Abstract
BACKGROUND
Large artery stiffness is a major risk factor for the development of hypertension and cardiovascular disease. Persistent prehypertension accelerates the progression of arterial stiffness.
METHODS
Forty-three unmedicated prehypertensive (systolic blood pressure (SBP) = 120–139mm Hg or diastolic blood pressure (DBP) = 80–89mm Hg) men and women and 15 normotensive time-matched control subjects (NMTCs; n = 15) aged 18–35 years of age met screening requirements and participated in the study. Prehypertensive subjects were randomly assigned to a resistance exercise training (PHRT; n = 15), endurance exercise training (PHET; n = 13) or time-control group (PHTC; n = 15). Treatment groups performed exercise training 3 days per week for 8 weeks. Pulse wave analysis, pulse wave velocity (PWV), and central and peripheral blood pressures were evaluated before and after exercise intervention or time-matched control.
RESULTS
PHRT and PHET reduced resting SBP by 9.6±3.6mm Hg and 11.9±3.4mm Hg, respectively, and DBP by 8.0±5.1mm Hg and 7.2±3.4mm Hg, respectively (P < 0.05). PHRT and PHET decreased augmentation index (AIx) by 7.5% ± 2.8% and 8.1% ± 3.2% (P < 0.05), AIx@75 by 8.0% ± 3.2% and 9.2% ± 3.8% (P < 0.05), and left ventricular wasted pressure energy, an index of extra left ventricular myocardial oxygen requirement due to early systolic wave reflection, by 573±161 dynes s/cm2 and 612±167 dynes s/cm2 (P < 0.05), respectively. PHRT and PHET reduced carotid–radial PWV by 1.02±0.32 m/sec and 0.92±0.36 m/sec (P < 0.05) and femoral–distal PWV by 1.04±0.31 m/sec and 1.34±0.33 m/sec (P < 0.05), respectively. No significant changes were observed in the time-control groups.
CONCLUSIONS
This study suggests that both resistance and endurance exercise alone effectively reduce peripheral arterial stiffness, central blood pressures, augmentation index, and myocardial oxygen demand in young prehypertensive subjects.
Keywords: arterial stiffness, augmentation index, blood pressure, exercise, hypertension, left ventricular wasted pressure energy, prehypertension.
Development of large artery stiffness has its origins in early adulthood.1 Arterial stiffness is a major contributing factor in the development of cardiovascular diseases, including myocardial infarction and heart failure, and is related to cardiovascular mortality.2 A hallmark of central arterial stiffness is the alterations in the common carotid intima-media layer, where the elastin is infiltrated with collagen and fibrin, decreasing the elastic properties of the vessel, which contributes to systolic hypertension, left ventricular hypertrophy, and impaired coronary perfusion and is primarily influenced by aging and mean arterial pressure.3,4 Elevated blood pressure (BP) causes vascular damage, plays a critical role in the pathogenesis of arterial stiffness, and should be specifically targeted to prevent arterial stiffening. Moreover, it has been suggested that arterial functions are impaired even at the prehypertension level and the functional relationship between BP and arterial stiffness is likely bidirectional.5–7
Approximately one-third of the US population aged ≥20 years (approximately 54 million Americans) exhibits prehypertension.8 This demographic is 11 times more likely to develop essential hypertension than normotensive persons.8–10 Prehypertension is defined as an untreated systolic BP (SBP) of 120–139mm Hg or diastolic BP (DBP) of 80–89mm Hg in persons that have not been told on 2 separate occasions by a health-care professional that he/she has hypertension.11 Prehypertension is not a disease category per se, and individuals with prehypertension are not candidates for drug therapy.11 According to the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7), people with prehypertension should practice lifestyle modification to prevent the progressive rise in BP and increased risk of cardiovascular disease.11 The foundation of the JNC7 recommendation is participation in regular physical activity for the treatment of prehypertension.11 The efficacy of endurance training to reduce BP and arterial stiffness has been well established, and resistance training is recommended as a complement to an endurance training regimen.12 However, the effects of resistance training on BP reduction are less described,12 and the effectiveness of either resistance or endurance exercise training to reduce the peripheral and central BP and arterial stiffness associated with young prehypertensive persons has been grossly underinvestigated. Therefore, in this randomized and time-controlled study, we tested the hypothesis that resistance and endurance exercise training would separately reduce peripheral arterial stiffness and improve indexes of central artery hemodynamics in young prehypertensive persons.
METHODS
Baseline status of subjects
Forty-three consecutive young (aged 18–35 years) and otherwise healthy subjects determined to be prehypertensive through BP screening were enrolled from the University of Florida and surrounding Gainesville, Florida, area. Additionally, 15 consecutive normotensive (SBP < 120mm Hg and DBP < 80mm Hg) young healthy subjects were also recruited to serve as a normotensive healthy nonexercising control group (NMTC; n = 15; 9 men and 6 women). All subjects (n = 58) were nonsmokers and considered to be novice exercisers who had not participated in a structured endurance and/or resistance training program in the past 6 months. Prehypertensive subjects were randomized to 1 of the following 3 groups: (i) resistance training (PHRT; n = 15; 11 men and 4 women), (ii) endurance training (PHET; n=13; 9 men and 4 women), and (iii) nonexercising time control (PHTC; n = 15; 10 men and 5 women). All subjects were studied before training and after 8 weeks of exercise treatment or control time period. The study was approved by the institutional review board of the University of Florida, and written informed consent was obtained from all patients.
Resting brachial BP screening
Before study enrollment, all subjects underwent BP screening to determine the presence or absence of prehypertension. BP measurements were performed according to the JNC7 guidelines.11 Briefly, subjects were screened on 3 separate visits during the same time of day and underwent at least 3 BP measurements per visit. After 20–30 minutes of rest, BP measurements were spaced by 5–10 minutes intervals on the left arm in a seated position using an automated oscillometric BP cuff (VSM MedTech, British Columbia, Canada). After meeting screening criteria, consent was obtained, and subjects were asked to report on a separate day to the Cardiovascular Laboratory in the Center for Exercise Science at the University of Florida. Prehypertensive subjects were included in the study when resting SBP was 120–139mm Hg or DBP was 80–89mm Hg on all 3 visits.
