passed [2]–[4]. Whereas the LMA Supreme™ is an inflatable device with an oesophageal drainage tube for suctioning gastric contents, with similar characteristics to the i-gel™: presence of a drain tube to separate the gastrointestinal tract from the respiratory tract and built-in bite block [4]–[6]. It differs from the i-gel™ in the following aspects: it is constructed of medical grade silicone, and has an inflatable cuff, a reinforced tip, and an elliptical, anatomically shaped, semi-rigid airway tube [4]. Their potential advantages include easier insertion, better airway protection, more effective ventilation, and better fiberoptic view of the glottis [3], [6], [7].
There has been a lot of interest in these two devices due to their acclaimed advantages, and there have been a number of studies in response to concerns regarding their effectiveness and safety [1], [5]–[9]. However, there have been conflicting results concerning oropharyngeal leak pressure, ease of insertion, and adverse effects of these two devices during anesthesia. Regarding to first attempt insertion success and mean oropharyngeal leak pressure, they were significantly higher in the LMA Supreme™ than the i-gel™ [10], but not in other studies [6]–[8]. To our knowledge, there has been no previous meta-analysis comparing these two devices. Thus, we conducted a meta-analysis comparing these two devices with a detailed evaluation of their effectiveness and safety during anesthesia.
Methods
We did this meta-analysis of available randomized controlled trials (RCTs) in accordance with the PRISMA guidelines [11].
Search Strategy
Systematic literature searches were conducted in PubMed, the Cochrane library, EMBASE, ISI Web of Knowledge, China Journal Full-text Database, Chinese Biomedical Database, Chinese Scientific Journals Full-text Database, CMA Digital Periodicals, and Google scholar. Search terms included laryngeal mask, LMA-S, LMA Supreme, i-gel. All searches were conducted in May 2012, and updated in December 2012. Reference lists of relevant reviews and eligible articles were hand-searched. A search of the ClinicalTrials.gov website was also conducted to identify RCTs which were completed but not yet published. Requests for original papers that were not published were made by contacting authors or principal investigators. All searches were conducted independently by two reviewers (JH Jiao and XG Chen); differences were resolved by discussion.
Inclusion and Exclusion Criteria
Reports potentially eligible for this meta-analysis had to meet the following criteria: they had to be RCTs, written in English or Chinese, and the studies needed to provide sufficient information to pool the effectiveness and safety of the LMA-Supreme™ and i-gel™. The outcomes that we evaluated included oropharyngeal leak pressure, grade 3 and 4 fiberoptic view, device insertion time, first attempt insertion success, ease of gastric tube insertion, blood on removal, and sore throat. Articles were excluded if they did not satisfy one or more inclusion criteria.
Study selection and data Extraction
Two reviewers (XF Cong and L Liu) independently assessed potential citations for inclusion; disagreements were resolved with a third reviewer (XG Chen). Data was extracted from each article using a standardized form by two independent reviewers (XM Wu and L Liu) to abstract the following information: country, patient characteristics (age, sex, etc), and treatment protocols (details of intervention & comparison, sample size, etc), and outcomes. Outcomes were extracted preferentially by intention to treat method. Any disagreements were resolved with a third reviewer (XG Chen).
Quality and evidence level assessment
Methodological quality was assessed by the Cochrane handbook 5.0 recommended standard [12]: randomization, blinding, concealed allocation, selective reporting, incomplete outcome data, and other biases. For evaluating the evidence levels of the outcomes, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which specifies four levels: high, moderate, low, and very low [13]. Two reviewers (XF Cong and JH Jiao) independently assessed quality for each RCT and evidence level for each outcome; disagreements were resolved with a third reviewer (XG Chen).
Data Analysis
RevMan 5.0 software was used to conduct the meta-analysis. We computed the relative risk (RR) with corresponding 95% confidence interval (CI) for dichotomous outcome data, and mean difference (MD) with its 95%CI for continuous variable. The percentage of variability across trials attributable to heterogeneity beyond chance was estimated with the I2 statistic, which was deemed significant when p was less than 0.05 or I-square was more than 50% [14]. Data was pooled using both the fixed-effect model and the random-effect model.
Important variables such as country, surgery or patients’ condition, sample size, pre-anesthetic medication, and general anesthesia methods could affect the pooled results. So we conducted mixed meta-regression regression (unrestricted maximum likelihood) to investigate their influences on the primary outcomes. The meta-regression was conducted using comprehensive meta-analysis software 2.0.
