Abstract
We estimated the incidence of watery diarrhea in the community before and after introduction of the pentavalent rotavirus vaccine in León, Nicaragua. A random sample of households was selected before and after rotavirus vaccine introduction. All children < 5 years of age in selected households were eligible for inclusion. Children were followed every 2 weeks for watery diarrhea episodes. The incidence rate was estimated as numbers of episodes per 100 child-years of exposure time. A mixed effects Poisson regression model was fit to compare incidence rates in the pre-vaccine and vaccine periods. The pre-vaccine cohort (N = 726) experienced 36 episodes per 100 child-years, and the vaccine cohort (N = 826) experienced 25 episodes per 100 child-years. The adjusted incidence rate ratio was 0.60 (95% confidence interval [CI] 0.40, 0.91) during the vaccine period versus the pre-vaccine period, indicating a lower incidence of watery diarrhea in the community during the vaccine period.
Introduction
Diarrheal diseases cause 1.3 million deaths of children each year and are an important cause of malnutrition, growth retardation, and developmental delays.1–3 Although child deaths caused by diarrhea have declined substantially since the introduction of oral rehydration therapy, there has been little change in diarrhea incidence in low and middle income countries (LMICs) since the 1950s.4
Vaccines against rotavirus, one of the most common causes of diarrhea,5 offer promise in reducing diarrhea incidence in LMIC settings. Rotavirus vaccines show excellent protection against rotavirus diarrhea in clinical trials conducted primarily in the industrialized world.6,7 Evaluations of the vaccines' effectiveness in LMICs report less robust protection.8–11 These evaluations have focused on the prevention of hospitalizations and severe diarrhea, although little is known about the vaccine's effect on diarrhea incidence in the community. However, the majority of rotavirus diarrhea cases are treated at home: 80–85% of cases do not receive treatment at any health facility.12
The goal of this study was to examine the childhood diarrhea incidence rate at the community level in Nicaragua, the first LMIC to introduce the pentavalent rotavirus vaccine (RV5, Rotateq®) in 2006. We examined watery diarrhea incidence in León, Nicaragua before and after RV5 introduction using active surveillance. Because rotavirus traditionally has a seasonal transmission pattern, we also examined changes in seasonal patterns of diarrhea incidence.
Methods
Setting.
The study was performed in the municipality of León, Nicaragua's second largest city, with an estimated 2010 population of 192,164. Municipal water treatment plants have been in continuous use in León since the 1990s. Improvements in child mortality have been made in recent decades,13 however, diarrhea remains among the most important causes of mortality and morbidity in children in León.14
Diarrhea incidence in Central America has two annual peaks; the dry season peak is attributed primarily to rotavirus infections, whereas the rainy season peak has traditionally been attributed to bacterial pathogens, such as enterotoxigenic Escherichia coli and Shigella spp.15–17 Overall, rotavirus was considered one of the most common causes of diarrhea among Nicaraguan children before the immunization program, isolated among 28% of children receiving care for diarrhea in health facilities.18
To reduce the burden of rotavirus diarrhea, Nicaragua implemented a rotavirus immunization program with RV5 in October 2006. Nicaraguan infants are offered the vaccine at the age of 2, 4, and 6 months as part of the country's Expanded Program on Immunization (EPI).
Sample selection.
Cohorts from two distinct periods were examined in the study: before implementation of the immunization program, or, “pre-vaccine” (December 2000 to March 2001, and July 2002 to January 2003; broken into two segments because of budgetary issues) and following implementation of the immunization program, or, “vaccine” (January 2010 to January 2011). The time periods were chosen to include an equal number of months during the rainy and dry seasons.
In each time period, a random sample of households was selected from the Health and Demographic Surveillance Site-León (HDSS-León). The HDSS-León provides longitudinal surveillance for a population-based sample of 28% of León's population, residing in 50 out of 208 randomly selected geographical clusters of equal population size19 (Figure 1). Children < 5 years of age in each sampled household were eligible for inclusion in the study. An “open cohort” design was used: children were excluded from the study after their fifth birthday, and new infants born into the household or children moving into the household during the study periods were eligible for inclusion.
Figure 1.
Map of León, Nicaragua: Geographic clusters and households included in HDSS-León surveillance site.
Data collection and study instruments.
Female field workers visited sampled households every 2 weeks and performed household interviews. The interview was conducted with the mother or with the child's caretaker if the mother was unavailable. Quality control of the interviews and data collection was performed by the field supervisor with systematic and random evaluations.