Exercise and time control
At study entry, prehypertensive subjects were randomly assigned, using a computer random number generator, to a group that performed resistance exercise training, a group that performed endurance training, or a nonexercising time-control group. The PHRT and PHET groups performed exercise training 3 days per week for 1 hour. Duration of the exercise program was 8 weeks. The resistance exercise training regimen consisted of 2 sets of 8–12 repetitions to volitional fatigue on 7 variable resistance machines (MedX, Ocala, FL) chosen to exercise all major muscle groups: leg extension, leg curl, leg press, lat pull down, chest press, overhead press, and biceps curl. When 12 repetitions were achieved on the second set, the training weight was increased 5% at the next training session. Recovery time between sets and exercises was 2–3 minutes. Subjects randomly assigned to the endurance training group were oriented to the Quinton endurance exercise treadmill and underwent a symptom limited graded exercise test to determine peak oxygen consumption and peak heart rate before and after 8 weeks of exercise training. A 3-day familiarization period for resistance exercise training and endurance training was instituted during the first week of training and set at 60% of each subjects predetermined 1-repetition maximum or peak oxygen consumption and maximum heart rate, respectively. The endurance training regimen consisted of interval treadmill walking/running to maintain a heart rate that was between 65%–85% of their predetermined maximum exercising heart rate. Walking/running intervals were standardized for PHET participants and consisted of 3 minutes of walking at a speed and incline at 65% of maximum heart rate and 2 minutes of running at a speed and incline at 85% of maximum heart rate for a total of nine 5-minute intervals. All subjects performed a 10-minute warm-up at low speed with no incline, then 45 minutes of aerobic or resistance exercise, followed by 5 minutes of cool-down, 3 days per week. Both PHTC and NMTC groups remained sedentary and refrained from initiating a structured exercise training program for 8 weeks. All subjects were instructed to maintain their current nutritional levels.
Pulse wave analysis
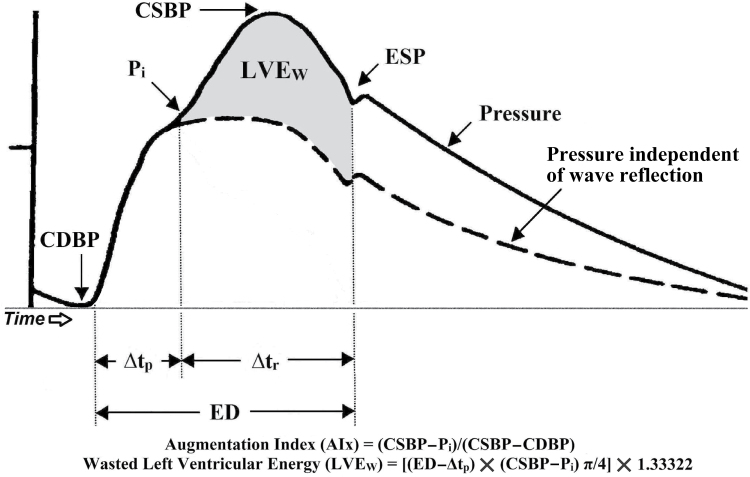
After a 15-minute rest period in a supine position, heart rate and brachial artery BP measurements were performed in triplicate in the left arm, and an average of the 3 heart rate and BP measurements were used for resting values. The assessment of arterial wave reflection characteristics were performed noninvasively using the SphygmoCor Pulse Wave Analysis Px system and SCOR-2000 Version 6.31 software (AtCor Medical, Sydney, Australia). A typical aortic pressure waveform synthesized from radial pulse pressure using applanation tonometry and the generalized transfer function is shown in Figure 1.13,14 The following pulse wave analysis parameters, related to the amplification and temporal characteristics of the reflecting wave, were used as dependent variables in this study: central aortic SBP, central aortic DBP, mean arterial pressure (MAP), end systolic pressure, ejection duration (ED), augmentation index (AIx), AIx normalized to a heart rate of 75 beats per minute (AIx@75) and ∆tp. ED is a measure of time, in milliseconds, of the duration of each cardiac systole.15 MAP was obtained from an integration of the waveform. End systolic pressure is defined as the pressure at the end of systole, which is the pressure at the end of ED.16 AIx, expressed as a percentage, characterizes augmentation of central pressures and is defined as reflected wave amplitude divided by pulse pressure.17,18 ∆tp is the round trip travel time of the forward traveling wave from the ascending aorta to the major reflection site and back and is measured from the foot of the forward traveling pressure wave to the foot of the reflected wave.
Figure 1.
A typical central aortic pressure waveform synthesized from the radial artery pressure waveform using applanation tonometry, with superimposed waveform of aortic blood flow. The dotted line is representative of the theoretical aortic pressure waveform independent of wave reflection. The line labeled “flow” is a representative waveform of aortic blood flow. Augmentation index (AIx) is the ratio of augmented pressure (central aortic systolic blood pressure (CSBP) − pressure at the first inflection point marking the onset of reflected aortic pressure wave return from the periphery (Pi) and central aortic pulse pressure (CSBP − central aortic diastolic blood pressure (CDBP). Wasted left ventricular pressure energy (LVEW) is directly related to augmented pressure (CSBP − Pi) and to the time duration of the reflected aortic pressure wave, (ejection duration (ED) − round trip travel time of the reflected pressure wave to the major peripheral reflecting site and back to the aorta (∆tp). Abbreviations: ∆tr, systolic duration of the reflected aortic pressure wave; ESP, end systolic pressure. Reproduced from reference 19 with permission of the Society for Experimental Biology and Medicine.
