Abstract
Methods for recombinant production of eukaryotic membrane proteins, yielding sufficient quantity and quality of protein for structural biology, remain a challenge. We describe here, expression and purification optimisation of the human SERCA2a cardiac isoform of Ca2+ translocating ATPase, using Saccharomyces cerevisiae as the heterologous expression system of choice. Two different expression vectors were utilised, allowing expression of C-terminal fusion proteins with a biotinylation domain or a GFP- His8 tag. Solubilised membrane fractions containing the protein of interest were purified onto Streptavidin-Sepharose, Ni-NTA or Talon resin, depending on the fusion tag present. Biotinylated protein was detected using specific antibody directed against SERCA2 and, advantageously, GFP-His8 fusion protein was easily traced during the purification steps using in-gel fluorescence. Importantly, talon resin affinity purification proved more specific than Ni-NTA resin for the GFP-His8 tagged protein, providing better separation of oligomers present, during size exclusion chromatography. The optimised method for expression and purification of human cardiac SERCA2a reported herein, yields purified protein (> 90%) that displays a calcium-dependent thapsigargin-sensitive activity and is suitable for further biophysical, structural and physiological studies. This work provides support for the use of Saccharomyces cerevisiae as a suitable expression system for recombinant production of multi-domain eukaryotic membrane proteins.
Introduction
Cellular calcium homeostasis is regulated by a large number of proteins, with the Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA) pumps as key players in this process. SERCA pumps are integral membrane proteins responsible for Ca2+ uptake from the cytosol into the sarcoplasmic/endoplasmic reticulum (SR/ER), using the energy derived from ATP hydrolysis to fuel the ion translocation. This process is vital for preserving low intracellular calcium levels in a resting cell, a prerequisite for the use of calcium as a secondary messenger to control essential cellular processes such as muscle relaxation/contraction, cell signalling and apoptosis. Human SERCA (hSERCA) pumps belong to the P-type ATPase superfamily and are encoded by three different genes (ATP2A1, ATP2A2 and ATP2A3), each with its splice variants, giving rise to SERCA1a-b, SERCA2a-c and SERCA3a-f isoforms. All SERCAs have a total molecular weight of about 110 kDa and share a similar general structural organization, possessing an integral transmembrane domain and a large cytoplasmic domain. The cytoplasmic domain contains three distinct subdomains; the nucleotide binding domain (N), the phosphorylation domain (P) and the actuator domain (A). These subdomains are jointly responsible for ATP binding and hydrolysis, and serve as the motor driving ion translocation through long-range intra-molecular interactions with the integral membrane domain. The integral membrane domain consists of ten, or for SERCA2b eleven, transmembrane (TM) helices and is responsible for calcium binding and translocation [1,2].
All SERCA isoforms are homologous; the main amino-acid sequence differences are located at the C-terminal ends. Functionally, SERCA isoforms have different affinities for Ca2+ and different enzymatic turn-over rates [3–5].
Mammalian SERCA1a isoform is present mainly in adult fast-twitch muscle and mammalian SERCA1b is found in foetal muscle tissue, whereas mammalian SERCA3 isoforms (a-f) have been found to be expressed in various tissues: heart, skin, platelets and pancreas [6–10]. Rabbit SERCA1a (rSERCA1a) has been extensively studied since the 1960s [11–15], its abundance in muscle tissue has made it the source of choice for functional and structural analyses and it has been used as a benchmark for recombinant eukaryotic protein expression and purification from S. cerevisiae [16–18].
Three human SERCA2 isoforms have been identified: hSERCA2a, found mainly in the heart and slow-twitch muscle [19,20]; hSERCA2b, ubiquitously expressed and is present in neurons and epidermis [21]; and hSERCA2c is found in pancreatic, hepatic and mesenchymal cells [20,22,23]. Genetic engineering experiments revealed that SERCA2b has an extra transmembrane domain compared to SERCA1a and a longer C-terminal luminal tail, providing the highest affinity for Ca2+ and the lowest enzymatic turn-over [24]. Physiologically, SERCA2a is the main Ca2+ translocating isoform in the heart, its activity being controlled by other important proteins such as phospholamban and sarcolipin [25].
Numerous studies have shown a correlation between SERCA2a down-regulation and heart failure [26–28], with specific mutations in hSERCA2a and hSERCA2b leading to skin diseases without affecting heart activity [29,30]. Recently, a biotechnology company, Celladon (www.celladon.net) has over-expressed SERCA2a protein by transgenic technologies and is currently performing phase II clinical trials for humans suffering from heart failure, with positive results.
To study hSERCA2a in greater detail and to understand the complex nature of the physiological regulation in the heart, a method is required for expression and purification of hSERCA2a, in sufficient quantities and purity, suitable for in depth functional and structural characterisation, similar to methods developed for other complex membrane proteins [18,31,32].
We present herein an optimised route for recombinant production of hSERCA2a in S. cerevisiae purified by two successive purification steps to obtain enzymatically active protein suitable for crystallisation trials and biophysical characterisation. The functional analysis of the purified protein demonstrates Ca2+-dependent ATP consumption and thapsigargin inhibition, confirming that the recombinant purified protein is correctly folded and active.
Materials and Methods
Materials
All reagents used were obtained from Sigma-Aldrich, UK unless stated otherwise. Restriction enzymes and polymerase were purchased from New England Biolabs and Promega. Talon resin was from Clontech and Ni-NTA super-flow resin was from Qiagen. Streptavidin Sepharose High Performance resin was from GE Healthcare and Thrombin from Calbiochem. Complete EDTA free protease inhibitor was from Roche. 1,2 –dioleoyl-sn-Glycero-3-Phosphocholine (DOPC) was from Avanti Polar Lipids. Dodecyl-β-D-maltoside (DDM) was from Glycon Biochemicals. Octaethylene glycol monododecyl ether (C12E8) was from Nikko Chemicals. 4-12% Tris-Glycine SDS-PAGE precast gels were from Invitrogen. Bio-RadDC Protein assay was from Bio-Rad Laboratories. cDNA for human SERCA2a (NM_001681) was a gift from Anne-Marie Lompré (Inserm UMRS956/Université Pierre et Marie Curie). Antibodies were purchased from Santa Cruz Biotechnologies.
Equipment
Centrifugal filter concentrators were from Amicon-Millipore. TosoHaas TSK-gel G3000SWXL gel filtration column was from Hichrom, UK. LAS-1000-3000 charged-coupled device (CCD) Imaging system was from Raytek Scientific. Polypropylene 96-well round bottom, clear plates were from Greiner. 96-well black, optical bottom plates were from Nunc. SpectraMax M2e microplate reader was from Molecular Devices. 5mL Ni-NTA His-trap columns were from GE Healthcare. NanoPhotometer was from Implen. Cell disruptor was from Constant Systems. iBlot Nitrocellulose membranes and iBlot™ Dry Gel Transfer Device were from Invitrogen. Tetra-detector was from Viscotek.
