Abstract
The study of relapsing fever borreliae in Africa has long suffered from the use of non-specific laboratory tools for the direct detection of these spirochetes in clinical and vector specimens. Accordingly, Borrelia hispanica, Borrelia crocidurae, Borrelia duttonii, and Borrelia recurrentis have traditionally been distinguished on the basis of geography and vector and the unproven hypothesis that each species was exclusive to one vector. The recent sequencing of three relapsing fever Borrelia genomes in our laboratory prompted the development of more specific tools and a reappraisal of the epidemiology in Africa. Five additional potential species still need to be cultured from clinical and vector sources in East Africa to further assess their uniqueness. Here, we review the molecular evidence of relapsing fever borreliae in hosts and ectoparasites in Africa and explore the diversity, geographical distribution, and vector association of these pathogens for Africans and travelers to Africa.
Introduction
Relapsing fever borreliae are a group of ectoparasite-borne, fastidious bacteria responsible for various febrile presentations, most commonly malaria-like symptoms.1 In Africa, Arnould, a French Army doctor, first clinically described relapsing fever among prisoners near Constantine, Algeria in March 1866.2 Two years later, borreliae were observed microscopically by Obermeier in the blood of relapsing fever patients.3 This observation was confirmed by a subsequent study4 and borreliae were further detected in Ornithodoros moubata ticks.5 The role of lice in the transmission of what is currently called louse-borne relapsing fever was hypothesized6 and experimentally demonstrated7 in the early 20th century. Because relapsing fever borreliae remained uncultured, they were classified on the basis of their vectors and virulence in animal models.8 Indeed, Borrelia hispanica remained uncultured in axenic medium until 1976,9 Borrelia recurrentis until 1994,10 and Borrelia duttonii until 199911; Borrelia crocidurae, first described in musk shrew blood in Senegal in 1917,12 was only cultured in axenic medium in 1999.13 Other relapsing fever borreliae have been identified along the eastern border of Africa in Eurasia, chiefly Borrelia persica in Israel and the Palestinian territories,14 Iran15 and Uzbekistan and Tajikistan,16 and Borrelia microti in Iran.17 Because these species have not been reported in the African continent itself, they will not be discussed in this review.
In 2008–2011, our laboratory sequenced the genomes of three of the four known cultured Borrelia from Africa, i.e., B. duttonii, B. recurrentis,18 and B. crocidurae.19 Recent efforts to culture and sequence relapsing fever borreliae have provided new information for a reassessment of the diversity of these bacteria. In this review, we consider the molecular evidence of relapsing fever borreliae in hosts and ectoparasites in Africa and explore the diversity, geographical distribution, and vector association of these pathogens for Africa and travelers to Africa.
Clinical Features
The main symptom characteristic of relapsing fever borreliosis is recurrent febrile episodes interrupted by afebrile periods. For the louse-borne B. recurrentis, fever is accompanied in more than 90% of patients by tachycardia, headache, myalgia, and arthralgia and is less frequently accompanied by hepatosplenomegaly, epistaxis, petechial rash, and jaundice. For the tick-borne B. crocidurae, the disease is characterized by a fever, asthenia, and vomiting in some patients. Most infected patients experience 1 to 2 relapses; however, up to eight relapses have been observed. The clinical signs and density of Borrelia are not affected by the age or sex of the patient. Immunity after infection is not permanent and patients may be newly infected as soon as 6 months after recovery. However, no deaths from borreliae were recorded in Senegal over a period of 14 years.20 In this country, 0.9% of 1,340 children were smear positive,21 and real-time PCR for the 16S rRNA Borrelia gene detected borreliae in 27 (13%) of 206 samples from febrile patients in rural Senegal.22 The clinical features of B. duttonii infection have been well studied in Tanzania, in which the total mortality rate of the disease is ∼2.3%.23 Symptoms of this pathogen include fever, which is accompanied in more than 90% of cases by tachycardia, headache, myalgia, arthralgia, conjunctivitis, hepatomegaly, and splenomegaly, along with orange urine in a few cases.24 In Tanzania, investigators found a perinatal mortality caused by B. duttonii of 436/1,000 births.25 In North Africa, B. hispanica causes 20.5% of unexplained fever cases in Northwestern Morocco.26
Relapsing fever borrelioses are easily treatable by penicillin, doxycycline, and tetracycline27 but the Jarisch-Herxheimer reaction, characterized by an increased respiratory rate and drop in blood pressure, is a major side effect of antibiotic treatment, causing a mortality rate of up to 5%.28,29 This reaction is associated with the release of cytokines during the clearance of borreliae from the blood.30 A few studies indicated that the administration of antibodies against tumor necrosis factor-alpha does prevent Jarisch-Herxheimer reaction during relapsing fever,31,32 however such expensive medications are not currently available in African countries where relapsing fevers are endemic. Because field experience in Africa has indicated that relapsing fevers are readily cured by antibiotics, there has been no need for the in vitro susceptibility testing of antibiotics. Nevertheless, ceftriaxone clears B. duttonii in a mouse model of infection.33
Investigating Relapsing Fever Borreliae In Africa
Phenotypic analyses.