At least 3 consecutive measurements were performed per time point, and the average of the 3 highest quality recordings, defined as an in-device quality index of >90% (derived from an algorithm including average pulse height variation, diastolic variation, and maximum rate of rise of the peripheral waveform) were used for analysis. In our laboratory, reproducibility has been established in young, healthy men with a mean coefficient of variation of 6.5%, 2.1%, 2.4%, and 2.4% for aortic AIx, ∆tp, central SBP and DBP, respectively.19
The central aortic pressure wave is composed of a forward traveling wave, generated by left ventricular ejection, and a reflected wave that is returning to the ascending aorta from the periphery.20 Additional calculations derived from pulse wave analysis included left ventricular wasted pressure energy (LVEW) and the tension–time index. LVEW is a component of extra myocardial oxygen requirement that is due to early systolic wave reflection.21 LVEW can be estimated in dynes s/cm2 as [(ED − ∆tp)(CSBP − Pi)π/4]1.33,322, where Pi is the first inflection point marking the onset of reflected aortic pressure wave return from the periphery (Figure 1). The tension–time index, a marker of left ventricular work and myocardial oxygen demand, was calculated as (ED × heart rate × mean aortic SBP)/1,000, and is expressed in mm Hg s/min. Arterial tonometry measurements took approximately 30 minutes per lab visit, and the assessment of central pressure waves is described in detail by Nichols and Singh.16
Pulse wave velocity
To determine PWV, pressure waveforms were recorded using a SphygmoCor Pulse Wave Velocity Px system and SCOR-2000 version 6.31 software (AtCor Medical) at the following 3 sets of sites sequentially: carotid to radial, carotid to femoral, and femoral to dorsalis pedis. PWV between measuring sites was used as an indirect measure of regional arterial stiffness. Central PWV (in the mostly elastic aorta) was evaluated using the carotid–femoral data and peripheral PWV (in the more muscular conduits) using the femoral–dorsalis pedis and carotid–radial data. The reliability of PWV between the different regions was established by sequential measurements on young men on 3 separate days. The mean coefficient of variation for carotid–radial, carotid–femoral, and femoral–dorsalis pedis were 4.5%, 2.1%, and 5.3%, respectively.22
Exercise tests
PHET subjects performed maximum graded exercise tests (GXT) on a treadmill using a Bruce protocol before and after 8 weeks of exercise to determine initial exercise training intensity and cardio-respiratory fitness level. Primary measurements were total exercise duration and peak oxygen consumption. Criteria for termination of the gradfed exercise tests included respiratory exchange ratio > 1, a heart rate equal to age-predicted maximum heart rate, plateau of oxygen consumption, and volitional fatigue.
Muscle strength was assessed by determining 1 repetition maximum in all PHRT subjects using variable resistance MedX training equipment for leg and chest press before and after 8 weeks of exercise to determine initial exercise training intensity and post-training strength level.
Statistical analysis
Analysis of variance (ANOVA) was used to analyze baseline group differences between the PHRT, PHET, PHTC, and NMTC groups. Continuous variable data are presented as mean ± SEM. All data were tested for normal distribution with the Shapiro-Wilk test for normality. An alpha level of P < 0.05 was required for statistical significance. ANOVA with repeated measures were used to evaluate changes in primary and secondary dependent variables and all other data. When a statistically significant group-by-time interaction was observed, within-group comparisons between time point and between groups were analyzed with ANOVA and Tukey post hoc analysis. All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Chicago, IL).
RESULTS
All subjects completed the entire exercise treatment or matched time-control period without adverse events. Table 1 contains the baseline characteristics for all participants. The prehypertensive and the normotensive groups did not differ at baseline with respect to age, height, weight, body mass index, or resting heart rate. After randomization, the prehypertensive groups did not differ at baseline with respect to resting peripheral BPs (peripheral SBP or DBP) or central BPs (central aortic SBP or central aortic DBP) (Table 1). By design, the prehypertensive groups had significantly higher baseline peripheral SBP and peripheral DBP compared with normotensive time controls at study entry (Table 1). Peripheral and central MAP and peripheral and central pulse pressure (PPP and APP) were also elevated in the prehypertensive groups at baseline compared with the normotensive group (Table 2). There was no demonstrable main effect of sex on the hemodynamic changes in response to exercise training.
Table 1.
Resting subject characteristics before and after exercise training and time control
| Characteristics | PHRT (n = 15) | PHET (n = 13) | PHTC (n = 15) | NMTC (n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Age, y | 21.1±0.6 | — | 20.1±0.9 | — | 21.6±0.8 | — | 21.6±0.7 | — |
| Height, cm | 174.8±2.4 | — | 177.0±2.0 | — | 180.1±2.7 | — | 173.3±2.4 | — |
| Weight, kg | 84.2±4.7 | 85.0±4.9 | 86.7±3.6 | 84.1±2.3 | 87.8±4.3 | 88.5±4.6 | 80.9±3.4 | 81.7±3.4 |
| BMI, kg/m2 | 27.4±1.3 | 27.5±1.4 | 28.7±1.4 | 28.3±1.6 | 27.0±1.1 | 27.2±1.2 | 24.5±0.9 | 25.6±0.8 |
| HR, bpm | 63±3.4 | 61±2.6 | 64±4.4 | 56±3.6 | 60±2.8 | 58±2.9 | 57±2.1 | 58±1.9 |
| PSBP, mm Hg | 130±3* | 121±2*,** | 132±3* | 120±2*,** | 130±3* | 130±4* | 111±4 | 112±4 |
| ASBP, mm Hg | 114±2* | 104±2*,** | 114±2* | 103±2*,** | 114±1* | 111±2* | 94±2 | 94±2 |
| PDBP, mm Hg | 80±2* | 72±2*,** | 81±1* | 74±2*,** | 81±2* | 81±2* | 67±2 | 68±2 |
| ADBP, mm Hg | 80±2* | 73±2** | 81±2* | 74±2** | 81±1* | 78±2* | 68±2 | 68±2 |
Values are mean ± SEM. There were no significant differences between prehypertensive groups at baseline (P > 0.05). Significance values are reported from between group and between time point–repeated measures analysis of variance and Tukey post hoc analysis.
Abbreviations: ADBP, aortic diastolic blood pressure; ASBP, aortic systolic blood pressure; BMI, body mass index; HR, heart rate; NMTC, normotensive time control; PDBP, peripheral diastolic blood pressure; PHET, prehypertensive subjects randomized to endurance training; PHRT, prehypertensive subjects randomized to resistance training; PHTC, prehypertensive subjects randomized to time control; PSBP, peripheral systolic blood pressure.
*P < 0.05 vs. normotensive control values at same time point; **P < 0.05 vs. pretreatment values.
Table 2.
