Abstract
Two cases of melioidosis at a residence in rural northern Australia were linked to the unchlorinated domestic bore (automated well) water supply, which was found to have a high concentration of Burkholderia pseudomallei. Using multilocus sequence typing, clinical B. pseudomallei isolates from both cases were identical to an isolate from the bore water supply. A simple UV sterilizer reduced B. pseudomallei from the domestic water supply to undetectable levels. We have shown that UV treatment is highly effective for remediation of water contaminated with B. pseudomallei and recommend its consideration in households where individuals may be at heightened risk of contracting melioidosis.
Burkholderia pseudomallei is an environmental bacterium that is the etiological agent of melioidosis, a disease endemic in northern Australia and Southeast Asia.1 Melioidosis is an emerging public health burden in the tropical Northern Territory, Australia, with > 780 documented cases in the last 23 years and a dramatic rise in cases in the past 3 years.2,3 In October 2012, B. pseudomallei was classified as a Tier 1 select agent by the Centers for Disease Control and Prevention (CDC) because of its potential for bioweaponization, high mortality rate, lack of available vaccine, and non-specific disease presentation. Percutaneous inoculation is considered the most common route of infection; however, the potentially important roles of inhalation and ingestion are increasingly being recognized.1,2
Four outbreaks of melioidosis in Australia have been associated with B. pseudomallei contaminated water supplies. A large outbreak in a piggery in Queensland was attributed to a contaminated unchlorinated water supply from a local river.4 Between 1994 and 1996, four deaths were linked to an unchlorinated bore water supply in a remote community of the Northern Territory.5 Bores are automated (pumped) water wells. In 1999, a B. pseudomallei contaminated water supply with a broken chlorination system caused an outbreak of melioidosis and three deaths in Western Australia.6 More recently, a cluster of parrot deaths was attributed to the water supply within an aviary in Darwin, Northern Territory.7 A recent survey of 55 unchlorinated bore water supplies in the Darwin area revealed that 33% were culture positive for B. pseudomallei.8 Currently, there are an estimated 2,600 domestic water bores in the rural Darwin region (Northern Territory Government, unpublished data), of which the majority are unchlorinated because of concerns about taste, by-products, cost, and maintenance. Alternative, cost-effective solutions are needed to decrease the load of B. pseudomallei in bore water supplies in melioidosis-endemic locations and to reduce the potential for infections and outbreaks.
The disinfecting properties of UV have been shown for various waterborne pathogens such as Escherichia coli, Salmonella typhimurium, and Legionella pneumophila.9 Burkholderia pseudomallei was shown to be UV susceptible at levels similar to other soil bacteria.10 However, there is a lack of knowledge regarding both the practical performance of UV-emitting devices in small water supplies and their usefulness for B. pseudomallei disinfection. In the current study, we investigated the effectiveness of UV irradiation in reducing B. pseudomallei load in a domestic bore water supply linked to two clinical cases of melioidosis. This study was approved by the Human Research Ethics Committee of the Northern Territory Department of Health and the Menzies School of Health Research (HREC 02/38).
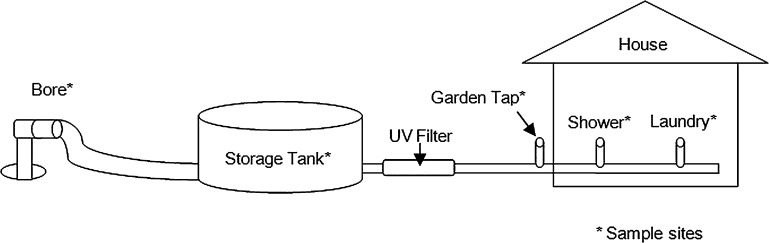
The study site was located on a house block in the Darwin rural area supplied with unchlorinated bore water, suspected of being the source of two clinical cases of melioidosis that occurred within a 3-month period in early 2012. Both patients were potentially inoculated through traumatic leg wounds that were washed with water from the domestic supply. Water sampling was conducted during two visits in June and July 2012. The first sampling was performed 2 weeks before the installation of a UV sterilizer, and the second sampling was performed 3 weeks after UV irradiation (UV-Guard, Sydney, NSW, Australia, model: SLT40, Peak flow rate: 2.7 m3/h [45 L/min]) had commenced. The sterilizer was installed between the tank and the home water supply. Water was collected from multiple sites upstream and downstream of the sterilizer including the bore, storage tank, garden tap, shower, and laundry (Figure 1).
Figure 1.
Schematic of house water supply including sampling sites.
Burkholderia pseudomallei culture detection was performed by 0.22 μm filtering of 500 mL aliquots of water followed by filter incubation in Ashdown's broth as previously described.8 Isolate confirmation was performed using a real-time polymerase chain reaction assay targeting a B. pseudomallei-specific 115 bp segments within the type III secretion system.11 Quantitative culture was based on spreading 100 μL of the water sample onto Ashdown's agar and incubating for 48 h at 37°C.
All sampling points were culture positive for B. pseudomallei before UV irradiation (Table 1). Following installation, all sampling points downstream of the UV sterilizer (shower, garden tap, and laundry) were B. pseudomallei culture negative, indicating successful decontamination of the house water supply. Furthermore, sampling points upstream of the UV filter (bore and tank) remained positive after installation of the filter, confirming that decontamination of the water supply was attributable to UV irradiation.
Table 1.
Detection of Burkholderia pseudomallei from the bore water supply by culture*
| Sample site† | Pre UV filter | Post UV filter |
|---|---|---|
| Bore | Pos | Pos |
| Storage tank | Pos | Pos |
| Garden tap | Pos | Neg |
| Shower | Pos | Neg |
| Laundry | Pos | Neg |
Neg = negative; Pos = positive.