In order to find the influence of methodological quality on the outcomes, we also pooled results using quality effect model by metaXL software. And the Q index (Qi) was calculated according to suggested quality scoring system for both experimental and prospective observational studies [15]. Publication bias was assessed by visually inspecting a funnel plot. The small-study effect in terms of publication bias was also estimated using Egger's linear regression test [16].
Results
Search results
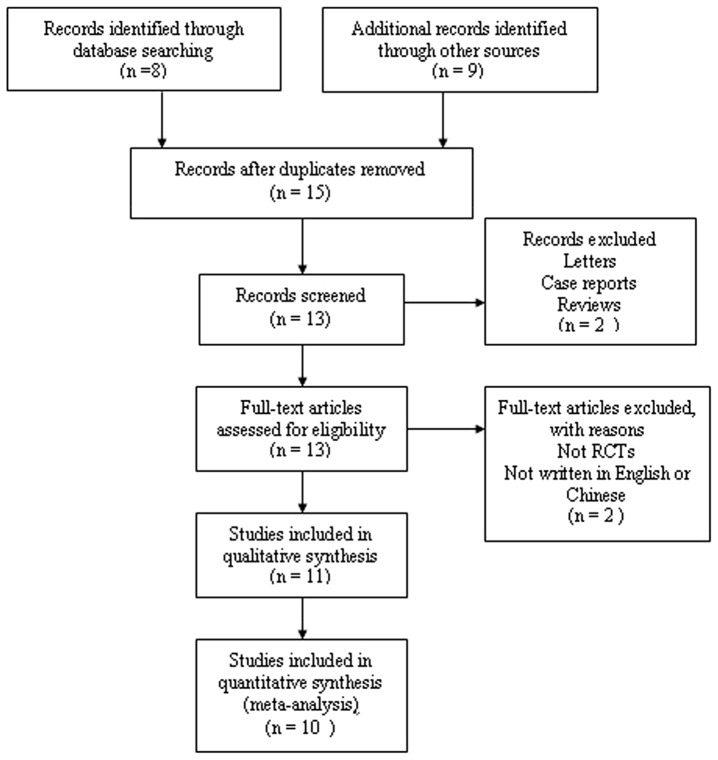
A total of 168 records were identified after comprehensive searches. No records were derived from ClinicalTrials.gov and contacting authors. Based on screening titles and abstracts, we excluded duplicates (n = 42), studies that did not include both LMA-S and i-gel (n = 54), and studies that were not RCTs (n = 50). Then based on reading full-texts we excluded studies those did not included both LMA-S and i-gel (n = 7) and studies that were not RCTs (n = 5). Finally, ten studies [1], [4], [8]–[10], [17]–[21] were included. The progress for study selection is shown in Figure 1.
Figure 1. Flow chart demonstrating the process for inclusion in the meta-analysis.
Characteristics of included trials
Ten studies were from China (n = 3), Germany (n = 2), Italy (n = 1), Singapore (n = 1), Switzerland (n = 1), The Netherlands (n = 1), and Malaysia (n = 1).
The surgeries the patients underwent were elective surgery ( 2 studies), laparoscopic gynecological surgery (2 studies ), elective peripheral or superficial surgery (1 study), spontaneously breathing anesthetized patients (1 study), laparoscopic gynecological and laparoscopic cholecystectomy (1 study), paralyzed anesthetized patient (1 study), laparoscopic cholecystectomy (1 study), and breast surgery (1 study). The sample size ranged from 30 to 150 with a total number of 860. And other information was presented in Table 1.
Table 1. Summary of included studies.