Pulse wave analysis before and after exercise training and time control
| Hemodynamics | PHRT(n = 15) | PHET(n = 13) | PHTC(n = 15) | NMTC(n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| PMAP, mm Hg | 94±2* | 85±2** | 94±2* | 86±2** | 95±2* | 94±2* | 80±2 | 81±2 |
| PPP, mm Hg | 54±3* | 49±3*,** | 51±3* | 46±4*,** | 51±3* | 50±3* | 43±3 | 44±3 |
| AMAP, mm Hg | 95±2* | 86±2** | 97±2* | 88±2** | 96±1* | 93±2* | 79±2 | 80±2 |
| APP, mm Hg | 34±1* | 31±1* | 32±1* | 29±1** | 33±1* | 32±1* | 26±1 | 26±1 |
| AgBP, mm Hg | 4±1* | 1±1** | 4±1* | 1±1** | 4±1* | 4±1* | 0±1 | 0±1 |
| ∆tp, msec | 156.6±8.1* | 173.0±8.7*,** | 159.5±8.2* | 172.6±9.0*,** | 157.9±7.5* | 160.1±8.2* | 196.8±8.2 | 194.4±9.0 |
| ∆tr, msec | 176.1±7.5* | 163.9±8.6* | 172.4±7.7* | 165.7±8.9* | 173.6±7.0* | 172.3±8.0* | 144.2±7.8 | 145.2±8.9 |
| ED, msec | 332±5.3 | 339±4.7 | 331±5.4 | 337±4.9 | 333±4.9 | 329±4.4 | 341±5.4 | 340±4.9 |
| ASTTI | 2,135±89* | 1,889±77*,** | 2,286±99* | 1,928±86*,** | 2,186±83* | 2,100±73* | 1,689±92 | 1,692±80 |
| DPTI | 3,586±75* | 3,340±67* | 3,516±84* | 3,323±75* | 3,487±71* | 3,452±63* | 3,071±78 | 3,092±69 |
| SEVR, % | 165.9±8.7* | 180.1±8.8** | 159.8±9.7* | 175.4±9.8** | 162.1±8.2* | 164.2±8.2* | 184.6±9.1 | 186.4±9.1 |
Values are mean ± SEM. Significance values are reported from between-group and between–time point repeated measures analysis of variance and Tukey post hoc analysis.
Abbreviations: AgBP, augmented blood pressure; AMAP, aortic mean arterial pressure; APP, aortic pulse pressure; ASTTI, aortic systolic tension time index; DPTI, diastolic pressure tension index; ED, ejection duration; HR heart rate; NMTC, normotensive time control; PHET, prehypertensive subject randomized to endurance training; PHRT prehypertensive subject randomized to resistance training; PHTC, prehypertensive subject randomized to time control; PMAP, peripheral mean arterial pressure; PPP, peripheral pulse pressure; SEVR, subendocardial viability ratio; ∆tp, round trip travel time of reflected pressure wave from ascending aorta to peripheral reflecting sites and back; ∆tr, systolic duration of reflected wave.
*P < 0.05 vs. normotensive control values at same timepoint; **P < 0.05 vs. pretreatment values.
Blood pressure
There were no significant peripheral or central BP changes in the time-control groups. The PHRT and PHET groups exhibited reduced resting peripheral and central SBPs and DBPs from baseline after exercise training but peripheral SBP, central aortic SBP, peripheral DBP, and central aortic DBP remained elevated above those of the NMTC group (Table 1). Additionally, the PHRT and PHET groups exhibited reductions in peripheral and central MAP after exercise training to pressure levels not different from the NMTC group (Table 2). Peripheral pulse pressure was significantly reduced in the PHRT and PHET groups from baseline but remained elevated above that of the NMTC group (Table 2). Central pulse pressure was reduced in the PHET group only. However, augmented BP was reduced in both PHRT and PHET groups to similar levels as the NMTC group.
Pulse wave analysis
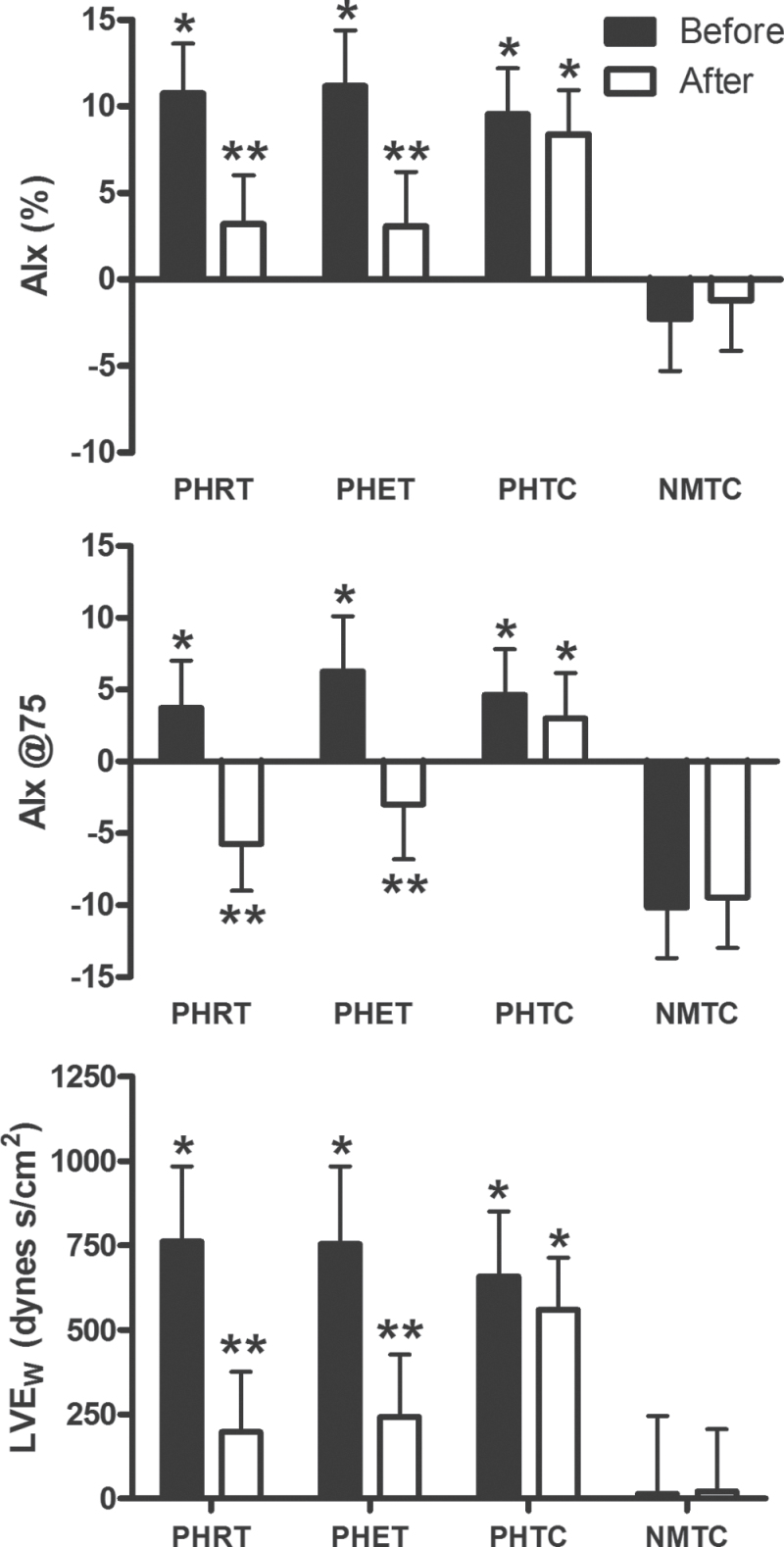