The study instrument contained information on diarrhea episodes and clinical characteristics as reported by the mother or caretaker, family characteristics, household characteristics, breastfeeding history, and during the vaccine period only, a detailed rotavirus vaccine history taken from the child's immunization card.
At each interview, the field worker asked about all diarrhea episodes within the past 14 days, and collected information on the presence of vomiting, fever, and the presence of visible blood in the stool. A clinical definition of watery diarrhea suggestive of rotavirus infection was developed based on the literature, and defined as a diarrhea episode with a maximum of four or more bowel movements per 24 hour period, with the presence of vomiting or fever (or both), and without any blood in the stool.20–25 An episode was considered to be new if the participant experienced at least 3 days without an episode before the onset of a new episode. In addition to diarrhea episodes, breastfeeding status, and rotavirus vaccine status were also updated at each household visit.
The study was approved by the Institutional Review Boards of the Universidad Nacional Autónoma de Nicaragua, León and the University of North Carolina at Chapel Hill. Informed consent was obtained from a parent or legal guardian of each participant.
Statistical analysis.
Mixed effects linear and logistic regression models were used to compare participant characteristics in the pre-vaccine and vaccine periods. The mixed effects models allowed for possible correlation between multiple participants living in the same household. The incidence rate of watery diarrhea was estimated as the number of episodes per 100 child-years of exposure time, and was stratified by age group (0–23 months, 24–59 months). The exposure time was estimated at the time each child was followed during the study and at-risk for a watery diarrhea episode; days with watery diarrhea were not included in the exposure time. Official rainfall data from León's regional airport26 were used to assign each month of the study as either rainy (≥ 6 mm per month) or dry (< 6 mm per month).
A mixed effects Poisson regression model was fit to compare incidence rates in the pre-vaccine and vaccine periods. The mixed effects model allowed for possible correlation between multiple participants living in the same household as well as multiple measurements for each individual, and also allowed for adjustment for potential confounders. Incidence rates were compared between the rainy and dry seasons. Furthermore, in the vaccine period, incidence rates were compared between fully or partially immunized children and unimmunized children. These results were stratified by age group because of potential differences in the vaccine's effect by age group.
Results
A total of 726 children were enrolled during the pre-vaccine period and followed for 306.9 years of exposure time; 826 children were enrolled in the vaccine period and followed for 610.6 years of exposure time. Characteristics of children during the pre-vaccine and vaccine periods are shown in Table 1. Households observed during the pre-vaccine period had fewer indoor toilets compared with the vaccine period (46.7% versus 80.1%, P < 0.001) and mothers of children in the vaccine period were slightly more likely to be employed (33.3% versus 40.0%, P < 0.001) and have completed a basic level of education (82.4% versus 90.1%, P = 0.007). In all, 78.1% of vaccine period children were eligible to receive the rotavirus vaccine based on having a birthday on or after August, 2006. Upon study entry, 67.3% of all children in the vaccine period had received at least one dose of the rotavirus vaccine; among children < 24 months of age, 86.6% had received at least one dose of vaccine, whereas among children 24 to 60 months of age, 53.2% of children had received at least one dose of vaccine.
Table 1.
Characteristics of the participants in the pre-vaccine and vaccine periods
| Characteristic | Pre-vaccine N = 726 | Vaccine N = 826 | P value for difference* |
|---|---|---|---|
| Personal characteristics | |||
| Age (mean in months [SD]) | 25.3 [17.3] | 28.9 [16.7] | < 0.001 |
| Sex, % male | 51.2% (372/726) | 50.4% (416/826) | 0.73 |
| Breastfeeding upon study enrollment | 36.4% (247/679)† | 34.0% (281/826) | 0.34 |
| Received at least one dose of rotavirus vaccine | 0.0% (0/726) | 67.3% (556/826) | < 0.001 |
| Household characteristics | |||
| Indoor municipal water source | 94.1% (683/726) | 97.0% (801/826) | 0.05 |
| Indoor toilet | 46.7% (339/726) | 80.1% (662/826) | < 0.001 |
| Concrete or tile floor (versus dirt floor) | 72.9% (529/726) | 77.2% (638/826) | 0.008 |
| Family characteristics | |||
| Mother completed fourth grade | 82.4% (598/726) | 90.1% (744/826) | 0.007 |
| Mother employed | 33.3% (242/726) | 40.0% (330/826) | < 0.001 |
P values based on mixed effects logistic or linear regression models with each characteristic as the outcome and an indicator for vaccine period as the sole covariate.
