Abstract
Between 1993 and 2011, a total of 371 intestinal amebiasis (IA), caused by Entamoeba histolytica cases were compared with 1,113 shigellosis (randomly selected) patients of icddr,b, excluding co-infections (rotavirus and Vibrio cholerae) in two age stratums: 0–14 years of age and ≥ 15 years of age. The number of IA and shigellosis cases gradually reduced over the study period. In multivariate analysis, individuals 0–14 years of age, slum dwellers (odds ratio [OR] 3.51; 95% confidence interval [CI] 1.69–7.24; P < 0.001), red blood cell (0.44 [0.24–0.86] 0.016), fecal leukocytes (0.17 [0.07–0.33] < 0.001), and alkaline stool (0.16 [0.07–0.36] < 0.001) were independently associated with IA; and among individuals ≥ 15 years of age, living in the slum area (1.88 [1.12–3.14] 0.016), watery stool (2.21 [1.37–3.55] 0.001), use of antimicrobials before visiting hospital (0.67 [0.46–0.99] 0.047), red blood cell (0.45 [0.22–0.94] 0.036), and fecal leukocytes (0.21 [0.12–0.35] < 0.001) in stool were independently associated with IA. Socio-demographic and clinical characteristics of IA and shigellosis varied distantly from each other.
Introduction
Intestinal amebiasis, caused by the Entamoeba histolytica (EH) parasite is the second leading cause of death from parasitic illness worldwide1; annually, it is estimated that 500 million people are infected with this parasite, leading to 40,000–100,000 deaths worldwide. As a result, intestinal amebiasis remains an important public health concern, particularly in developing countries.1,2 The global incidence rate of diarrhea caused by EH has been reported to be 0.08/child-year,3 although the burden of disease differs greatly among countries as a result of differences in population, socioeconomic, and water and sanitation characteristics.4 The transmission of EH is thought to be dependent on demographic, environmental, socioeconomic, and personal hygiene behaviors.5 The clinical presentation of intestinal amebiasis varies from asymptomatic colonization to intestinal amebiasis and extraintestinal amoebeasis, which is most commonly in the form of liver and lung abscesses.1,6 A 3-year study reported intestinal amebiasis in 2.2% and shigellosis in 5.3% of preschool children.7 A review of recent literature reveals that shigellosis remains a major contributor to the diarrheal disease burden in developing countries, including Bangladesh.8 As a result of similar clinical characteristics of shigellosis to intestinal amebiasis, clinical differentiation between both is often difficult. However, these clinical characteristics often evident in case of shigellosis.9 There is also a lack of recent evidence of comparative clinical and socio-demographic data on intestinal amebiasis and shigellosis from Bangladesh. Therefore, we performed analyses to compare socio-demographic and clinical characteristics of these two conditions using a large database of an urban diarrheal disease hospital of Bangladesh.
Materials and Methods
Study site, population, and source of data.
Established in 1962, the Dhaka Hospital of icddr,b, located in Dhaka, the capital city of Bangladesh provided care and treatment to people with diarrheal diseases. The majority of the patients came from urban and peri-urban Dhaka.
During the last 20 years, over 100,000 diarrheal patients annually receive free care and treatment from this hospital. A Diarrheal Disease Surveillance System (DDSS) was established in 1979 that systematically samples patients—4% of all patients from 1979 through 1995, followed by 2% of all the patients since 1996. The DDSS currently collects information on clinical, epidemiological, and demographic characteristics of feeding practices, particularly of infants and young children and drug and fluid therapy use at home of every 50th patient, regardless of age, sex, disease severity, or socioeconomic status through an administered structured questionnaire. A trained research assistant interviews either the patients themselves or caregivers in the case of young children. Extensive microbiological assessments of fecal samples (culture, enzyme-linked immunosorbent assay [ELISA], and microscopy) are routinely performed to identify diarrheal pathogens. This activity provides valuable information to hospital clinicians in guiding their decision-making processes and enables the detection of emerging new pathogens. It also allows for the early identification of outbreaks and therefore alerting host governments to take appropriate preventive and control measures. The system also monitors changes in patient characteristics and antimicrobial susceptibility of common bacterial pathogens. For this analysis, relevant information was collected from the electronic database of the DDSS for the period 1993–2011.
