Abstract
In this large-scale longitudinal study conducted in rural Southern India, we compared a presence/absence hydrogen sulfide (H2S) test with quantitative assays for total coliforms and Escherichia coli as measures of water quality, health risk, and water supply vulnerability to microbial contamination. None of the three indicators showed a significant association with child diarrhea. The presence of H2S in a water sample was associated with higher levels of total coliform species that may have included E. coli but that were not restricted to E. coli. In addition, we observed a strong relationship between the percent positive H2S test results and total coliform levels among water source samples (R2 = 0.87). The consistent relationships between H2S and total coliform levels indicate that presence/absence of H2S tests provide a cost-effective option for assessing both the vulnerability of water supplies to microbial contamination and the results of water quality management and risk mitigation efforts.
Introduction
Diarrheal diseases, including severe illnesses such as cholera and dysentery, are primarily caused by the ingestion of diarrhea-causing microbial pathogens, which include specific viruses, bacteria, and protozoa. Diarrheal pathogens are concentrated in the feces of infected individuals; as a result, their transmission is promoted by environmental fecal contamination. Approximately 88% of diarrhea cases worldwide are attributable to pathogen transmission by fecal contamination of water, food, or hands; these cases result in about 1.5 million deaths each year, mostly among children < 5 years of age.1
Water quality improvements can reduce diarrhea,2–5 and monitoring microbial water quality is a primary activity of safe water management.6 The diversity of diarrheal pathogens renders direct pathogen testing impractical, therefore water providers and public health surveillance agencies rely on water quality indicators to assess microbial drinking water quality: process indicators monitor the efficacy of water treatment and the integrity of distribution networks; fecal indicators directly identify the presence of fecal contamination.7 Most national regulations, which are generally based on World Health Organization (WHO) guidelines, specify the broad group of coliform bacterial species (total coliforms) as process indicators; fecal indicators include thermotolerant coliforms, a subset of the total coliform group, and Escherichia coli, a subset of the thermotolerant coliforms.8
Disease outbreaks, however, have been associated with water that was free of fecal indicators,9 and in tropical climates, fecal indicators may persist in the environment in the absence of recent fecal contamination.10–12 These observations reflect the variable relationships observed between water quality indicators and actual fecal pathogens,13,14 which may also contribute to the weak and inconsistent associations between water quality indicators and diarrheal disease.2,13,15–19
Most of the validated methods for quantifying total coliforms, thermotolerant coliforms, and E. coli in drinking water are costly and require both dedicated facilities and specialized training. To promote on-site microbial water testing in resource-poor settings, Manja and others20 developed a simple presence/absence test for hydrogen sulfide (H2S)-producing bacteria, based on the observation that coliform bacteria in drinking water were consistently associated with H2S production. Improved versions of the presence/absence H2S test have gained popularity as low-cost assays for fecal contamination. For example, the Indian government has supported the use of H2S tests for community-level monitoring of common water supplies, accompanied by laboratory-based confirmation of positive results.21
A recent systematic review showed that the diagnostic accuracy of the H2S test for the established fecal indicators, thermotolerant coliforms and E. coli, varied between studies and that the sources of this variation were not obvious.22 Culture-based and molecular analysis of environmental water samples, however, have shown strong relationships between positive H2S tests and fecal microorganisms, including coliform and non-coliform species.23,24
To directly evaluate the H2S test as a drinking water management tool in low-resource settings, our study focused on two objectives: 1) to conduct a comparative analysis of drinking water quality indicators (H2S, total coliforms, and E. coli); and 2) to estimate the associations between the water quality indicators and diarrhea among children < 5 years of age. We conducted this analysis as part of a 12-month longitudinal cohort study in rural Southern India, designed to measure the effect of a combined sanitation, water supply, and hygiene intervention program on child health.25
Materials and Methods
Study setting.
This study was conducted between January 2008 and April 2009 in 25 rural villages in the Tiruchirappalli district of Tamil Nadu, India. The study population mainly worked in agriculture (64% of households in the cohort). The climate is tropical and subject to heavy rainfall during seasonal monsoons (August–December). In all study villages, drinking water was provided by pumping groundwater into overhead storage tanks and then distributing it to public and private taps. Qualitative interviews suggested that chlorination of the drinking water was infrequent and, in most villages, probably limited to disease outbreaks. During the study, 93% of households relied on public or private taps for all of their daily water use (drinking, cooking, bathing, and washing).
Study design.
Details of the study design and population have been reported.25 In brief, the study included 12 villages that received a combined water supply, sanitation, and hygiene education intervention between 2003 and 2007, and 13 matched control villages that did not receive the intervention. We enrolled a random sample of up to 50 households with children < 60 months of age per village from a complete listing of all households in the villages in 2008 after the completion of the intervention. We visited households monthly over 12 months to collect water samples and caregiver-reported child health symptoms.
Water sample collection and testing.
Beginning in the third month of the cohort study (Round 3), field staff collected water samples concurrently with diarrhea surveillance. The 25 study villages had between one and seven water sources, and all village sources were tested in survey Rounds 3–12. Field staff collected 125 mL of water from these sources according to local water retrieval practices.
Field staff also collected household drinking water samples from a random, rotating sample during the study. Households within each village were randomly allocated into four groups: two of the groups were measured in survey Rounds 3 and 5, and the other two groups were measured in survey Rounds 4 and 6. Households in one of the four groups were tested in each survey round thereafter (Rounds 7–12). Household water samples were collected in 125 mL sterilized plastic bottles in a fashion that mimicked each household's water retrieval practices: by dipping a household cup into the vessel to transfer the water, by pouring water from the storage container into the sample container, or, if a household did not store drinking water, by retrieving water directly from the tap. Along with the water samples, field staff recorded basic characteristics of the water conditions at the time of collection (such as storage container type). The field team transported all the water samples to the laboratory in coolers containing ice. In the laboratory, samples were stored at 4°C before conducting assays for total coliforms, E. coli and H2S. All assays were performed within 24 hr of sample collection. Autoclaved tap water samples were used as negative controls for each Round of water quality analysis.
Samples collected during survey Round 3 were diluted 1:10 before the analysis of total coliform and E. coli levels. In survey Rounds 4–12, the laboratory increased the dilution to 1:100 to optimize total coliform enumeration. After dilution, 100 mL of sample water was passed through a 0.45 μM membrane filter, which was then placed onto a rehydrated membrane nutrient pad impregnated with HiCrome Coliform medium with sodium lauryl sulfate (HiMedia MF026; HiMedia, Mumbai, India). The filters and nutrient pads were incubated at 37°C for 24 hr. HiCrome coliform medium contains two chromogenic substrates, Salmon-gal and X-glu. All coliform bacterial species produce the enzyme β-galactosidase, which cleaves Salmon-gal to generate red-purple colonies. Escherichia coli produce an additional enzyme, β-glucuronidase, which cleaves X-glu: the combination of Salmon-gal and X-glu cleavage by E. coli generates dark blue-green colonies. The number of red-purple colonies was counted and recorded as total coliform bacteria. The number of blue-green colonies was counted and recorded as E. coli.