6% of respondents chose not to answer this question.
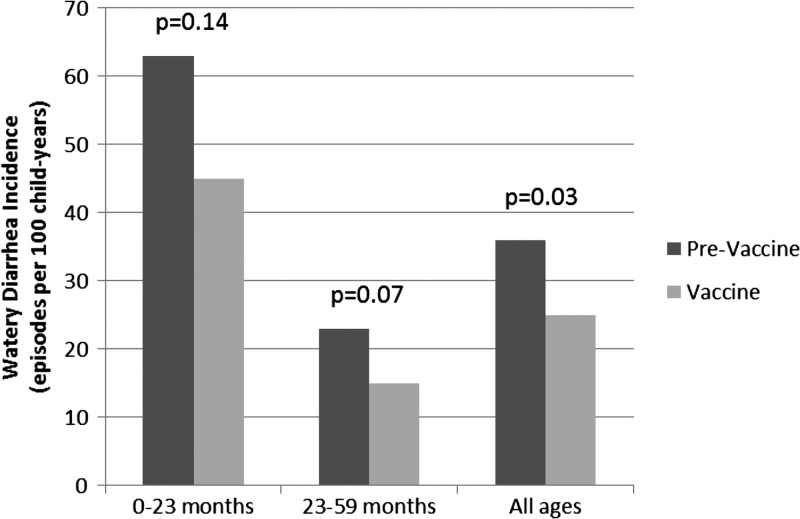
The crude watery diarrhea incidence rate was 35.8 episodes per 100 child-years in the pre-vaccine period and 24.9 episodes per 100 child-years in the vaccine period (Table 2). Incidence rates stratified by age group are shown in Figure 2; among both age groups there was a lower watery diarrhea incidence rate in the vaccine period.
Table 2.
Watery diarrhea in pre-vaccine and vaccine periods
| Pre-vaccine | Vaccine | |
|---|---|---|
| Number of households | 411 | 530 |
| Number of child participants | 726 | 826 |
| Numbers of children with watery diarrhea episodes | 91 | 117 |
| Total number of watery diarrhea episodes | 110 | 152 |
| Person-years in the study | 306.9 | 610.6 |
| Overall watery diarrhea incidence (episodes per 100 child-years) | 35.8 | 24.9 |
Figure 2.
Watery diarrhea incidence by age group.
Table 3 shows the adjusted incidence rate ratios (IRRa) of watery diarrhea in the vaccine versus pre-vaccine periods, as estimated by the mixed effects Poisson regression model. In the model, we adjusted for sex, breastfeeding, maternal education, maternal employment status, living in a household with a dirt floor, indoor municipal water source, season, and indoor toilet. Among all ages, the IRRa was 0.60 (95% confidence interval [CI] 0.40, 0.91) in the vaccine period as compared with the pre-vaccine period (“overall effect”). Among children < 24 months of age the IRRa was 0.63 (95% CI 0.37, 1.05), whereas among children between 24 and 59 months of age the IRRa was 0.56 (95% CI 0.32, 1.07). Among unimmunized children only, the IRRa was 0.27 (95% CI: 0.13, 0.60) in the vaccine period as compared with the pre-vaccine period; this result is consistent with an indirect effect of the immunization program, but could also be caused by other differences between the pre-vaccine and vaccine period. During the vaccine period, there were no significant differences in watery diarrhea incidence rate observed between immunized or partially unimmunized children as compared with unimmunized children, stratified by age group (“direct effect”). In both time periods, there was also no difference in diarrhea incidence detected between the rainy seasons as compared with the dry season.
Table 3.
Adjusted incidence rate ratios* (IRRa) of watery diarrhea in the vaccine versus pre-vaccine periods
| Age group | IRRa | 95% confidence intervals |
|---|---|---|
| 0–24 months | 0.63 | 0.37, 1.05 |
| 24–59 months | 0.56 | 0.32, 1.07 |
| All ages | 0.60 | 0.40, 0.91 |
Adjusted by sex, breastfeeding, household water source, indoor toilet, dirt floor, season, maternal education, and maternal employment.
