Abstract
Melioidosis is among the most common causes of septicemia in Thailand, but data on economic burden are limited. We describe the economic impact of bacteremic melioidosis hospitalizations in two Thailand provinces during 2006–2008. Costs are presented in US dollars ($1 = 30.49 Thai Baht). The average annual incidence of bacteremic melioidosis cases per 100,000 persons in Sa Kaeo and Nakhon Phanom was 4.6 and 14.4, respectively. The annual cost of bacteremic melioidosis hospitalizations from the societal perspective, including direct and indirect costs, was $152,159 in Sa Kaeo and $465,303 in Nakhon Phanom. The average cost per fatal case was $14,182 and $14,858 in Sa Kaeo and Nakhon Phanom, respectively. In addition to the high morbidity and mortality, the substantial economic burden of melioidosis further supports the need for investments to identify improved prevention and control strategies for melioidosis.
Introduction
Melioidosis is known to be endemic in tropical areas between latitudes 20°N and 20°S, predominantly in southeast Asia, northern Australia, Papua New Guinea, India, southern China, Hong Kong, and Taiwan.1 It has been estimated that around 2,000 to 3,000 cases of melioidosis occur each year in Thailand.2 The annual incidence in northeastern provinces of the country has been estimated to be 12.6–14.9 cases per 100,000 persons.3,4
The high melioidosis incidence in endemic countries likely carries a high economic burden. Melioidosis therapy requires intensive antimicrobial treatment in the acute phase as well as prolonged eradication treatment. In addition to this expensive antimicrobial regimen, costs are especially high for patients requiring intensive care unit admission, which account for 38% of hospitalized patients with melioidosis.5 Furthermore, melioidosis has a high mortality rate,4 which could lead to substantial economic impact to society in terms of productivity losses. Although data are available on the economic impact of other high-burden diseases in Thailand (e.g., dengue fever6 and tuberculosis7), we were unable to identify any previously published data on the economic burden of melioidosis in Thailand.
We aim to describe the economic burden of bacteremic melioidosis from the societal perspective, including direct and indirect costs related to morbidity and mortality, using data from one eastern and one northeastern province in Thailand.
Material and Methods
Setting.
This study was conducted in two Thailand provinces, Sa Kaeo in the eastern region of the country with a 2007 population of 531,884 and Nakhon Phanom in the northeast with a population of 738,184. The 2007 per capita income in Sa Kaeo was 56,092 Baht ($1,839), and the 2007 per capita income in Nakhon Phanom was 30,244 Baht ($992) (Table 1). Both provinces have primarily agrarian-based economies and share common occupations, including farming and raising livestock.
Table 1.
Characteristics of the study population in Sa Kaeo and Nakhon Phanom provinces, Thailand, 2006–2008
| Sa Kaeo | Nakhon Phanom | Thailand | |||
|---|---|---|---|---|---|
| Population in 2007* | 531,884 | 738,184 | 63,038,247 | ||
| Male sex (%) | 50 | 50 | 49 | ||
| Per capita income in Thai Baht ($US) | 56,092 ($1,840) | 30,244 ($992) | 129,089 ($4,234) | ||
| All cases | Charge data† available, n (%) | All cases | Charge data† available, n (%) | ||
| Total number of case-patients | 73 | 52 | 319 | 141 | |
| Median age in years (range) | 52 (3–77) | 52 (3–76) | 52 (2–85) | 52 (2–84) | |
| Age group (years) | |||||
| 0–14 | 2 (2.7) | 2 (3.8) | 9 (2.8) | 6 (4.3) | |
| 15–64 | 55 (75) | 39 (75) | 254 (80) | 105 (74) | |
| ≥ 65 | 16 (22) | 11 (21) | 56 (18) | 30 (21) | |
| ICU admission | 25 (34) | 10 (19) | 91 (29) | 41 (29) | |
| Male sex | 45 (62) | 30 (58) | 178 (56) | 77 (55) | |
| Fatal outcome | 27 (37) | 20 (38) | 95 (30) | 33 (23) | |
Office of the National Economic and Social Development Board, Office of the Prime Minister.