N = 5 for all sample sites.
Quantitative culture revealed that the highest B. pseudomallei load was found in the bore water supply after installation of the UV sterilizer, which yielded 475 colony forming units (CFU)/mL. The storage tank had a considerably smaller B. pseudomallei load of 20 CFU/mL. Burkholderia pseudomallei favors growth in acidic, low salt conditions12,13 and the bore water was more acidic (pH 6.2–6.7) and less saline (EC 0.022–0.032 mS/cm) than the storage tank and house samples (pH 7.4–7.9) (EC 0.103–0.134 mS/cm).
Multilocus sequence typing (MLST)14 was performed on clinical and environmental B. pseudomallei isolates associated with the bore water supply. Isolates from the two clinical cases of melioidosis and the storage tank were found to be the same sequence type (ST 325); this finding implicated the bore water supply as the likely source of infection and in support of this, B. pseudomallei isolates cultured from soil around the house were different STs (ST 109 and 333).
To our knowledge, this is the first report of successful disinfection of a B. pseudomallei contaminated domestic water supply using a UV sterilizer. Clinical isolates from two occupants of the property who developed melioidosis were matched by MLST to those found in the bore water supply, indicating that the contaminated bore water was the likely source of their infection. Based on our results, we recommend that households in melioidosis-endemic locations supplied with untreated bore water could consider installation of a UV sterilizer, especially if occupants have risk factors for melioidosis such as diabetes. Further work is needed to determine the effectiveness of UV filters to eliminate B. pseudomallei from other water supplies including those with turbid or iron-rich water.
Footnotes
Financial support: This work was supported by project grants from the Australian National Health and Medical Research Council. DG and BGS were funded by Wellcome Trust grant WT089472.
Authors' addresses: Evan McRobb, Mirjam Kaestli, Mark Mayo, Erin P. Price, Derek S. Sarovich, and Bart J. Currie, Menzies School of Health Research, Darwin, Australia, E-mails: evan.mcrobb@menzies.edu.au, mirjam.kaestli@menzies.edu.au, mark.mayo@menzies.edu.au, erin.price@menzies.edu.au, derek.sarovich@menzies.edu.au, and bart@menzies.edu.au. Daniel Godoy and Brian G. Spratt, Imperial College, London, UK, E-mails: d.godoy@outlook.com and b.spratt@imperial.ac.uk.
References
- 1.Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–1044. doi: 10.1056/NEJMra1204699. [DOI] [PubMed] [Google Scholar]
- 2.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parameswaran U, Baird RW, Ward LM, Currie BJ. Melioidosis at Royal Darwin Hospital in the big 2009–2010 wet season: comparison with the preceding 20 years. Med J Aust. 2012;196:345–348. doi: 10.5694/mja11.11170. [DOI] [PubMed] [Google Scholar]
- 4.Ketterer PJ, Webster WR, Shield J, Arthur RJ, Blackall PJ, Thomas AD. Melioidosis in intensive piggeries in south eastern Queensland. Aust Vet J. 1986;63:146–149. doi: 10.1111/j.1751-0813.1986.tb02953.x. [DOI] [PubMed] [Google Scholar]
- 5.Currie BJ, Mayo M, Anstey NM, Donohoe P, Haase A, Kemp DJ. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am J Trop Med Hyg. 2001;65:177–179. doi: 10.4269/ajtmh.2001.65.177. [DOI] [PubMed] [Google Scholar]
- 6.Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O'Reilly L, Cameron B. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–59. doi: 10.3201/eid0601.000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hampton V, Kaestli M, Mayo M, Choy JL, Harrington G, Richardson L, Benedict S, Noske R, Garnett ST, Godoy D, Spratt BG, Currie BJ. Melioidosis in birds and Burkholderia pseudomallei dispersal, Australia. Emerg Infect Dis. 2011;17:1310–1312. doi: 10.3201/eid1707.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayo M, Kaesti M, Harrington G, Cheng AC, Ward L, Karp D, Jolly P, Godoy D, Spratt BG, Currie BJ. Burkholderia pseudomallei in unchlorinated domestic bore water, tropical Northern Australia. Emerg Infect Dis. 2011;17:1283–1285. doi: 10.3201/eid1707.100614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrotta MJ, Bekdash F. UV disinfection of small groundwater supplies. J Am Water Works Assoc. 1998;90:71–81. [Google Scholar]
- 10.Sagripanti JL, Levy A, Robertson J, Merritt A, Inglis TJ. Inactivation of virulent Burkholderia pseudomallei by sunlight. Photochem Photobiol. 2009;85:978–986. doi: 10.1111/j.1751-1097.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 11.Novak RT, Glass MB, Gee JE, Gal D, Mayo MJ, Currie BJ, Wilkins P. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J Clin Microbiol. 2006;44:85–90. doi: 10.1128/JCM.44.1.85-90.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglis TJ, Sagripanti JL. Environmental factors that affect the survival and persistence of Burkholderia pseudomallei. Appl Environ Microbiol. 2006;72:6865–6875. doi: 10.1128/AEM.01036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draper AD, Mayo M, Harrington G, Karp D, Yinfoo D, Ward L, Haslem A, Currie BJ, Kaestli M. Association of the melioidosis agent Burkholderia pseudomallei with water parameters in rural water supplies in Northern Australia. Appl Environ Microbiol. 2010;76:5305–5307. doi: 10.1128/AEM.00287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, Spratt BG. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–2079. doi: 10.1128/JCM.41.5.2068-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]