| Study | Country | N | Age (year) | Weight (Kg) | Surgery or patients’ condition | ASA grading | Pre-anesthetic medication | General anesthesia | Primary measurement | how OP leak pressures were obtained | Definition for the inflation protocols for the LMA-Supreme |
| Cao 2012 [19] | China | 50 | 20–60 | 50–80 | Laparoscopic gynecological and Cholecystectomy | I–II | atropine+ midazolam | fentanyl+propofol | oropharyngeal leak pressure | using the technique of static equilibrium | inflated by volume |
| Chew 2010 [8] | Malaysia | 90 | 16–55 | N/A | Spontaneously breathing anesthetized patients | I–II | N/A | fentanyl+propofol+sevoflurane | oropharyngeal leak pressure | using the technique of static equilibrium | inflated by volume |
| Eschertzhuber 2012 [18] | Germany | 30 | 20–70 | N/A | Paralyzed anesthetized patient | I–II | midazolam | fentanyl+propofol+rocuronium | oropharyngeal leak pressure | using the technique of static equilibrium | inflated by volume |
| Li 2011 [20] | China | 120 | 30–60 | 45–90 | Laparoscopic Cholecystectomy | I–II | midazolam + atropine | fentanyl+vecuronim | oropharyngeal leak pressure | using the technique of static equilibrium | inflated by volume |
| Ragazzi 2012 [10] | Italy | 80 | 18+ | N/A | Breast surgery | I–III | without | remifentanil+propofol | insertion success rate | unclear | unclear |
| Russo 2012 [1] | Germany | 120 | 18+ | N/A | Elective surgery | I–II | midazolam | sufentanil+propofol | oropharyngeal leak pressure | determined as a function of cuff pressure for the supraglottic airway devices with inflatable cuffs | inflated by volume |
| Teoh 2010 [4] | Singapore | 100 | 20–80 | 30+ | Laparoscopic gynecological | I–II | without | fentanyl+propofol+atracurium | oropharyngeal leak pressure | using the technique of static equilibrium | unclear |
| Theiler 2009 [17] | Switzerland | 60 | 18–80 | 50+ | Elective surgery | I–III | midazolam | fentanyl+propofol+remifentanil | Overall success rate | unclear | unclear |
| Van Zundert 2012 [9] | The Netherlands | 150 | 18–80 | 50–120 | elective peripheral or superficial surgery | I–II | N/A | fentanyl+propofol+sufentanil | ease of insertion | using the technique of static equilibrium | inflated by volume |
| Yu 2011 [21] | China | 60 | 21–64 | 45–90 | Laparoscopic gynecological | I–III | N/A | propofol+remifentanil+rocuronium | oropharyngeal leak pressure | using the technique of static equilibrium | inflated by volume |
Quality and evidence level assessment
Ten studies mentioned randomization, but only seven studies reported the details of randomization, and four studies reported the details of concealed allocation. Four studies mentioned blinding, and only three mentioned who were blinded to. And other information was presented in Table 2.
Table 2. Quality assessment of included studies.
| randomization | concealed allocation | blinding | incomplete outcome data | selective reporting | other biases | Qi | |
| Cao 2012 | mentioned | unclear | unclear | unclear | unclear | unclear | 0.75 |
| Chew 2010 | mentioned | yes, sealed opaque envelopes | no | unclear | unclear | yes, conflict of interest | 0.83 |
| Eschertzhuber 2012 | mentioned | unclear | yes | unclear | unclear | unclear | 0.92 |
| Li 2011 | yes, random number | unclear | unclear | unclear | unclear | unclear | 0.75 |
| Ragazzi 2012 | yes, random number | unclear | unclear | unclear | unclear | unclear | 0.83 |
| Russo 2012 | yes, random number | yes, sealed opaque envelopes | unclear | unclear | unclear | no | 0.83 |
| Teoh 2010 | yes, random number | yes, sealed opaque envelopes | blinded to patients | unclear | unclear | yes, conflict of interest | 0.96 |
| Theiler 2009 | yes, random number | unclear | blinded to operators | unclear | unclear | yes, conflict of interest | 0.88 |
| Van Zundert 2012 | yes, random number | yes, sealed opaque envelopes | blinded to observer | unclear | unclear | unclear | 0.96 |
| Yu 2011 | yes, random number | unclear | unclear | unclear | unclear | unclear | 0.83 |
For the seven outcomes we evaluated, the evidence levels for two outcomes (grade 3 and 4 fiberoptic view and device insertion time) were very low, and the evidence levels for the rest were low, due to high risk of bias, high heterogeneity, or small sample size (Table 3).
Table 3. Meta-analysis results for each outcome.
