Abstract
Data from a prospective study of 3,319 children ages 2 months to 5 years admitted with febrile illness to a Tanzanian district hospital were analyzed to determine the relationship of blood glucose and mortality. Hypoglycemia (blood sugar < 2.5 mmol/L and < 45 mg/dL) was found in 105 of 3,319 (3.2%) children at admission, and low-normal blood glucose (2.5–5 mmol/L and 45–90 mg/dL) was found in 773 of 3,319 (23.3%) children. Mortality was inversely related to admission blood sugar; compared with children with an admission blood glucose of > 5 mmol/L, the adjusted odds of dying were 3.3 (95% confidence interval = 2.1–5.2) and 9.8 (95% confidence interval = 5.1–19.0) among children with admission blood glucose 2.5–5 and < 2.5 mmol/L, respectively. Receiver operating characteristic (ROC) analysis suggested an optimal cutoff for admission blood sugar of < 5 mmol/L in predicting mortality (sensitivity = 57.7%, specificity = 75.2%). A cutoff for admission blood glucose of < 5 mmol/L represents a simple and clinically useful predictor of mortality in children admitted with severe febrile illness to hospital in resource-poor settings.
Introduction
The association between hypoglycemia (blood glucose < 2.5 mmol/L and < 45 mg/dL) and severe infection, particularly malaria, is widely recognized,1–4 and it has been estimated that between 1.8% and 7.3% of children admitted to the hospital with febrile illness in sub-Saharan Africa are hypoglycemic.5,6 True hypoglycemia is a well-known cause of altered consciousness that may require emergency treatment with glucose because of the exclusive dependence of the brain on glucose metabolism.7 However, independent of its effect on brain function, low blood glucose is also associated with poor clinical outcome in severe infection, and the level of blood sugar may serve as an accessible and objective indicator of severity that could be used to prioritize care in resource-limited settings.
Although the blood glucose cutoff of < 2.5 mmol/L for administering glucose as an emergency measure to avert brain damage is relatively well-established, two recent studies have questioned whether the same cutoff is optimal for predicting poor outcome. A study of 418 children with severe malaria in Mali showed that children with low glycemia (defined as 2.2–4.4 mmol/L and 39.6–79.2 mg/dL) had increased mortality.8 These data are supported by a larger retrospective review of the patient records of children admitted to a hospital in Kenya.9
To complement these studies, we have analyzed data from a large prospective observational study of children admitted to a hospital with febrile disease in an area of high Plasmodium falciparum transmission to determine the mortality and other clinical features associated with a range of blood glucose cutoffs and establish the optimal level of blood sugar to predict mortality in children with and without falciparum malaria.
Methods
The study took place in a district hospital in northeast Tanzania serving a largely rural population in an area highly endemic for P. falciparum. These data were collected as part of a study of the etiology of febrile disease in pediatric admissions. Full details of the study have been published elsewhere.10
Children admitted during daytime hours over a 1-year period with fever or a history of fever ages 2 months to 13 years were screened for eligibility and enrolled in the study after consent procedures. Children under 5 years of age were included in this analysis, because these children represent the group most at risk of malaria and infectious diseases as well as hypoglycemia. This age group includes the majority of admissions. Children with chronic disease other than human immunodeficiency virus (HIV) or malnutrition and children admitted for surgery were excluded. Children were assessed using a standardized interview and examination including pulse oximetry. Height and weight were recorded, and venous blood was taken for aerobic culture (BactAlert, Biomerieux), bedside tests for P. falciparum histidine-rich protein 2 (HRP2) (Paracheck; Orchid Biomedical, Mumbai, Maharashtra, India), hemoglobin concentration, glucose (Hemocue; Anglholm, Skane, Sweden), and lactate (Lactate-Pro; Arkray Inc, Kyoto, Kyoto, Japan). Blood glucose was measured photometrically using the Hemocue 201+ system,11 with opened microcuvette containers stored at 2–8°C and container contents disposed of within 1 month of opening. Giemsa-stained blood slides were double read independently, and discordant slides were resolved with a third reader. HIV status was tested in all children by serology (Capillus HIV-1, HIV-2 Test; Trinity Biotech, Bray, co Wicklow, Ireland; Determine HIV-1/2 Test; Abbott Laboratories, IL), with discordant results resolved by HIV-1 enzyme-linked immunosorbent assay (ELISA; Vironistika UniForm II Plus-O Test; BioMérieux, NC).12 Children under 18 months of age with positive serology results were tested for HIV-1 RNA (Abbott Real-Time m2000 System; Abbott Molecular, IL).
Cerebrospinal fluid (CSF) was obtained by lumbar puncture according to hospital protocols (any one of history of multiple or partial seizures, history of seizures in children ages under 6 months or over 6 years, confusion or reduction in conscious level, bulging fontanelle, or neck stiffness). CSF and positive blood cultures were cultured on commercial agar and identified using standard methods. Children were considered to have invasive bacterial disease (IBD) if blood or CSF cultures were positive for pathogenic organisms. Children with hypoglycemia were treated with glucose according to World Health Organization (WHO) guidelines.3 After the initial assessment, children received routine hospital care administered by the hospital staff; no record was made of any repeat blood glucose measurements. All results were communicated with hospital staff when available.