Hospital charge data were collected from the NHSO and used to calculate direct medical cost.
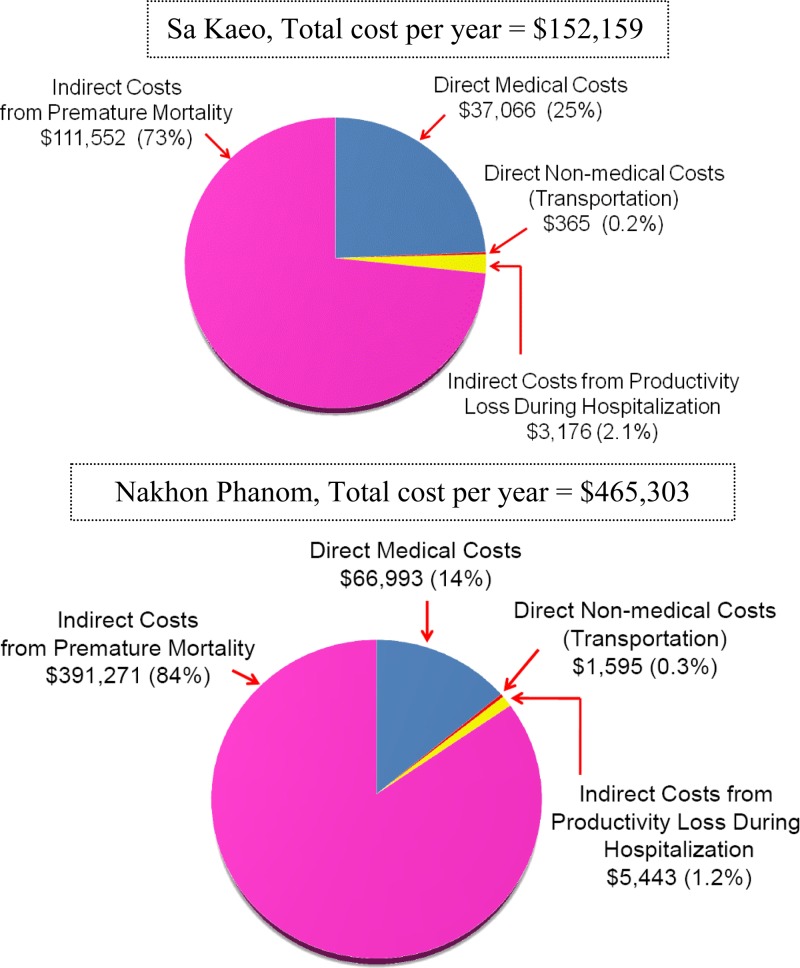
Figure 1.
Average annual cost of hospitalized bacteremic melioidosis cases from the societal perspective – Sa Kaeo and Nakhon Phanom, Thailand, 2006–2008.
Study sites.
In 2005, we established active population-based surveillance for bloodstream infections in all hospitals in Sa Kaeo (1 provincial and 7 district hospitals), an area not usually thought of as highly endemic for melioidosis, and Nakhon Phanom (1 provincial and 11 district hospitals), an area known to have high melioidosis rates.4,5 Automated blood culture systems (BacT/ALERT 3D, bioMérieux Inc., Durham, NC) were implemented in the provincial hospital in each province to enhance detection of blood-borne pathogens in patients hospitalized for community-acquired pneumonia or suspected sepsis.8 Blood cultures from all other hospitals in each province were processed at provincial hospitals within 24 hours. Cultures positive for Burkholderia pseudomallei were confirmed by the National Institutes of Health, Thailand Ministry of Public Health. The enhanced laboratory capacity and surveillance system allowed estimation of population-based incidence of invasive bacterial infections,8 including melioidosis.4 We defined a case of bacteremic melioidosis as blood culture-confirmed B. pseudomallei infection in a patient hospitalized in Sa Kaeo or Nakhon Phanom from January 1, 2006 to December 31, 2008.