An undiluted 30 mL volume of each sample was also analyzed for H2S producing bacteria, using the HiH2S test kit (HiMedia K020; HiMedia, Mumbai, India). Samples were left to incubate at room temperature for 24 hr and, if room temperature fell below 30°C, for an additional 12 hr. Samples were recorded as positive for H2S, if they turned black.
Diarrhea measurement.
The primary health outcome in this study was caregiver-reported diarrhea during the 7 days before the visit among children < 5 years of age at enrollment; we defined diarrhea as three or more loose or watery stools in 24 hr or a single stool with blood or mucus.26,27 Field interviewers collected caregiver-reported symptoms during each monthly home visit with the aid of a health calendar.28
Statistical analysis.
Water quality descriptive analyses.
We summarized total coliform measurements using quantitative measures. The distribution of total coliform levels was right-skewed, therefore we imputed samples below the lower detection limit at 0.1 and conducted the analysis on the log10 scale. We calculated mean total coliform levels (on the log10 scale) and the proportion of positive H2S samples by village source and household characteristics.
Comparison of H2S to other indicators.
Analyses that examined lower concentrations of E. coli (i.e., < 10 colony forming units (CFUs)/100 mL) were restricted to the 727 samples from Round 3 when the detection limit was 10 CFUs/100 mL. All other analyses included all samples from survey Rounds 3–12. We estimated the sensitivity and specificity of H2S for total coliform and E. coli contamination based on the proportion of water samples that were positive for H2S within pre-specified ranges of total coliform concentrations (< 100, 100–199, 200–499, 500–999, 1,000–4,999, 5,000–9,999, ≥ 10,000 CFUs/100 mL) and E. coli concentrations (< 10, 10–99, ≥ 100 CFUs/100 mL). We also calculated the positive predictive value (PPV) and negative predictive value (NPV) of H2S for total coliform and E. coli contamination > 100 CFUs/100 mL.29
Association between water quality indicators and child diarrhea.
We estimated the increase in diarrhea risk associated with a positive H2S test result or with higher concentrations of total coliforms or E. coli from drinking water samples collected concurrently with disease surveillance. In our analysis, we only included diarrhea surveillance that was paired with a household water sample. Our parameter of interest for each comparison was the prevalence ratio (PR), comparing diarrhea prevalence among children at higher exposure levels of the water quality indicators with children in the baseline category. For H2S, we compared diarrhea risk associated with a positive test versus a negative test; for total coliforms we compared diarrhea risk at concentrations > 10,000 CFUs/100 mL to concentrations below that value; for E. coli we compared diarrhea risk at concentrations of 101–1,000 and > 1,000 CFUs/100 mL to concentrations ≤ 100 CFUs/100 mL. For binary exposures (H2S, total coliforms), we modeled the probability of diarrhea using a log-binomial model:
where Y is a binary indicator of a child's diarrhea status, A is a categorical variable of the water quality indicator, and W is a vector of potentially confounding characteristics. We modeled the association similarly for E. coli, but included two categories of exposure: A1 = I(E. coli 101–1,000 CFUs/100 mL) and A2 = I(E. coli > 1,000 CFUs/100 mL).
The exponentiated coefficient estimates for the exposure variables in the models (A) estimate the PR.30 In adjusted models, W included fixed effects for survey round and village and potentially confounding child (age, age squared, sex, currently breastfeeding) and household characteristics: water source, confirmed baseline boiling, water storage container type, latrine ownership, dedicated location to wash hands with water and soap, housing characteristics (soil floor, thatch roof), durable goods ownership (television, motorcycle, mobile phone), socioeconomic characteristics (has a bank account, participation in a Self Help Group, household head works in agriculture, Scheduled Caste membership, education level of the child's primary caregiver). All analyses (descriptive, PPV/NPV, and regression models) estimated standard errors and 95% confidence intervals (CIs) using the robust sandwich estimator, clustered at the village level.31
As a robustness check, we repeated the analyses that estimated the association between water quality indicator levels and child health using two caregiver-reported respiratory symptoms (constant cough, congestion/coryza) that were unlikely to be caused by contaminated water but could be biased by similar confounding or measurement error as diarrhea and could, thus, serve as negative control outcomes.32
Human subjects research protection.
All data collection followed protocols approved by the institutional review boards at the University of California, Berkeley, CA and Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India. All study participants provided informed consent.
Results
Study population and sample collection.
We enrolled 900 households and 1,284 children < 5 years of age. Of these, 1,220 (95%) children completed the 12 months of follow-up, and the study included 14,259 child-weeks of observation. Table 1 summarizes child and household characteristics in the study population. All households had access to an improved water source as defined by the WHO/United Nations Children's Fund (UNICEF) Joint Monitoring Program, with 93% of households obtaining water from public or private taps fed by ground water-supplied overhead tanks.33
Table 1.
Child and household characteristics in the 25 study villages
| Mean | (SD) | |
|---|---|---|
| Child characteristics (N = 1,284*) | ||
| Age at enrollment, mo | 28.5 | (18.3) |
| Female, % | 49.8 | |
| Currently breastfeeding, % | 32.1 | |
| Diarrhea, last 7 days, % | 1.8 | |
| Fever, last 7 days, % | 11.8 | |
| Cough, last 7 days, % | 8.1 | |
| Congestion, last 7 days, % | 31.6 | |
| Household characteristics (N = 900) | ||
| Mother's age, y | 26.8 | (5.0) |
| Caregiver's education, % | ||
| None | 14.6 | |
| Primary | 40.3 | |
| Secondary | 30.8 | |
| Post-secondary | 14.1 | |
| Missing | 0.2 | |
| Primary water source, % | ||
| Public tap | 63.8 | |
| Private tap | 28.8 | |
| Public or private well | 7.4 | |
| Boil drinking water, % | 16.8 | |
| Own private toilet, % | 41.6 | |
| Hand washing location, water and soap present, % | 31.2 | |
| Human/animal feces observed in living area, % | 26.8 | |
| Work in agriculture, % | 63.7 | |
| Scheduled caste, % | 13.0 | |
| Women members in Self-Help Group, % | 34.4 | |
| House has soil floor, % | 31.4 | |
| House has thatch roof, % | 24.4 | |
| Total persons in house | 4.8 | (1.3) |
| Total rooms in house | 2.7 | (1.6) |
| House has electricity, % | 90.0 | |
| Own their house, % | 92.9 | |
| Have bank account, % | 21.7 | |
| Own radio, % | 55.9 | |
| Own television, % | 65.3 | |
| Own mobile phone, % | 32.7 | |
| Own motorcycle/scooter, % | 25.3 | |
| Own bicycle, % | 76.3 | |
| Own mosquito net, % | 13.3 | |
N = 14,259 child-weeks of observation for symptom prevalence estimates.