Discussion
Using active community surveillance in León, Nicaragua, we observed a 40% lower incidence rate of watery diarrhea episodes suggestive of rotavirus infection in the vaccine period as compared with the pre-vaccine period. This reduction may be attributable to an overall protective effect of the immunization program on immunized and unimmunized children. The findings correlate with an observed decline in rotavirus prevalence among children seeking care for diarrhea in primary care clinics in León following the vaccine's introduction (14.0% in the pre-vaccine period versus 3.5% in the vaccine period).27
The reduction in incidence observed among unimmunized children provides evidence for herd protection conferred by rotavirus immunization programs, as noted in prior studies.28 Surprisingly, the reduction in incidence among unimmunized children (IRR = 0.27, 95% CI 0.13, 0.60) was greater than among the population as a whole (IRR = 0.60, 95% CI 0.40, 0.91). An additional analysis found no differences in IRR among unimmunized children living in neighborhoods with low versus high vaccine coverage. Although our findings were unexpected, a pattern of greater reductions in rotavirus diarrhea incidence among age groups with low versus high vaccine coverage was previously observed in a United States study of rotavirus diarrhea hospitalizations in the years before and after vaccine introduction.29 In our study of a population with high vaccine coverage, it is possible that our sample size of unimmunized children was not large enough to detect a true difference in incidence among unimmunized children as compared with immunized children in the vaccine period. Alternatively, it is conceivable that rotavirus immunization could increase the risk of infection by a different diarrheal pathogen, which would provide the unimmunized children an advantage within this highly immunized population; however, there is no biological explanation for this alternate hypothesis.
We did not observe a statistically significant direct effect of the vaccine following introduction, which may be the result of strong herd protection effects afforded by the immunization program in a setting with high vaccine coverage. A similar phenomenon was observed by Ali and others30 in an analysis of herd immunity conferred by killed oral cholera vaccines. To further investigate the direct effect of the rotavirus vaccine, we examined the dose effect by estimating age-stratified incidence rates among those who had received one, two, or three doses of vaccine. Among all age groups, the incidence rate was lower among those who had received the full course of vaccine than those who had received only two doses. However, among infants, the incidence rate among those receiving the full course was no different than those receiving just one dose. This could be explained by different exposures to enteric pathogens among infants who had received one dose as compared with those who had completed the full course; the majority of infants who had received just one dose were two to three months of age, were breastfed, and were less likely to interact with their environment as compared with older infants, who had received the full course.
This study highlights the importance of health and demographic surveillance systems in assessing disease rates in a population after an intervention. As the majority of diarrhea cases are typically treated at home, they would not be captured by more commonly used hospital-based surveillance systems.
Our study has several limitations. First, as an observational study, we cannot prove that differences in incidence rates between the pre-vaccine and vaccine periods were caused by the immunization program and not by other factors. We did observe certain improvements in living standards between the two time periods. Although we included these socioeconomic and household variables in our regression model, we did not find them to be associated with watery diarrhea episodes. It is possible, however, that factors associated with improvements in living standards that we did not measure may have contributed to the overall decrease in diarrhea incidence rate observed in the vaccine period. Second, this report does not include laboratory data on the causes of diarrhea episodes, but instead used a clinically defined watery diarrhea definition based on characteristics of rotavirus diarrhea reported in the literature. Clearly, this definition is not as specific as laboratory analysis, but allows a narrowing of diarrhea cases that are more likely to be caused by rotavirus infection, and was feasible in a community setting. As this study was conducted in the community, the definition relied on the mother's or caretaker's reports of fever during the episode. Prior studies have shown that mothers detect fevers in their children with a high sensitivity, although with a lower specificity than when a thermometer is used by a health provider.31,32 Therefore, our definition may have overestimated the incidence of episodes. However, if the rate of overestimation was similar between the pre-vaccine and vaccine periods, then our estimated IRR would be biased towards the null, suggesting the true reduction in incidence associated with the introduction of the rotavirus vaccine may be even greater than indicated by this analysis. Finally, as rotavirus transmission can vary from year to year, we cannot rule out that differences in watery diarrhea incidence noted in the two time periods may be caused by such year-to-year variability.
In summary, the changes in watery diarrhea incidence observed in the two time periods support an overall and indirect effect of the rotavirus immunization program at the community level. Future studies should attempt to identify the etiologies of diarrhea that persist in the community following vaccine introduction, to better design prevention programs and further reduce the burden of childhood diarrhea.
ACKNOWLEDGMENTS
We thank Magdaly Torres, Sophia Giebultowicz, the home interviewers, and the participating families for their contributions to this study.
Footnotes
Financial support: Becker-Dreps is supported by 4K01TW008401-04 from the Fogarty International Center at the National Institutes of Health. This study received funding from the Thrasher Research Fund (vaccine period) and NeTropica, SIDA/SAREC (pre-vaccine period).