Data collection.
Medical records of bacteremic melioidosis case-patients were reviewed for demographic and clinical characteristics, length of hospital stay, and outcome at the time of discharge. Patients who were discharged in moribund condition were categorized as fatal cases. For patients with unconfirmed vital status at discharge, outcome was considered unknown.
Cost estimation.
The total cost attributable to hospitalized bacteremic melioidosis cases was calculated from the societal perspective and included three types of costs: direct medical costs, direct non-medical costs, and indirect costs because of morbidity and pre-mature mortality.
Direct medical costs.
Hospital charge data for case-patients were obtained from the Central Office of Healthcare Information and the National Health Security Office (NHSO), Thailand. We linked each case hospitalization to the corresponding record in the NHSO database to obtain hospital charge information. We estimated direct medical cost by converting charge data to costs using a cost-to-charge ratio of 0.88 and 0.93 for provincial and district hospitals, respectively.9 All costs in 2006–2008 were converted to 2011 values using the consumer price index for medical care of Thailand.10 Because links to NHSO charge data could not be made for all cases, an average direct medical cost per case was calculated using the cases with NHSO charge data available. This average cost was then applied to all hospitalized bacteremic melioidosis cases identified by province to estimate the total direct medical costs. The direct medical costs were stratified by case patients with and without comorbidities based on International Classification of Diseases Tenth Revision (ICD-10)–coded discharge diagnoses.
Direct non-medical costs.
Direct non-medical costs generally include expenditures (e.g., travel costs, lodging, and meals) that result from the illness but are not part of the direct purchasing of medical services. For this analysis, we estimated direct non-medical costs based only on transportation costs related to hospitalization using data from the National Health and Welfare Survey (National Statistics Office, Thailand Ministry of Information and Communication Technology).11 Data on other direct non-medical (e.g., lodging and meals) were not available.
Indirect costs.
Indirect costs were divided into costs related to morbidity and costs related to pre-mature mortality. The human capital approach was used for estimating indirect costs.12 This approach estimates the loss of future earnings based on the assumption that vacant positions will never be replaced and that society is impacted by the productivity loss while affected persons are out of the work force. Morbidity-related indirect costs were estimated as the lost productivity among patients and caregivers during the time of hospitalization.13 Each patient was assumed to have one productive caregiver during hospitalization. Morbidity-related indirect costs were calculated for patients ages 15–64 years (the group considered to have earning power) and caregivers as the number of days missed from work during hospitalization multiplied by the average daily individual income using income data from the National Statistics Office.14
Future productivity losses from pre–mature death were calculated by multiplying the number of fatal cases by the estimated future earnings by age group and sex using data available from the International Health Policy Program, which uses a 3% annual discounting rate.15,16
Sensitivity analysis.
We performed sensitivity analyses by varying the annual discount rate for future productivity from 0% to 6% to assess the impact on cost estimates.17
Statistical analysis.
Data were double entered and validated using Microsoft Access version 2003 (Redmond, WA). SPSS for Windows version 15 (SPSS Inc., Chicago, IL) was used for data analysis. Continuous variables with normal distributions were summarized as means and standard deviations (SD). Continuous variables were compared using Student's t test or for variables with non-Gaussian distributions, the Mann–Whitney test. All costs were converted to US dollars ($1 = 30.49 Thai Baht as of September of 2011) and presented as means ± SD.
Ethical considerations.
The study was approved by the Ethical Review Committees of the Department of Disease Control, Thailand Ministry of Public Health and the Faculty of Tropical Medicine, Mahidol University as well as an Institutional Review Board of the Centers for Disease Control and Prevention (CDC; Protocol number 5694).
Results
From January of 2006 to December of 2008, 392 hospitalized cases of bacteremic melioidosis were identified, including 73 cases (27 deaths) in Sa Kaeo and 319 cases (95 deaths) in Nakhon Phanom (Table 1). The average annual incidence of hospitalized bacteremic melioidosis cases per 100,000 persons in Sa Kaeo and Nakhon Phanom was 4.6 (4.2 in 2006, 4.7 in 2007, and 4.8 in 2008) and 14.4 (11.7 in 2006, 17.9 in 2007, and 13.6 in 2008), respectively.