Hypoglycemia was defined as blood glucose < 2.5 mmol/L, raised blood lactate was defined as > 5 mmol/L, severe anemia was defined as hemoglobin < 5 g/dL, and hypoxia was defined as oxygen saturation of < 90% on room air. Severe acute malnutrition (SAM) was defined as any one of following variables: visible severe wasting, bilateral pedal edema, weight for height Z score of less than −3, or mid-upper arm circumference of less than 11.5 cm. Shock was defined as any two of the following factors: severe tachycardia for age, temperature gradient, or capillary refill of greater than 3 seconds. Altered consciousness was defined as a Blantyre coma score < 5 on admission. For the purposes of this analysis, children were classified as having severe illness if they presented with SAM, severe respiratory distress (lower chest indrawing), deep breathing, shock, altered consciousness, prostration, or reported more than two convulsions in the previous 24 hours.
Admission outcomes (death or discharge) were recorded. Data were scanned using Teleforms (Verity software) and analyzed using Stata (Stata Version 11). Proportions were tested by χ2 tests. Logistic regression was used to estimate odd ratios (ORs) and 95% confidence intervals (95% CIs) for the association between hypoglycemia and other variables, with adjusted odds ratios (AORs) adjusting for confounders in the final multivariate logistic regression model. Wilcoxon rank sum was used to compare the median time to death in children with different levels of blood glucose. Receiver operating characteristics (ROCs) from the logistic regression were used to plot the sensitivity and specificity for mortality, blood glucose measures alone, and these factors as part of a model of factors with significant association with mortality.
The study was approved by the Ethics Committees of the London School of Hygiene and Tropical Medicine, United Kingdom (LSHTM Ethics 2087) and the National Institute for Medical Research, Tanzania (NIMR/HQ/R.8a/Vol.IX/392). Written informed consent to participate was obtained from the parent or guardian of each child in the study.
Results
There were 3,327 children ages 2 months to 5 years enrolled in the study over the 1-year period; data on 3,319 children (99.8%; admission blood glucose recording was missing in 8 children) were available for analysis. There were 170 fatalities (case fatality rate [CFR] = 5.1%). P. falciparum was detected by slide microscopy in 2,032 children (61.2%), and invasive bacterial disease was shown in 316 children (9.5%). Hypoglycemia (blood glucose < 2.5 mmol/L) was present on admission in 105 children (3.2%), and another 773 children (23.3%) had a blood glucose between 2.5 and 5 mmol/L (45 and 90 mg/dL). Hypoglycemia was more common in children with a positive blood slide for asexual P. falciparum asexual parasites (slide-positive; 80/2,032, 3.9%) than slide-negative children (25/1,287, 1.9%, P = 0.001). Among children who died, 44 of 170 children (25.9%) were hypoglycemic at admission, and another 54 children (31.8%) had a blood glucose between 2.5 and 5 mmol/L.
Factors associated with low blood glucose.
Altered consciousness was found in 59 of 105 hypoglycemic children (56.2%) and 73 of 773 children (9.4%) with admission blood glucose between 2.5 and 5 mmol/L. Of 46 hypoglycemic children without altered consciousness, 10 children (22%) showed either deep breathing or jaundice. Table 1 shows factors associated with hypoglycemia in children with and without malaria. In children with malaria, hypoglycemia was associated with age over 1 year (AOR = 2.84, P = 0.01), deep breathing (AOR = 8.55, P < 0.001), raised lactate (AOR = 5.36, P < 0.001), hypoxia (AOR = 3.02, P = 0.03), invasive bacterial disease (AOR = 1.21, P = 0.054), and hemoglobin as a continuous variable (AOR = 1.16, P = 0.03 for each 1 mg/dL increase in hemoglobin). In children without malaria, hypoglycemia was associated with age (AOR = 3.03, P = 0.04), deep breathing (AOR = 3.48, P = 0.03), visible jaundice (AOR = 12.32, P = 0.008), and invasive bacterial disease (AOR = 2.46, P = 0.06) (Table 1).
Table 1.