Strain and plasmid
S. cerevisiae W303.1b/GAL4-2 (a, leu2, his3, trp1::TRP1-GAL10-Gal4, ura3, ade2-1, can R, cir+) strain and pYeDP60-SERCA1a-BAD expression vector (AmpR, ura, ade, OriBact, thrombin cleavage site, Biotin Acceptor Domain) were a gift from Dr Christine Jaxel (Institut de Biologie et de Technologies de Saclay, France) as previously described [17]. S. cerevisiae FGY217 (MATa, ura3-52, lys2Δ201, pep4Δ) strain and expression plasmid pRS426-Gal1-GFP (ura3, Gal1, Sma1, 8His, yEGFP, AmpR) were a gift from Dr. Konstantinos Beis (Membrane Protein Laboratory, Imperial College, London) previously described in [33].
Cloning human SERCA2a
pYeDP60-SERCA1a-BAD vector was linearised using EcoR1 and Sma1 restriction enzymes, removing SERCA1a gene. hSERCA2a gene, previously mutated for an EcoR1 restriction site, was cloned into the pYeDP60-BAD vector by standard T4DNA ligation and transformed into E. coli. The clones were tested by colony PCR and DNA sequencing. pYeDP60-hSERCA2a-BAD vector was prepared and used for transformation in yeast using a standard protocol [34].
hSERCA2a cDNA was amplified using the following primers:
Forward (5’) ATTAGAATTCTAGTATGGAGAACGCGCACACC(3’) and
Reverse (5’) ATTACCCGGGAGCAGCAGTAGATCCTCTTGGAACCAAACCACCTTCCA
GTATTGCAGGTTCCAGGTAG (3’).
hSERCA2a gene was cloned into the pRS426-Gal1-GFP-His 8 vector by homologous recombination in S. cerevisiae. The vector was linearised using Sma1 enzyme, providing the blunt ends needed for homologous recombination. The hSERCA2a PCR product was mixed with the linearised vector and used for direct transformation into S. cerevisiae FGY217, as described previously [35].
hSERCA2a cDNA was amplified using the following primers:
Forward (5’) A C C C C G G A T T C T A G A A C T A G T G G A T C C C C CATGGAGAACGCGCACACC (3’) and
Reverse (5’) A A A T T G A C C T T G A A A A T T A A A T T T T C C C CCTCCAGTATTGCAGGTTCC (3’) - the underlined 15 base pair sequence corresponds to the blunt ends of the linearised vector.
All buffers and containers used were sterile and freshly prepared. The obtained transformants were platted on minimal media agar plates (0.1% w/v casaminoacids, 0.7% w/v yeast nitrogen base without amino acids, 2% w/v glucose, 2% w/v agar) and grown for 48 hours at 30°C. The clones were tested by PCR and DNA sequencing.
Expression
The expression level of hSERCA2a in S. cerevisiae was tested using pYeDP60 and pRS426 vectors in small scale minimal media cultures (0.1% w/v casaminoacids, 0.7% w/v yeast nitrogen base without amino acids, 2% w/v glucose). Overnight cultures were used to inoculate 10mL minimal media containing 0.1% w/v glucose at a final OD600 0.12. After complete consumption of the glucose, i.e. when reaching OD600 of about 0.6, the expression was induced by 2% w/v galactose. Cells were harvested and processed as in [35] and [18]. Western blotting and in-gel-fluorescence (for the GFP-construct) analysis were used to detect hSERCA2a expression.
For large scale cultures 2.5 L baffled Tunair flasks were used, each flask containing 1 L media. An overnight hSERCA2a minimal media (2% glucose) pre-culture (300 ml) grown at 30°C and 280 rpm shaking was used to inoculate 12L of rich media (2% w/v tryptone, 2% w/v yeast extract, 1% w/v glucose, 2.7% v/v ethanol) or minimal media (0.1% w/v casaminoacids, 0.7% w/v yeast nitrogen base without amino acids and 0.1 glucose % w/v) at a starting OD600 of 0.12.
Rich media cultures were grown for 36 hours at 30°C and 260 rpm shaking. The culture temperature was lowered to 18°C and protein expression was induced by addition of 2% w/v galactose, followed by a second induction with 2% w/v galactose after 15 hours. This methodology was based on previous optimisation of yeast recombinant protein expression established for rabbit SERCA1a [16,18], which showed that two inductions increased the expression level of the target protein. Cells were harvested 6 hours after final induction in 1 L centrifuge bottles in a Sorvall Evolution RC centrifuge (10 min at 5000gav).
Minimal media cultures were grown for approximately 7 hours at 30°C until OD600 reached 0.6 and were induced with 2% w/v galactose. Cells were harvested 20 hours after induction, as described above. Cell pellets were frozen using liquid nitrogen and stored at -80°C [35].
Fluorescence size exclusion chromatography
The methodology used for fluorescence size exclusion chromatography was as previously described [35].
Purification
Membrane preparation
Pelleted cells were resuspended in 100mL TES buffer/L culture (50 mM Tris-HCl pH 7.5, 1 mM EDTA, 0.6 M sorbitol, 0.1 M KCl) supplemented with protease inhibitor (1 tablet for each 100 ml buffer), 1 mM PMSF and 5 mM β-ME and passed through the cell disruptor (Constant Systems) three times: once at 30 and twice at 35 Kpsi. Unbroken cells and cell debris were removed by centrifugation for 10 minutes at 15000gav using JLA16.250 rotor. Membranes containing hSERCA2a were pelleted by ultracentrifugation at 135000gav (41000 rpm) for two hours using the Type 45 Ti rotor in a Beckman Coulter Optima L100XP ultracentrifuge. The membranes were resuspended and washed in presolubilisation buffer (50 mM MOPS pH 7.0, 100 mM KCl, 1 mM CaCl2, 20% glycerol, 5 mM β-ME) and pelleted again by ultracentrifugation for 1 hour at 135000gav. Membranes were resuspended in 20 mL HEPES buffer/L culture (20 mM HEPES pH 7.5, 0.3 M sucrose, 0.1 mM CaCl2), frozen using liquid nitrogen and stored at -80°C.