Cost of bacteremic melioidosis in Sa Kaeo.
Among 73 bacteremic melioidosis cases identified in Sa Kaeo, hospital charge information from the National Health Insurance Database was available for 52 (71%) cases. The age and sex distributions were similar for cases with and without charge data available (Table 1). Among 52 cases with charge data available, the physician discharge diagnosis was melioidosis in 23 (44%) patients, whereas 29 (56%) patients had other diagnoses (diabetes mellitus [14/29, 48%], renal disease [7/29, 24%], lung disease [12/29, 41%], and heart disease [4/29, 14%]). Thirty-two of 52 (62%) case-patients were discharged alive and 20 (39%) died in hospital. Ten (19%) of 52 cases were admitted to an intensive care unit (ICU).
The average annual direct medical costs attributable to hospitalized bacteremic melioidosis cases in Sa Kaeo were $37,066 ($16,187 among fatal cases and $20,876 among non-fatal cases). Patients with a discharge diagnosis of melioidosis (N = 23) had higher average direct medical costs than patients with other discharge diagnoses (N = 29): $2,149 versus $1,027 (P = 0.05). Of 23 cases with a melioidosis discharge diagnosis, 6 patients had diabetes listed as an additional discharge diagnosis, and for these cases, the average annual direct medical costs were $1,435 (SD = $1,463). The average annual direct non-medical costs for transportation were $365. The average annual indirect costs totaled $114,729 ($3,176 attributable to productivity losses during hospitalization and $111,552 attributable to pre-mature mortality) (Table 2).
Table 2.
Average annual cost in US dollars of bacteremic melioidosis in Sa Kaeo and Nakhon Phanom, 2006–2008
| Cost items | Sa Kaeo | Nakhon Phanom | ||||
|---|---|---|---|---|---|---|
| Cost/case (USD ± SD) | n | Average cost/year (USD) | Cost/case (USD ± SD) | n | Average cost/year (USD) | |
| Direct medical cost* | ||||||
| All cases | 1,523 ± 1,862 | 52 | 37,066 | 630 ± 572 | 141 | 66,993 |
| Fatal cases | 1,730 ± 2,455 | 20 | 16,187 | 690 ± 609 | 33 | 17,178 |
| Non-fatal cases | 1,394 ± 1,401 | 32 | 20,876 | 636 ± 578 | 99 | 47,475 |
| Direct non-medical cost | ||||||
| Transportation cost | 15 | 73 | 365 | 15 | 319 | 1,595 |
| Indirect costs for productivity losses during hospitalization† | ||||||
| All cases | 77 ± 70 | 124 | 3,176 | 29 ± 25 | 573 | 5,443 |
| Fatal cases | 42 ± 67 | 50 | 693 | 11 ± 9 | 175 | 651 |
| Non-fatal cases | 106 ± 59 | 74 | 2,611 | 37 ± 25 | 398 | 4,870 |
| Indirect costs for productivity losses because of pre-mature death‡ | ||||||
| Cost of pre-mature death | 12,395 ± 9,836 | 27 | 111,552 | 14,142 ± 7,973 | 83 | 391,271 |
| Total | 152,159 | 465,303 | ||||
Direct medical cost per case was estimated from cases with hospital charge data available from the Central Office of Healthcare Information and the NHSO and presented as rounded whole US dollars ± SD. Average direct medical cost per year was calculated as the average cost per case times the total number of cases divided by 3 years.
Productivity losses from morbidity are calculated only for patients ages 15–64 years during hospitalization (Sa Kaeo, N = 51; Nakhon Phanom, N = 254) and one productive caregiver per patient (Sa Kaeo, N = 73; Nakhon Phanom, N = 319).
Productivity losses for pre-mature mortality are calculated using age- and sex-specific discounted earnings16 for fatal cases when the patient would have been ages 15–64 years (Sa Kaeo, N = 27; Nakhon Phanom, N = 83).