Association of blood glucose < 2.5 mmol/L with clinical or laboratory factors by univariable and multivariable analyses according to blood slide result
| Slide positive for P. falciparum (N = 2,032) | Slide negative for P. falciparum (N = 1,287) | |||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | Adjusted OR** (95% CI) | Adjusted P | OR (95% CI) | P | Adjusted OR∥ (95% CI) | Adjusted P | |
| Age 12–60 months | 2.01 (1.06–3.83) | 0.03 | 2.84 (1.27–6.35) | 0.01 | 2.04 (0.85–4.93) | 0.1 | 3.03 (1.06–8.69) | 0.04 |
| ≥ 30 days ill | * | * | * | * | 12.09 (2.47–59.1) | < 0.001 | 6.39 (0.73–55.8) | 0.09 |
| > 7 days of fever | * | * | * | * | 2.48 (0.83–7.38) | 0.1 | 1.96 (0.46–8.33) | 0.3 |
| SAM† | * | * | * | * | 1.80 (0.53–6.12) | 0.3 | 0.52 (0.09–3.04) | 0.5 |
| Deep breathing | 14.0 (8.72–22.6) | < 0.001 | 8.55 (4.45–16.4) | < 0.001 | 8.13 (3.39–19.5) | < 0.001 | 3.48 (1.12–10.79) | 0.03 |
| Hypoxia‡ | 8.86 (4.03–19.5) | < 0.001 | 3.02 (1.12–8.17) | 0.03 | 3.00 (0.68–13.2) | 0.15 | 1.32 (0.24–7.26) | 0.7 |
| Severe anemia | 2.30 (1.43–3.70) | < 0.001 | 1.54 (0.63–3.78) | 0.3 | 2.03 (0.75–5.51) | 0.16 | 0.32 (0.08–1.28) | 0.1 |
| Shock | 8.60 (4.51–16.4) | < 0.001 | 2.04 (0.83–4.98) | 0.12 | 2.15 (0.28–16.5) | 0.46 | 0.90 (0.09–8.84) | 0.9 |
| Hemoglobin (per 1 mg/dL increase) | 0.86 (0.78–0.95) | 0.002 | 1.16 (1.01–1.33) | 0.03 | 0.83 (0.71–0.96) | 0.012 | 1.11 (0.92–1.34) | 0.3 |
| Parenteral antimalarial§ | 1.17 (0.50–2.75) | 0.7 | 0.56 (0.18–1.74) | 0.3 | 3.26 (1.18–9.00) | 0.02 | 1.40 (0.36–5.43) | 0.6 |
| Jaundice | 4.93 (1.84–13.2) | < 0.001 | 0.99 (0.27–3.548 | 0.9 | 28.5 (6.69–121.2) | < 0.01 | 12.32 (1.91–79.5) | 0.008 |
| HIV | 0.69 (0.09–5.13) | 0.72 | 0.23 (0.02–2.30) | 0.2 | 1.40 (0.32–6.03) | 0.6 | 1.33 (0.28–6.39) | 0.7 |
| Invasive bacterial disease | 2.59 (1.26–5.35) | < 0.001 | 1.21 (1.00–1.46) | 0.054 | 4.05 (1.81–9.05) | 0.001 | 2.46 (0.97–6.18) | 0.06 |
| Lactate > 5 mmol/L | 8.41 (5.13–13.8) | < 0.001 | 5.36 (2.80–10.3) | < 0.001 | 13.81 (5.89–32.4) | < 0.001 | 7.85 (2.95–20.9) | < 0.001 |
| > 50,000 pf/μL | 2.05 (1.30–3.22) | < 0.001 | 1.32 (0.76–2.29) | 0.3 | – | – | – | – |
| Recent malaria¶ | – | – | – | – | 1.71 (0.77–3.77) | 0.19 | 0.87 (0.34–2.12) | 0.7 |
No cases of hypoglycemia in children with positive slides and fever > 7 days, ill for ≥ 1 month, or SAM.
SAM indicated by any one of following symptoms: visible severe wasting, bilateral pedal edema, weight for height Z score < −3, or mid-upper arm circumference < 11.5 cm.
SaO2 < 90%.
Reported as given in this febrile episode before attendance at the hospital.
Positive for HRP by rapid test with negative slide.
Adjusted for age, deep breathing, invasive bacterial disease, lactate > 5 mmol/L, hypoxia, and hemoglobin for children with positive slide.
Adjusted for age, deep breathing, invasive bacterial disease, lactate > 5 mmol/L, and jaundice for children with negative slide.
Relationship of CFR to blood glucose level.
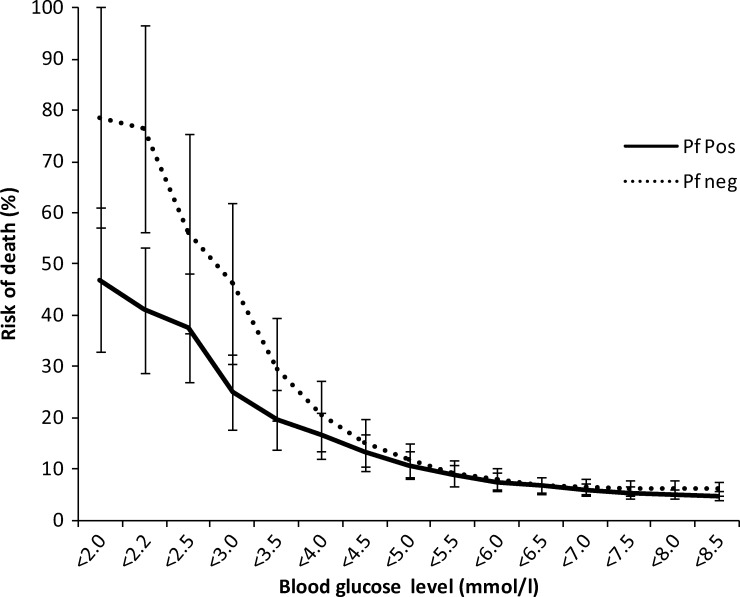
CFR in children with hypoglycemia (44/105, 41.9%) was higher than in children with blood glucose ≥ 2.5 mmol/L (126/3,214, 3.9%, OR = 17.68, P < 0.001). Children with an admission blood sugar of 2.5–5.0 mmol/L had a higher CFR (54/773, 7.0%) than those children with blood glucose ≥ 5.0 mmol/L (72/2,441, 2.9%, P < 0.001). Decreasing blood glucose was associated with a rise in CFR up to a blood glucose level of 4–5 mmol/L for both slide-positive and -negative children. Correspondingly, compared with children with admission blood glucose ≥ 5 mmol/L, children with admission blood glucose between 2.5 and 5 mmol/L had a higher odds of death, regardless of whether they were slide-positive for malaria (OR = 2.59, 95% CI = 1.53–4.38, P < 0.001) or slide-negative (OR = 2.24, 95% CI = 1.35–3.70, P = 0.002). In children with a blood glucose < 2.2 mmol/L (39.6 mg/dL), CFR was significantly higher in slide-negative children (13/17, 76.5%) than slide-positive children (25/61, 41.0%, P = 0.01) (Figure 1).