Field staff collected and tested 695 village source water samples and 3,026 household drinking water samples during the study. Household water quality samples were collected concurrently with 4,166 child-weeks (H2S) and 4,152 child-weeks (total coliform and E. coli) of diarrhea surveillance. Of the 900 households in the study, field staff collected water samples three (N = 422) or four (N = 435) times from 95% of households during the follow-up period.
Water quality descriptive analyses.
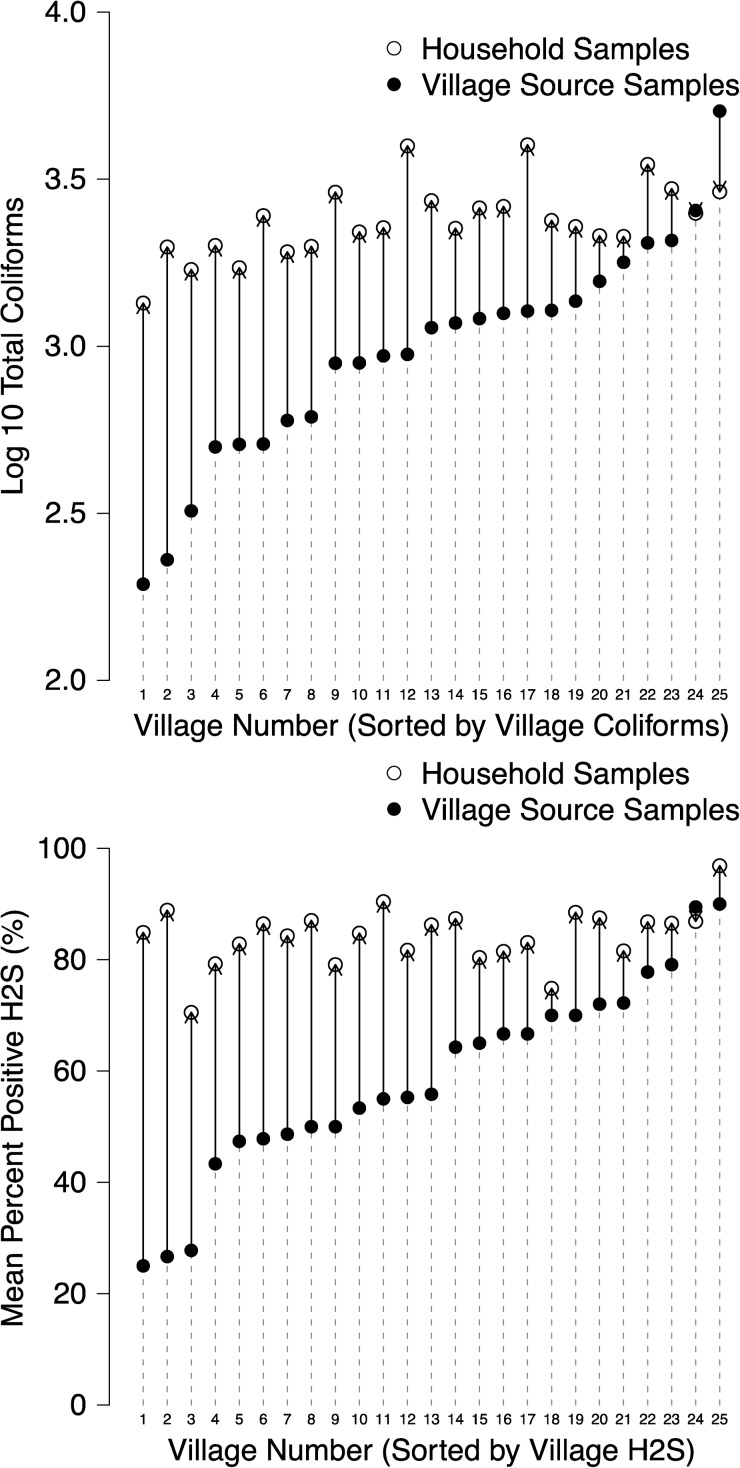
On average, source water quality was best when obtained directly from hand pumps (mean Log10 total coliforms 2.579, SE 0.099; percent positive H2S 36.3%, SE 4.8%) and poorest in the smaller, public mini-tanks (mean Log10 total coliforms 3.286, SE 0.071; percent positive H2S 75.4%, SE 3.8%) (Table 2). When we aggregated water quality data by village, we observed a consistent deterioration in microbial water quality between village source and household drinking water samples, and, although we observed heterogeneity in village source water quality, household water quality was uniformly poor across villages based on both total coliform and H2S indicators (Figure 1). Self-reported boiling was infrequent in this study population: 9.6% of samples (291 of 3,026) were reported to be boiled (Table 2). Boiled water was of higher quality based on total coliform and H2S indicators, but contamination levels in reportedly boiled samples remained high (mean log10 total coliforms 3.162, SE 0.060; percent positive H2S 66.7%, SE 3.6%) (Table 2).
Table 2.
Summary of total coliform and H2S indicators for village source and household water samples measured over a 12-month period in rural Tamil Nadu, India
| Water sample | N | Log10 total coliforms | H2S proportion positive | ||
|---|---|---|---|---|---|
| Characteristics | Mean | SE | Mean | SE | |
| Village source samples | |||||
| Overall | 695 | 2.974 | 0.062 | 0.586 | 0.034 |
| Village water source | |||||
| Hand pump (tube well) | 201 | 2.579 | 0.099 | 0.363 | 0.048 |
| Large overhead tank | 304 | 3.059 | 0.070 | 0.641 | 0.042 |
| Public mini-tank | 122 | 3.286 | 0.071 | 0.754 | 0.038 |
| Other | 68 | 3.194 | 0.126 | 0.691 | 0.058 |
| Household samples | |||||
| Overall | 3,026 | 3.386 | 0.023 | 0.843 | 0.010 |
| Household water source | |||||
| Private tap | 875 | 3.381 | 0.026 | 0.826 | 0.013 |
| Public tap | 1,920 | 3.385 | 0.029 | 0.848 | 0.013 |
| Private well | 78 | 3.487 | 0.088 | 0.962 | 0.015 |
| Public well | 129 | 3.396 | 0.079 | 0.860 | 0.032 |
| Other | 24 | 3.286 | 0.128 | 0.542 | 0.090 |
| Storage time, hours | |||||
| 0–1 | 71 | 3.326 | 0.087 | 0.803 | 0.042 |
| 2–3 | 106 | 3.296 | 0.077 | 0.821 | 0.036 |
| 4–5 | 949 | 3.478 | 0.033 | 0.863 | 0.009 |
| 6–7 | 1,254 | 3.391 | 0.031 | 0.829 | 0.018 |
| 8–9 | 499 | 3.262 | 0.052 | 0.856 | 0.024 |
| ≥ 10 | 139 | 3.277 | 0.083 | 0.849 | 0.039 |
| Unknown | 8 | 3.039 | 0.160 | 0.375 | 0.124 |
| Storage container type | |||||
| Directly from tap | 11 | 2.920 | 0.286 | 0.455 | 0.136 |
| Narrow mouth | 488 | 3.303 | 0.036 | 0.814 | 0.019 |
| Wide mouth | 2,527 | 3.404 | 0.025 | 0.850 | 0.010 |
| Self-reported boiling | |||||
| Not boiled | 2,735 | 3.410 | 0.025 | 0.862 | 0.011 |
| Boiled | 291 | 3.162 | 0.069 | 0.667 | 0.036 |
Figure 1.