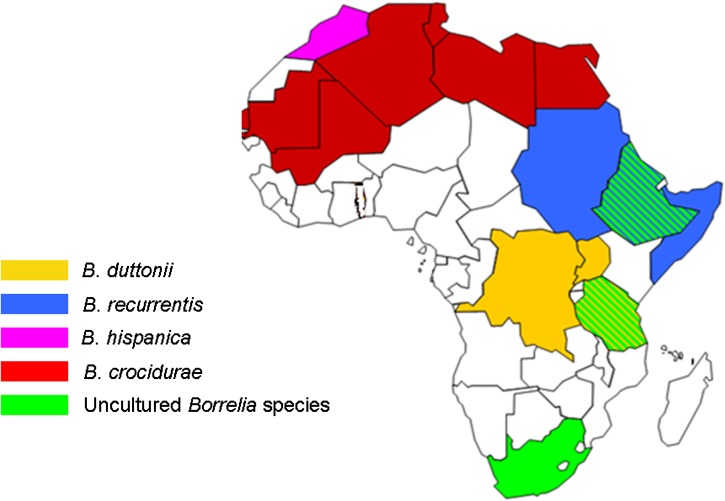
The relapsing fever borreliae species were initially distinguished on the basis of geography and vector34; this classification was based on a cospeciation hypothesis that postulated that only one relapsing fever Borrelia species could be found in a particular host and vector in a given geographic area. However, recent demonstrations of the coexistence of B. duttonii and B. crocidurae in Togo35 and of B. crocidurae and B. hispanica in North Africa36 suggest that the previous geographical distribution studies were not comprehensive. Further microscopic observation of relapsing fever borreliae isolated from vectors and the blood of patients after Giemsa staining did not help to distinguish the species, nor did observations of characteristic morphology and motility by dark field microscopy, phase contrast microscopy, and electron microscopy.10 The various relapsing fever borreliae exhibit similar sizes and morphology. Further phenotypic characterization of relapsing fever borreliae isolated and cultured by xenoisolation in mice or in axenic, artificial culture medium37 is also unhelpful. In particular, matrix-assisted laser desorption ionization time-of-flight mass spectrometry identification has not been reported for relapsing fever borreliae, in contrast with other spirochetes.38 Phenotypic traits, therefore, do not discriminate between cultured African relapsing fever borreliae (Figure 1).
Figure 1.
Geographical distribution of African relapsing fever of the Borrelia species.
Genetic analyses.