Figure 1.
Risk of death by admission blood glucose according to the presence of asexual parasites of P. falciparum in the admission blood slide. Risk of death overall was 4.4% for slide-positive and 6.2% for slide-negative children. Bars represent 95% CIs.
Factors associated with mortality are shown in Table 2. In the multivariate model, deep breathing was dropped, because it was strongly correlated with raised blood lactate levels (> 5 mmol/L); also, hemoglobin was no longer strongly associated with mortality. After adjusting for age, hypoxia, shock, altered consciousness, SAM, invasive bacterial disease, and malaria infection, a strong association remained between mortality and hypoglycemia; compared with children with an admission blood glucose of > 5 mmol/L, the adjusted odds of dying were 3.3 (95% CI = 2.1–5.2) and 9.8 (95% CI = 5.1–19.0) among children with admission blood glucose 2.5–5 and < 2.5 mmol/L, respectively. In the adjusted model, the association between blood glucose of 2.5–5 mmol/L and mortality was similar in slide-positive (AOR = 3.52, 95% CI = 1.79–6.93, P < 0.001) and negative children (AOR = 3.58, 95% CI = 1.81–7.05, P < 0.001) compared with children with admission blood glucose ≥ 5 mmol/L. Mortality in children with jaundice and hypoglycemia was six of eight children (75%).
Table 2.
Association of clinical parameters with mortality by univariable and multivariable analysis
| OR | 95% CI | P | AOR | Adjusted 95% CI | Adjusted P | |
|---|---|---|---|---|---|---|
| Age (months) | ||||||
| 2–11 | 1 | – | – | 1 | – | – |
| 12–23 | 0.70 | 0.49–1.00 | 0.05 | 0.45 | 0.28–0.76 | 0.002 |
| 24–60 | 0.44 | 0.29–0.66 | < 0.001 | 0.30 | 0.17–0.52 | < 0.001 |
| Hypoxia* | 11.03 | 6.63–18.36 | < 0.001 | 3.50 | 1.66–7.38 | 0.001 |
| Deep breathing | 12.04 | 8.53–17.00 | < 0.001 | † | † | † |
| Shock‡ | 6.23 | 3.68–10.54 | < 0.001 | 2.39 | 1.06–5.40 | 0.036 |
| Altered consciousness§ | 16.20 | 11.62–22.59 | < 0.001 | 7.36 | 4.56–11.88 | < 0.001 |
| Severe acute malnutrition¶ | 5.70 | 3.61–9.00 | < 0.001 | 5.90 | 3.16–11.03 | < 0.001 |
| Blood glucose (mmol/L) | ||||||
| > 5 | 1 | 1 | ||||
| 2.5–5 | 2.47 | 1.72–3.55 | < 0.001 | 3.29 | 2.08–5.20 | < 0.001 |
| < 2.5 | 23.73 | 15.09–37.33 | < 0.001 | 9.84 | 5.09–19.04 | < 0.001 |
| Blood lactate > 5 mmol/L | 9.75 | 6.93–13.73 | < 0.001 | 4.73 | 2.93–7.66 | < 0.001 |
| Hemoglobin < 5 g/dL | 2.32 | 1.65–3.27 | < 0.001 | 0.67 | 0.39–1.17 | 0.16 |
| Invasive bacterial disease | 4.81 | 3.39–6.83 | < 0.001 | 3.30 | 2.00–5.46 | < 0.001 |
| P. falciparum positive | 0.70 | 0.51–0.95 | 0.02 | 0.52 | 0.32–0.87 | 0.011 |
SaO2 < 90%.
Raised lactate used rather than deep breathing in the model.
Any two of severe tachycardia for age, temperature gradient, or capillary refill > 3 seconds.
Blantyre score < 5.
SAM indicated by any one of following symptoms: visible severe wasting, bilateral pedal edema, weight for height Z score < −3, or mid-upper arm circumference < 11.5 cm.