Solubilisation
The membranes were thawed and diluted to 10 mg/ml total membrane protein in solubilisation buffer (50 mM MOPS pH 7.0, 100 mM KCl, 1mM CaCl2, 20% v/v glycerol, 5 mM β-ME and 1.5:1 w:w ratio DDM: total membrane protein) and mixed for 1 hour at 4°C. Unsolubilised material was removed by ultracentrifugation for 45 min at 135000gav in a Type 45 Ti rotor in a Beckman Coulter Optima L100XP ultracentrifuge.
Affinity purification
Ni-NTA super-flow or Talon resin was pre-equilibrated with the solubilisation buffer and incubated with the solubilised material; 1 ml resin/1L culture, for one hour at 4°C while on a mixer roller, in the presence of 20 or 15 mM imidazole, respectively, for each resin type, adjusting the pH to 7 to minimise unspecific binding. The resin was then poured into a Bio-Rad glass column and left to settle under gravitational force.
Ni-NTA resin was washed with 30x column volumes of buffer (50 mM MOPS pH 7, 100 mM KCl, 1 mM CaCl2, 20% glycerol, 5 mM β-Me, 0.5 mg/ml DDM) and 50 mM imidazole. Elution of bound protein was performed with three column volumes of buffer containing 250 mM imidazole. Similarly, Talon resin was washed with 20x column volumes washing buffer and 15 mM imidazole and bound protein was eluted with two column volumes of buffer containing 150 mM imidazole.
The eluted protein fraction was incubated with TEV-His6 protease (0.5 mg TEV protease/L culture used for purification) for GFP-His8 tag cleavage and left overnight at 4°C while dialysing (dialysis membrane cut-off of 12 kDa; dialysis buffer: 50 mM MOPS, pH 7, 100 mM KCl, 1 mM CaCl2, 15% v/v glycerol, 0.25 mg/ml DDM). Since imidazole and DTT are not compatible with the His-Trap column, dialysis was performed to remove excess imidazole and DTT, the latter of which is initially present in the TEV protease buffer. The sample was passed twice through a 5 mL Ni-NTA His-Trap column, previously equilibrated with dialysis buffer to remove the cleaved GFP-His8 tag and the TEV-His6 protease.
Streptavidin-Sepharose affinity purification was performed as described previously [18] [10]. Briefly, solubilised material was mixed overnight at 4°C with Streptavidin-Sepharose resin to allow binding of protein via the biotinylated tag, at a ratio hSERCA2a: slurry resin of 1 mg: 2 mL, where hSERCA2a concentration was assumed to be 1% w/w of total membrane protein concentration, based on previous purification of rSERCA1a [17] [30]. The resin was washed with a high-salt buffer (50 mM MOPS-Tris pH 7, 1 M KCl, 20% glycerol (v/v), 1 mM CaCl2 and 0.5 mg/ml DDM) and the proteins were eluted in low salt buffer (50 mM MOPS-Tris pH 7, 100 mM KCl, 20% glycerol (v/v), 2.5 mM CaCl2 and 0.5 mg/ml DDM) after two short incubations with 25U of Thrombin per ml of settled resin used. Thrombin cleavage action was quenched by addition of 1 mM PMSF. The elution was frozen in the presence of 40% glycerol (v/v), using liquid nitrogen and stored at -80°C until further use.
Size Exclusion Chromatography
Eluted protein from the affinity purification step was concentrated at 4°C and 2600gav in an Eppendorf 5804R bench-top centrifuge, Swing-bucket rotor A-4-44, to 500 μl using 50 or 100 kDa cut-off Millipore centrifugal concentrators. The total protein concentration prior to gel filtration was about 3 mg/ml, as determined by A280 method. The sample was loaded onto a TosoHaas TSK-gel G3000SWXL GSK gel filtration column, previously equilibrated with gel filtration buffer (50 mM MOPS pH 7, 80 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM β-Me, 0.25 mg/ml DDM or 0.5 mg/ml C 12E8). The chromatography was performed at 4°C using a flow of 0.3 ml/minute and the elution was collected in 0.5 mL fractions.
SERCA2a detection
Ca2+-ATPase protein presence was monitored by SDS-PAGE separation followed by Coomassie brilliant blue staining, in-gel-fluorescence analysis, and/or Western blotting analysis using specific antibodies directed against hSERCA2. Precast 4-12% Tris-Glycine gels were used to detect the presence of hSERCA2a-GFP, throughout the purification steps. Samples were mixed in equal volumes with the loading buffer without boiling, which permitted in-gel-fluorescence visualisation of the fusion GFP-tagged protein by measuring excitation at 460 nm and emission at 515 nm. Also, the fluorescence signal of GFP-membrane protein fusion was monitored in solution using a microplate spectrofluorometer using an excitation wavelength of 488 nm, while measuring emission at 512 nm [35].
Protein concentration estimation
Total membrane protein concentration was determined using a Bio-RadDC Protein assay, using bovine serum albumin as standard. Purified protein concentration was determined using a NanoDrop Spectrophotometer, measuring the absorbance at 280 nm and using an extinction coefficient at 280 nm of 99945 M-1cm-1 for hSERCA2a; or by SDS-PAGE quantification, using known amounts of native rabbit SERCA1a as standard. The software used to quantify the SERCA2a yield was ImageJ [36].
ATPase activity measurement
The functional assay used is based on an ATP-NADH enzyme coupled assay and involved measuring, spectrophotometrically, the decrease of NADH absorbance at 340 nm [37,38], which is related to ATP consumption. The reaction buffer contained 50 mM TES pH 7.5, 100 mM KCl, 7 mM MgCl2, 0.56/0.28 mg/ml DDM/DOPC mix; 5 mM ATP, 1 mM phosphoenolpyruvate (PEP), 0.2 mg/ml lactate dehydrogenase [39], 0.4 mg/ml protein kinase from rabbit (PK), 1 mM NADH, 1.1 mM EGTA. To determine if the activity observed was Ca2+-dependent, the assay was performed at different free Ca2+ concentrations (0.0073, 0.0164, 0.042, 0.062, 0.089, 0.20, 0.47, 0.74, 1.1, 2.3, 7.1, 19.6, 34.9, 49.3, 98.8 µM and 1.11mM), which were calculated using http://maxchelator.stanford.edu/CaMgATPEGTA-TS.htm programme [40]. For each enzymatic reaction, 150 𝜇L of reaction buffer were incubated at 37°C and the reaction was triggered by adding 5-10 µg purified protein. The reaction was quenched with EGTA or thapsigargin (TG). The data were analysed using SigmaPlot Systat software. The slope obtained after quenching was subtracted from the slope obtained after addition of the protein to eliminate any contaminant activity. Specific activity was determined over time intervals where the change in absorbance was linear minus any background activity observed after calcium removal or TG inhibition.