Authors' addresses: Sylvia Becker-Dreps, Department of Family Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, E-mail: sbd@unc.edu. Marlon Meléndez, Centers for Disease Control and Prevention, Central American Region, Universidad del Valle, Guatemala City, Guatemala, E-mail: marlonmelendezrodriguez@gmail.com. Lan Liu and Michael G. Hudgens, Department of Biostatistics, UNC-Gillings School of Global Public Health, Chapel Hill, NC, E-mails: lanl@live.unc.edu and mhudgens@email.unc.edu. Luis Enrique Zambrana, Centro de Investigación en Demografía y Salud (CIDS), León, Nicaragua, E-mail: lamarcoleta@gmail.com. Margarita Paniagua and Félix Espinoza, Departmento de Microbiología y Parasitología, Facultad de Sciencias Médicas, Universidad Nacional Autónoma de Nicaragua, León (UNAN-León), León, Nicaragua, E-mails: paniaguagaitan@yahoo.com.mx and espinozafelix08@gmail.com. David J. Weber, Division of Infectious Diseases, University of North Carolina at Chapel Hill Chapel Hill, NC, E-mail: Dweber@unch.unc.edu. Mercedes Cáceres, Departmento de Microbiología y Parasitología, Facultad de Sciencias Médicas, Universidad Nacional Autónoma de Nicaragua, León (UNAN-León), León, Nicaragua, E-mail: merkaceres2001@yahoo.com.mx. Carina Källeståll, IMCH, Women's and Children's Health, Uppsala University, Uppsala University Hospital, Sweden, E-mail: carina.kallestall@kbh.uu.se. Douglas R. Morgan, Division of Hepatology and Gastroenterology, University of North Carolina at Chapel Hill Chapel Hill, NC, E-mail: douglas_morgan@med.unc.edu. Rodolfo Peña, Centro de Investigación e Intervenciones en Salud León, Nicaragua, E-mail: rpenag58@gmail.com.
References
- 1.World Health Organization (WHO) Child mortality by cause among children aged 1–59 months. 2008. http://www.who.int/healthinfo/statistics/mortality_child_cause/en/index.html Available at. Accessed January 10, 2013.
- 2.Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, Guyatt H, Lima AM, Guerrant RL. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25:513–520. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 3.Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, Guerrant RL. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 4.Kosek BC, Guerrant RL. The global burden of diarrhea disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 5.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200((Suppl 1)):S9–S15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 6.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, Shinefield HR, Christie CD, Ylitalo S, Itzler RF, Coia ML, Onorato MT, Adeyi BA, Marshall GS, Gothefors L, Campens D, Karvonen A, Watt JP, O'Brien KL, DiNubile MJ, Clark HF, Boslego JW, Offit PA, Heaton PM. Rotavirus Efficacy and Safety Trial (REST) Study Team Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O'Ryan M. Human Rotavirus Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 8.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, Lewis K, Coia ML, Attah-Poku M, Ojwando J, Rivers SB, Victor JC, Nyambane G, Hodgson A, Schödel F, Ciarlet M, Neuzil KM. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 9.Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, Han HH, Neuzil KM. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 10.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schödel F, Steele AD, Neuzil KM, Ciarlet M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 11.Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, Gonzalez A, Malespin O, Amador JJ, Umaña J, Balmaseda A, Perez MC, Gentsch J, Kerin T, Hull J, Mijatovic S, Andrus J, Parashar U. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 12.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass IR. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez W, Peña R, Persson LÅ, Källestål C. Tracking progress towards equitable child survival in a Nicaraguan community: neonatal mortality challenges to meet the MDG 4. BMC Public Health. 2011;11:455. doi: 10.1186/1471-2458-11-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicaraguan Ministry of Health (MINSA) Epidemiological Bulletin; 2011–2012. http://www.minsa.gob.ni/index.php?option=com_remository&Itemid=52&func=startdown&id=8281 Available at. Accessed January 10, 2013.