Relatively few genetic loci, such as the single-copy chromosomal 16S rRNA and flabB genes39 and the 16S-23S ribosomal RNA intergenic spacer (IGS), have been used to discriminate between the various African relapsing fever borreliae.40 Genetic comparisons18,19 indicate that the 16S rRNA gene exhibits two to six nucleotide differences among the four cultured species; these differences are scattered along the whole 16S rRNA gene sequence for each species, resulting in a high overall sequence similarity of 99.7–99.9% between the four species.41 Moreover, interrogation of GenBank database indicates that these signatures are not consistent over the 37 B. crocidurae 16S rRNA gene sequences recorded in GenBank, resulting in a < 1% 16S rRNA gene sequence intraspecies variation. The same observation is true for B. duttonii. For the 1,008-bp flaB gene, genetic comparison indicates that only two nucleotides differentiate B. duttonii from B. recurrentis, whereas 10 nucleotides differentiate B. crocidurae from B. duttonii and B. recurrentis. GenBank database consultation indicates that these flaB sequence signatures are not conserved across different strains. The flaB sequences in 18 B. duttonii strains contain 31 single nucleotide polymorphisms with 11 non-synonymous mutations. Five flaB sequences from B. crocidurae contain 11 single nucleotide polymorphisms with two non-synonymous mutations. For B. recurrentis the flaB sequences of 21 strains contain only one synonymous mutation. In addition several flaB sequences with 100% similarity are labeled with equal confidence as B. duttonii or B. recurrentis. For the 1,002-bp glpQ gene, genetic analysis indicates that 9 positions, located in a 200-bp hot spot, differentiate B. crocidurae from B. duttonii and B. recurrentis with a similarity level of 96%; only one position differentiates B. duttonii from B. recurrentis. The sequence signature differentiating B. crocidurae from B. duttonii and B. recurrentis is conserved across the glpQ sequences that have been deposited into GenBank.
Further discrimination between borreliae could be achieved by analyzing intergenic spacers. The IGS showed an interspecies sequence variability of 92–99%, making it a potential target to identify borreliae from vectors and from blood.40 The IGS sequence-based phylogenetic tree clustered B. crocidurae and B. hispanica into one clade and also generated a clade comprising B. duttonii and B. recurrentis. Indeed, IGS sequencing in Tanzania mixed four B. duttonii types with two B. recurrentis types.40 Because sequencing a single spacer may be inaccurate, we recently developed a multispacer sequence typing (MST) approach and showed that sequencing five appropriate intergenic spacers accurately identified cultured relapsing fever borreliae and revealed diversity among them.42
These data indicate that no single sequence accurately discriminates between relapsing fever Borrelia and that a combination of multiple sequencing is required. The MST can achieve this goal because sequencing both the glpQ gene and the IGS is suitable for differentiating B. crocidurae and B. hispanica from B. duttonii and B. recurrentis, as illustrated in samples from Morocco and Tunisia.26–39 Conventional PCR with or without sequencing and real-time PCR targeting these genomic regions have been used to document borreliae in reservoirs, ectoparasites, and clinical specimens.43–46
Relapsing fever borreliae with cultured representatives in Africa.
Borrelia crocidurae flagellin sequences have been detected in patients in Senegal and Mauritania.21 Accordingly, B. crocidurae has in turn been detected in travelers returning from Senegal in both France47,48 and Italy.49 In Tunisia, B. crocidurae was documented by 16S rRNA gene and ITS sequencing in 15% of Ornithodoros erraticus ticks but not in human patients.39 The B. crocidurae 16S rRNA gene sequence has also been identified in patients in Mali, Gambia, and Togo.13,35,41 The latter observation was unexpected because the tick vector has not been documented in this country (Diatta G, personal communication). In Togo, blood smear examinations were negative and B. crocidurae and B. duttonii were detected using nested PCR in the absence of negative controls, a procedure with a high risk of cross-contamination and false positive results. Therefore, the presence of B. crocidurae and B. duttonii in Togo remains unconfirmed. In North Africa, B. hispanica has been documented by the 16S rRNA gene and IGS sequencing, and O. erraticus is the known vector for this pathogen.26 Additionally, Borrelia merionesi was recently detected in Morocco.36
An analysis of the unpublished B. duttonii 16S rRNA sequences in GenBank shows five sequences from Tanzania, two sequences from Zaire, one sequence from Rwanda, and three sequences without known geographical origin. A similar analysis for the B. duttonii flaB sequences shows 38 from Tanzania and 10 sequences without known origin. The 34 16S–23S sequences from this species were collected in Tanzania. Borrelia recurrentis has been confirmed in the East African countries of Ethiopia, Rwanda, Sudan, and Zaire.50,51 The disease was once distributed worldwide, however it has recently become less prevalent and more geographically restricted to African countries. Ethiopia and Sudan remain hotspot regions.