Results for AIx, AIx@75, and LVEW are presented in Figure 2. Pulse wave analysis demonstrated an elevated AIx in the PHRT, PHET, and PHTC groups (10.73% ± 2.90%, 11.17% ± 3.24%, and 9.56% ± 2.65%, respectively) when compared with the NMTC group (−2.29% ± 3.00%) (P < 0.01) at baseline. AIx@75 was higher in the PHRT, PHET, and PHTC groups compared with the normotensive group (3.73% ± 3.29%, 6.27% ± 3.84%, 4.63% ± 3.19%, and −10.15% ± 3.54%, respectively; P < 0.05). Likewise, LVEW was elevated in the prehypertensive groups above the normotensive group at baseline (689±168 dynes s/cm2, 727±174 dynes s/cm2, 648±153 dynes s/cm2, and 8±174 dynes s/cm2, respectively; P < 0.01). AIx, AIx@75, and LVEW were reduced with exercise training in both the PHRT (to 3.20% ± 2.81%, −5.73% ± 3.27%, and 126±154 dynes s/cm2, respectively; P < 0.05) and PHET (to 3.08% ± 3.14%, −3.00% ± 3.82%, and 175±160 dynes s/cm2, respectively; P < 0.05) groups after exercise training to similar levels as the normotensive group (−1.21% ± 2.91%, −9.46% ± 3.51%, and −25±160 dynes s/cm2, respectively; P > 0.05) (Figure 2). ∆tp was increased in the PHRT and PHET groups (Table 2). Aortic systolic tension time index was reduced in the PHRT and PHET groups, resulting in an increase of the subendocardial viability ratio (Table 2). The diastolic pressure tension index was elevated in all prehypertensive groups at baseline, however, no significant changes occurred in any group (Table 2). There were no changes in the results of pulse wave analysis observed in either the PHTC or NMTC groups.
Figure 2.
Absolute values for augmentation index (AIx), AIx normalized to a heart rate of 75 beats per minute (AIx@75), and wasted left ventricular pressure energy (LVEw) are presented. P values are from within-group repeated measures analysis of variance and Tukey post hoc analysis of between-group and between–time point differences in absolute values. * indicates statically significant differences (P < 0.05) between post-treatment and pretreatment values within each group; ** indicates statistically significant (P < 0.05) differences between prehypertensive subjects randomized to resistance training (PHRT), prehypertensive subjects randomized to endurance training (PHET), and prehypertensive subjects randomized to nonexercising time control (PHTC) groups vs. normotensive time-matched control subjects (NMTC) group at the same experimental time point. Data are expressed as mean ± SEM.
Arterial stiffness
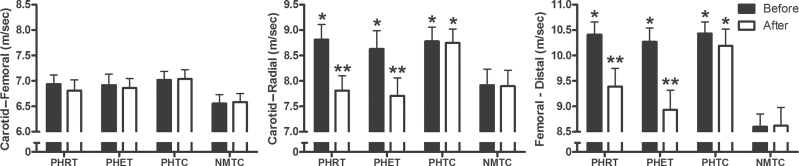
The results of central and peripheral PWV are presented in Figure 3. Results of PWV analysis demonstrated increased transit times in both the carotid–radial and femoral–dorsalis pedis measurements for the PHRT, PHET, and PHTC groups (8.81±0.30 m/sec, 8.63±0.36 m/sec, 8.78±0.28 m/sec, respectively; 10.41±0.25 m/sec, 10.27±0.27 m/sec, 10.43±0.23 m/sec, respectively; P < 0.05) compared with the normotensive group (7.92±0.31 m/sec and 8.60±0.25 m/sec, respectively; P < 0.05). There was no main effect difference between any prehypertensive group and the NMTC group in carotid–femoral artery PWV (6.94±0.18 m/sec, 6.92±0.20 m/sec, and 7.02±0.17 m/sec, respectively, vs. 6.55±0.18 m/sec; P = 0.56). Further, there were no changes observed in carotid–femoral PWV after exercise training (6.94±0.18 m/sec to 6.81±0.18 m/sec; and 6.86±0.20 m/sec to 6.92±0.20 m/sec, respectively; P > 0.05). Both carotid–radial and femoral–dorsalis pedis PWV (peripheral indices of arterial stiffness) were decreased after training in the PHRT (7.81±0.30 m/sec and 9.39±0.36 m/sec, respectively; P < 0.05) and PHET (7.71±0.35 m/sec and 8.93±0.39 m/sec, respectively; P < 0.05) groups. No significant changes were observed in carotid–femoral, carotid–radial, and femoral–dorsalis pedis PWV after time control.
Figure 3.