The overall average cost per year of bacteremic melioidosis in Sa Kaeo was $152,159, of which 75% was attributed to indirect costs (Figure 1). The average cost of bacteremic melioidosis per fatal and non-fatal case was $14,181 and $1,515, respectively. The average cost of bacteremic melioidosis cases with and without ICU admission was $18,481 per case (direct cost per case = $2,722 and indirect cost per case = $15,692) and $14,533 per case (direct cost per case = $1,256 and indirect cost per case = $13,210), respectively.
Cost of bacteremic melioidosis in Nakhon Phanom.
In Nakhon Phanom, 141 (44%) of 319 cases had hospital charge data available. The age and sex distribution was similar for cases with and without charge data available (Table 1). Melioidosis was specified as at least one discharge diagnosis for all cases in Nakhon Phanom. Of 141 case-patients with charge data available, 119 (84%) case-patients also had at least one comorbid condition noted among the discharge diagnoses: diabetes mellitus (66/119, 56%), renal disease (40/119, 34%), lung disease (29/119, 24%), liver disease (16/119, 13%), musculoskeletal disease (10/119, 8%), or heart disease (6/119, 5%). Ninety-nine (70%) of 141 case-patients (72 [51%] patients ages 15–64 years) were discharged alive, and 33 case-patients (27 [82%] patients ages 15–64 years) had fatal outcomes. Of 33 deaths, 10 deaths (30%) occurred among patients discharged in moribund condition; 9 of 141 (6.4%) case-patients were transferred to other healthcare facilities, and outcomes could not be determined. Forty-two (30%) cases required ICU care.
The average annual direct medical costs attributable to hospitalized bacteremic melioidosis cases in Nakhon Phanom were $66,993 ($17,178 among fatal cases and $47,475 among non-fatal cases). Bacteremic melioidosis case-patients with discharge diagnoses that included melioidosis and comorbid conditions (N = 119) had higher direct medical costs than those case-patients with melioidosis alone (N = 22): $678 versus $369 (P < 0.01). Of 119 case-patients with comorbid conditions, 25 case-patients (21%) had diabetes, and the direct medical costs among this group were $526 (SD = 507) per case. The annual direct non-medical cost for transportation was $1,595. The annual indirect costs were $396,715 ($5,443 attributable to productivity losses during hospitalization and $391,271 attributable to pre-mature mortality) (Table 2).
The overall average cost per year of bacteremic melioidosis in Nakhon Phanom was $465,303, and the majority (85%) of costs was attributable to indirect costs (Figure 1). The average cost of bacteremic melioidosis per fatal case and non-fatal case were $14,859 and $688, respectively. The average cost of bacteremic melioidosis with and without ICU admission was $17,197 per case (direct cost per case = $956 and indirect cost per case = $16,241) and $13,369 per case (direct cost per case = $517 and indirect cost per case = $12,852), respectively.
Sensitivity analysis.
Setting the discount rates to 0% and 6% (compared with the base case of 3%) changed the total annual cost of bacteremic melioidosis in Sa Kaeo to $185,150 (22% increase) and $131,141 (14% decrease), respectively. In Nakhon Phanom, varying the discount rate changed the total annual cost estimates to $589,766 (27% increase) and $388,408 (17% decrease), respectively.
Discussion
In addition to the high disease burden, our study documents the substantial economic impact of bacteremic melioidosis in Thailand, even in eastern Thailand, an area not previously known to be endemic.4 The total annual cost of hospitalized cases of bacteremic melioidosis was estimated as $152,159 in Sa Kaeo and $465,303 in Nakhon Phanom. The majority of costs was attributable to the indirect costs from pre-mature mortality (75% and 85% of total costs in Sa Kaeo and Nakhon Phanom, respectively). The average cost per fatal case in Sa Kaeo and Nakhon Phanom was 2.8 and 2.7 times higher than Thailand's gross domestic product (GDP) per capita ($4,972 in 2011),18 respectively, whereas the average cost per case with ICU admission was 3.7 and 4.5 times higher than Thailand's GDP per capita.