The genetic diversity is variable among relapsing fever species. The Borrelia recurrentis MST-based study showed that cultured strains collected over 10 years had two types; B. duttonii was more variable with four types; and B. crocidurae strains obtained in two closed rural villages in Senegal had more variability (8 MST) and exhibited geographical clustering.42
Uncultured relapsing fever borreliae in Africa.
In addition to the classified species and the new species described in Tanzania,52 gene sequencing has provided evidence for three new yet uncultured Borrelia species in Africa. The flaB gene sequencing of spirochetes observed in the blood of two penguins (Spheniscus demersus) revealed the presence of a novel relapsing fever Borrelia.45 Indeed, this 327-bp flaB gene sequence shared 99% sequence similarity with that of Borrelia sp. K64, which was previously detected in Carios sawaii ticks removed from seabirds in Japan.53 Phylogenetic analyses included the penguin Borrelia sp. in a well-supported clade along with Borrelia sp. K64 and Borrelia turicatae. In Ethiopia, 16S rRNA real-time PCR screening of Amblyomma cohaerens ticks followed by flaB gene sequencing suggested the presence of a potentially new Borrelia species in three males, three females, and two nymphs (8 of 109; 7.3%). This 344-bp flaB gene sequence shared only 85–86% sequence similarity with B. duttonii, B. recurrentis, and B. crocidurae.54 Further phylogenetic analysis of a 297-bp portion of the sequence showed that the new Borrelia species formed a separate branch distinct from the Lyme disease and recurrent fever groups.54 Another potentially new species was described in Rhipicephalus evertsi ticks from Nigeria based on 16S rRNA gene sequencing.55
Perspectives
The data reviewed in this work indicate that at least 10 different relapsing fever borreliae have been documented in Africa, including five different borreliae in humans and five different borreliae in nonhuman hosts. The former include the pathogens classified as B. hispanica, B. crocidurae, B. duttonii, and B. recurrentis. Parallel to these results, the huge diversity of B. crocidurae strains has been illustrated using post-genomic MST genotyping.42 A few borreliae, however, have been identified from African sources, indicating the absolute necessity of effective laboratory tools for precisely identifying African relapsing fever borreliae. The sequencing of complete genomes should be extended to B. hispanica to allow the creation of such post-genomic tools after they have been validated on a set of well-documented strains, as has previously been conducted for the recA gene in B. recurrentis.56 These techniques may include repeat sequence-based tools with increased sensitivity and specificity. It will be important to develop these tests in a format that is compatible with the point-of-care approach.57 Such point-of-care diagnostic tests will allow for a better appraisal of the prevalence of different relapsing fevers, their associated clinical features, and their epidemiology by the documentation of hosts and vectors, aiding in the effective control of the pathogens.
Disclaimer: The authors declare that they have no conflict of interests.
Footnotes
Financial support: This study was supported by a 2008 grant from the French National Research Agency (ANR) for the project ANR-08-MIEN-000 BORETIC.
Authors' addresses: Haitham Elbir, Didier Raoult, and Michel Drancourt, Aix Marseile Université - URMITE UMR63 CNRS 7872, IRD 198 Inserm 1095, Marseille, France, E-mails: haythamalbur@hotmail.com, didier.raoult@gmail.com, and michel.drancourt@univ-amu.fr.