Results and Discussion
Recombinant expression of hSERCA2a in S. cerevisiae
We have successfully achieved expression of hSERCA2a in S. cerevisiae using two different constructs (Table 1). One construct was designed with a cleavable C-terminal biotin acceptor domain (hSERCA2a-BAD) and a second construct with a cleavable C-terminal His-tagged green fluorescence protein (hSERCA2a-GFP-His8). An estimated expression level of up to 5 mg of solubilised hSERCA2a-GFP-His8 per litre of culture was obtained, which is comparable to that reported for heterologous expression of rSERCA1a isoform using various constructs in different cell lines, including mammalian and S. cerevisiae [16–18,41].
Table 1. Human cardiac SERCA2a protein yield obtained per 1L of culture S. cerevisiae using minimal and rich media.
| SERCA construct | Total membrane protein (g) |
Estimated Ca2+ ATPase
|
||
|---|---|---|---|---|
| Solubilised material (mg) | After affinity purification (mg) | After SEC (µg) | ||
| SERCA2a-GFP-His8 | 1.1 | 5.0 | 0.40 | 100 |
| SERCA2a-BAD | 1.0 | n/d | 0.15 | 65 |
| SERCA2a-GFP-His8 minimal media | 0.2 | 1.2 | 0.18 | 50 |
The yields were estimated from SDS-Page gels and Western Blots, using ImageJ software [36,70]. The cultures were grown in rich media unless specified otherwise. Data was estimated for each construct on at least two batches of protein sample, using rabbit SERCA1a and free GFP as control standards; n/d- not determined.
Given the comparable yields for the two constructs, the hSERCA2a-GFP-His8 construct offers two main advantages: a) the purification is more cost effective as the resin for affinity chromatography can be re-used and b) the GFP-fusion protein can easily be monitored throughout the expression and purification steps by in-gel fluorescence, with the GFP detection being as sensitive as or better than Western blot detection using a hSERCA2 specific antibody. An example of assessment of hSERCA2a-GFP-His8 expression levels in different clones using in-gel fluorescence and Western blotting techniques is shown in Figure 1.
Figure 1. Small scale expression test for hSERCA2a-GFP-His8 using different clones.
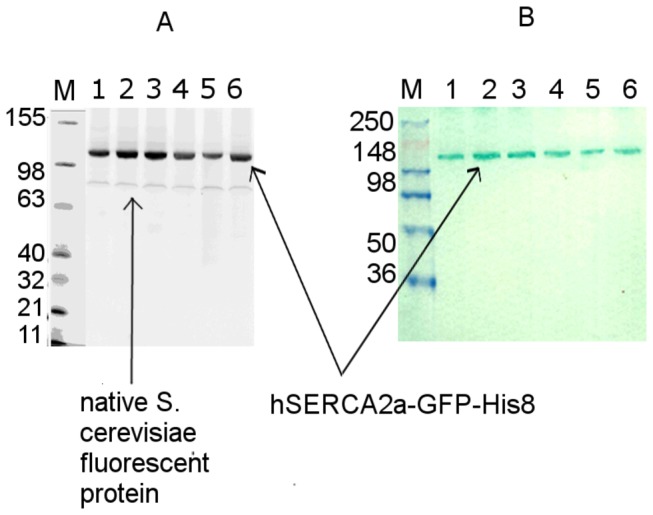
A. SDS-PAGE 4-12% Tris-Glycine in gel fluorescence analysis. M-fluorescent ladder, 1-6 different hSERCA2a-GFP-His8 clones; B. Western Blotting using specific hSERCA2 antibodies, MM – prestained protein ladder.
To ensure the highest possible expression levels of functional hSERCA2a-GFP-His8, we used rich medium and lowered the temperature to 18°C, before two steps of galactose induction of protein expression [16]. In addition, the use of baffled flasks for cell growth significantly improves media aeration and allows routine yields of 50-60 g of yeast per litre culture to be obtained. We also tested hSERCA2a-GFP-His8 expression levels in minimal medium and determined expression levels above 1mg L-1 culture (Table 1) of solubilised protein. Minimal medium cell culture growth led to much lower cell mass yield (<10g L-1 culture).
Previous studies have shown that chemical chaperons such as DMSO (2.5% v/v), histidine (0.04% w/v) or biotin (0.2̶2 mg/ml) can improve the expression level of recombinant proteins [33,42]. To further increase hSERCA2a expression, we performed small scale tests in the presence of DMSO and histidine for the hSERCA2a-GFP-His8 construct or biotin for the hSERCA2a-Biotin construct. Addition of these chemicals was performed concomitant with galactose induction. No visible difference was observed in the expression levels (data not shown), as previously reported for rSERCA1a in the case of biotin addition [8].
Isolation of hSERCA2a-Biotin
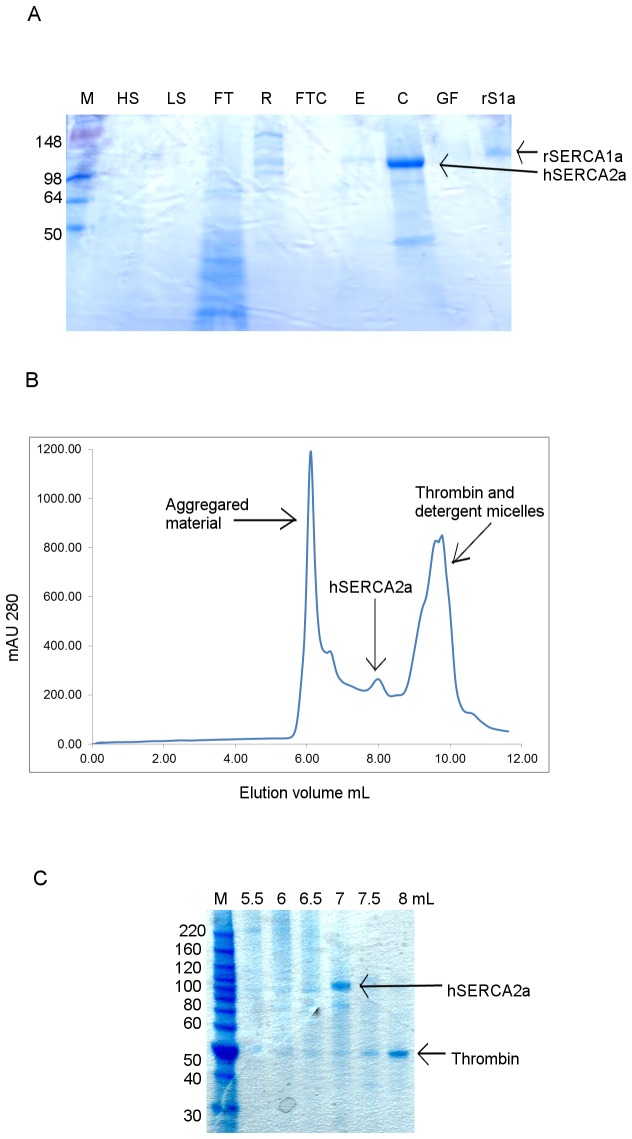
Purification of hSERCA2a-Biotin, using its biotinylated tag, produced pure and functionally active protein. The resulting purified protein is shown in Figure 2. Yields of approximately 150 µg of purified hSERCA2a per litre culture were obtained after affinity chromatography and 65 µg per litre after size exclusion chromatography (SEC), Table 1. This is slightly lower compared to the yield obtained for rSERCA1a, using the same approach [18], and comparable to the yield obtained for purified hSERCA2a by Magro et al. [43], using surface active maghemite nanoparticles for purification (65 𝜇g/L at >90% purity versus 125 𝜇g/L at 70% purity, respectively). However, the hSERCA2a protein obtained in this study is nearly pure (> 90%) following SEC (Figure 2C) and is shown to be functionally active.