- 15.de Oliveira LH, Danovaro-Holliday MC, Andrus JK, de Fillipis AM, Gentsch J, Matus CR, Widdowson MA. Rotavirus Surveillance Network Sentinel hospital surveillance for rotavirus in Latin American and Caribbean countries. J Infect Dis. 2009;200((Suppl1)):S131–S139. doi: 10.1086/605060. [DOI] [PubMed] [Google Scholar]
- 16.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487–1496. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization (WHO) Readings on Diarrhea: Student Manual. Geneva: WHO; 1992. [Google Scholar]
- 18.Espinoza F, Paniagua M, Hallander H, Hedlund KO, Svensson L. Prevalence and characteristics of severe rotavirus infections in Nicaraguan children. Ann Trop Paediatr. 1997;17:25–32. doi: 10.1080/02724936.1997.11747859. [DOI] [PubMed] [Google Scholar]
- 19.Peña R, Pérez W, Meléndez M, Kallestal C, Persson LA. The Nicaraguan Health and Demographic Surveillance Site, HDSS-Leon: a platform for public health research. Scand J Public Health. 2008;36:318–325. doi: 10.1177/1403494807085357. [DOI] [PubMed] [Google Scholar]
- 20.Staat MA, Azimi PH, Berke T, Roberts N, Bernstein DI, Ward RL, Pickering LK, Matson DO. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J. 2002;21:221–227. doi: 10.1097/00006454-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Salinas B, González G, González R, Escalona M, Materán M, Schael IP. Epidemiologic and clinical characteristics of rotavirus disease during five years of surveillance in Venezuela. Pediatr Infect Dis J. 2004;23((Suppl)):S161–S167. doi: 10.1097/01.inf.0000142465.25992.c3. [DOI] [PubMed] [Google Scholar]
- 22.Abugalia M, Cuevas L, Kirby A, Dove W, Nakagomi O, Nakagomi T, Kara M, Gweder R, Smeo M, Cunliffe N. Clinical features and molecular epidemiology of rotavirus and norovirus infections in Libyan children. J Med Virol. 2011;83:1849–1856. doi: 10.1002/jmv.22141. [DOI] [PubMed] [Google Scholar]
- 23.Wiegering V, Kaiser J, Tappe D, Weissbrich B, Morbach H, Girschick HJ. Gastroenteritis in childhood: a retrospective study of 650 hospitalized pediatric patients. Int J Infect Dis. 2011;15:e401–e407. doi: 10.1016/j.ijid.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez WJ, Kim HW, Arrobio JO, Brandt CD, Chanock RM, Kapikian AZ, Wyatt RG, Parrott RH. Clinical features of acute gastroenteritis associated with human reovirus-like agent in infants and young children. J Pediatr. 1977;91:188–193. doi: 10.1016/S0022-3476(77)80810-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nokes DJ, Abwao J, Pamba A, Peenze I, Dewar J, Maghenda JK, Gatakaa H, Bauni E, Scott JA, Maitland K, Williams TN. Incidence and clinical characteristics of group A rotavirus infections among children admitted to hospital in Kilifi, Kenya. PLoS Med. 2008;5:e153. doi: 10.1371/journal.pmed.0050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicaraguan Institute of Geological Studies (INETER) Daily Rainfall Summary. León; Nicaragua: 2001–2011. [Google Scholar]
- 27.Becker-Dreps S, Paniagua M, Zambrana LE, Bucardo F, Hudgens MG, Weber DJ, Morgan DR, Espinoza F. Rotavirus prevalence in the primary care setting in Nicaragua after universal infant rotavirus immunization. Am J Trop Med Hyg. 2011;85:957–960. doi: 10.4269/ajtmh.2011.11-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopman BA, Payne DC, Tate JE, Patel MM, Cortese MM, Parashar UD. Post-licensure experience with rotavirus vaccination in high and middle income countries; 2006 to 2011. Curr Opin Virol. 2012;2:434–442. doi: 10.1016/j.coviro.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, Chappell J, Curns AT, Wikswo M, Tate JE, Lopman BA, Parashar UD. New Vaccine Surveillance Network (NVSN) Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 U.S. Counties, 2006–2009. Clin Infect Dis. 2011;53:245–253. doi: 10.1093/cid/cir307. [DOI] [PubMed] [Google Scholar]
- 30.Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Rao M, Holmgren J, Clemens JD. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 31.Graneto JW, Soglin DF. Maternal screening of childhood fever by palpation. Pediatr Emerg Care. 1996;12:183–184. doi: 10.1097/00006565-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Teng CL, Ng CJ, Nik-Sherina H, Zailinawati AH, Tong SF. The accuracy of mother's touch to detect fever in children: a systematic review. J Trop Pediatr. 2008;54:70–73. doi: 10.1093/tropej/fmm077. [DOI] [PubMed] [Google Scholar]