References
- 1.Cutler SJ, Abdissa A, Trape JF. New concepts for the old challenge of African relapsing fever borreliosis. Clin Microbiol Infect. 2009;15:400–406. doi: 10.1111/j.1469-0691.2009.02819.x. [DOI] [PubMed] [Google Scholar]
- 2.Ministére de la Guerre, Direction du Service de Santé . L'œuvre du Service de Santé Militaire en Algérie 1830–1930. Charles Lavauzelle; Paris: 1931. pp. 246–289. [Google Scholar]
- 3.Obermeier O. Vorkommen, feinster eine Eigenbewegung zeigender Fäden im Blute von Recurrenskranken. Zentralblatt für die medizinischen Wissenschaften. 1873;11:145–147. [Google Scholar]
- 4.Cook AR. Relapsing fever in Uganda. J Trop Med Lond. 1904;7:24–26. [Google Scholar]
- 5.Dutton JE, Todd JL. The Nature of Human Tick Fever in the Eastern Part of Congo Free State. Liverpool, England: Liverpool School of Tropical Medicine; 1905. [Google Scholar]
- 6.Mackie FP. The part played by Pediculus corporis in the transmission of relapsing fever. BMJ. 1907;2:1706–1709. doi: 10.1136/bmj.2.2450.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolle C, Blaizot L, Conseil E. Etude sur la fièvre récurrente. Arch Inst Pasteur Tunis. 1913;8:1–93. [Google Scholar]
- 8.Davis GE. A Symposium on Relapsing Fever in the Americas. Washington, DC: American Association for the Advancement of Science; 1942. Species unity or plurality of the relapsing fever spirochetes. American Association for the Advancement of Science; pp. 41–47. [Google Scholar]
- 9.Kelly RT. Borrelia. Bergey's Manual of Systematic Bacteriology. Baltimore, MD: The Williams & Wilkins Co; 1984. pp. 57–62. [Google Scholar]
- 10.Cutler SJ, Akintunde CO, Moss J, Fukunaga M, Kurtenbach K, Talbert A, Zhang H, Wright DJ, Warrell DA. Successful in vitro cultivation of Borrelia duttonii and its comparison with Borrelia recurrentis. Int J Syst Bacteriol. 1999;49:1793–1799. doi: 10.1099/00207713-49-4-1793. [DOI] [PubMed] [Google Scholar]
- 11.Cutler SJ, Fekade D, Hussein K, Knox KA, Melka A, Cann K, Emilianus AR, Warrell DA, Wright DJ. Successful in-vitro cultivation of Borrelia recurrentis. Lancet. 1994;343:242. doi: 10.1016/s0140-6736(94)91032-4. [DOI] [PubMed] [Google Scholar]
- 12.Leger A. Spirochete de la musaraigne (Crocidura stampflii) Bull Soc Pathol Exot. 1917;1917:225–250. [Google Scholar]
- 13.van Dam AP, van Gool T, Wetsteyn JC, Dankert J. Tick-borne relapsing fever imported from West Africa: diagnosis by quantitative buffy coat analysis and in vitro culture of Borrelia crocidurae. J Clin Microbiol. 1999;37:2027–2030. doi: 10.1128/jcm.37.6.2027-2030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safdie G, Farrah IY, Yahia R, Marva E, Wilamowski A, Sawalha SS, Wald N, Schmiedel J, Moter A, Göbel UB, Bercovier H, Abdeen Z, Assous MV, Fishman Y. Molecular characterization of Borrelia persica, the agent of tick borne relapsing fever in Israel and the Palestinian Authority. PLoS ONE. 2010;5:e14105. doi: 10.1371/journal.pone.0014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moemenbellah-Fard MD, Benafshi O, Rafinejad J, Ashraf H. Tick-borne relapsing fever in a new highland endemic focus of western Iran. Ann Trop Med Parasitol. 2009;103:529–537. doi: 10.1179/136485909X451852. [DOI] [PubMed] [Google Scholar]