Sampling.
For this analysis, we studied patients of either sex, segregated into two age stratum: 0 to 14 years of age and 15 years of age and above, who attended the hospital between 1993 and 2011 inclusive.
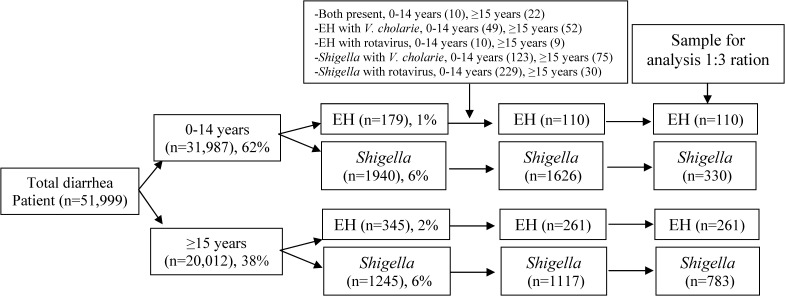
A positive stool microscopy for vegetative EH patients constituted intestinal amebiasis and those with culture proven Shigella served as a comparison group (shigellosis). We selected a ration of 1:3 (comparison group) to increase the statistical power of our analyses. The comparison group was identified by a computer-generated random list using SPSS (version 15.0; SPSS Inc., Chicago, IL) from all individuals. Age stratified randomization was done for the age group 0–14 years (0 to < 1 year; 1 to < 5 years, and 5 to 14 years). In total, 51,999 patients were enrolled into the DDSS during the study period, of which 31,987 were 0–14 years of age (considered as the pediatric age group) and the rest were 15 years of age or older. Among the 0–14 year olds, 179 of 31,987 (0.56%), and 345 of 20,012 (1.72%) of patients aged 15 years or older had intestinal amebiasis. In total, 3,185 patients had culture-confirmed shigellosis during the period and 1,940 of these children were younger than 15 years and 1,245 were 15 years of age or older. Co-infections such as EH with Shigella, 0–14 years (N = 10), ≥ 15 years (N = 22); EH with Vibrio cholerae, 0–14 years (N = 49), ≥ 15 years (N = 52); EH with rotavirus, 0–14 years (N = 10), ≥ 15 years (N = 9); Shigella with V. cholerae, 0–14 years (N = 123), ≥ 15 years (N = 75); Shigella with rotavirus, 0–14 years (N = 229), ≥ 15 years (N = 30) were noted and excluded those mixed infections from the analyses. Thus, we finally had 110 analyzable cases and 330 controls in the age group 0–14 years and 261 cases and 783 controls in the age group 15 years and above (Figure 1).
Figure 1.
Sampling flow chart for the analysis (1993–2011).
Definition.
Diarrhea was defined as passage of abnormally loose or watery stools three times or more within the last 24 hours. Intestinal amebiasis was defined as individuals having a positive stool microscopy for vegetative EH10 (It was difficult to detect Entamoeba dispar in stool microscopy; however, in most of the cases, this parasite is nonpathogenic and remains asymptomatic,11 and we included all the diarrhea patients in the analysis); and shigellosis was defined as stool culture yielding growth of any member of Shigella spp. Individuals with no formal schooling were considered as illiterate. Pneumonia was defined as presence of adventitious sound (rhonchi or crepitation) in the lungs.12
Data analysis.
Data analyses were done using the Statistical Package for Social Sciences (SPSS) Windows (Version 15.2) and Epi Info (version 6.0, USD, Stone Mountain, GA). For categorical variables, differences in the proportions were compared by the χ2 test and a probability of < 0.05 was considered as statistically significant. Strength of association was determined by estimating the odds ratio (OR) and its 95% confidence interval (CI). Finally, logistic regression analyses were performed, after adjusting for the co-variantes that were significantly associated with univariate analysis.
Ethical consideration.
This study was approved by the RRC (Research Review Committee) and ERC (Ethical Review Committee) of icddr,b. All individuals were enrolled and their stool specimens were collected for microbiological assessment once they gave consent.