Diabetes mellitus is known to be the common comorbidity related to melioidosis,19,20 and its prevalence is increasing worldwide, including in Thailand.21 Diabetes alone results in substantial cost to society. According to a previous study in one northeastern Thailand province, the average annual cost of illness in 2008 per diabetic patient from a societal perspective was $881, which was 21% of Thailand's GDP per capita.22 The direct medical cost alone of bacteremic melioidosis in a patient with diabetes is two times that amount.
In Sa Kaeo, an area outside the highly endemic northeast, only 44% of hospitalized bacteremic melioidosis cases had melioidosis specified as a discharge diagnosis based on ICD-10 coding in the National Health Insurance Database. Infrequent use of melioidosis as a discharge diagnosis likely led to underestimation of the true direct medical costs, because ICD-10 coding strongly influences the direct medical cost calculation.23 In our study, bacteremic melioidosis patients with an ICD-10–coded discharge diagnosis of melioidosis had direct medical costs two times higher than those patients without the discharge diagnosis, although all patients were hospitalized with blood culture-confirmed B. pseudomallei infection. Underuse of the melioidosis coding would also lead to underestimation of disease burden from surveillance systems that rely on discharge diagnosis. More precise coding for melioidosis cases would improve the accuracy of cost as well as disease burden estimates.
This study was strengthened by the presence of active surveillance for bloodstream infections in all hospitals in the two provinces, which has improved detection of bacteremic melioidosis4 and allowed for more robust cost estimates. Additionally, we included a province in a region not previously considered highly endemic, documenting the economic impact beyond northeastern Thailand.
An important limitation of this study was the inclusion of costs associated with only melioidosis cases identified in hospital. Our surveillance also only captured bacteremic melioidosis cases, which have previously been estimated to account for only 50–60% of all melioidosis cases.24,25 Furthermore, we included only one direct non-medical cost (transportation) but did not have data on other costs, such as costs related to follow-up visits, meals, lodging, long-term treatment, and intangibles (pain and mental suffering). We also did not have costs incurred at other healthcare facilities before hospitalization or if transferred after discharge. Cost calculations in this study, therefore, represent a minimum estimate of the total economic impact of melioidosis overall. Finally, we did not have primary income data for each case-patient, and therefore, we relied on average income data from a national database, which may not have always reflected income levels in the two study provinces.
Although the human capital approach is recommended for indirect cost calculations in Thailand,12 this method is known to overestimate indirect costs, because lost productivity from morbidity or mortality can be recovered by others in the workforce.26 Other methods that can be used are the friction-cost and the willingness to pay (WTP) methods. The friction-cost method assumes that the productivity lost from morbidity or pre-mature mortality can be eliminated after a new employee is trained and can replace the former employee who experienced the disease in question. Indirect costs are assumed to occur only during the time that it takes to replace an employee (friction period). We do not have sufficient information on when the friction period starts and ends, and this method can underestimate indirect costs.26 The WTP method calculates indirect costs based on estimates of how much money individuals would be prepared to pay to prevent morbidity or pre-mature mortality from the condition. However, this method is affected by individual income; individuals with lower incomes tend to be willing to pay less than individuals with higher incomes.12
Our study revealed the substantial economic impact of melioidosis from the societal perspective and demonstrated that the impact reaches regions of Thailand not historically considered to have a high melioidosis burden. Early case detection and proper treatment of melioidosis are required to decrease disease severity and prevent pre-mature mortality, which accounted for the greatest economic losses. These data underscore the need for effective melioidosis prevention and control strategies in Thailand and can serve policymakers in their efforts to make the best use of public health resources.