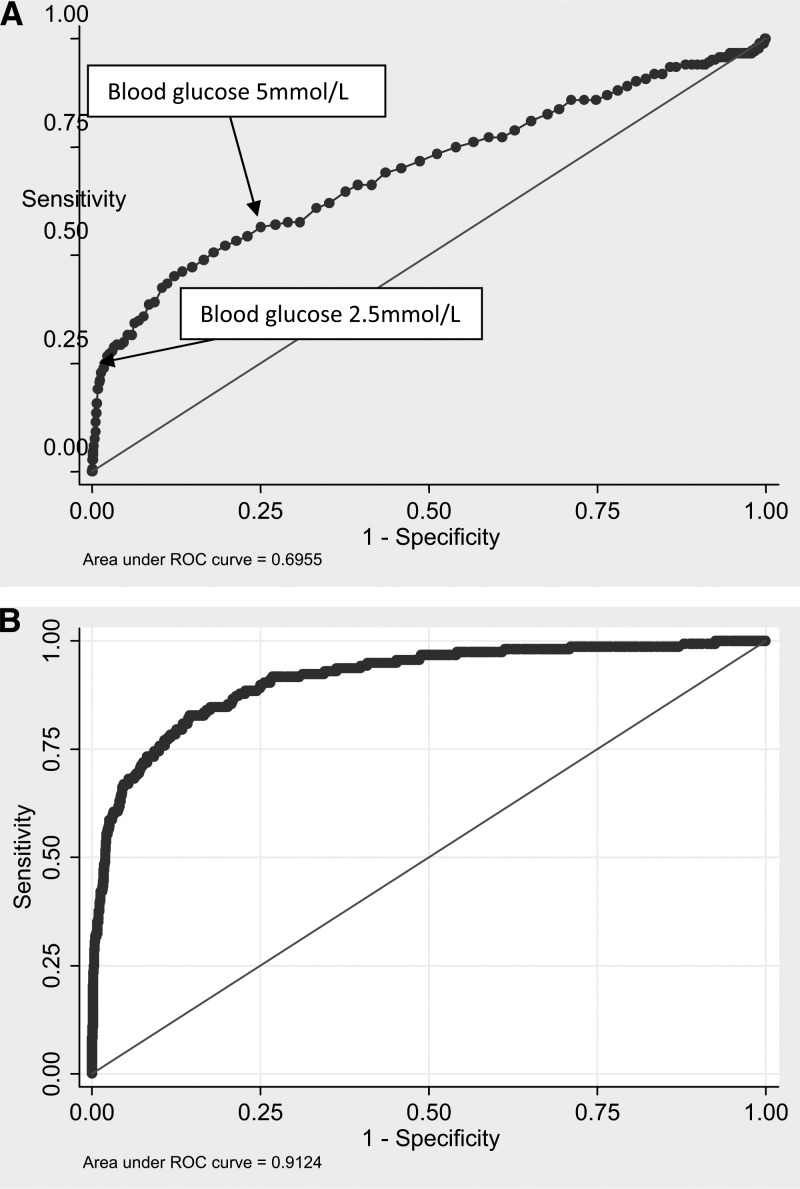
Figure 2A shows that, by itself, a blood glucose cutoff of less than 2.5 mmol/L predicted 25% of the deaths, with a specificity of 90% (Figure 2A), and that a cutoff of 5.0 mmol/L has 60% sensitivity and 75% specificity. In the multivariate model, with other signs and symptoms in addition to blood glucose, the prediction is more accurate, increasing the area under the curve from 70% to over 90% (Figure 2B). The addition of hypoglycemia or blood glucose < 5 mmol/L to clinical markers of severe illness improved the proportion of deaths that were identified from 79.4% to 81.8% and 88.8%, respectively (Table 3).
Figure 2.
ROC curves for blood glucose as a predictor of mortality in children admitted to the hospital. (A) Univariate association. (B) Blood glucose as one factor in a logistic regression model including age, blood lactate, presence of severe anemia, deep breathing, altered consciousness, severe malnutrition, hypoxia, malaria infection, presence of invasive bacterial disease, and coma.
Table 3.
Case fatality rate and proportion of deaths identified using clinical features alone or in conjunction with varying blood glucose thresholds
| Case fatality rate | Proportion of deaths identified (%) | |
|---|---|---|
| Clinical features* alone | 16.7% (135/806) | 79.4 |
| Clinical features + blood glucose < 2.5 mmol/L | 16.7% (139/833) | 81.8 |
| Clinical features + blood glucose < 5 mmol/L | 10.7% (151/1,409) | 88.8 |
Any one of SAM, severe respiratory distress, deep breathing, shock, prostration, altered consciousness, jaundice, or history of multiple convulsions.
In 157 of 170 children (92.4%) who had time of death recorded, the median time to death in children with hypoglycemia (9 hours and 51 minutes) was significantly lower than the median time to death for children with blood glucose ≥ 5 mmol/L (28 hours and 46 minutes, P = 0.004) but did not differ significantly from children with blood glucose of 2.5–5.0 mmol/L (15 hours and 17 minutes, P = 0.44).
Bacterial disease and hypoglycemia.