Figure 2. Purification of hSERCA2a-Biotin fusion protein using Streptavidin-Sepharose resin.
A. Coomassie stained 12% Tris-Glycine SDS PAGE gel from purification, M- protein ladder, HS-high salt wash, LS-low salt wash, FT- flow-through after binding to Streptavidin resin, R-resin with bound hSERCA2a, FTC- flow-through concentrated, E – elution from Streptavidin resin, C- concentrated sample prior to gel filtration, GF- elution after gel filtration, rS1a- rabbit SERCA1a, 1µg. B. HPLC-SEC profile for hSERCA2a after Streptavidin affinity purification using 12L culture. DDM detergent was exchanged on gel filtration column with C12E8 detergent. C. Coomassie stained 4-12% Bis-Tris SDS PAGE gel. SEC fractions obtained for hSERCA2a purified with Streptavidin resin.
The biotin-fusion protein purification methodology required the use of new Streptavidin-Sepharose resin for each purification, due to the strong interaction of biotin with streptavidin resin. Thus, alternative approaches to express and purify hSERCA2a from S. cerevisiae were investigated in order to reduce the cost of purification. Immobilized metal affinity chromatography (IMAC) purification is more economical and can be useful for membrane protein purification [44,45]. However, a GFP-His fusion tag additionally offers the ability to follow easily the over-expressed protein during the expression and purification steps [35].
Isolation of hSERCA2a-GFP-His8
Purification of hSERCA2a-GFP-His8 was performed initially using Ni-NTA batch-mode binding [35]. The yield of hSERCA2a after affinity purification was improved to 0.4 mg per litre of culture (Table 1). Despite the higher yield, more than half of the solubilised material is not bound to the resin but is recovered in the flow-through after the affinity step, as observed using in-gel fluorescence (Figure 3B, lane FT versus S). Further, poor separation of the monomer and oligomerised/aggregated fraction during size exclusion chromatography was observed (Figure 3C). Oligomers are expected when SEC is performed in the presence of C12E8 [11]. Note that the contribution of light scattering in OD for protein aggregates is high but in fact it corresponds to a relatively low amount of protein, as demonstrated in Figure 3D (see lane 6.0 and 6.5 mL). However, the protein following SEC chromatography is almost pure, as shown by Coomassie Blue stained gel (Figure 3A, lane GF). Purification of hSERCA2a-GFP-His8 using minimal medium yielded approximately 180 µg of purified hSERCA2a per litre culture, after affinity purification (Table 1). This is less than half compared to the yield when using rich medium. The benefit of using minimal medium, besides being selective for SERCA2 vector containing cells, is that it provides a reduced amount of membranes at the start of the purification, thus requiring less detergent for solubilisation than when using rich medium. The final yield of hSERCA2a after SEC, obtained using minimal media, is approximately 50 µg per litre of culture, which means a lower yield per litre culture than when using rich media but a higher yield per wet cell weight. Also, minimal media resulted in a lower amount of total membrane protein loaded on the affinity resin, which can be important to reduce the amount of unspecific bound material.
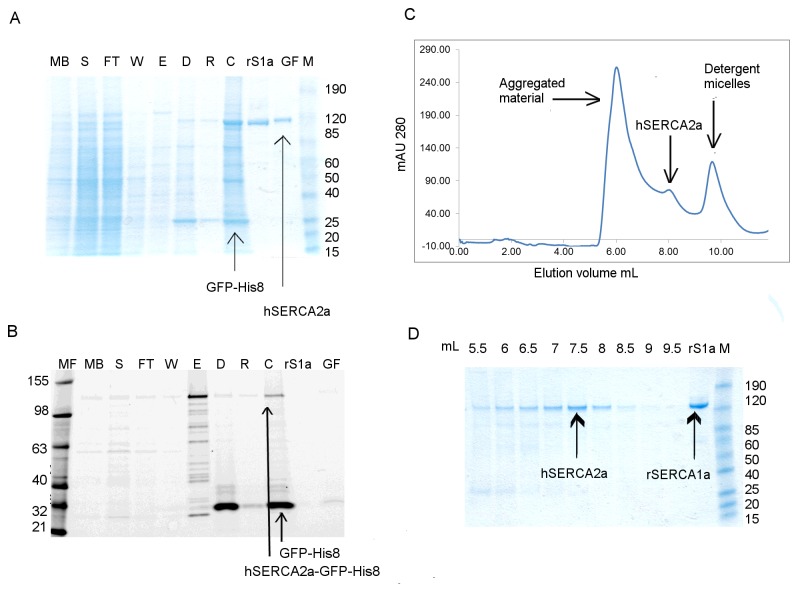
Figure 3. Purification of hSERCA2a-GFP-His8 using Ni-NTA affinity chromatography.
Purification was done in the presence of DDM only throughout all steps, including SEC. Protein was obtained using rich media. A. Coomassie stained SDS-PAGE gel; B. In gel fluorescence 12% Tris-Glycine SDS-PAGE gel. MF- fluorescent protein ladder; MB- diluted membrane fraction; S- solubilised fraction; FT- flow-through after binding; W- wash fraction; E- elution; D- sample after cleavage with TEV protease and dialysis; R- sample after Ni-NTA rebinding after tag cleavage; C- sample concentrated using 50 kDa cut-off filter concentrator, before gel filtration; S1a- rabbit SERCA1a; GF- fraction containing human SERCA2a after gel filtration; M- prestained protein ladder. C. HPLC-SEC profile for hSERCA2a purified using Ni-NTA super-flow resin. D. Coomassie stained 4-12% Tris-Glycine SDS PAGE gel for SEC fractions obtained for purification of hSERCA2a using Ni-NTA super-flow resin.