Results
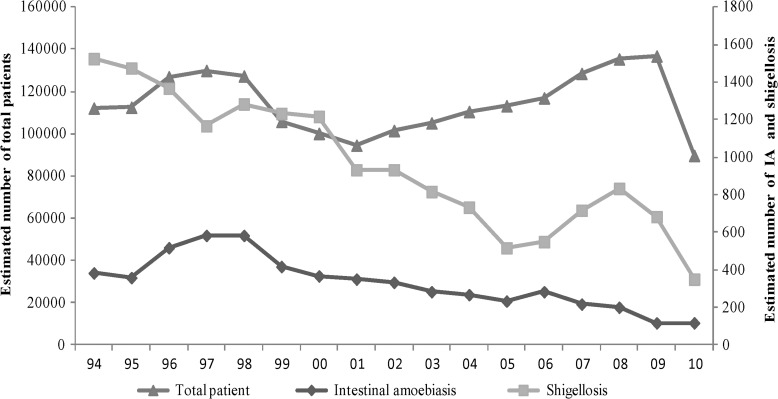
During the study period (1993–2011), patients with intestinal amebiasis represented 1% (N = 371) of all patients enrolled in DDSS (N = 51,999) with diarrhea. The number of intestinal amebiasis and shigellosis cases gradually reduced over the study period (Figure 2). Mean age among 0–14 years was similar among children infected with intestinal amebiasis (6.24 versus 5.59 years; P = 0.121) and shigellosis, and so for the 15 years and above age group (36.42 versus 35.95, P = 0.706) (data not presented).
Figure 2.
Yearly distribution of the moving average of the estimated number of total attending patients, intestinal amebiasis (IA), and shigellosis.
In patients aged 0–14 years, maternal and paternal illiteracy rates were higher in cases of intestinal amebiasis compared with cases of shigellosis (Table 1). Among infants that stopped breast feeding within 1 month of age, 80% and 27% were infected with EH and Shigella, respectively (P = 0.682; Table 1). The likelihood of intestinal amebiasis was greater than shigellosis among slum dwellers not using sanitary latrines for defecation (Table 1). Patients with intestinal amebiasis presented more often with watery stool. However, fever and abdominal pain were less often among amebiasis children than those with shigellosis. Moreover, intestinal amebiasis cases often presented to the hospital within 24 hours of diarrhea onset but less frequently received antimicrobial at home before attending the hospital than those of shigellosis (Table 1). A lesser proportion of patients with intestinal amebiasis had presence of red blood cell (RBC), leukocytes/hpf (11 to > 50) and macrophage/hpf (1 to10) in their stool microscopy than the patients with shigellosis (Table 1). A greater proportion of patients with intestinal amebiasis had an alkaline stool pH than patients with shigellosis among the pediatric age group.
Table 1.
Characteristics and stool microscopic examination of patients with intestinal amebiasis and shigellosis 0–14 years of age
| Characteristic | Intestinal amebiasis; N = 110 (%) | Shigellosis; N = 330 (%) | Unadjusted OR (95% CI) P value | Adjusted OR (95% CI)* |
|---|---|---|---|---|
| Male sex | 67 (61) | 204 (62) | 0.96 (0.60, 1.54) 0.954 | – |
| Illiterate mother | 75 (68) | 165 (50) | 2.14 (1.33, 3.47) 0.001 | 1.15 (0.58, 2.27) 0.673 |
| Illiterate father | 55 (50) | 124 (38) | 1.66 (1.05, 2.63) 0.028 | 0.97 (0.51, 1.84) 0.920 |
| Stopped breast feeding within 1 mo of age | 16/20 (80) | 29/106 (27) | 1.51 (0.42, 5.85) 0.682 | – |