Village-level mean water quality indicators in village source and household samples. Total coliforms (top panel) and H2S (bottom panel). Arrows between points indicate the direction of change between household sample means and village sample means. N = 3,026 household samples; N = 695 village source samples.
Comparisons of H2S to other microbial water quality indicators.
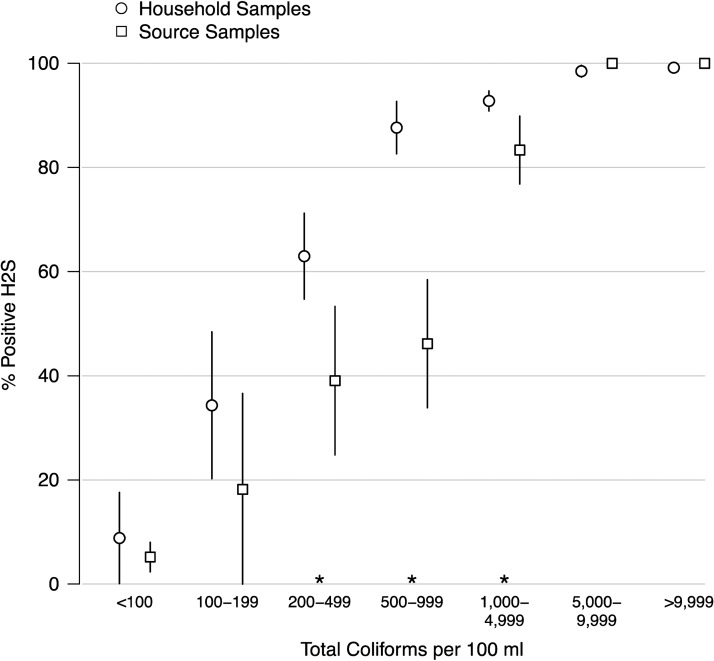
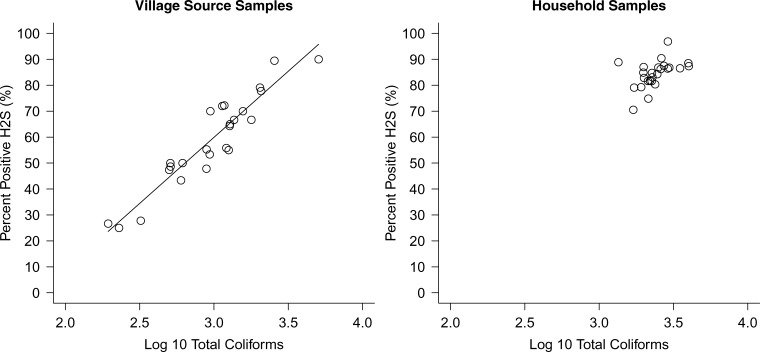
Our comparisons of H2S test results and total coliform contamination showed that H2S was increasingly sensitive to total coliform levels that fell in the intermediate to high range in this setting: 90% (328 of 363) of village source samples and 96% (2,148 of 2,232) of household drinking water samples that contained total coliform CFUs ≥ 1,000/100 mL were also H2S positive (Figure 2 ). For total coliform concentrations between 200 and 4,999 CFUs/100 mL, however, H2S was consistently more sensitive to total coliform contamination in household samples than in village source samples (Figure 2). H2S also showed specificity for total coliforms, with the proportion of positive H2S tests dropping to 10% in samples containing < 100 CFUs/100 mL total coliforms (Figure 2). Village-level aggregations of water quality data showed a strong relationship between the percentage of positive H2S tests and mean total coliform levels for village source samples in the concentrations observed (R2 = 0.87); household samples did not vary greatly in their average levels of contamination (Figure 3 ).
Figure 2.
Percent positive H2S tests by total coliform concentration for household and village source samples. Vertical lines are robust 95% confidence intervals. Asterisks (*) mark statistically different means between household and village source samples at the 95% confidence level. N = 3,026 household samples; N = 695 village source samples.
Figure 3.
Relationship between the percentage of H2S positive tests and log10 total coliform concentration for village source samples (left) and household samples (right) using mean concentrations at the village level. N = 695 village source samples; N = 3,026 household samples. The fitted line in the village source samples is a weighted least squares fit with parameters H2S = −92.9 + 51.0 × log10TC, R2 = 0.87. The parameters fit including both village (left) and household samples (right) was similar: H2S = −90.2 + 51.3 × log10TC, R2 = 0.81, and the fit was not greatly improved using a logistic instead of linear model.
In contrast to total coliforms, H2S was sensitive to low levels of E. coli contamination: 96.4% (95% CI 89.9, 99.3) of household water samples with 10–99 E. coli CFUs/100 mL and 100% (95% CI 91.4, 100.0) of household water samples with ≥ 100 E. coli CFUs/100 mL tested positive for H2S (Table 3). The H2S test, however, was not specific for E. coli contamination: 53.3% (95% CI 48.7, 57.9) of household water samples containing < 10 CFUs/100 mL were also positive for H2S (Table 3).
Table 3.
H2S positive test results for different Escherichia coli concentrations in survey round 3*
| Escherichia coli (CFUs/100 mL) | N | H 2S+ | % Positive | 95% CI |
|---|---|---|---|---|
| Village source samples | ||||
| < 10 | 126 | 3 | 2.4 | (0.5, 6.8) |
| 10–99 | 8 | 0 | 0.0 | (0.0, 36.9) |
| ≥ 100 | 3 | 1 | 33.3 | (0.8, 90.6) |
| Household samples | ||||
| < 10 | 465 | 248 | 53.3 | (48.7, 57.9) |
| 10–99 | 84 | 81 | 96.4 | (89.9, 99.3) |
| ≥ 100 | 41 | 41 | 100.0 | (91.4, 100.0) |
Results are stratified by village source and household source water samples.
CFU = colony forming units; CI = confidence interval.