Optimising the purification of hSERCA2a-GFP-His8
After several optimisation tests (using higher salt concentration in purification buffers and varying the detergent concentration used during solubilisation), Talon resin was used in an attempt to improve the yield during the affinity purification step (Figure 4). Talon resin is an IMAC resin, similar to Ni-NTA, but charged with cobalt ions instead of nickel. It is described to bind His-tagged proteins with higher specificity than nickel-charged resins, resulting in isolation of His-tagged proteins with higher purity and lower metal ion leakage (Co2+ ions are more tightly bound to NTA than Ni2+ ions) [46]. As anticipated, the Talon resin proved to be more specific than the Ni-NTA resin. Binding at 15 mM imidazole and at pH 7 was essential for obtaining a cleaner fraction, as binding at 5 or 10 mM imidazole led to higher unspecific binding. Following TEV-His6 protease cleavage, the protein was passed twice through the Ni-NTA His trap column to remove uncleaved material and any contaminants bound to the affinity resin. We observed that passing the sample twice, rather than once, through the Ni-NTA column improved the purity of the sample (Figure S1A, lane R1 and R2). The yield of purified protein, for the hSERCA2a-GFP-His8 construct, following the second purification step (SEC-HPLC), was 100 µg hSERCA2a per litre of culture (Table 1).
Figure 4. Talon resin affinity purification of hSERCA2a-GFP-His8.
Protein was obtained using minimal media. A. Coomassie stained SDS-PAGE gel; B. In gel fluorescence 12% Tris-Glycine SDS-PAGE gel, MF- fluorescent protein ladder; MB- membranes fraction; S- solubilised fraction; FT- flow-through after binding to Talon resin; W- wash fraction; E- elution; D- sample after cleavage with TEV protease and dialysis; R- sample after Ni-NTA reverse binding; C- sample concentrated, before gel filtration; rS1a- rabbit SERCA1a at; C- fraction containing human SERCA2a concentrated before gel filtration; W2- eluted material from Ni-NTA His trap after reverse Ni-NTA purification; M- protein ladder. C. HPLC-SEC profile for hSERCA2a purified using Talon resin. Protein was concentrated with 100 kDa cut-off filter concentrator and DDM detergent was exchanged on gel filtration column with C12E8 detergent. D. Coomassie stained 4-12% Tris-Glycine SDS PAGE gel for SEC fractions obtained for purification of hSERCA2a using Talon super-flow resin.
Purification of hSERCA2 tagged only with the deca-histidine (-His10) tag rather than the GFP-octa-histidine (-GFP-His8) tag was also tried, but no improvement was observed in the purification profile during size exclusion chromatography (Figure S2), suggesting no effect of the size of the histidine tag or of the presence of GFP on aggregation.
The main difficulty with the purification of hSERCA2a-GFP-His8, using Ni-NTA resin, is the low level of binding of the solubilised protein to the metal-affinity matrix. This was observed by in-gel fluorescence analysis of the flow-through sample after binding (Figure 3B). No improvement was observed even when performing a longer binding step or when rebinding of unbound material was tried (data not shown). There are several potential factors contributing to the low recovery observed. A fraction of the expressed protein may not be correctly inserted into the membrane and hence misfolded to some extent, resulting in shielding the GFP-His-tag. Alternatively, the GFP-His-tag may only be partially accessible without any misfolding of the fusion protein, as suggested in a comparable study [13]. The latter hypothesis is sustained by our current results, such that high fluorescence intensity of GFP-fusion protein (Figure 3B) indicates correct folding of the protein, in agreement with previous work using GFP [33,35,47]. However, this does not eliminate the possibility of partial unfolding of the protein during the extraction from the membranes. From this perspective, any or all of these factors may lead to reduced recovery. One might argue that the poor binding of hSERCA2a-GFP-His8 may be due to low binding affinity of the fusion protein for the metal resin. Nevertheless, a step gradient elution of GFP tagged protein from Ni-NTA resin revealed that the protein starts to elute at and above 75 mM imidazole, indicating strong binding and proper folding of the expressed protein. In this respect, the poor affinity could be considered an advantage as it allows us to eliminate misfolded and probably inactive enzymes leading to optimal purification of properly folded hSERCA2a-GFP-His8.
Aggregation properties and effect on purification
To address the possibility that aggregation of hSERCA2a-GFP-His8 was induced by the solubilisation procedure, various DDM: total membrane protein concentration ratios were tried. We observed that lowering the ratio from 3:1 to 1:1 does not affect the recovery of hSERCA2a-GFP-His8 during the affinity chromatography step. Fluorescence size exclusion chromatography (FSEC) analysis [48] was used to investigate whether using DDM, C12E8 or a lipid-like detergent ,FC12, in the absence or presence of cholesterol hemissuccinate salt could improve the yield of recovered protein. FC12 detergent and cholesterol have been shown to improve the solubility and stability of heterologous membrane proteins [35]. FSEC analysis showed that hSERCA2a-GFP-His8 solubilisation in the presence of FC12 and cholesterol presented the highest monodispersity (FigureS3A), although further large scale purification revealed that this detergent-lipid combination led to aggregates after solubilisation without any improvement in the yield of monodispersed sample after SEC (Figure S3B). This may be due to the high solubility capacity of the FC12 detergent.
The final purification step for both constructs (-Biotin and -GFP-His8) is size exclusion chromatography with detergent exchange from DDM to C12E8. Detergent exchange has been shown to be critical for native and recombinant SERCA1a crystallisation [15,49,50]. Figure 4C shows a typical SEC profile for hSERCA2a-GFP-His8 after purification, highlighting the two different populations observed i.e. an aggregated fraction (at ~5.5-6.5 mL) and a monomeric fraction (at ~7.5-8.0 mL). For all GFP-His8 purified samples we observed a third peak in the chromatograms (Figure 3C, from ~9.0–10.5 mL), which was assigned to correspond to detergent micelles [51] since it did not appear to contain protein, as observed by SDS-PAGE (Figure 3D). To further investigate the identity of this third peak, we used a Tetra-detector, which allows measurement of refractive index by light scattering of membrane protein in detergent solution [52]. The sensitivity of this method can reveal the presence of free detergent micelles and the ratio between protein and detergent present in the sample [51,53]. For comparison, we analysed the sample before SEC (concentrated using 50kDa cut-off filter concentrators) and the fraction corresponding to the monomeric fraction, after gel filtration. The refractive index peak corresponding to the detergent micelles is present only in the sample before SEC (Figure S4A) and not in the sample corresponding to the monomeric hSERCA2a (Figure S4B). This suggests that most of the detergent micelles were eliminated during the SEC step. We also observed that using 100 kDa cut-off concentrators instead of 50 kDa cut-off removes free detergent micelles from solution (Figure 4C), as indicated by refractive index measurement, at the expense of 12% protein loss as previously shown in similar experiments [54]. This point is critical, as final detergent concentrations need to be tightly controlled for crystallisation trials [49].