| Poor socio-economic status (median monthly family income ≤ US$40) | 66 (60) | 176 (53) | 1.31 (0.83, 2.08) 0.268 | – |
| Living in the slum | 31 (28) | 34 (10) | 3.42 (1.91, 6.11) < 0.001 | 3.51 (1.69, 7.24) 0.001 |
| Use of sanitary toilet | 46 (42) | 176 (53) | 0.63 (0.40, 1.00) 0.047 | 1.06 (0.60, 1.87) 0.834 |
| Did not receive vitamin A capsule, (under 5 years) | 76 (69) | 234 (71) | 0.92 (0.56, 1.51) 0.809 | – |
| History of measles within last 6 mo | 4 (4) | 19 (6) | 0.62 (0.17, 1.98) 0.536 | – |
| History of abdominal pain | 71 (65) | 236 (72) | 0.73 (0.45, 1.18) 0.208 | – |
| Fever (Temperature 37.8°C) | 7 (6) | 46 (14) | 0.42 (0.17, 1.01) 0.051 | 0.49 (0.17, 1.43) 0.195 |
| Duration of diarrhea (< 1 d) | 37 (38) | 82 (25) | 1.53 (0.93, 2.51) 0.094 | – |
| Watery stool (lack of mucus/blood) | 64 (58) | 107 (32) | 2.90 (1.82, 4.63) < 0.001 | 0.97 (0.54, 1.74) 0.935 |
| Dehydration (moderate/severe) | 26/96 (27) | 60/296 (20) | 1.46 (0.83, 2.57) 0.207 | – |
| Wasting (WHZ–2 z score) | 23/76 (30) | 88/277 (32) | 0.93 (0.52, 1.67) 0.911 | – |
| Stunting (HAZ–2 z score) | 38/76 (50) | 121/156 (44) | 1.29 (0.75, 2.21) 0.395 | – |
| Underweight (WAZ–2 z score) | 42/76 (55) | 162/277 (59) | 0.88 (0.51, 1.51) 0.709 | – |
| Use of antimicrobial before hospital visit | 55 (50) | 222 (67) | 0.49 (0.31, 0.77) < 0.001 | 0.77 (0.44, 1.33) 0.351 |
| Death | 1 (1) | 3 (1) | 1.00 | – |
| Pneumonia | 1 (2) | 10 (3) | 0.59 (0.09, 2.94) 0.738 | – |
| Red blood cell/ /hpf (1 to > 50) | 50 (45) | 252 (79) | 0.21 (0.13, 0.35) < 0.001 | 0.44 (0.24, 0.86) 0.016 |
| Fecal leukocytes/hpf (11 to > 50) | 62 (56) | 278 (82) | 0.18 (0.11, 0.31) < 0.001 | 0.17 (0.07, 0.33) < 0.001 |
| Macrophage/hpf (1 to 10) | 38 (34) | 238 (75) | 0.18 (0.11, 0.29) < 0.001 | 0.00 (0.00) 0.997 |
| pH (alkali) | 99 (90) | 257 (81) | 2.07 (1.00, 4.35) 0.049 | 0.16 (0.07, 0.36) < 0.001 |
Variables that were found to be significant in univeriate analysis were included in the multivariate model.
OR = odds ratio; CI = confidence interval.
On the other hand, patients 15 years of age and older, a higher proportion of patients in this age group with intestinal amebiasis and living in the slum presented with watery stool with dehydrating diarrhea and also commonly visited the hospital within 24 hours of diarrhea onset (Table 2). However, at least 71% patients with EH and Shigella had abdominal pain. Stool microscopic examination was found similar in the 15 years and above age group (Table 2).
Table 2.