Positive and negative predictive values of H2S for total coliform and E. coli contamination.
We analyzed the H2S test's ability to predict total coliform and E. coli contamination by comparing the levels of these indicator species between H2S positive and H2S negative water samples (Table 4). Almost all H2S positive samples contained ≥ 100 total coliform CFUs/100 mL: the PPV was 99% for both village source and household samples (Table 4). Only about 50% of H2S negative samples, however, contained < 100 total coliform CFUs/100 mL: the NPV for total coliforms < 100 CFUs/100 mL was 46% for village source samples and 51% for household samples (Table 4). In contrast, a positive H2S result was not highly predictive of E. coli contamination: the PPV of the H2S test for E. coli concentrations ≥ 100 CFUs/100 mL was 17% for village samples and 23% for household samples (Table 4). The NPV of the H2S test for < 100 CFUs/100 mL E. coli was 97% for both village source and household water samples (Table 4).
Table 4.
Positive predictive value (PPV) and negative predictive value (NPV) of the H2S test for village and household water samples using different indicators and a cutoff point of 100 CFU/100 mL, measured over a 12-month period in rural Tamil Nadu, India*
| Indicator cutoff, sample type | N H2S+ | PPV % | (95% CI) | N H2S− | NPV % | (95% CI) |
|---|---|---|---|---|---|---|
| Total coliforms > 100 CFU/100 mL | ||||||
| Village samples | 407 | 99 | (98, 100) | 287 | 46 | (36, 55) |
| Household samples | 2,546 | 99 | (98, 100) | 469 | 51 | (45, 56) |
| Escherichia coli > 100 CFU/100 mL | ||||||
| Village samples | 407 | 17 | (10, 24) | 287 | 97 | (95, 99) |
| Household samples | 2,546 | 23 | (20, 25) | 469 | 97 | (96, 99) |
CI = confidence interval; CFU = colony forming units.
Microbial water quality indicator associations with child diarrhea.
Diarrhea prevalence among children < 5 years of age was 1.8% over the entire study period, 2.4% in the 4,166 random subsample of visits that included water testing, and 1.4% in household visits from the same time points but without water sample collection (2.4% versus 1.4%, P < 0.0001; seasonally adjusted difference = 2.3% versus 1.8%, P = 0.06). We observed slightly higher diarrhea prevalence at times with a positive H2S result compared with a negative H2S result (2.5% versus 2.1%), but found no evidence for an association between a positive H2S test result and child diarrhea (adjusted PR = 1.13; 95% CI 0.53, 2.40) (Table 5). In addition, we did not identify associations between high levels of total coliforms (> 10,000 CFUs/100 mL) and child diarrhea (adjusted PR = 1.01, 95% CI 0.57, 1.80), and although we observed higher diarrhea prevalence at higher concentrations of E. coli, the PRs were not different from 1.0 at the 95% confidence level (Table 5). When we re-estimated the association between E. coli levels and negative control respiratory symptoms, we found a similar increase in risk associated with higher E. coli concentrations, which suggests that at least part of the association observed between E. coli and diarrhea could be the result of incomplete confounding control (Table 6).
Table 5.
Association between water quality indicator levels and diarrhea among children < 5 years of age measured over a 12-month period in rural Tamil Nadu, India*
| Indicator level | N† | Diarrhea % | Unadjusted PR (95% CI) | Adjusted PR (95% CI) |
|---|---|---|---|---|
| H2S | ||||
| Negative | 662 | 2.1 | Ref | Ref |
| Positive | 3,504 | 2.5 | 1.17 (0.54, 2.56) | 1.13 (0.53, 2.40) |
| Total coliforms | ||||
| ≤ 10,000 | 3,150 | 2.6 | Ref | Ref |
| > 10,000 | 1,002 | 1.9 | 0.73 (0.45, 1.17) | 1.01 (0.57, 1.80) |
| Escherichia coli | ||||
| ≤ 100 | 3,509 | 2.3 | Ref | Ref |
| 101–1,000 | 488 | 2.7 | 1.14 (0.58, 2.23) | 1.23 (0.67, 2.28) |
| > 1,000 | 155 | 3.9 | 1.66 (0.65, 4.21) | 2.16 (0.82, 5.71) |
PR = prevalence ratio; CI = confidence interval; Ref = reference group.
N = child weeks of observation collected from 1,284 children.
Table 6.
The association between water quality indicators and negative control respiratory outcomes among children < 5 years of age measured over a 12-month period in rural Tamil Nadu, India*
| Indicator level | N† | Cough % | Adjusted PR (95% CI) | Congestion/coryza % | Adjusted PR (95% CI) |
|---|---|---|---|---|---|
| H2S | |||||
| Negative | 662 | 8.5 | Ref | 32.9 | Ref |
| Positive | 3,504 | 7.1 | 0.93 (0.70, 1.24) | 31.2 | 0.97 (0.85, 1.10) |
| Total coliforms | |||||
| ≤ 10,000 | 3,150 | 6.8 | Ref | 30.0 | Ref |
| > 10,000 | 1,002 | 9.1 | 1.21 (0.94, 1.55) | 36.2 | 1.09 (0.98, 1.21) |
| E. coli | |||||
| ≤ 100 | 3,509 | 7.3 | Ref | 31.1 | Ref |
| 101–1,000 | 488 | 6.8 | 0.89 (0.60, 1.30) | 32.6 | 1.01 (0.88, 1.16) |
| > 1,000 | 155 | 11.0 | 1.42 (0.80, 2.55) | 38.7 | 1.15 (0.97, 1.38) |
PR = prevalence ratio; CI = confidence interval; Ref = reference group.
N = child weeks of observation collected from 1,284 children.
Discussion
Summary of main results.
In this large-scale, longitudinal study of water quality indicators and child health in rural Southern India, we found that microbial contamination of village water sources was common and that contamination levels increased after collection by households (Figure 1). The H2S test was sensitive to microbial water contamination measured by total coliforms at concentrations ≥ 1,000 CFUs/100 mL (Figure 2) and by E. coli at concentrations ≥ 10 CFUs/100 mL (Table 3). Aggregation of water quality measures at the village-level showed a strong relationship between the percentage of positive H2S tests and total coliform concentrations observed (Figure 3). Diarrhea prevalence was relatively low in this population (1.8% over 12 months), and we found no consistent association between water quality indicators and diarrhea among children < 5 years of age (Table 5).
Interpretations.
Comparison of H2S with total coliforms and E. coli.
Our analysis found that water samples with ≥ 1,000 CFUs/100 mL total coliforms and/or ≥ 10 CFUs/100 mL E. coli were likely to test positive for the presence of H2S (Figure 2, Table 3). On the other hand, only samples with low total coliforms concentrations (< 100 CFUs/100 mL) were likely to be negative for H2S (Figure 2): among household samples with low E. coli concentrations (< 10 CFUs/100 mL), the proportion of H2S positive samples was 53% (Table 3). In addition, the PPV of the H2S test was high for total coliforms and low for E. coli (Table 4). Together, these results suggest that the presence of H2S in a water sample was associated with total coliform species (at levels ≥ 1,000 CFUs/100 mL) that may have included E. coli but that were not restricted to E. coli.