ACKNOWLEDGMENTS
We are grateful for the contributions of the many study collaborators: Sa Kaeo and Nakhon Phanom Provincial Health Offices, surveillance officers, district and provincial hospitals in Sa Kaeo and Nakhon Phanom. The authors thank the Central Office of Healthcare Information, the Comptroller General's Department, and the National Health Security Office, Thailand, for the permission to use data on hospital charges. Finally, the authors thank Professor Dr. Wipada Chawakul for her expert advice and Dr. Robert Spengler for critical review. These data were presented in poster format at the International Conference on Emerging Infectious Diseases 2012 in Atlanta, GA (presentation #179, Session on Policy Implications and Infectious Diseases on March 12, 2012).
Disclaimer: Findings presented in this paper represent the views of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Footnotes
Financial support: Support for the blood culture systems was provided by Pneumococcal Vaccines Accelerated Development and Introduction Plan (PneumoADIP), which was funded by the Global Alliance for Vaccines and Immunization (GAVI) and based at the Johns Hopkins Bloomberg School of Public Health.
Authors' addresses: Saithip Bhengsri, Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, and International Emerging Infections Program, Global Disease Detection Regional Center, Thailand Ministry of Public Health—US Centers for Disease Control and Prevention Collaboration, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, E-mail: saithipb@th.cdc.gov. Jongkol Lertiendumrong and Kanjana Tisayaticom, International Health Policy Program, Ministry of Public Health, Nonthaburi, Thailand, E-mails: bljongkol@gmail.com and kanjana@ihpp.thaigov.net. Henry C. Baggett and Somsak Thamthitiwat, International Emerging Infections Program, Global Disease Detection Regional Center, Thailand Ministry of Public Health—US Centers for Disease Control and Prevention Collaboration, Department of Disease Control, Ministry of Public Health, Nonthaburi, Thailand, E-mails: kipb@th.cdc.gov and somsakt@th.cdc.gov. Wirongrong Chierakul, Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: kae@tropmedres.ac. Kittisak Tanwisaid, Nakhon Phanom Hospital, Nakhon Phanom, Thailand, E-mail: kittisak97@gmail.com. Somrak Chantra, Sa Kaeo Crown Prince Hospital, Sa Kaeo, Thailand, E-mail: somrakc@hotmail.com. Jaranit Kaewkungwal, Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, E-mail: jaranitk@biophics.org.
References
- 1.Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg. 2008;102((Suppl 1)):S1–S4. doi: 10.1016/S0035-9203(08)70002-6. [DOI] [PubMed] [Google Scholar]
- 2.Leelarasamee A. Melioidosis in Southeast Asia. Acta Trop. 2000;74:129–132. doi: 10.1016/s0001-706x(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg. 2010;82:1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhengsri S, Baggett HC, Jorakate P, Kaewpan A, Prapasiri P, Naorat S, Thamthitiwat S, Tanwisaid K, Chantra S, Salika P, Dejsirilert S, Peruski LF, Maloney SA. Incidence of bacteremic melioidosis in eastern and northeastern Thailand. Am J Trop Med Hyg. 2011;85:117–120. doi: 10.4269/ajtmh.2011.11-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanwisaid K, Surin U. Monthly rainfall and severity of melioidosis in Nakhon Phanom, northeastern Thailand. J Health Sci. 2008;17:363–375. [Google Scholar]
- 6.Clark DV, Mammen MP, Jr, Nisalak A, Puthimethee V, Endy TP. Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg. 2005;72:786–791. [PubMed] [Google Scholar]
- 7.Kamolratanakul P, Hiransuthikul N, Singhadong N, Kasetjaroen Y, Akksilp S, Lertmaharit S. Cost analysis of different types of tuberculosis patient at tuberculosis centers in Thailand. Southeast Asian J Trop Med Public Health. 2002;33:321–330. [PubMed] [Google Scholar]
- 8.Baggett HC, Peruski LF, Olsen SJ, Thamthitiwat S, Rhodes J, Dejsirilert S, Wongjindanon W, Dowell SF, Fischer JE, Areerat P, Sornkij D, Jorakate P, Kaewpan A, Prapasiri P, Naorat S, Sangsuk L, Eampokalap B, Moore MR, Carvalho G, Beall B, Ungchusak K, Maloney SA. Incidence of pneumococcal bacteremia requiring hospitalization in rural Thailand. Clin Infect Dis. 2009;48((Suppl 2)):S65–S74. doi: 10.1086/596484. [DOI] [PubMed] [Google Scholar]
- 9.Pramualratana PWS. Health Insurance Systems in Thailand. Nonthaburi, Thailand: Ministry of Public Health; Thailand: 2002. [Google Scholar]
- 10.Bureau of Trade and Economic Indicies, Thailand Customer Price Index. 2012. http://www.price.moc.go.th/price/cpi/index_new_all.asp Available at. Accessed January 15, 2012.