| Fixed-model | Random-model | Quality-effect model | Heterogeneity | Evidence level | |
| Oropharyngeal leak pressure | MD 0.46, 95%CI (–0.32 1.23) | MD 0.72, 95%CI (–1.10 2.53) | MD 0.61, 95%CI (–0.25 1.46) | I2 = 79%, P < 0.00001 | Low |
| Grade 3 and 4 fiberoptic view | RR 0.87, 95%CI (0.76 0.99) | RR 0.88, 95%CI (0.63 1.22) | RR 0.90, 95%CI (0.68 1.19) | I2 = 84%, P < 0.0001 | Very low |
| Device insertion time | MD –0.05, 95%CI (–1.16 1.06) | MD –1.30, 95%CI (–4.02 1.44) | MD –0.51, 95%CI (–1.76 0.75) | I2 = 75%, P = 0.003 | Very low |
| First attempt insertion success | RR 1.08, 95%CI (1.00 1.16) | RR 1.05, 95%CI (0.97 1.13) | RR 1.04, 95%CI (0.97 1.12) | I2 = 69%, P = 0.006 | Low |
| Ease of gastric tube insertion | RR 1.17, 95%CI (1.07 1.29) | RR 1.18, 95%CI (1.06 1.31) | RR 1.18, 95%CI (1.08 1.32) | I2 = 45%, P = 0.16 | Low |
| Sore throat | RR 2.55, 95%CI (1.59 4.09) | RR 2.45, 95%CI (1.50 3.99) | RR 2.36, 95%CI (1.43 3.88) | I2 = 0%, P = 0.53 | Low |
| Blood on removal | RR 0.62, 95%CI (0.32 1.22) | RR 0.61, 95%CI (0.27 1.41) | RR 0.58 95%CI (0.27 1.22) | I2 = 16%, P = 0.31 | Low |
Meta analysis results
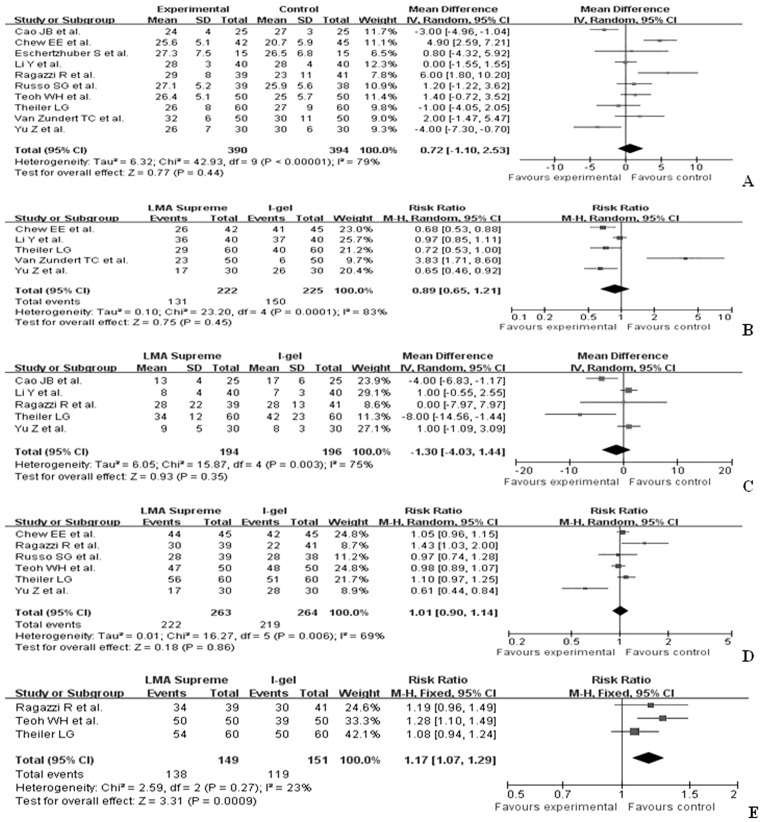
The pooled results revealed that there were similar oropharyngeal leak pressures during anesthesia between the LMA-Supreme™and the i-gel™ (WMD 0.72, 95%CI –1.10 2.53). There were no significant differences in device placement time (WMD –1.3, 95%CI –4.07 1.44), first attempt insertion success (RR 1.01, 95%CI 0.9 1.14), and grade 3 and 4 fiberoptic view (RR 0.89, 95%CI 0.65–1.21) between the LMA-Supreme™ group and the i-gel™ group. However, gastric tube insertion was significantly easier (RR 1.17, 95%CI 1.07 1.29) in the LMA-Supreme™than that in the i-gel™group during anesthesia (Figure 2).
Figure 2. Forest plot displaying the pooled summary performance of the LMA-Supreme™ versus the i-gel™: A) oropharyngeal leak pressure, B) grade 3 and 4 fiberoptic view, C) device insertion time, D) first attempt insertion success, E) ease of gastric tube insertion.
SD = standard deviation; IV = weighted mean difference; CI = confidence interval; df = degrees of freedom; Chi2 = chi-square statistic; p = p value; I2 = I-square heterogeneity statistic; Z = Z statistic; RR = risk ratio; WMD = weight mean difference.