Absolute values for carotid–radial, carotid–femoral, and femoral–distal pulse wave velocity are presented. P values are from within-group repeated measures analysis of variance and Tukey post hoc analysis of between-group and between-time point differences in absolute values. * indicates statically significant differences (P < 0.05) between post-treatment and pretreatment values within each group; ** indicates statistically significant (P < 0.05) differences between prehypertensive subjects randomized to resistance training (PHRT), prehypertensive subjects randomized to endurance training (PHET), and prehypertensive subjects randomized to nonexercising time control (PHTC) groups vs. normotensive time-matched control subjects (NMTC) group at the same experimental time point. Data are expressed as mean ± SEM.
DISCUSSION
To the best of our knowledge, this is the first randomized and normotensive-controlled study examining the independent effects of 8 weeks of resistance and endurance exercise training on arterial stiffness and central BPs in young prehypertensive subjects. The primary findings of this study are that 8 weeks of resistance or endurance exercise training are effective in reducing peripheral arterial stiffness, myocardial oxygen demand, and both peripheral and central BPs in young prehypertensive subjects.
BP reduction is the primary goal of prehypertension therapy.11 Our study demonstrates that sedentary young prehypertensive subjects exhibit increased peripheral and central aortic BPs when compared with age-matched normotensive persons. Further, 8 weeks of resistance or endurance training leads to significant reductions in resting peripheral and central SBP and DBP and evidence suggests that a 5–mm Hg reduction in SBP results in a 14%, 9%, and 7% decrease risk in stroke, coronary heart disease, and all-cause mortality, respectively.23 Therefore, the reductions in peripheral and central BP observed in our study may be clinically significant.
Results from our study also demonstrate that sedentary young prehypertensive subjects exhibit increased peripheral arterial stiffness when compared with age-matched normotensive persons; however, central aortic stiffness was not evident. Peripheral arterial stiffness, as measured by PWV, was higher in our prehypertensive groups for carotid–radial and femoral–dorsalis pedis arteries by approximately 10% and approximately 21%, respectively (Figure 3). These results are in agreement with a previous report from a large cohort study of American youth that prehypertensive subjects exhibit increased radial and foot PWV but central aortic PWV is within normotensive ranges.24 These findings are also similar to what has been reported in young prehypertensive subjects and some studies of older prehypertensive subjects.5,24 In this study, we observed no significant differences in carotid–femoral artery (central) PWV in young prehypertensive subjects when compared with matched normotensive control subjects. Based on our observations in young prehypertensive subjects and those of Zhu and colleagues,24 any reported increases in central PWV in young prehypertensive subjects using the ankle–brachial method appear to be the passive result of increased peripheral artery PWVs. Further, the increases in central PWV, left ventricular mass, and carotid artery stiffness observed in prehypertensive subjects aged >35 years may be a consequence of the undetected and/or unaltered elevated central pressures persisting over a decade or more.25,26
The comparison of the effects of resistance and endurance training on PWV in prehypertensive subjects has been understudied. The results of this study demonstrate that 8 weeks of resistance or endurance exercise training reduces peripheral arterial stiffness and central BPs. Resistance training reduced carotid–radial and femoral–dorsalis pedis artery PWV by 11%, and 10%, respectively (Figure 3). Similarly, endurance training reduced carotid–radial and femoral–dorsalis pedis artery PWV by 10% and 13%, respectively (Figure 3). These improvements in peripheral PWV resulted in values that were not significantly different from matched normotensive subjects.
Our results confirm previous reports indicating that resistance training is effective in reducing central BPs without altering central arterial stiffness in young men.27,28 Further, Heffernan and colleagues also observed in their cohort of young black and white men reduced peripheral microvascular resistance and increased resistance artery vasodilatory capacity after resistance training.27 Conversely, in a comparison of the effects of short-term (4 weeks) resistance and endurance exercise training in older (aged approximately 48 years) male and female pre- and stage-1 hypertensive subjects, Collier et al. found that endurance training decreased carotid–femoral and femoral–dorsalis pedis PWV, whereas resistance training resulted in significant PWV increases in both measures of arterial stiffness.29 The authors concluded that 4 weeks of resistance training increases arterial stiffness in older pre- and stage-1 hypertensive subjects,29 whereas we report in this study that 8 weeks of resistance and endurance training in young male and female prehypertensive subjects only results in reductions in peripheral PWV without change in central artery PWV, which is similar to other reports in younger participants training for longer than 4 weeks. Increased central arterial stiffness is a hallmark of alterations in the common carotid intima-media layer and is associated with aging, where the elastin is infiltrated with collagen and fibrin decreasing the elastic properties of the vessel.3,4 In a randomized interventional study, Miyachi and colleagues reported reduced central artery compliance after high-intensity resistance training, although there were no changes in carotid artery intimamedia thickness or carotid lumen diameter.30 Together, it is unlikely that structural changes in the arterial wall occurred over a 4-week period, and the increase in central and peripheral PWV may have been due to age-related alterations and/or an acute but transient peripheral vascular alteration to resistance training. For instance, when comparing chronic resistance trained subjects with age-, sex-, and BP-matched nonresistance-trained sedentary subjects with similar resting brachial artery flow-mediated dilation (a marker of vascular endothelial function), an acute bout of resistance exercise impairs endothelial function in the unconditioned subjects, whereas, endothelial function in the chronic resistance-trained subjects remains unchanged.31 Additionally, central arterial stiffness is influenced by endothelial function,32 brachial artery flow-mediated dilation is inversely related to central arterial stiffness,33 and reduced conduit artery endothelial function is associated with increased peripheral artery pulse wave velocities.34 Indeed, we recently demonstrated in the same cohort of subjects as in this study that both resistance and endurance training improves brachial artery flow-mediated dilation, increases nitric oxide bioavailability, and reduces circulating endothelin.35 In totality, these data suggest that chronic resistance training may not only protect against transient vascular dysfunction and the adverse effect of a resistance load in humans with elevated BP31 but also explain the transient arterial stiffness reported by a few investigators when measured in previously sedentary subjects shortly after beginning a resistance training program. Therefore, based on our previously reported findings35 and the results of this study, we suggest that reductions in peripheral PWV and central BPs in prehypertensive subjects after exercise training may be primarily due to improvements in endothelial function and vasoactive substances.