Characteristics and stool microscopic examination of patients with intestinal amebiasis and shigellosis 15 years of age and above
| Intestinal amebiasis N = 261 (%) | Shigellosis N = 783 (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI)* | |
|---|---|---|---|---|
| Male sex | 145 (56) | 501 (64) | 0.70 (0.52, 0.94) 0.018 | 1.09 (0.75, 1.61) 0.635 |
| Illiterate mother | 219 (84) | 617 (79) | 1.40 (0.95, 2.07) 0.089 | – |
| Illiterate father | 192 (74) | 523 (67) | 1.38 (1.00, 1.91) 0.049 | 0.85 (0.56, 1.29) 0.441 |
| Poor socio-economic status (median monthly family income ≤ US$40) | 132 (50) | 437 (56) | 0.80 (0.60, 1.07) 0.131 | – |
| Living in the slum | 49 (19) | 95 (12) | 1.37 (1.13, 2.48) 0.009 | 1.88 (1.12, 3.14) 0.016 |
| Use of sanitary toilet | 149 (57) | 430 (55) | 1.09 (0.82, 1.46) 0.589 | – |
| History of abdominal pain | 185 (71) | 620 (79) | 0.64 (0.46, 0.89) 0.007 | 1.18 (0.77, 1.81) 0.433 |
| Fever (temperature 37.8°C) | 14 (5) | 70 (9) | 0.58 (0.31, 1.08) 0.087 | – |
| Duration of diarrhea (< 1 d) | 148 (57) | 273 (35) | 2.45 (1.82, 3.29) < 0.001 | 1.08 (0.72, 1.64) 0.689 |
| Watery stool (lack of mucus/blood) | 219 (84) | 368 (47) | 5.88 (4.05, 8.56) < 0.001 | 2.21 (1.37, 3.55) 0.001 |
| Dehydration (moderate/severe) | 113/193 (59) | 236/651 (36) | 2.48 (1.77, 3.49) < 0.001 | 1.36 (0.91, 2.04) 0.130 |
| BMI (< 18.5) | 132 (54) | 367 (50) | 1.17 (0.87, 1.58) 0.329 | – |
| Use of antimicrobial prior to hospital visit | 123 (47) | 522 (67) | 0.43 (0.33, 0.60) < 0.001 | 0.67 (0.46, 0.99) 0.047 |
| Death | 1 (< 1) | 2 (< 1) | – | |
| Red blood cell/hpf (1 to > 50) | 120 (46) | 602 (77) | 0.25 (0.18, 0.34) < 0.001 | 0.45 (0.22, 0.94) 0.036 |
| Fecal leukocytes/hpf (11 to > 50) | 141 (54) | 672 (86) | 0.19 (0.13, 0.26) < 0.001 | 0.21 (0.12, 0.35) < 0.001 |
| Macrophage/hpf (1 to 10) | 81 (31) | 575 (74) | 0.16 (0.12, 0.22) < 0.001 | 0.18 (0.03, 1.43) 0.107 |
| pH (alkali) | 231 (88) | 669 (86) | 1.29 (0.81, 2.04) 0.304 | – |
Variables that were found to be significant in univeriate analysis were included in the multivariate model.
OR = odds ratio; CI = confidence interval; BMI = body mass index.
In logistic regression analysis of data of patients younger than 15 years, after controlling for factors that were significantly associated in an univariate analyses, living in slum areas was found to be significantly associated with intestinal amebiasis; however, alkaline stool pH and presence of any number of RBC and fecal leukocytes in stool microscopy were found to be protective for IA (Table 1). Conversely, living in slum areas and watery stool were the predictors for intestinal amebiasis; whereas, use of antimicrobials before attending the hospital, presence of RBC, leukocytes in stool were protective for IA for patients 15 years of age or older (Table 2).
Discussion
In Bangladesh, mothers are usually the primary caretakers of children. Lack of maternal knowledge and practices related to the importance of breast feeding and appropriate weaning practices and optimal hygienic behaviors often interfere with the proper care of children.13–15 On the other hand, fathers as the household heads are the primary decision makers for the family, particularly in health care-seeking behaviors and use of health care facilities. In univariate analysis, we noted the associations between lack of formal schooling of both parents with intestinal amebiasis. Living in conditions lacking safe water and adequate sanitation facilities, contaminated environment, and overcrowding also facilitate fecal-oral transmission of the diarrheal pathogens.16 We observed an independent association of intestinal amebiasis with a lack of formal schooling of mothers, slum dwelling, and poor socioeconomic background of the patients.
In this study, patients with intestinal amebiasis often presented with watery stool associated with dehydrating diarrhea.3,17 Patients attending with shigellosis or bacillary dysentery (bloody-mucoid stools) presented less often with clinical dehydration because they lose smaller quantities and frequency of stools.18 Although the frequency of abdominal pain was greater among patients with shigellosis who were 15 years of age or older, the frequency was almost the same among younger patients for both intestinal amebiasis and shigellosis.