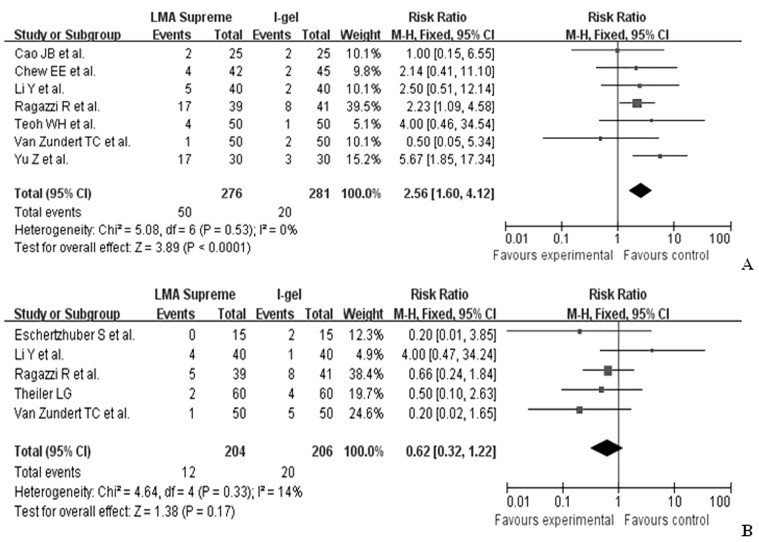
There was no significant difference in the presence of blood on device removal between the LMA-Supreme™ and the the i-gel™ (RR 0.62, 95%CI 0.32 1.22), but sore throat was more common in the LMA-Supreme™ group than that in the i-gel™ group (RR 2.56, 95%CI 1.60 4.12) (Figure 3).
Figure 3. Forest plot displaying the pooled summary of adverse effects of the LMA-Supreme™ versus the i-gel™: A) sore throat, B) blood on removal.
SD = standard deviation; IV = weighted mean difference; CI = confidence interval; df = degrees of freedom; Chi2 = chi-square statistic; p = p value; I2 = I-square heterogeneity statistic; Z = Z statistic; RR = risk ratio; WMD = weight mean difference.
Both fixed- and random- effects models were used to pool the results, and the results for seven outcomes were consistent (Table 3). We also took quality into account using quality effect models. And the results produced by quality effect models were also in accordance with the results by both fixed- and random- effects models (Table 3).
Meta-regression results
The results of meta-regression showed that coefficients for country, surgery or patients’ condition, sample size, pre-anesthetic medication and general anesthesia methods were not statistically significant. (Table 4)
Table 4. Meta-regression results of variables for oropharyngeal leak pressure.
| Coefficient | 95%CI | P | Residual Q | ||
| Country | 0.04 | –0.03 | 0.10 | 0.26 | 36.99 |
| Surgery or patients’ condition | 0.04 | –0.11 | 0.19 | 0.62 | 10.18 |
| Sample size | 0.01 | –0.00 | 0.01 | 0.17 | 10.30 |
| Pre-anesthetic medication | 0.08 | –0.09 | 0.24 | 0.35 | 10.02 |
| General anesthesia | –0.01 | –0.15 | 0.12 | 0.86 | 10.34 |
Publication bias
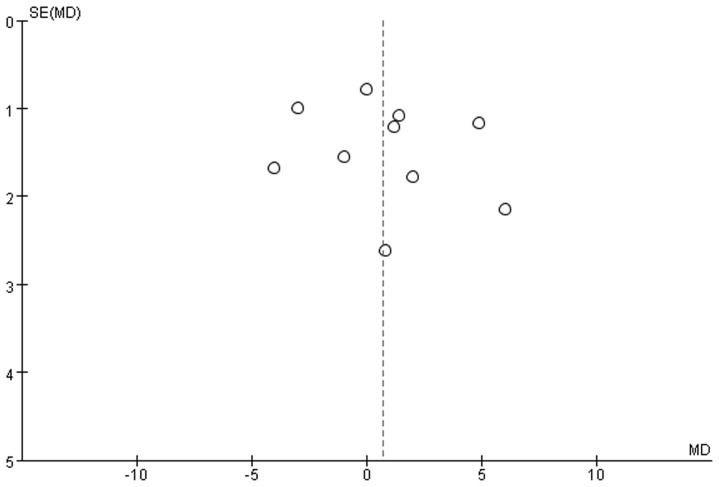
There was no significant publication bias based on funnel plot (Figure 4). Egger's test indicated that there was not a possibility of publication bias for oropharyngeal leak pressures (intercept –3.45, 95%CI –12.07 5.16, p = 0.38).