Enzymatic properties of the purified hSERCA2a
To characterise the functional properties of the purified hSERCA2a, the enzymatic activity was measured using a regenerative ATP-NADH enzyme coupled assay [38,55]. For all functional assays, only SEC-HPLC purified protein, which had previously undergone all purification steps (including cleavage of any affinity tag), was tested.
A typical spectroscopic trace, measuring NADH absorbance at 340 nm, is shown in Figure 5. Upon addition of hSERCA2a to the reaction mixture, a linear reduction in absorbance is observed indicative of NADH conversion to NAD+ as a consequence of ATP hydrolysis by the purified hSERCA2a. As expected, after addition of molar equivalent concentrations of EGTA (equivalent to the free Ca2+ concentration) or thapsigargin (equivalent to the protein concentration), ATP hydrolysis is quenched, confirming the presence of a calcium and thapsigargin dependent hSERCA2a.
Figure 5. Typical activity assay profile for purified recombinant human Ca2+ ATPase isoform 2a (hSERCA2a).

The protein was purified using -GFP-His8 tag and Talon resin affinity purification. Reaction buffer used was as in Materials and Methods; the reaction was triggered by adding 5 µg of purified protein. Addition of thapsigargin inhibits activity of purified protein. Calcium-dependent activity corresponds to the difference of slope before and after thapsigargin addition. Here, final calcium-dependent ATPase activity is about 3 µmol hydrolysed ATP/min/mg of hSERCA2a.
Specific enzymatic activity
ATPase calcium dependent analysis of hSERCA2a (from hSERCA2a-GFP-His8) revealed a variation in the specific enzymatic activity from 1 to 3 µmol min-1 mg-1 protein between different batches of purified protein. However, we observed that hSERCA2a, solubilised and purified in the presence of DDM, consistently showed lower specific activity compared to hSERCA2a solubilised in the presence of DDM and exchanged for C12E8, during the final SEC purification step. The presence of low amounts of DDM monomers, close to the Ca2+ translocating protein, may affect its enzymatic activity as demonstrated in previous studies on rabbit SERCA1a [56]. Thus, exchange of DDM for C12E8 during SEC purification is the most efficient way to remove DDM from the ATPase monomers. Another reason for the observed variability in specific activity may be the measurement of protein concentration in the eluted fractions, which is difficult to assess considering the low concentration.
Previously published data showed an enzymatic ATPase turnover value of 70 sec-1 for hSERCA2a, when expressed in HEK cells [57], 30 sec-1 when expressed in COS cells [3] and 35 sec-1 when obtained from natural source [3]. Our results have shown a turn-over rate of 2-5 sec-1, depending on the protein batch used. Assuming there is no inactive protein after purification, which is reasonably difficult to assess, the differences in ATPase turn-over rate between hSERCA2a expressed in HEK, COS cells, or retrieved from natural source and the values obtained in the present study could arise from the difference in activity between membrane embedded and detergent solubilised SERCA2a. Interestingly, these differences are not observed for SERCA1a when purified from natural source [58] or after heterologous expression [59,60]. The natural environment surrounding hSERCA2a is very different from the surrounding environment in this study. The major lipid found in heart tissue is phosphatidylcholine followed by phosphatidylethanolamine [61,62]. Exactly what part of hSERCA2a is embedded in the lipid bilayer is not known [63] and it is not clear to what extent it differs from the part embedded into a detergent micelle after solubilisation. Detergent solubilisation and extensive chromatography steps delipidate the protein and may cause a destabilising effect. In some cases, detergent purified rSERCA1a presents a higher activity upon relipidation (DOPC) [56,64]. Thus, the interactions between the lipids and hSERCA2a may be important for the protein to demonstrate maximum turnover [65].
Ca2+-dependent ATP hydrolysis
Calcium-dependence of the ATP hydrolysis using an ATP-NADH coupled assay was estimated as described above. As shown in Figure 6, we observed calcium dependent activation of hSERCA2a from which an apparent calcium affinity of approximately 0.6 μM for DDM-solubilised hSERCA2a was determined. This value is in agreement with previously published values for apparent calcium affinity, K0.5 values ranging from 0.2 to 0.9 μM [3,22,57,66,67], depending on the nature of the protein sample. There is a notable sharp decrease of activity at calcium concentrations higher than 100 µM. As observed for rSERCA1a, high calcium concentrations inhibit the pump due to: i) calcium affinity for the luminal binding sites is in the mM range and binding of calcium on the luminal side dramatically slows down the dephosphorylation step, and ii) Ca2+ATP can replace Mg2+ATP at the nucleotide binding site, resulting in deceleration of the phosphorylation step.
Figure 6. Normalised specific ATPase activity rate versus Ca2+ dependence for DDM solubilised hSERCA2a after HPLC-SEC purification.
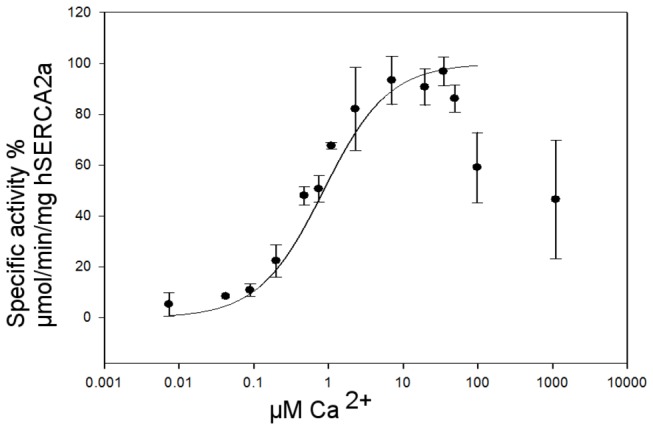
100% specific ATPase activity corresponds to 3 µmol hydrolysed ATP/min/mg of hSERCA2a. The results are the means of seven measurements, using protein obtained from three independent membrane preparations; error bars represent ±S.D.
Conclusions
We have succeeded in expressing and purifying functionally active, recombinant hSERCA2a using S. cerevisiae and we used the purified enzyme to characterise its functional properties. The hSERCA2a yield reported in this study, using the biotinylated tag, is comparable to that observed by Magro et al. [43]. Furthermore, our work has demonstrated a purification protocol for functionally active hSERCA2a.