Early cessation of breast feeding in infants has clear ramifications on health, nutrition, and disease morbidities, including diarrheal disease. Although the proportion of early discontinuation of breast feeding was higher among children with intestinal amebiasis, the values were not statistically significant because of inadequate sample size. Conversely, the proportion of children not receiving a vitamin A capsule was higher among both the groups. Children who did not receive vitamin A were very likely to have a lower serum retinol level along with reduced liver store of the vitamin A, making them immunocompromised,19 which is a greater problem among malnourished children.
A lesser proportion of patients with intestinal amebiasis had a history of taking antimicrobials at home than those with shigellosis in both age groups of patients. However, the proportion of shorter duration of diarrhea before a hospital visit (< 24 hours) was more observed among ameabiasis patients. Possible explanations of this may be a result of early reporting to the hospital (within 24 hours) because of dehydration and delayed manifestation of the bloody mucoid stool for intestinal amebiasis. Distribution of malnourished children and adults were similar in patients with intestinal amebiasis and shigellosis. At least 30% of the children were wasted; however, cases of stunting and underweight were 50% and 55%, respectively. Moreover, malnutrition was significantly associated with both the morbidities.
The presence of RBC and leukocytes/hpf (11 to > 50) in stools signifies an inflammatory process caused by invasive pathogens or may be the result of idiopathic ulcerative colitis, which is associated with loss of mucosal integrity and inflammatory response in the gut.20,21 Trophozoites of EH produce two types (nodular and irregular) of nonspecific lesions,22 whereas Shigella causes an invasive colitis involving the epithelium and lamina propria through a complex and intimate mechanism mediated by both microbial and host protein.23 In direct stool microscopy, the presence of RBC, leukocytes/hpf (11 to > 50), and macrophage were less frequently observed among cases with intestinal amebiasis.24 Host response disparities including impairment of cell-mediated immunity and under-nutrition, variations in the disease severity, repeated enteric infections, and mixed infections may influence the number of fecal leukocytes in the stools.25 The prevalence of shigellosis is much higher compared with intestinal amebiasis in Bangladesh and presence of any number of RBCs and ≥ 20 fecal leukocytes and presence of macrophages in stool microscopy is associated with shigellosis.26 The EH secretes proteinase, which dissolves host tissues, kills host cells on contact, and engulfs RBCs1 and causes lysis of leukocytes. Therefore, their presence and numbers in stool are less than those in shigellosis24; these factors may explain our observations. The number of fecal leukocytes depends on the anatomic site of involvement and the extent of the inflammatory process rather than the etiology.27
There were an increasing number of patients with Shigella but no alteration for EH from 2006 to 2009. We did not have any ready explanation for such an increasing trend; however, the increased number of pediatric patients with a higher prevalence of rotavirus might reflect the increased number of patients over this period.28 On the other hand, the increased prevalence of malnutrition from 2005 to 2010 might have accelerated the prevalence of shigellosis.28
Hospital-based data, however, might not adequately represent the population at large with a possible bias toward patients with intestinal amebiasis who seek care less often from hospital facilities. On the other hand, we considered only vegetative EH in stool microscopic examination not by ELISA or PCR, excluding co-infection such as V. cholerae and rotavirus, but not enterotoxigenic Escherichia coli (ETEC) because of the unavailability of data; however, prevalence of ETEC as co-infection was very low (with EH [< 1%] and Shigella [< 1%]) (Faruque AS, personal communication), which might not influence our analysis; and also did not exclude the non-pathogenic and non-hematophagus Entamoeba dispar. However, our strengths were that we followed an unbiased systematic sampling regardless of age, sex, nutrition status, disease severity, or socioeconomic context and used a large data set for this analysis. High-quality laboratory performance of the Clinical Microbiology Laboratory of icddr,b for detecting EH by stool microscopy was our strength. This is the only laboratory that has international accreditation (ISO 15189:2007) in Bangladesh.
Although the proportion of intestinal amebiasis has decreased,29 it still continues to be a major health problem because of the morbidities and nutritional and economic consequences. Improvement of livelihoods with proper knowledge might interrupt the transmission and therefore reduce the burden of disease. However, the distinct clinical and cost-effective stool microscopic features of IA from those with shigellosis might help to initiate appropriate treatment. At the same time, emphasis needs to be placed on low-cost sanitation and hygiene practices. In addition, an increase in the rational use of antimicrobials is urgently required for taking appropriate measures to control these continued public health challenges among the poorer slum-dwelling population.