- 16.Colin de Verdiere N, Hamane S, Assous MV, Sertour N, Ferquel E, Cornet M. Tickborne relapsing fever caused by Borrelia persica, Uzbekistan and Tajikistan. Emerg Infect Dis. 2011;17:1325–1327. doi: 10.3201/eid1707.101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naddaf SR, Ghazinezhad B, Bahramali G, Cutler SJ. Phylogenetic analysis of the spirochete Borrelia microti, a potential agent of relapsing fever in Iran. J Clin Microbiol. 2012;50:2873–2876. doi: 10.1128/JCM.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lescot M, Audic S, Robert C, Nguyen TT, Blanc G, Cutler SJ, Wincker P, Couloux A, Claverie JM, Raoult D, Drancourt M. The genome of Borrelia recurrentis, the agent of deadly louse-borne relapsing fever, is a degraded subset of tick-borne Borrelia duttonii. PLoS Genet. 2008;4:e1000185. doi: 10.1371/journal.pgen.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbir H, Gimenez G, Robert C, Bergström S, Cutler SJ, Raoult D, Drancourt M. Complete genome sequence of Borrelia crocidurae. J Bacteriol. 2012;194:3723–3724. doi: 10.1128/JB.00118-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vial L, Diatta G, Tall A, Ba el H, Bouganali H, Durand P, Sokhna C, Rogier C, Renaud F, Trape JF. Incidence of tick-borne relapsing fever in West Africa: longitudinal study. Lancet. 2006;368:37–43. doi: 10.1016/S0140-6736(06)68968-X. [DOI] [PubMed] [Google Scholar]
- 21.Trape JF, Duplantier JM, Bouganali H, Godeluck B, Legros F, Cornet JP, Camicas JL. Tick-borne borreliosis in West Africa. Lancet. 1991;23:473–475. doi: 10.1016/0140-6736(91)93404-w. [DOI] [PubMed] [Google Scholar]
- 22.Parola P, Diatta G, Socolovschi C, Mediannikov O, Tall A, Bassene H, Trape JF, Raoult D, 2011. Tick-borne relapsing fever borreliosis, rural senegal. Emerg Infect Dis. 17:883–885. doi: 10.3201/eid1705.100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayegga E, Ljøstad U, Mygland A, Monstad P. Absence of focal neurological involvement in tick-borne relapsing fever in northern Tanzania. Eur J Neurol. 2005;12:449–452. doi: 10.1111/j.1468-1331.2005.01003.x. [DOI] [PubMed] [Google Scholar]
- 24.Dupont HT, La Scola B, Williams R, Raoult D. A focus of tick-borne relapsing fever in southern Zaire. Clin Infect Dis. 1997;25:139–144. doi: 10.1086/514496. [DOI] [PubMed] [Google Scholar]
- 25.Jongen VH, van Roosmalen J, Tiems J, van Holten J, Wetsteyn JC. Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstet Gynecol Scand. 1997;76:834–838. doi: 10.3109/00016349709024361. [DOI] [PubMed] [Google Scholar]
- 26.Sarih M, Garnier M, Boudebouch N, Bouattour A, Rihani A, Hassar M, Gern L, Postic D, Cornet M. Borrelia hispanica relapsing fever, Morocco. Emerg Infect Dis. 2009;15:1626–1629. doi: 10.3201/eid1510.090403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos JM, Malmierca E, Reyes F, Tesfamariam A. Results of a 10-year survey of louse-borne relapsing fever in southern Ethiopia: a decline in endemicity. Ann Trop Med Parasitol. 2008;102:467–469. doi: 10.1179/136485908X300887. [DOI] [PubMed] [Google Scholar]
- 28.Bryceson AD, Parry EH, Perine PL, Warrell DA, Vukotich D, Leithead CS. Louse-borne relapsing fever. Q J Med. 1970;39:129–170. [PubMed] [Google Scholar]
- 29.Warrell DA, Pope HM, Parry EH, Perine PL, Bryceson AD. Cardiorespiratory disturbances associated with infective fever in man: studies of Ethiopian louse-borne relapsing fever. Clin Sci. 1970;39:123–145. doi: 10.1042/cs0390123. [DOI] [PubMed] [Google Scholar]