Our analysis of the NPV of the H2S test, however, showed that a large fraction of H2S negative water samples also contained total coliforms ≥ 100 CFUs/100 mL, although, they were unlikely to contain E. coli ≥ 100 CFUs/100 mL (Table 4). The low NPV of the H2S test for total coliforms < 100 CFUs/100 mL is likely explained by the poor sensitivity of the H2S test for total coliform levels < 1,000 CFUs/100 mL (Figure 2). The high NPV of H2S for E. coli < 100 CFUs/100 mL may be caused by the relatively high percentage of samples with low levels of E. coli contamination (Table 3).
We also observed that under conditions where repeated H2S presence/absence tests were recorded, the percentage that were positive for H2S provided a quantitative measure of contamination that is strongly related to log10 total coliform concentrations (Figure 3). Our results suggest that in source monitoring applications, repeated presence/absence H2S samples could be averaged to provide a quantitative measure of source water quality.
Differences between H2S sensitivity for total coliforms in source versus household samples.
The higher sensitivity of the H2S test for total coliform contamination in household water samples versus village source samples suggests that the composition of total coliform species changed after water was collected and stored in households (Figure 2). This change in total coliform composition might be linked to the consistent increase in total coliform levels after collection (Figure 1), which likely included household-level fecal contamination resulting from poor hygiene and water handling practices. An increase in fecal contamination after collection was also indicated by the increase in the proportion of water samples with ≥ 10 E. coli CFUs/100 mL from 8.0% (11 of 137) in village source samples to 21% (125 of 590) in household samples (Table 3). Consequently, we hypothesize that the higher sensitivity of the H2S test for total coliforms in household samples is caused by a greater proportion of fecal coliforms in these samples. Our hypothesis assumes that fecal coliforms are more likely to include H2S producers than environmental coliforms, which is supported by a recent report of the consistent detection of multiple species of fecal microorganisms in H2S positive environmental water samples.24 A study that directly compares H2S test results with fecal microorganism levels in village source and household water samples could test this hypothesis directly.
Poor microbial water quality was not associated with child diarrhea.
In this study, we found no consistent association between water quality indicators and diarrhea among children < 5 years of age (Table 5). A lack of association between water quality indicators and child diarrhea is consistent with findings from studies in Bangladesh,15 Pakistan,17 and Ecuador19 but stands in contrast to findings from studies in the Philippines16 and Cambodia.18 These variable results between study sites are not unexpected given regional differences in the non-fecal contributions to microbial indicator levels and in the relative importance of drinking water as a disease transmission pathway.19 Our observation of low child diarrhea prevalence despite poor microbial water quality suggests that although the ground water sources in the study region are exposed to environmental microbial contamination, basic protection from fecal contamination may be sufficient to prevent the majority of water source-transmitted disease risk.34 The consistent increase in household-level microbial contamination (Figure 1), however, indicates that water contamination during storage remains a potential transmission pathway for pathogens that could facilitate rapid disease spread during a diarrheal disease outbreak.
Is the H2S test appropriate for monitoring microbial water quality in low resource settings?
As shown in this and other studies, microbial water quality indicators are often poor surrogates for the actual health risks associated with drinking water.2,14,19 Given the diversity of pathogens that cause diarrhea and the multiple exposure pathways to these pathogens (drinking water, food, hands, and other contaminated surfaces), the inconsistent associations between a limited set of microbial water quality indicators and disease risk is not surprising. It is likely that accurate evaluations of the health risks associated with a drinking water supply will require direct assessments of pathogen levels. Methods for detecting and quantifying the full spectrum of waterborne pathogens, however, are difficult to implement in low-resource settings, and water quality management is increasingly focused on minimizing risk by reducing the vulnerability of drinking water supplies to contamination.35,36
Culture-based and molecular analysis of the bacteria present in positive H2S tests indicate that H2S production is correlated with species of fecal origin, however these species are not limited to the coliform group.23,24,37 Nevertheless, many evaluations of the H2S test as a microbial water quality indicator, including this one, have evaluated H2S test results based on their correspondence with assays for total coliforms, fecal coliforms, or E. coli.22,38 Interpreting the results of these evaluations is difficult because they do not consider the impact of non-coliform fecal species on H2S test results. For example, positive H2S test results in the absence of detectable coliform contamination are classified as “false positives,” despite the possibility that non-coliform fecal bacteria may have contributed to H2S production.22,38–40 In addition, culture-based assays for coliform species, particularly methods developed for field use, do not always produce comparable results.19 It is likely that differences in the microbial ecology of water supplies and in the performance of coliform assays have both contributed to the variable associations reported between H2S and coliform test results.22
Despite the challenges of evaluating H2S as a fecal indicator based solely on comparisons with coliform assays, our results show that in rural southern India, H2S was associated with higher levels of total coliform contamination (Figure 2). In addition, the percentage of positive H2S water samples from water sources showed a strong relationship with total coliform levels (Figure 3). Total coliforms are the accepted process indicators for water quality management: rather than providing a direct measure of health risks, they are used to determine the vulnerability of water supplies to possible pathogen contamination.7 However, quantitative comparisons of coliform contamination between different water supplies or before and after supply improvements are often not feasible in low-resource settings because of the cost and complexity of approved diagnostic methods. Our results indicate that presence/absence H2S tests provide a viable alternative for assessing both water supply vulnerabilities and improvement efforts.
Limitations.
A limitation of our water collection approach was that field staff collected water samples during child disease surveillance visits. This design and analysis assumes that water quality conditions, as measured at the time illness is ascertained, reflect water quality conditions that led to the illness episode. This design limitation is shared by all other studies that have estimated the association between water quality indicators and child health to date.16–19 The lack of temporal ordering between exposure and outcome could be subject to bias, if caregivers boil water or change their water management in response to the onset of illness.
In such a scenario, the association between water contamination and illness could be biased toward the null. In this study, 21.8% of drinking water samples were reportedly boiled when collected during visits when study children had diarrhea versus 9.7% of samples collected during visits without diarrhea (P < 0.0001 for difference). Although our adjusted estimates of the PR conditioned on whether the water was reportedly boiled (in an attempt to block this potential pathway), regression adjustment is an imperfect solution to this problem and residual bias could exist. Indeed, water samples were also more likely to be reported boiled when children had cough (17.3% versus 9.4%, P = 0.012) and congestion/coryza (13.1% versus 8.5%, P = 0.001), yet we observed a positive association between household water quality and these negative control outcomes suggesting the potential for residual bias (Table 6).