Figure 4. Funnel plot of randomized controlled trials.
Discussion
Summary of finding
This meta-analysis summarized evidence in published RCTs concerning the effectiveness and safety of the LMA-Supreme™ vs. the i-gel™ during anesthesia. The LMA-Supreme™ was similar to the i-gel™in oropharyngeal leak pressure, first attempt insertion success, device insertion time, grade 3 and 4 fiberoptic view and blood on removal. However, LMA-Supreme™was associated with easier gastric tube insertion and more sore throat than the i-gel™. Country, surgery or patients’ condition, sample size, pre-anesthetic medication, and general anesthesia did not affect the pooled results of the oropharyngeal leak pressure between the LMA-Supreme™and the i-gel™. However, the methodological quality of included studies was of high risk and the evidence levels for outcomes were low or very low.
Six RCTs in this analysis [1], [4], [8], [10], [18], [22] demonstrated positive trends which is consistent with the pooled result of oropharyngeal leak pressures in our meta-analysis. The oropharyngeal leak pressures were similar for both devices based on these six RCTs and the results of this meta-analysis. However, other studies [19], [21] have suggested that the i-gel™ provided a higher oropharyngeal leak pressure than the LMA-Supreme™. On the other hand, Chew et al. [8] and Ragazzi et al. [10] demonstrated that oropharyngeal leak pressures were significantly greater with the LMA-Supreme™ than those with the i-gel™.
Our meta-analysis showed no significant difference on number of grade 3 and 4 fiberoptic view between the i-gel™ and the LMA-Supreme™. This suggests that these two devices might function similarly as a conduit in difficult airway management and failed intubation. The LMA-Supreme™ has an anatomically shaped airway tube, a drain tube (DT), a modified inflatable cuff, an integral bite block, and a fixation tab [23]. The DT provides access to gastric contents [24] and is helpful for gastric tube insertion, but we found that there were no significant differences in first attempt insertion success rates and speed of insertion, which means that the two devices a appeared equally easy to insert in patients under anesthesia [22], [25].
Regarding to safety, there was not a significant difference in blood on removal between the two devices. However, sore throat was more in LMA-Supreme™than that in the i-gel ™. The I-gel ™ has non-inflatable cuff seals that fit anatomically against perilaryngeal structures [26], but the LMA-Supreme™ has been designed with an inflatable cuff and a reinforced tip. Yu et al. [9] and Ragazzi et al. [4] reported that sore throat was more common in the LMA-Supreme™ group than that in the i-gel™ group, which is consistent with our meta-analysis result. This complication may be related to the inflatable cuff of the LMA-Supreme™ compressing microvascular structures and terminal nerve endings in these tissues [2].
In conclusion, both the LMA-Supreme™ and the i-gel™ were similarly successful and rapidly inserted. However, gastric tube insertion was much easier with the LMA-Supreme™ than that with the i-gel™. Sore throat was more common with the LMA-Supreme™ than that with the i-gel™.
Strength and limitation
To the best of our knowledge, this is the first meta-analysis specifically evaluating the effectiveness and safety of the LMA-Supreme™ and the i-gel™ during anesthesia. However, there were several limitations of this study: (1) Although we conducted meta-regression of different surgeries, countries, sample size, pre-anesthetic medication, and general anesthesia methods, country, surgery or patients’ condition, sample size, pre-anesthetic medication and general anesthesia methods did not affect the results of oropharyngeal leak pressure. Many factors including patient characteristics, depths of anaesthesia, successes of insertion, paralyzed statuses and the settings could affect the anesthetic effects. However, we did not conduct meta-regression of these factors due to limited data.
(2) Our results revealed that there might be heterogeneity. These factors we mentioned above probably contributed to the heterogeneity between studies. It was difficult to adequately scrutinize the heterogeneity of outcomes between studies, as the nuanced differences between the studies were not defined or appreciated. The heterogeneity across studies was clinical heterogeneity and might be the biggest issue in this meta-analysis. The end points are faulted and have inherent error that cannot be quantified, which would lower the reliability of the data among studies. In this condition, it is hard to judge that the effects observed is the true effect or not. More often, the effects could be affected by the clinical heterogeneity among studies and the influence of clinical heterogeneity for end points should be investigated. However, we could not investigate their influence by conducting subgroup analysis or meta-regression analysis due to limited data.