- 30.Cooper PJ, Fekade D, Remick DG, Grint P, Wherry J, Griffin GE. Recombinant human interleukin-10 fails to alter proinflammatory cytokine production or physiologic changes associated with the Jarisch-Herxheimer reaction. J Infect Dis. 2000;181:203–209. doi: 10.1086/315183. [DOI] [PubMed] [Google Scholar]
- 31.Fekade D, Knox K, Hussein K, Melka A, Lalloo DG, Coxon RE, Warrell DA. Prevention of Jarisch-Herxheimer reactions by treatment with antibodies against tumor necrosis factor alpha. N Engl J Med. 1996;335:311–315. doi: 10.1056/NEJM199608013350503. [DOI] [PubMed] [Google Scholar]
- 32.Coxon RE, Fekade D, Knox K, Hussein K, Melka A, Daniel A, Griffin GG, Warrell DA. The effect of antibody against TNF alpha on cytokine response in Jarisch-Herxheimer reactions of louse-borne relapsing fever. QJM. 1997;90:213–221. doi: 10.1093/qjmed/90.3.213. [DOI] [PubMed] [Google Scholar]
- 33.Larsson C, Lundqvist J, Bergström S. Residual brain infection in murine relapsing fever borreliosis can be successfully treated with ceftriaxone. Microb Pathog. 2008;44:262–264. doi: 10.1016/j.micpath.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Barbour AG, Hayes SF. Biology of Borrelia species. Microbiol Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nordstrand A, Bunikis I, Larsson C, Tsogbe K, Schwan TG, Nilsson M, Bergström S. Tickborne relapsing fever diagnosis obscured by malaria, Togo. Emerg Infect Dis. 2007;13:117–123. doi: 10.3201/eid1301.060670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diatta G, Souidi Y, Granjon L, Arnathau C, Durand P, Chauvancy G, Mané Y, Sarih M, Belghyti D, Renaud F, Trape JF. Epidemiology of tick-borne borreliosi in Morocco. PLoS Negl Trop Dis. 2012;6:e1810. doi: 10.1371/journal.pntd.0001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly R. Cultivation of Borrelia hermsii. Science. 1971;173:443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- 38.Djelouadji Z, Roux V, Raoult D, Kodjo A, Drancourt M. Rapid MALDI-TOF mass spectrometry identification of Leptospira organisms. Vet Microbiol. 2012;158:142–146. doi: 10.1016/j.vetmic.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Bouattour A, Garnier M, M'Ghirbi Y, Sarih M, Gern L, Ferquel E, Postic D, Cornet M. Borrelia crocidurae infection of Ornithodoros erraticus (Lucas, 1849) ticks in Tunisia. Vector Borne Zoonotic Dis. 2010;10:825–830. doi: 10.1089/vbz.2009.0151. [DOI] [PubMed] [Google Scholar]
- 40.Cutler SJ, Bonilla EM, Singh RJ. Population structure of East African relapsing fever Borrelia spp. Emerg Infect Dis. 2010;16:1076–1080. doi: 10.3201/eid1607.091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ras NM, La Scola B, Postic D, Cutler SJ, Rodhain F, Baranton G, Raoult D. Phylogenesis of relapsing fever Borrelia spp. Int J Syst Bacteriol. 1996;46:859–865. doi: 10.1099/00207713-46-4-859. [DOI] [PubMed] [Google Scholar]
- 42.Elbir H, Gimenez G, Sokhna C, Bilcha KD, Ali J, Barker SC, Cutler SJ, Raoult D, Drancourt M. Multispacer sequence typing relapsing fever borreliae in Africa. PLoS Negl Trop Dis. 2012;6:e1652. doi: 10.1371/journal.pntd.0001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cutler SJ, Abdissa A, Adamu H, Tolosa T, Gashaw A. Borrelia in Ethiopian ticks. Ticks Tick Borne Dis. 2012;3:14–17. doi: 10.1016/j.ttbdis.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Scott JC, Wright DJ, Cutler SJ. Typing African relapsing fever spirochetes. Emerg Infect Dis. 2005;11:1722–1729. doi: 10.3201/eid1111.050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yabsley MJ, Parsons NJ, Horne EC, Shock BC, Purdee M. Novel relapsing fever Borrelia detected in African penguins (Spheniscus demersus) admitted to two rehabilitation centers in South Africa. Parasitol Res. 2012;110:1125–1130. doi: 10.1007/s00436-011-2602-2. [DOI] [PubMed] [Google Scholar]