Blood/CSF culture was positive for pathogenic organisms in 316 children; 311 of 316 children (98.4%) had positive blood cultures alone, and 20 of 316 children (6.3%) had positive CSF results, with 15 of 316 children (4.7%) having positive findings in blood and CSF. Positive culture was significantly more common in hypoglycemic children (20/105, 19.1%) than children with a blood glucose > 2.5 mmol/L (396/3,214, 9.2%, P = 0.001). Children with blood glucose of 2.5–5.0 mmol/L had a similar rate of invasive bacterial disease (74/773, 9.6%) to children with a higher blood glucose (222/2,441, 9.1%, P = 0.688). In children with invasive bacterial disease, there was an increased mortality in children with hypoglycemia (OR = 21.0, 95% CI = 7.21–61.20, P < 0.001) and children with blood glucose 2.5–5 mmol/L (OR = 3.43, 95% CI = 1.69–6.98, P = 0.001).
Looking at specific bacterial pathogens, Haemophilus influenzae had the strongest association with hypoglycemia (OR = 5.5, 95% CI = 2.2–14.1, P < 0.001). Non-typhi Salmonella was the most commonly isolated organism in both hypoglycemic (10/20, 50% of organisms) and non-hypoglycemic children (150/319, 47%). Invasive Streptococcus pneumoniae was not significantly associated with hypoglycemia (OR = 0.7, P = 0.7) (Table 4).
Table 4.
Association of specific invasive bacterial isolates with hypoglycemia
| Number (%) of total (N = 316) | Number (%) in hypoglycemia (N = 20) | OR for hypoglycemia (95% CI) | P* | |
|---|---|---|---|---|
| Non-typhi Salmonella | 156 (49.4) | 10 (50) | 2.35 (1.21–4.57) | 0.01 |
| S. pneumonae | 47 (14.9) | 1 (5) | 0.74 (0.11–5.28) | 0.7 |
| H. influenzae | 36 (11.4) | 5 (25) | 5.54 (2.17–14.14) | 0.001 |
| Escherichia coli | 23 (7.3) | 0 | – | |
| S. aureus | 14 (4.4) | 0 | – | |
| S. typhi | 5 (1.6) | 0 | – | |
| Other Gram negative | 27 (8.5) | 3 (15) | 4.29 (1.35–13.67) | 0.01 |
| Other Gram positive | 8 (2.5) | 1 (5) | 4.90 (0–31.04) | 0.1 |
Comparison with children with no invasive bacterial disease.
Discussion
Our results show an inverse relationship between mortality and admission blood glucose. The optimum balance between sensitivity and specificity in defining a useful cutoff for increased mortality risk was well above values normally regarded as defining hypoglycemia and also above the level set as a WHO criteria of severe malaria.7,13 Thus, children admitted with blood glucose levels usually considered to be in the low-normal range (2.5–5 mmol/L) were almost 2.5 times more likely to die as those children with higher blood glucose, regardless of malaria blood slide result. This finding is consistent with results from a smaller study of children with severe malaria in Mali,8 although the cutoff proposed in the Mali study was slightly higher at 6.1 mmol/L (109.8 mg/dL). Similarly, a retrospective review of admissions in Kenya showed that the odds of dying were increased twofold in children with an admission blood glucose < 5 mmol/L.9
It is unclear whether the association of excess mortality in children and low-normal blood glucose represents a causal link or residual confounding. It is possible that the association between low-normal blood sugar and mortality that we observed could simply be because of the increased risk of true hypoglycemia later in the admission; in children with malaria, one-half of all post-admission cases of hypoglycemia occurred in children who were hypoglycemic at admission in one study.14 We found no published data exploring an association between low-normal blood sugar on admission and true hypoglycemia occurring later in the admission. Additional observational studies exploring the relationship between sequential blood sugar readings and mortality in children are indicated. Although hypoglycemia occurred less frequently in children with non-malarial illness, it was associated with a high case fatality in this group, and studies exploring the excess mortality associated with low blood glucose should involve children with and without malaria.
In children with malaria, we found an almost threefold increased risk of invasive bacterial disease if the blood sugar on admission was < 5 mmol/L, and there was a similar trend in children without malaria (although among these children, the association did not reach statistical significance). The association of hypoglycemia with bacterial sepsis in children is well-documented,1,6,15,16 and low sugar is a candidate to act as an indicator for antimicrobial treatment in children with severe malaria. This information is particularly important in African hospitals, where over one-half of all pediatric deaths occur in the first 48 hours of admission17 and admission assessments are often cursory.18,19
In a logistic model, we found a strong association between hypoglycemia and jaundice in children with negative malaria slide results. Given the extremely high mortality observed in this group, the association may be an agonal failure of hepatic gluconeogenesis. As noted previously,14,20,21 a strong association was also observed between hypoglycemia and acidosis (represented by hyperlactatemia) and its clinical correlate of deep breathing. Where blood glucose testing is available, it is most commonly restricted to children with altered conscious level. Broadening the testing strategy to include those children with deep breathing and jaundice in addition to those children with altered consciousness would still have failed to identify one-third of children with hypoglycemia.
The pathogenesis of hypoglycemia in infection is incompletely understood. In adults with severe malaria, there is an estimated rise of 50% in the basal metabolism of glucose22; however, in children with severe malaria, reduced production of glucose, possibly caused by a fasting state and poor glycogen reserves, may be more important.23 Very few studies have explored glucose metabolism in children with non-malarial infection, and it is difficult to determine whether the finding of a strong association of hypoglycemia with H. influenzae group B but not S. pneumoniae represents a true pathophysiological link.