ACKNOWLEDGMENTS
The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: Hospital surveillance was funded by icddr,b and the Government of the People's Republic of Bangladesh through IHP-HNPRP. icddr,b acknowledges with gratitude the commitment of the Government of the People's Republic of Bangladesh to the icddr,b's research efforts. icddr,b also gratefully acknowledges the following donors that provide unrestricted support to the Centre's research efforts: Australian Agency for International Development (AusAID), Government of the People's Republic of Bangladesh, Canadian International Development Agency (CIDA), Embassy of the Kingdom of the Netherlands (EKN), Swedish International Development Cooperation Agency (Sida), Swiss Agency for Development and Cooperation (SDC), and Department for International Development, UK (DFID).
Authors' addresses: Sumon Kumar Das, Mohammod Jobayer Chisti, Mohammad Abdul Malek, Mohammed Abdus Salam, Tahmeed Ahmed, Abu Syed Golam Faruque, and Dinesh Mondal, International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), E-mails: sumon@icddrb.org, chisti@icddrb.org, mamalek@icddrb.org, masalm@icddrb.org, tahmeed@icddrb.org, gfaruque@icddrb.org, and din63d@icddrb.org.
References
- 1.Stanley SL., Jr Amoebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 2.WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28–29 January, 1997. Epidemiol Bull. 1997;18:13–14. [PubMed] [Google Scholar]
- 3.Haque R, Mondal D, Kirkpatrick BD, Akther S, Farr BM, Sack RB, Petri WA., Jr Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am J Trop Med Hyg. 2003;69:398–405. [PubMed] [Google Scholar]
- 4.Al-Harthi S, Jamjoom M. Diagnosis and differentiation of Entamoeba infection in Makhah Al Mukarramah using microscopy and stool antigen detection kits. J Med Sci. 2007;2:15–20. [Google Scholar]
- 5.Norhayati M, Fatmah MS, Yusof S, Edariah AB. Intestinal parasitic infections in man: a review. Med J Malaysia. 2003;58:296–305. quiz 306. [PubMed] [Google Scholar]
- 6.Petri WA, Jr, Mondal D, Peterson KM, Duggal P, Haque R. Association of malnutrition with amebiasis. Nutr Rev. 2009;67((Suppl 2)):S207–S215. doi: 10.1111/j.1753-4887.2009.00242.x. [DOI] [PubMed] [Google Scholar]
- 7.Haque R, Huston CD, Hughes M, Houpt E, Petri WA., Jr Amebiasis. N Engl J Med. 2003;348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- 8.Bardhan P, Faruque AS, Naheed A, Sack DA. Decrease in shigellosis-related deaths without Shigella spp.-specific interventions, Asia. Emerg Infect Dis. 2010;16:1718–1723. doi: 10.3201/eid1611.090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Amebiasis. Wkly Epidemiol Rec. 1997;72:97–100. [Google Scholar]
- 11.Haque R, Neville LM, Hahn P, Petri WA., Jr Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J Clin Microbiol. 1995;33:2558–2561. doi: 10.1128/jcm.33.10.2558-2561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chisti MJ, Huq S, Das SK, Malek MA, Ahmed T, Faruque AS, Salam MA. Predictors of severe illness in children under age five with concomitant infection with pneumonia and diarrhea at a large hospital in Dhaka, Bangladesh. Southeast Asian J Trop Med Public Health. 2008;39:719–727. [PubMed] [Google Scholar]