- 46.Reller ME, Clemens EG, Schachterle SE, Mtove GA, Sullivan DJ, Dumler JS. Multiplex 5′ nuclease-quantitative PCR for diagnosis of relapsing fever in a large Tanzanian cohort. J Clin Microbiol. 2011;49:3245–3249. doi: 10.1128/JCM.00940-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poirier P, Lebuisson A, Menager C, Moulin F, Dupouy Camet J. Fever in a 7-year-old girl returning from Mali. Clin Infect Dis. 2008;47:1490–1491. doi: 10.1086/593096. [DOI] [PubMed] [Google Scholar]
- 48.Million M, Cazorla C, Doudier B, La Scola, B, Parola P, Drancourt M, Brouqui P. Molecular identification of Borrelia crocidurae in a patient returning from Senegal. BMJ Case Rep. 2009 doi: 10.1136/bcr.06.2008.0298. doi:pii: bcr06.2008.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tordini G, Giaccherini R, Corbisiero R, Zanelli G. Relapsing fever in a traveller from Senegal: determination of Borrelia species using molecular methods. Trans R Soc Trop Med Hyg. 2006;100:992–994. doi: 10.1016/j.trstmh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Cobey FC, Goldbarg SH, Levine RA, Patton CL. Short report: detection of Borrelia (relapsing fever) in rural Ethiopia by means of the quantitative buffy coat technique. Am J Trop Med Hyg. 2001;65:164–165. doi: 10.4269/ajtmh.2001.65.164. [DOI] [PubMed] [Google Scholar]
- 51.De Jong J, Wilkinson RJ, Schaeffers P, Sondorp HE, Davidson RN. Louse-borne relapsing fever in southern Sudan. Trans R Soc Trop Med Hyg. 1995;89:621. doi: 10.1016/0035-9203(95)90414-x. [DOI] [PubMed] [Google Scholar]
- 52.Mitani H, Talbert A, Fukunaga M. New World relapsing fever Borrelia found in Ornithodoros porcinus ticks in central Tanzania. Microbiol Immunol. 2004;48:501–505. doi: 10.1111/j.1348-0421.2004.tb03545.x. [DOI] [PubMed] [Google Scholar]
- 53.Takano A, Muto M, Sakata A, Ogasawara Y, Ando S, Hanaoka N, Tsurumi M, Sato F, Nakamura N, Fujita H, Watanabe H, Kawabata H. Relapsing fever spirochete in seabird tick, Japan. Emerg Infect Dis. 2009;15:1528–1530. doi: 10.3201/eid1509.090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mediannikov O, Abdissa A, Socolovschi C, Diatta G, Trape JF, Raoult D. Detection of a new Borrelia species in ticks taken from cattle in southwest Ethiopia. Vector Borne Zoonotic Dis. 2013;13:266–269. doi: 10.1089/vbz.2011.0874. [DOI] [PubMed] [Google Scholar]
- 55.Reye AL, Arinola OG, Hübschen JM, Muller CP. Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl Environ Microbiol. 2012;78: 2562–2568. doi: 10.1128/AEM.06686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cutler SJ, Rinky IJ, Bonilla EM. Does RecA have a role in Borrelia recurrentis? Clin Microbiol Infect. 2011;17:195–197. doi: 10.1111/j.1469-0691.2010.03249.x. [DOI] [PubMed] [Google Scholar]
- 57.Cheikh S, Mediannikov O, Fenollar F, Bassene H, Diatta G, Tall A, Trape JF, Drancourt M, Raoult R. Point-of-Care laboratory of pathogen diagnosis in rural Senegal. PLoS Negl Trop Dis. 2013;7:e1999. doi: 10.1371/journal.pntd.0001999. [DOI] [PMC free article] [PubMed] [Google Scholar]