(3) Although we have tried to access to un-published results, we did not include any unpublished studies. (4) For oropharyngeal leak pressure using supraglottic airway devices, it depends significantly on the body habitus, the experience of the operator and assistant, and the depth of anaesthesia. But none of our included studies reported the influence of body habitus and the experience of the operator and assistant on oropharyngeal leak pressure. Failure to include such clinically important measures limits the usefulness of this entire manuscript. So in the future, better RCTs should include such data. (5) Due to small sample size of included studies, high heterogeneity across the included studies and high risk of bias of included studies, the evidence level for the evidence was low. (6) Although the personal preference might influence the choice of LMA, there is no data to quantify how much personal preference influenced the choice of LMA.
Implication for research and practice
The sample sizes of included studies were not large enough, ranging from 30 to 150. The number of participants in clinical research should always be large enough to provide a sufficiently precise answer to the research question posed, so in the future the sample size for the RCTs comparing LMA-Supreme™ with i-gel™should be large enough to detect the small differences.
The methodological quality for all included studies was not high, as some important methodological items were not well conducted or reported, such as concealed allocation. All the studies mentioned randomization, but not all studies mentioned the details of randomization methods and concealed allocation. So in the future, important methodological items such as randomization, concealed allocation and blinding should be well conducted and reported.
At the same time, none of included studies mentioned the influences of patient characteristics, depths of anaesthesia, successes of insertion, paralyzed statuses and the settings. Only one study reported the effectiveness of LMA-Supreme™ vs. i-gel™in paralyzed patients, and its result was consistent with the pooled results, so we thought paralyzed status did not affect the results. Whether these factors affect the the true effects of LMA should be well discussed in the future RCTs. Meanwhile, studies that quantify how much personal preference influenced the choice of LMA should also be conducted.
For practice, our meta-analysis showed that the LMA-Supreme™ was similar to the i-gel™in oropharyngeal leak pressure, first attempt insertion success, device insertion time, grade 3 and 4 fiberoptic view and blood on removal with easier gastric tube insertion and more sore throat. So in the future, anesthetists should choose different devices according to the real situations.
Conclusion
This meta-analysis summarized evidence in published RCTs concerning the effectiveness and safety of the LMA-Supreme™ vs. the i-gel™ during anesthesia. The LMA-Supreme™ was similar to the i-gel™in oropharyngeal leak pressure, first attempt insertion success, device insertion time, grade 3 and 4 fiberoptic view and blood on removal. However, gastric tube insertion was easier and sore throat was more in LMA-Supreme™than those in the i-gel™. Even with the limitations, we feel the conclusions of this meta-analysis are clinically useful for the consideration of anesthetists.
Supporting Information
PRISMA 2009 Flow Diagram.
(DOC)
PRISMA 2009 Checklist.
(DOC)
Acknowledgments
Thanks to Jingpu Shi, Ph.D, Professor of the Department of Clinical Epidemiology and Evidence Medicine in the First Affiliated Hospital of China Medical University. Thanks to the editors and anonymous reviewers. We thank Carl Borromeo for reviewing our paper, Mercedes Susan Mandell and other editors for working on our paper.
Funding Statement
No current external funding sources for this study.