Limitations of our study include that we had no laboratory gold standard measure for blood glucose. Although the Hemocue system performed well in normoglycemic and hypoglycemic adults with minimal bias,11,24 we are not aware of any published studies validating its use in severely ill African children. Nonetheless, clinicians in resource-limited settings are unlikely to have access to laboratory gold standards and will be reliant on point-of-care diagnostics for the foreseeable future. As already discussed, the absence of serial blood sugar readings in our study limited our ability to describe more fully the relationship between blood sugar and mortality.
It is reasonable to question whether an improvement in the identification of children with poor outcome from 79.4% to 88.8% achieved by testing all children and using a cutoff of blood glucose < 5 mmol/L (Table 3) is worthwhile given the costs involved in testing. This answer will depend on the local costs and availability of the tests, whether such a system could facilitate early discharge or different levels of care, and whether the excess mortality can be effectively addressed.
In conclusion, our data highlight the importance of measuring blood glucose in malarial and non-malarial febrile illness. Our data support two cutoffs for low blood glucose: one cutoff for determining the need for urgent glucose therapy to treat a physiological state that may be because of hypoglycemia and one cutoff for indicating increased mortality. Determination of blood glucose can act as a useful marker of increased mortality in children with severe febrile illness. Additional studies are required to determine if the excess mortality in children with low admission blood glucose (above the recognized cutoff for hypoglycemia) could be reduced through specific interventions.
ACKNOWLEDGMENTS
We would like to thank the staff and patients of Teule Hospital for their cooperation during this study. The following individuals were involved in data collection: Aikande Shoo, Halima Mohammed, Charles Mgaya, Selina Wycliffe, Emmanuel Swai, Edward Mtili, Walii Msuya, Christina Kemi, Stella Emmanuel, Rosalia Marwa, and Simphorosa Silaye. Anne B. Morrissey, Denise Dekker, and Susan C. Morpeth provided advice and support for microbiology.
Disclaimer: None of the funders had a role in the design, analysis, or interpretation of results.
Footnotes
Financial support: This work was supported by European Commission Europaid Grant Code SANTE/2004/078-607. B.N. was supported by grants from the Berkeley Fellowship, Sir Halley Stewart Trust, and Pfizer Pharmaceuticals. H.H. received support from the European Commission under a COFAS Marie Curie Fellowship. Pfizer Pharmaceuticals provided equipment and consumables for microbiology. Abbott Diagnostics provided reagents for HIV testing.
Authors' addresses: Behzad Nadjm, Oxford University Clinical Research Unit, National Hospital for Tropical Diseases, Hanoi, Vietnam, E-mail: bnadjm@yahoo.com. George Mtove, National Institute for Medical Research, Amani Centre, Muheza, Tanga, United Republic of Tanzania, E-mail: mtoveg2002@gmail.com. Ben Amos, Microbiology, Teule Hospital, Muheza, Tanga, United Republic of Tanzania, E-mail: bennsal@teule.org.uk. Helena Hildenwall, Division of Global Health, Karolinska Institute, Stockholm, Sweden, E-mail: helena.hildenwall@ki.se. Anne Najjuka, Frank Mtei, and Jim Todd, Joint Malaria Programme, Moshi, United Republic of Tanzania, E-mails: juxus33@gmail.com, mailfrankmt@gmail.com, and Jim.Todd@lshtm.ac.uk. Hugh Reyburn, Department of Disease Control, London School of Hygiene and Tropical Medicine, London, UK, E-mail: hugh.reyburn@lshtm.ac.uk.
References
- 1.Sperling MA. Hypoglycemia. In: Nelson WE, editor. Nelson Textbook of Paediatrics. Philadelphia, PA: WB Saunders Company; 1996. [Google Scholar]
- 2.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G, Snow R. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 3.White NJ, Miller KD, Marsh K, Berry CD, Turner RC, Williamson DH, Brown J. Hypoglycaemia in African children with severe malaria. Lancet. 1987;1:708–711. doi: 10.1016/s0140-6736(87)90354-0. [DOI] [PubMed] [Google Scholar]
- 4.Ssekitoleko R, Jacob ST, Banura P, Pinkerton R, Meya DB, Reynolds SJ, Kenya-Mugisha N, Mayanja-Kizza H, Muhindo R, Bhagani S, Scheld WM, Moore CC. Hypoglycemia at admission is associated with inhospital mortality in Ugandan patients with severe sepsis. Crit Care Med. 2011;39:2271–2276. doi: 10.1097/CCM.0b013e3182227bd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osier FH, Berkley JA, Ross A, Sanderson F, Mohammed S, Newton CR. Abnormal blood glucose concentrations on admission to a rural Kenyan district hospital: prevalence and outcome. Arch Dis Child. 2003;88:621–625. doi: 10.1136/adc.88.7.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigauque B, Roca A, Mandomando I, Morais L, Quinto L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardaji A, Nhalungo D, Soriano-Gabarro M, Flannery B, Menendez C, Levine MM, Alonso PL. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses with Limited Resources. Geneva: World Health Organization; 2005. [PubMed] [Google Scholar]