- 13.Senarath U, Agho KE, Akram DE, Godakandage SS, Hazir T, Jayawickrama H, Joshi N, Kabir I, Khanam M, Patel A, Pusdekar Y, Roy SK, Siriwardena I, Tiwari K, Dibley MJ. Comparisons of complementary feeding indicators and associated factors in children aged 6–23 months across five South Asian countries. Matern Child Nutr. 2012;8((Suppl 1)):89–106. doi: 10.1111/j.1740-8709.2011.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel A, Pusdekar Y, Badhoniya N, Borkar J, Agho KE, Dibley MJ. Determinants of inappropriate complementary feeding practices in young children in India: secondary analysis of National Family Health Survey 2005–2006. Matern Child Nutr. 2012;8((Suppl 1)):28–44. doi: 10.1111/j.1740-8709.2011.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caruso B, Stephenson R, Leon JS. Maternal behavior and experience, care access, and agency as determinants of child diarrhea in Bolivia. Rev Panam Salud Publica. 2010;28:429–439. doi: 10.1590/s1020-49892010001200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Paz MG, de Almeida MF, Gunther WM. Diarrhea in children and sanitation and housing conditions in periurban areas in the city of Guarulhos, SP. Rev Bras Epidemiol. 2012;15:188–197. [PubMed] [Google Scholar]
- 17.Haque R, Mondal D, Karim A, Molla IH, Rahim A, Faruque AS, Ahmad N, Kirkpatrick BD, Houpt E, Snider C, Petri WA., Jr Prospective case-control study of the association between common enteric protozoal parasites and diarrhea in Bangladesh. Clin Infect Dis. 2009;48:1191–1197. doi: 10.1086/597580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molla AM, Rahman M, Sarker SA, Sack DA, Molla A. Stool electrolyte content and purging rates in diarrhea caused by rotavirus, enterotoxigenic E. coli, and V. cholerae in children. J Pediatr. 1981;98:835–838. doi: 10.1016/s0022-3476(81)80863-3. [DOI] [PubMed] [Google Scholar]
- 19.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 20.Khan AI, Huq S, Malek MA, Hossain M, Talukder KA, Faruque AS, Salam MA. Analysis of fecal leukocytes and erythrocytes in Shigella infections in urban Bangladesh. Southeast Asian J Trop Med Public Health. 2006;37:747–754. [PubMed] [Google Scholar]
- 21.Harris JC, Dupont HL, Hornick RB. Fecal leukocytes in diarrheal illness. Ann Intern Med. 1972;76:697–703. doi: 10.7326/0003-4819-76-5-697. [DOI] [PubMed] [Google Scholar]
- 22.Espinosa-Cantellano M, Martinez-Palomo A. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin Microbiol Rev. 2000;13:318–331. doi: 10.1128/cmr.13.2.318-331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speelman P, McGlaughlin R, Kabir I, Butler T. Differential clinical features and stool findings in shigellosis and amoebic dysentery. Trans R Soc Trop Med Hyg. 1987;81:549–551. doi: 10.1016/0035-9203(87)90402-0. [DOI] [PubMed] [Google Scholar]
- 25.Stoll BJ, Glass RI, Banu H, Huq MI, Khan MU, Ahmed M. Value of stool examination in patients with diarrhoea. Br Med J (Clin Res Ed) 1983;286:2037–2040. doi: 10.1136/bmj.286.6383.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan A, Huq S, Hossain M, Talukder K, Malek M, Faruque A. Presumptive shigellosis: clinical and laboratory characteristics of Bangladeshi patients. Scand J Infect Dis. 2005;37:96–100. doi: 10.1080/00365540510026823. [DOI] [PubMed] [Google Scholar]
- 27.Pickering LK, DuPont HL, Olarte J, Conklin R, Ericsson C. Fecal leukocytes in enteric infections. Am J Clin Pathol. 1977;68:562–565. doi: 10.1093/ajcp/68.5.562. [DOI] [PubMed] [Google Scholar]
- 28.Das SK, Faruque AS, Chisti MJ, Malek MA, Salam MA, Sack DA. Changing trend of persistent diarrhoea in young children over two decades: observations from a large diarrhoeal disease hospital in Bangladesh. Acta Paediatr. 2012;101:e452–e457. doi: 10.1111/j.1651-2227.2012.02761.x. [DOI] [PubMed] [Google Scholar]
- 29.Stoll BJ, Glass RI, Huq MI, Khan MU, Holt JE, Banu H. Surveillance of patients attending a diarrhoeal disease hospital in Bangladesh. Br Med J (Clin Res Ed) 1982;285:1185–1188. doi: 10.1136/bmj.285.6349.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]