Furthermore, diarrhea prevalence was significantly higher in the random subsample of household visits where water samples were collected, after adjusting for season (2.3% versus 1.8%), despite the water sample collection occurring at the end of the interview. This suggests that caregivers may have over reported diarrhea in visits with water sample collection, leading to possible misclassification bias in the outcome. A more rigorous design would collect water samples and then measure child illness in the subsequent 7–14 days to avoid a potential reversal of the cause and its effect and to reduce the chance for biased outcome measurements.
The low prevalence of child diarrhea in our study population limited the statistical power of this study to estimate associations between microbial water quality indicators and diarrhea. Although caregiver-reported illness could be subject to under-reporting,27 we validated the community-based diarrhea surveillance with clinic records in the study villages.25
With respect to water quality analysis, we conducted a few initial side-by-side comparisons of our total coliform and E. coli diagnostics with Coliscan membrane filter medium (Micrology Laboratories CMFK2; Micrology Laboratories, Goshen, Indiana). However, we did not conduct a thorough comparison with approved methods (e.g., Standard Methods in the United States) for detecting total coliforms and E. coli. Similarly, we did not compare the performance of our selected H2S assay with other H2S tests. As a result, it is possible that poor performance of our reagents generated under- or over-estimates of the levels of each indicator. Nevertheless, we note that relative levels would have remained consistent through our study.
We also note that as a result of variability in water characteristics, sample handling, and analysis, assaying a single water sample will not always provide an accurate measurement of microbial water quality. We attempted to reduce the effects of this measurement variability by analyzing a large number of water samples over a 12-month study period. The consistent relationships that we observed between source and household water quality and between total coliform levels and H2S presence suggest that measurement errors were limited. Nevertheless, it remains possible that inaccuracies in water quality results reduced our ability to detect associations between indicator levels and diarrhea, particularly since diarrhea prevalence was low.
Conclusions.
Child diarrhea prevalence was surprisingly low in our study population (1.8%) despite poor microbial drinking water quality, as measured by H2S presence and by total coliforms and E. coli indicator levels. These findings suggest that waterborne pathogens were not a principal cause of diarrhea during the study period. However, the poor microbial drinking water quality indicated that water supplies were vulnerable to fecal contamination and could facilitate pathogen transmission during disease outbreaks. The relationships between total coliform contamination and H2S test results indicate that verified presence/absence H2S tests provide simple and cost-effective alternatives to quantitative total coliform assays, both for assessing the vulnerability of water supplies to fecal contamination and for evaluating water quality management efforts. Because of the increasing emphasis on maintaining drinking water quality through risk mitigation, our results support the use of presence/absence H2S tests for water supply management in low-resource settings.
ACKNOWLEDGMENTS
The Imayam College of Arts and Sciences, Thuraiyur, generously provided laboratory facilities for the duration of this study. Water.org and Gramalaya facilitated research in villages where they had conducted water/sanitation/hygiene interventions. Professor Kara Nelson, University of California, Berkeley, provided insightful comments on initial interpretations of our results. We especially thank the field research team from Tiruchirappalli, Tamil Nadu for their effort and diligence in data collection.
Footnotes
Financial support: This study was supported by a grant from the Open Square Foundation to The Aquaya Institute.
Authors' addresses: Ranjiv S. Khush, The Aquaya Institute, San Francisco, CA, E-mail: ranjiv@aquaya.org. Benjamin F. Arnold, School of Public Health, University of California, Berkeley, CA, E-mail: benarnold@berkeley.edu. Padma Srikanth and Suchithra Sudharsanam, Department of Microbiology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India, E-mails: srikanth_padma@rediffmail.com and suchisanam_84@yahoo.com. Padmavathi Ramaswamy and Prabhakar Ramaprabha, Department of Physiology, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India, E-mails: drpadmavathi@yahoo.com and abyrr@yahoo.com. Natesan Durairaj, Kalpana Balakrishnan, and Paramasivan Rajkumar, Department of Environmental and Health Engineering, Sri Ramachandra Medical College and Research Institute, Chennai, Tamil Nadu, India, E-mails: n_durai2008@yahoo.com, kalpanasrmc@gmail.com, and rkpk16@yahoo.co.in. Alicia G. London, San Francisco, CA, E-mail: alicialgray@gmail.com. John M. Colford Jr., School of Public Health, University of California, Berkeley, CA, E-mail: jcolford@berkeley.edu.
References
- 1.Prüss-Üstün A, Bos R, Gore F, Bartram J. Safer Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health. Geneva: WHO; 2008. [Google Scholar]
- 2.Gundry S, Wright J, Conroy R. A systematic review of the health outcomes related to household water quality in developing countries. J Water Health. 2004;2:1–13. [PubMed] [Google Scholar]
- 3.Cutler D, Miller G. The role of public health improvements in health advances: the twentieth-century United States. Demography. 2005;42:1–22. doi: 10.1353/dem.2005.0002. [DOI] [PubMed] [Google Scholar]
- 4.Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM. Water, sanitation, and hygiene interventions to reduce diarrhea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 5.Clasen T, Schmidt W-P, Rabie T, Roberts I, Cairncross S. Interventions to improve water quality for preventing diarrhea: systematic review and meta-analysis. BMJ British Medical Journal. 2007;334:782–792. doi: 10.1136/bmj.39118.489931.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . Guidelines for Drinking-Water Quality. Fourth edition. Geneva: World Health Organization; 2011. [Google Scholar]
- 7.Ashbolt N, Grabow W, Snozzi M. Indicators of microbial water quality: water quality. Fewtrell L, Bartram J, eds. Water Quality: Guidelines, Standards and Health. London: World Health Organization/IWA Publishing; 2001. pp. 289–314. [Google Scholar]
- 8.Rahman Z, Crocker J, Chang K, Khush R, Bartram J. A comparative assessment of institutional frameworks for managing drinking water quality. Journal of Water Sanitation Hygiene and Develop. 2011;1:242–258. [Google Scholar]