- 8.Willcox ML, Forster M, Dicko MI, Graz B, Mayon-White R, Barennes H. Blood glucose and prognosis in children with presumed severe malaria: is there a threshold for ‘hypoglycaemia’? Trop Med Int Health. 2010;15:232–240. doi: 10.1111/j.1365-3156.2009.02444.x. [DOI] [PubMed] [Google Scholar]
- 9.Achoki R, Opiyo N, English M. Mini-review: management of hypoglycaemia in children aged 0–59 months. J Trop Pediatr. 2010;56:227–234. doi: 10.1093/tropej/fmp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, Kimera J, Msuya W, Mtei F, Dekker D, Malahiyo R, Olomi R, Crump JA, Whitty CJ, Reyburn H. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ. 2010;340:c1350. doi: 10.1136/bmj.c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stork AD, Kemperman H, Erkelens DW, Veneman TF. Comparison of the accuracy of the HemoCue glucose analyzer with the Yellow Springs Instrument glucose oxidase analyzer, particularly in hypoglycemia. Eur J Endocrinol. 2005;153:275–281. doi: 10.1530/eje.1.01952. [DOI] [PubMed] [Google Scholar]
- 12.Mayhood MK, Afwamba IA, Odhiambo CO, Ndanu E, Thielman NM, Morrissey AB, Shao JF, Wells Pence B, Crump JA. Validation, performance under field conditions, and cost-effectiveness of Capillus HIV-1/HIV-2 and determine HIV-1/2 rapid human immunodeficiency virus antibody assays using sequential and parallel testing algorithms in Tanzania. J Clin Microbiol. 2008;46:3946–3951. doi: 10.1128/JCM.01045-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Guidelines for the Treatment of Malaria 2010. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 14.English M, Wale S, Binns G, Mwangi I, Sauerwein H, Marsh K. Hypoglycaemia on and after admission in Kenyan children with severe malaria. QJM. 1998;91:191–197. doi: 10.1093/qjmed/91.3.191. [DOI] [PubMed] [Google Scholar]
- 15.Mtove G, Amos B, von Seidlein L, Hendriksen I, Mwambuli A, Kimera J, Mallahiyo R, Kim DR, Ochiai RL, Clemens JD, Reyburn H, Magesa S, Deen JL. Invasive salmonellosis among children admitted to a rural Tanzanian hospital and a comparison with previous studies. PLoS One. 2010;5:e9244. doi: 10.1371/journal.pone.0009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassat Q, Guinovart C, Sigauque B, Mandomando I, Aide P, Sacarlal J, Nhampossa T, Bardaji A, Morais L, Machevo S, Letang E, Macete E, Aponte JJ, Roca A, Menendez C, Alonso PL. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop Med Int Health. 2009;14:1011–1019. doi: 10.1111/j.1365-3156.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- 17.Berkley JA, Ross A, Mwangi I, Osier FH, Mohammed M, Shebbe M, Lowe BS, Marsh K, Newton CR. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ. 2003;326:361. doi: 10.1136/bmj.326.7385.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.English M, Esamai F, Wasunna A, Were F, Ogutu B, Wamae A, Snow RW, Peshu N. Assessment of inpatient paediatric care in first referral level hospitals in 13 districts in Kenya. Lancet. 2004;363:1948–1953. doi: 10.1016/S0140-6736(04)16408-8. [DOI] [PubMed] [Google Scholar]
- 19.Reyburn H, Mwakasungula E, Chonya S, Mtei F, Bygbjerg I, Poulsen A, Olomi R. Clinical assessment and treatment in paediatric wards in the north-east of the United Republic of Tanzania. Bull World Health Organ. 2008;86:132–139. doi: 10.2471/BLT.07.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.English M, Sauerwein R, Waruiru C, Mosobo M, Obiero J, Lowe B, Marsh K. Acidosis in severe childhood malaria. QJM. 1997;90:263–270. doi: 10.1093/qjmed/90.4.263. [DOI] [PubMed] [Google Scholar]
- 21.Taylor TE, Borgstein A, Molyneux ME. Acid-base status in paediatric Plasmodium falciparum malaria. Q J Med. 1993;86:99–109. [PubMed] [Google Scholar]
- 22.Binh TQ, Davis TM, Johnston W, Thu LT, Boston R, Danh PT, Anh TK. Glucose metabolism in severe malaria: minimal model analysis of the intravenous glucose tolerance test incorporating a stable glucose label. Metabolism. 1997;46:1435–1440. doi: 10.1016/s0026-0495(97)90144-x. [DOI] [PubMed] [Google Scholar]
- 23.Zijlmans WC, van Kempen AA, Serlie MJ, Sauerwein HP. Glucose metabolism in children: influence of age, fasting, and infectious diseases. Metabolism. 2009;58:1356–1365. doi: 10.1016/j.metabol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Watkinson PJ, Barber VS, Amira E, James T, Taylor R, Young JD. The effects of precision, haematocrit, pH and oxygen tension on point-of-care glucose measurement in critically ill patients: a prospective study. Ann Clin Biochem. 2012;49:144–151. doi: 10.1258/acb.2011.011162. [DOI] [PubMed] [Google Scholar]