Hypertension, as determined from standard brachial artery sphygmomanometry, is a well-established cardiovascular risk factor. However, peripheral BP measurements obtained by standard brachial artery techniques are not always a reliable measure of ascending aorta pressure.16 In fact, data from the Strong Heart Study have shown that noninvasively obtained central arterial pressure is more strongly related to cardiovascular outcomes and central pressure is a stronger stimulus to left ventricular hypertrophy than brachial pressure.36 Our study confirms that young sedentary prehypertensive subjects exhibit increased aortic pulse pressure, MAP, and augmented pressure when compared with matched normotensive subjects. Importantly, our study also demonstrates that 8 weeks of resistance or endurance exercise training is effective in reducing resting central pulse pressure, MAP, and augmented pressure. Further, the improvements in aortic pressure wave characteristics observed in the PHRT and PHET groups resulted in decreases in LVEW and estimated myocardial oxygen demand (aortic systolic tension time index).
LVEW is an index of extra myocardial oxygen requirement that is due to early systolic wave reflection and depends on the amplitude of central aortic pressure augmentation and systolic duration of the pressure wave.21 Increased LVEW resulting from enhanced wave reflection contributes to the development of left ventricular hypertrophy.21 Prehypertensive subjects not only exhibit increased left ventricular mass and wall abnormalities but also display a significantly greater age-related increase in left ventricular wall thickness and increased incidence of left ventricular remodeling compared with normotensive individuals.37–39 The timing and amplitude of the reflected wave to ascending aortic pulse pressure can be estimated by aortic AIx. Results from our study demonstrate that young prehypertensive subjects exhibit elevated AIx, AIx@75, and LVEW when compared with normotensive subjects. These data in young prehypertensive subjects are in agreement with findings in older prehypertensive subjectss.5 Gedliki and colleagues observed significant increases in AIx in slightly older (aged 30±6 years) prehypertensive subjects when compared with age-matched normotensive control subjects.5 In our study, increases in baseline resting aortic pressure wave characteristics resulted in increased LVEW in prehypertensive subjects (Figure 2). We speculate that chronic exposure to the increased LVEW observed in young prehypertensive subjects may contribute to the increased left ventricular mass reported in older prehypertensive subjects. The results of our study demonstrate that 8 weeks of both resistance and endurance exercise reduce AIx, AIx@75, and LVEW in prehypertensive subjects and the reductions in LVEW after exercise are primarily due to reductions in augmented pressure (Table 2).
In conclusion, this study demonstrates that short-term (8weeks) resistance or endurance training are independently efficacious in reducing the elevated peripheral arterial stiffness, central BP, AIx, and myocardial oxygen demand exhibited in young prehypertensive subjects. Based on the findings recently reported on the same cohort of prehypertensive subjects and the observations from this study and others, we reason that the reductions in peripheral arterial stiffness in young prehypertensive subjects after resistance or endurance training may be explained by the improvements in endothelial function and vasoactive agents, whereas the reductions in AIx, central pressures, and left ventricular pressure energy are secondary to decreases in peripheral arterial stiffness.
It is essential to investigate the effects of resistance and endurance training on sympathetic nervous system (SNS) and parasympathetic nervous system (PNS) modulation in young prehypertensive subjects. Following a study of older pre- and stage-1 hypertensive subjects, Collier and colleagues suggested that aerobic and resistance training similarly reduce resting BP but through differing mechanisms.40 Indeed, after just 4 weeks, aerobic training improved the autonomic nervous system by increasing vagal tone and reducing sympathovagal balance, whereas resistance training decreased sympathetic modulation of peripheral vessels while decreasing parasympathetic modulation of the heart.40 Indices of sympathetic and parasympathetic modulation were not measured in our study and, consequently, the effects of SNS and PNS modulation in response to exercise cannot be ruled out as a contributing potential mechanism.
Additionally, it is important to investigate any possible sex or racial differences in response to exercise in young prehypertensive subjects. This study was not statistically powered to detect sex or racial differences, and, therefore, no demonstrable main effect of sex or race on hemodynamics after exercise intervention is reported.
Finally, it is critical to investigate the exercise response dependent upon systolic vs. diastolic prehypertension. Unfortunately, roughly 70% of the prehypertensive participants in this study exhibited combined systolic and diastolic prehypertension, 100% exhibited systolic prehypertension, and this study lacked the statistical power to determine differences based on the presence of diastolic prehypertension as a covariable post hoc.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported, in part, by a National Institutes of Health predoctoral training grant (NIH 5-T32-HL083810-04) awarded to D.T.B. by the University of Florida Hypertension Center.