- 9.MacKenzie WR, Schell WL, Blair KA, Addiss DG, Peterson DE, Hoxie NJ, Kazmierczak JJ, Davis JP. Massive outbreak of waterborne cryptosporidium infection in Milwaukee, Wisconsin: recurrence of illness and risk of secondary transmission. Clin Infect Dis. 1995;21:57–62. doi: 10.1093/clinids/21.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Solo-Gabriele HM, Wolfert MA, Desmarais TR, Palmer CJ. Sources of Escherichia coli in a coastal subtropical environment. Appl Environ Microbiol. 2000;66:230–237. doi: 10.1128/aem.66.1.230-237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera SC, Hazen TC, Toranzos GA. Isolation of fecal coliforms from pristine sites in a tropical rain forest. Appl Environ Microbiol. 1988;54:513–517. doi: 10.1128/aem.54.2.513-517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiménez L, Muñiz I, Toranzos GA, Hazen TC. Survival and activity of Salmonella typhimurium and Escherichia coli in tropical freshwater. J Appl Bacteriol. 1989;67:61–69. doi: 10.1111/j.1365-2672.1989.tb04955.x. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council . Health Effects Assessment. Committee on Indicators for Waterborne Pathogens; Board on Life Sciences; Water Science and Technology Board, ed. Indicators for Waterborne Pathogens. Washington, DC: The National Academies Press; 2005. pp. 53–108. [Google Scholar]
- 14.Wu J, Long SC, Das D, Dorner SM. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health. 2011;9:265–278. doi: 10.2166/wh.2011.117. [DOI] [PubMed] [Google Scholar]
- 15.Henry FJ, Huttly SR, Patwary Y, Aziz KM. Environmental sanitation, food and water contamination and diarrhea in rural Bangladesh. Epidemiol Infect. 2009;104:253–259. doi: 10.1017/s0950268800059422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moe CL, Sobsey MD, Samsa GP, Mesolo V. Bacterial indicators of risk of diarrheal disease from drinking-water in the Philippines. Bull World Health Organ. 1991;69:305–317. [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen PK, Jayasinghe G, Van der Hoek W, Cairncross S, Dalsgaard A. Is there an association between bacteriological drinking water quality and childhood diarrhea in developing countries? TM & IH. 2004;9:1210–1215. doi: 10.1111/j.1365-3156.2004.01329.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown JM, Proum S, Sobsey MD. Escherichia coli in household drinking water and diarrheal disease risk: evidence from Cambodia. Water Sci Technol. 2008;58:757–763. doi: 10.2166/wst.2008.439. [DOI] [PubMed] [Google Scholar]
- 19.Levy K, Nelson KL, Hubbard A, Eisenberg JN. Rethinking indicators of microbial drinking water quality for health studies in tropical developing countries: case study in northern coastal Ecuador. Am J Trop Med Hyg. 2012;86:499–507. doi: 10.4269/ajtmh.2012.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manja K, Maurya M, Rao K. A simple field test for the detection of fecal pollution in drinking water. Bull World Health Organ. 1982;60:797–801. [PMC free article] [PubMed] [Google Scholar]
- 21.Rajiv Gandhi National Drinking Water Mission . Guidelines for National Rural Drinking Water Quality Monitoring and Surveillance Programme. New Delhi: Department of Drinking Water Supply, Ministry of Rural Development, Government of India; 2006. [Google Scholar]
- 22.Wright JA, Yang H, Walker K, Pedley S, Elliott J, Gundry SW. The H2S test versus standard indicator bacteria tests for fecal contamination of water: systematic review and meta-analysis. TM & IH. 2012;17:94–105. doi: 10.1111/j.1365-3156.2011.02887.x. [DOI] [PubMed] [Google Scholar]
- 23.Castillo G, Duarte R, Ruiz Z, Marucic MT, Honorato B, Mercado R, Coloma V, Lorca V, Martins MT, Dutka BJ. Evaluation of disinfected and untreated drinking water supplies in Chile by the H2S paper strip test. Water Res. 1994;28:1765–1770. [Google Scholar]
- 24.McMahan L, Grunden AM, Devine AA, Sobsey MD. Evaluation of a quantitative H2S MPN test for fecal microbes analysis of water using biochemical and molecular identification. Water Res. 2012;46:1693–1704. doi: 10.1016/j.watres.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 25.Arnold BF, Khush RS, Ramaswamy P, London AG, Rajkumar P, Ramaprabha P, Durairaj N, Hubbard AE, Balakrishnan K, Colford JM. Causal inference methods to study nonrandomized, preexisting development interventions. Proc Natl Acad Sci USA. 2010;107:22605–22610. doi: 10.1073/pnas.1008944107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baqui AH, Black RE, Yunus M, Hoque AR, Chowdhury HR, Sack RB. Methodological issues in diarrheal diseases epidemiology: definition of diarrheal episodes. Int J Epidemiol. 1991;20:1057–1063. doi: 10.1093/ije/20.4.1057. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt W-P, Arnold BF, Boisson S, Genser B, Luby SP, Barreto ML, Clasen T, Cairncross S. Epidemiological methods in diarrhea studies–an update. Int J Epidemiol. 2011;40:1678–1692. doi: 10.1093/ije/dyr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldman N, Vaughan B, Pebley AR. The use of calendars to measure child illness in health interview surveys. Int J Epidemiol. 1998;27:505–512. doi: 10.1093/ije/27.3.505. [DOI] [PubMed] [Google Scholar]
- 29.Altman DG, Bland JM. Diagnostic tests 2: predictive values. BMJ. 1994;309:102. doi: 10.1136/bmj.309.6947.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 31.Freedman DA. Statistical models for causation: what inferential leverage do they provide? Eval Rev. 2006;30:691–713. doi: 10.1177/0193841X06293771. [DOI] [PubMed] [Google Scholar]
- 32.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WHO/UNICEF Joint Monitoring Program . Progress on Drinking Water and Sanitation: 2012 Update. New York: UNICEF & WHO: 2012. [Google Scholar]
- 34.Lloyd B, Bartram J. Surveillance solutions to microbiological problems in water quality control in developing countries. Water Sci Technol. 1991;24:61–75. [Google Scholar]
- 35.Howard G. Water safety plans for small systems: a model for applying HACCP concepts for cost-effective monitoring in developing countries. Water Sci Technol. 2003;47:215–220. [PubMed] [Google Scholar]
- 36.Davison A, Howard G, Stevens M, Callan P. Water Safety Plans. Geneva: WHO; 2004. [Google Scholar]
- 37.Ratto A, Dutka B, Vega C, Lopez C, El-Shaarawi A. Potable water safety assessed by coliphage and bacterial tests. Water Res. 23. 1989:253–255. [Google Scholar]
- 38.Sobsey M, Pfaender F. Evaluation of the H2S Method for Detection of Fecal Contamination of Drinking Water. Geneva: WHO; 2002. [Google Scholar]
- 39.Roser DJ, Ashbolt N, Ho G, Mathew K, Nair J, Ryken-Rapp D, Toze S. Hydrogen sulphide production tests and the detection of groundwater fecal contamination by septic seepage. Water Sci Technol. 2005;51:291–300. [PubMed] [Google Scholar]
- 40.Gupta SK, Sheikh MA, Islam MS, Rahman KS, Jahan N, Rahman MM, Hoekstra RM, Johnston R, Ram PK, Luby S. Usefulness of the hydrogen sulfide test for assessment of water quality in Bangladesh. J Appl Microbiol. 2008;104:388–395. doi: 10.1111/j.1365-2672.2007.03562.x. [DOI] [PubMed] [Google Scholar]