References
- 1. Russo SG, Cremer S, Galli T, Eich C, Brauer A, et al. (2012) Randomized comparison of the i-gel, the LMA Supreme, and the Laryngeal Tube Suction-D using clinical and fibreoptic assessments in elective patients. BMC Anesthesiol 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levitan RM, Kinkle WC (2005) Initial anatomic investigations of the I-gel airway: a novel supraglottic airway without inflatable cuff. Anaesthesia 60: 1022–1026. [DOI] [PubMed] [Google Scholar]
- 3. Gatward JJ, Cook TM, Seller C, Handel J, Simpson T, et al. (2008) Evaluation of the size 4 i-gel airway in one hundred non-paralysed patients. Anaesthesia 63: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 4. Teoh WH, Lee KM, Suhitharan T, Yahaya Z, Teo MM, et al. (2010) Comparison of the LMA Supreme vs the i-gel in paralysed patients undergoing gynaecological laparoscopic surgery with controlled ventilation. Anaesthesia 65: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 5. van Zundert A, Brimacombe J (2008) The LMA Supreme—a pilot study. Anaesthesia 63: 209–210. [DOI] [PubMed] [Google Scholar]
- 6. Timmermann A, Cremer S, Eich C, Kazmaier S, Brauer A, et al. (2009) Prospective clinical and fiberoptic evaluation of the Supreme laryngeal mask airway. Anesthesiology 110: 262–265. [DOI] [PubMed] [Google Scholar]
- 7. Cook TM, Gatward JJ, Handel J, Hardy R, Thompson C, et al. (2009) Evaluation of the LMA Supreme in 100 non-paralysed patients. Anaesthesia 64: 555–562. [DOI] [PubMed] [Google Scholar]
- 8. Chew EFF, Hashim NHM, Wang CY (2010) Randomised comparison of the LMA Supreme(trademark) with the I-Gel(trademark) in spontaneously breathing anaesthetised adult patients. Anaesth Intensive Care 38: 1018–1022. [DOI] [PubMed] [Google Scholar]
- 9. Van Zundert TC, Brimacombe JR (2012) Similar oropharyngeal leak pressures during anaesthesia with i-gel, LMA-ProSeal and LMA-Supreme Laryngeal Masks. Acta Anaesthesiol Belg 63: 35–41. [PubMed] [Google Scholar]
- 10. Ragazzi R, Finessi L, Farinelli I, Alvisi R, Volta CA (2012) LMA Supreme vs i-gel—a comparison of insertion success in novices. Anaesthesia 67: 384–388. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration website. Available: http://www.cochrane.org/training/cochrane-handbook/Accessed 2012 Jan 5.
- 13. Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, et al. (2006) An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 174: 605–614. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doi SA, Thalib L (2008) A quality-effects model for meta-analysis. Epidemiology 19: 94–100. [DOI] [PubMed] [Google Scholar]
- 16. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Theiler LG, Kleine-Brueggeney M, Kaiser D, Urwyler N, Luyet C, et al. (2009) Crossover comparison of the laryngeal mask supreme and the i-gel in simulated difficult airway scenario in anesthetized patients. Anesthesiology 111: 55–62. [DOI] [PubMed] [Google Scholar]
- 18. Eschertzhuber S, Brimacombe J, Hohlrieder M, Keller C (2009) The Laryngeal Mask Airway Supreme(TM)- a single use laryngeal mask airway with an oesophageal vent. A randomised, cross-over study with the Laryngeal Mask Airway ProSeal(TM) in paralysed, anaesthetised patients. Anaesthesia 64: 79–83. [DOI] [PubMed] [Google Scholar]
- 19. CAO J-B, WANG P, MI W-D, ZHANG H (2012) Effect of laryngeal mask i-gel on airway of patients undergoing laparoscopic surgery. J Chinese PLA Postgrad Med Sch 33: 362–366. [Google Scholar]
- 20. Li Y, Liu J, Li C, Zhang W, Yu D (2011) Comparison of effectiveness of the ProSeal laryngeal mask, the Supreme laryngeal mask and the i-gel laryngeal mask with airway management for laparoscopic cholecystectomy in patients. Chin J Anesthesiol 31: 1146–1148. [Google Scholar]
- 21. Yu Z, Hu Z, Zuo M, Shi Y (2011) Efficacy of laryngeal mask airway i-gel in patients undergoing laparoscopic gynecological surgery. Chin J Anesthesiol 31: 223–225. [Google Scholar]
- 22. Tiesmeier J, Emmerich M (2011) Successful use of the I-gel supraglottic airway as a conduit for blind pre-hospital intubation during resuscitation. Resuscitation 82: 1358–1359. [DOI] [PubMed] [Google Scholar]
- 23. Verghese C, Ramaswamy B (2008) LMA-Supreme—a new single-use LMA with gastric access: a report on its clinical efficacy. Br J Anaesth 101: 405–410. [DOI] [PubMed] [Google Scholar]
- 24. Sharma V, Verghese C, McKenna PJ (2010) Prospective audit on the use of the LMA-Supreme for airway management of adult patients undergoing elective orthopaedic surgery in prone position. Br J Anaesth 105: 228–232. [DOI] [PubMed] [Google Scholar]
- 25. Mann V, Spitzner T, Schwandner T, Mann ST, Muller M, et al. (2012) The effect of a cervical collar on the seal pressure of the LMA Supreme: a prospective, crossover trial. Anaesthesia 67: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 26. Helmy AM, Atef HM, El-Taher EM, Henidak AM (2010) Comparative study between I-gel, a new supraglottic airway device, and classical laryngeal mask airway in anesthetized spontaneously ventilated patients. Saudi J Anaesth 4: 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Flow Diagram.
(DOC)
PRISMA 2009 Checklist.
(DOC)