REFERENCES
- 1. Ferreira I, van de Laar RJ, Prins MH, Twisk JW, Stehouwer CD. Carotid stiffness in young adults: a life-course analysis of its early determinants: the Amsterdam Growth and Health Longitudinal Study. Hypertension 2012; 59:54–61 [DOI] [PubMed] [Google Scholar]
- 2. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37:1236–1241 [DOI] [PubMed] [Google Scholar]
- 3. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation 2003; 107:490–497 [DOI] [PubMed] [Google Scholar]
- 4. Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol 2003; 28:204–212 [DOI] [PubMed] [Google Scholar]
- 5. Gedikli O, Kiris A, Ozturk S, Baltaci D, Karaman K, Durmus I, Baykan M, Celik S. Effects of prehypertension on arterial stiffness and wave reflections. Clin Exp Hypertens 2010; 32:84–89 [DOI] [PubMed] [Google Scholar]
- 6. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012; 308:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gedikli O, Kiris A, Yilmaz H, Ozturk S, Baykan M, Durmus I, Karaman K, Karahan C, Celik S. The relationship between endothelial damage and aortic augmentation index. Clin Exp Hypertens 2010; 32:29–34 [DOI] [PubMed] [Google Scholar]
- 8. Greenlund KJ, Croft JB, Mensah GA. Prevalence of heart disease and stroke risk factors in persons with prehypertension in the United States, 1999–2000. Arch Intern Med 2004; 164:2113–2118 [DOI] [PubMed] [Google Scholar]
- 9. Chobanian AV. Prehypertension revisited. Hypertension 2006; 48:812–814 [DOI] [PubMed] [Google Scholar]
- 10. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’, Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:480–486 [DOI] [PubMed] [Google Scholar]
- 11. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572 [DOI] [PubMed] [Google Scholar]
- 12. Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 2004; 36:533–553 [DOI] [PubMed] [Google Scholar]
- 13. Gallagher D, Adji A, O’, Rourke MF. Validation of the transfer function technique for generating central from peripheral upper limb pressure waveform. Am J Hypertens 2004; 17:1059–1067 [DOI] [PubMed] [Google Scholar]
- 14. Martin JS, Casey DP, Gurovich AN, Beck DT, Braith RW. Association of age with timing and amplitude of reflected pressure waves during exercise in men. Am J Hypertens 2011; 24:415–420 [DOI] [PubMed] [Google Scholar]
- 15. Pauca AL, O’, Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38:932–937 [DOI] [PubMed] [Google Scholar]
- 16. Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 2002; 17:543–551 [DOI] [PubMed] [Google Scholar]
- 17. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000; 525:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Manipulation of ascending aortic pressure and flow wave reflections with the Valsalva maneuver: relationship to input impedance. Circulation 1981; 63:122–132 [DOI] [PubMed] [Google Scholar]
- 19. Martin JS, Beck DT, Gurovich AN, Braith RW. The acute effects of smokeless tobacco on central aortic blood pressure and wave reflection characteristics. Exp Biol Med (Maywood) 2010; 235:1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Casey DP, Beck DT, Braith RW. Progressive resistance training without volume increases does not alter arterial stiffness and aortic wave reflection. Exp Biol Med (Maywood) 2007; 232:1228–1235 [DOI] [PubMed] [Google Scholar]
- 21. Hashimoto J, Nichols WW, O’, Rourke MF, Imai Y. Association between wasted pressure effort and left ventricular hypertrophy in hypertension: influence of arterial wave reflection. Am J Hypertens 2008; 21:329–333 [DOI] [PubMed] [Google Scholar]
- 22. Casey DP, Beck DT, Nichols WW, Conti CR, Choi CY, Khuddus MA, Braith RW. Effects of enhanced external counterpulsation on arterial stiffness and myocardial oxygen demand in patients with chronic angina pectoris. Am J Cardiol 2011; 107:1466–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens 2010; 28:384–388 [DOI] [PubMed] [Google Scholar]
- 24. Zhu H, Yan W, Ge D, Treiber FA, Harshfield GA, Kapuku G, Snieder H, Dong Y. Cardiovascular characteristics in American youth with prehypertension. Am J Hypertens 2007; 20:1051–1057 [DOI] [PubMed] [Google Scholar]
- 25. Tomiyama H, Hashimoto H, Matsumoto C, Odaira M, Yoshida M, Shiina K, Nagata M, Yamashina A, Doba N, Hinohara S. Effects of aging and persistent prehypertension on arterial stiffening. Atherosclerosis 2011; 217:130–134 [DOI] [PubMed] [Google Scholar]
- 26. Kim SH, Cho GY, Baik I, Lim SY, Choi CU, Lim HE, Kim EJ, Park CG, Park J, Kim J, Shin C. Early abnormalities of cardiovascular structure and function in middle-aged Korean adults with prehypertension: the Korean Genome Epidemiology Study. Am J Hypertens 2011; 24:218–224 [DOI] [PubMed] [Google Scholar]
- 27. Heffernan KS, Fahs CA, Iwamoto GA, Jae SY, Wilund KR, Woods JA, Fernhall B. Resistance exercise training reduces central blood pressure and improves microvascular function in African American and white men. Atherosclerosis 2009; 207:220–226 [DOI] [PubMed] [Google Scholar]
- 28. Rakobowchuk M, McGowan CL, de Groot PC, Bruinsma D, Hartman JW, Phillips SM, MacDonald MJ. Effect of whole body resistance training on arterial compliance in young men. Exp Physiol 2005; 90:645–651 [DOI] [PubMed] [Google Scholar]
- 29. Collier SR, Kanaley JA, Carhart R, Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens 2008; 22:678–686 [DOI] [PubMed] [Google Scholar]
- 30. Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance: a randomized intervention study. Circulation 2004; 110:2858–2863 [DOI] [PubMed] [Google Scholar]
- 31. Jurva JW, Phillips SA, Syed AQ, Syed AY, Pitt S, Weaver A, Gutterman DD. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. J Am Coll Cardiol 2006; 48:588–589 [DOI] [PubMed] [Google Scholar]
- 32. Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension 2004; 44:112–116 [DOI] [PubMed] [Google Scholar]
- 33. Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol 2003; 92:395–399 [DOI] [PubMed] [Google Scholar]
- 34. McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006; 48:602–608 [DOI] [PubMed] [Google Scholar]
- 35. Beck DT, Casey DP, Martin JS, Emerson BD, Braith RW. Exercise training improves endothelial function in young prehypertensives. Exp Biol Med (Maywood) 2013; 238:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203 [DOI] [PubMed] [Google Scholar]
- 37. Di Bello V, Talini E, Dell’, Omo G, Giannini C, Delle Donne MG, Canale ML, Nardi C, Palagi C, Dini FL, Penno G, Del Prato S, Marzilli M, Pedrinelli R. Early left ventricular mechanics abnormalities in prehypertension: a two-dimensional strain echocardiography study. Am J Hypertens 2010; 23:405–412 [DOI] [PubMed] [Google Scholar]
- 38. Markus MR, Stritzke J, Lieb W, Mayer B, Luchner A, Doring A, Keil U, Hense HW, Schunkert H. Implications of persistent prehypertension for ageing-related changes in left ventricular geometry and function: the MONICA/KORA Augsburg study. J Hypertens 2008; 26:2040–2049 [DOI] [PubMed] [Google Scholar]
- 39. Drukteinis JS, Roman MJ, Fabsitz RR, Lee ET, Best LG, Russell M, Devereux RB. Cardiac and systemic hemodynamic characteristics of hypertension and prehypertension in adolescents and young adults: the Strong Heart Study. Circulation 2007; 115:221–227 [DOI] [PubMed] [Google Scholar]
- 40. Collier SR, Kanaley JA, Carhart R, Frechette V, Tobin MM, Bennett N, Luckenbaugh AN, Fernhall B. Cardiac autonomic function and baroreflex changes following 4 weeks of resistance versus aerobic training in individuals with pre-hypertension. Acta Physiologica 2009; 195:339–348 [DOI] [PubMed] [Google Scholar]