- 11.National Statistical Office, Ministry of Information and Communication Technology of Thailand . The 2007 Health and Welfare Survey. 2007. http://web.nso.go.th/en/survey/hw/hw.htm Available at. Accessed December 1, 2011. [Google Scholar]
- 12.Riewpaiboon A. Measurement of costs. J Med Assoc Thai. 2008;91((Suppl 2)):S28–S37. [PubMed] [Google Scholar]
- 13.Liljas B. How to calculate indirect costs in economic evaluations. Pharmacoeconomics. 1998;13:1–7. doi: 10.2165/00019053-199813010-00001. [DOI] [PubMed] [Google Scholar]
- 14.National Statistical Office, Ministry of Information and Communication Technology of Thailand . Per Capita Income of Population by Region and Province: 2000–2010. 2011. http://service.nso.go.th/nso/nso_center/project/search_center/23project-th.htm Available at. Accessed December 12, 2011. [Google Scholar]
- 15.Permsuwan U, Guntawongwan K, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thai. 2008;91((Suppl 2)):S53–S58. [PubMed] [Google Scholar]
- 16.International Health Policy Program, Bureau of Policy and Strategy, Ministry of Public Health, Thailand Research on Health Plan Investment. 2012. http://ihppthaigov.net/publication/publication_research_show.php?id=91 Available at. Assessed June 16, 2012.
- 17.Edejer TT-T. World Health Organization . Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva: World Health Organization; 2003. [Google Scholar]
- 18.The World Bank . GDP per Capita (Current USD) Thailand. 2013. http://data.worldbank.org/indicator/NY.GDP.PCAP.CD/ Available at. Accessed January 21, 2013. [Google Scholar]
- 19.Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, Budhsarawong D, Mootsikapun P, Wuthiekanun V, Teerawatasook N, Lulitanond A. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408–413. doi: 10.1086/520223. [DOI] [PubMed] [Google Scholar]
- 20.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee S, Riewpaiboon A, Piyauthakit P, Riewpaiboon W, Boupaijit K, Panpuwong N, Archavanuntagul V. Cost of diabetes and its complications in Thailand: a complete picture of economic burden. Health Soc Care Community. 2011;19:289–298. doi: 10.1111/j.1365-2524.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- 23.Pongpirul K, Walker DG, Winch PJ, Robinson C. A qualitative study of DRG coding practice in hospitals under the Thai Universal Coverage scheme. BMC Health Serv Res. 2011;11:71. doi: 10.1186/1472-6963-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suputtamongkol Y, Hall AJ, Dance DA, Chaowagul W, Rajchanuvong A, Smith MD, White NJ. The epidemiology of melioidosis in Ubon Ratchatani, northeast Thailand. Int J Epidemiol. 1994;23:1082–1090. doi: 10.1093/ije/23.5.1082. [DOI] [PubMed] [Google Scholar]
- 25.Currie BJ, Fisher DA, Howard DM, Burrow JN, Lo D, Selva-Nayagam S, Anstey NM, Huffam SE, Snelling PL, Marks PJ, Stephens DP, Lum GD, Jacups SP, Krause VL. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis. 2000;31:981–986. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 26.van den Hout WB. The value of productivity: human-capital versus friction-cost method. Ann Rheum Dis. 2010;69((Suppl 1)):i89–i91. doi: 10.1136/ard.2009.117150. [DOI] [PubMed] [Google Scholar]