The differences in purification yield between that previously reported for rabbit SERCA1a [18] and hSERCA2a obtained during this work using S. cerevisiae, may be explained by the differences in the amino-acid sequences of the two proteins, particularly those present in the C-terminal tail region. It would be interesting to estimate the level of in vivo biotinylation for the hSERCA2a-Biotin, which could reveal the amount of properly folded protein [17,50].
Alternatively, the differences in purification yield observed could be due to the particular post-translational modifications of hSERCA2a, as recent heart failure research revealed that SUMOylation by SUMO-1 of hSERCA2a contributes to its stability [68]. It may be that the SUMO-like machinery present in S. cerevisiae is not completely equivalent to that in human [69]. The SUMOylation modification of SERCA2a could be potentially sustained in yeast through the SUMO-1 homolog SMT3 (suppressor of mif two 3 (macrophage migration inhibitory factor, glycosylation-inhibiting factor)). However, currently there are no published data available to confirm that SUMOlylation of the heterologous expressed protein takes place. Future work could involve Western blotting analysis of heterologously expressed hSERCA2a proteins with double-labelling against SERCA proteins and SMT3 to check for SUMOylation in yeast. If no SUMOylation is found, then a heterologous co-expression of SUMO-1 and SERCA2 should be considered.
With regard to the solubilisation detergent, as tested in the case of the hSERCA2a-GFP-His8 construct, no increase in the yield of purified protein was observed when using a smaller detergent: total membrane protein ratio. Thus, it can be concluded that the initial 3:1 ratio used did not contribute to the high aggregation observed during SEC. Further, based on the result obtained with FC12, it may be that usage of a higher solubility capacity detergent may lead to aggregated fraction solubilisation rather than the solubilisation of the active form of the protein. Further work would be necessary to more fully understand the effect of different detergents or lipids on human cardiac Ca2+-ATPase stability [58].
Purified hSERCA2a (from hSERCA2a-GFP-His8 construct) showed calcium-dependent and thapsigargin-sensitive activity. The calcium K0.5 for hSERCA2a of 0.6 μM found herein is within previously reported values [57]. A significant difference observed in the turn-over rate between previously purified samples of SERCA2a may be explained by the different lipid content, since previous data were measured on vesicular microsomes which contain some natural lipids associated with SERCA2, whereas SERCA2a purified from S. cerevisiae was not reconstituted into liposomes and was analysed in the presence of detergent. Another plausible explanation for the differences in turn-over rate could be the presence of inactive protein in the final purified sample. Finally and importantly, are the diversity of ATPase assay conditions (e.g. functional assay, temperature, pH, buffer composition), which may explain the significant differences in the enzymatic activities reported. It is noteworthy that, although the turn-over rate for hSERCA2a was different than when expressed using other systems (HEK, COS, natural source), the values obtained here are very close to the specific enzymatic activities obtained for rSERCA1a expressed and purified from S. cerevisiae [16,17].
The optimised protocol outlined in this work is easily extended to other SERCA isoforms and useful for the production of high quality recombinant active protein for further analysis to study interactions between SERCAs and their physiologically relevant partners. The resulting protein is suitable for crystallisation trials and subsequent structural analysis. Furthermore, the method outlined may prove useful generally for the recombinant production of other multi-domain eukaryotic membrane proteins.
Supporting Information
Purification optimisation: two Ni-NTA reverse binding steps for hSERCA2a-GFP-His8. Coomassie stained 4-12% Tris-Glycine SDS PAGE gel: M- protein ladder, E- eluted protein, D- sample after cleavage with TEV protease and dialysis, R1- after first Ni-NTA reverse binding, R2- after second Ni-NTA reverse binding.
(TIF)
Comparison of SEC purification profile of hSERCA2a-His10 and hSERCA2a-GFP-His8. A. SEC profile for hSERCA2a-His10. B. SEC profile for hSERCA-GFP-His8. Affinity purification was performed using Talon resin. The purification protocol did not involve washing the membranes prior to solubilisation. The protein was concentrated with 50KDa cut-off filter concentrators prior to SEC purification.
(TIF)
Detergent screening for hSERCA2a-GFP-His8. A. Fluorescence size exclusion chromatography profile for hSERCA2a-GFP-His8 solubilised in the presence of 2% w/v cholesterol hemisuccinate salt and 1% w/v FC12 or 1% w/v β-DDM. The same volume of solubilised hSERCA2-GFP-His8 was loaded onto a Superose 6 10/300 column for each detergent screen. The eluted fractions were analysed using a SpectraMax spectrophotometer, as described in Materials and Methods. Aggregation peak corresponds to the void volume. B. HPLC-SEC profile for large scale purification of hSERCA2a-GFP-His, in the presence of FC12 and hemisuccinate salt cholesterol.
(TIF)
Tetra-detector analysis of purified hSERCA2a before and after SEC. The construct used for this analysis was hSERCA2a-GFP-His8. A. Protein sample analysis before SEC. The profile obtained is comparable to the SEC profiles obtained for hSERCA2a. The data shows two UV peaks after the void volume, the first peak (at 13.83mL) corresponds to hSERCA2a monomer and the second peak (at 16.31mL) presence a refractive index trace, indicating excess detergent micelles. B. Protein sample analysis after SEC. The protein fraction eluted from SEC, corresponding to the monomer form of hSERCA2a, was run on the Tetra-detector. The result indicates that the concentrated detergent micelles are separated and eliminated during SEC. The refractive index peak corresponds to the protein-detergent complex.
(TIF)
Acknowledgments
We would like to thank Anne Marie Lompré for the kind gift of human SERCA2a cDNA, Christine Jaxel for providing us with pYeDP60-rabbit SERCA1a-BAD vector and, Konstantinos Beis for the pRS462-Gal1-GFP vector and for useful discussions.
Funding Statement
This work was supported by the University of Reading Endowment Trust Faculty Studentship (MRC DTG) and Diamond Light Source. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Toyoshima C (2009) How Ca2+-ATPase pumps ions across the sarcoplasmic reticulum membrane. Biochim Biophys Acta 1793: 941-946. doi:10.1016/j.bbamcr.2008.10.008. PubMed: 19010358. [DOI] [PubMed] [Google Scholar]
- 2. Møller JV, Olesen C, Winther A-ML, Nissen P (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q Rev Biophys 43: 501-566. doi:10.1017/S003358351000017X. PubMed: 20809990. [DOI] [PubMed] [Google Scholar]
- 3. Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH (1992) Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem 267: 14483-14489. PubMed: 1385815. [PubMed] [Google Scholar]
- 4. Misquitta CM, Mack DP, Grover AK (1999) Sarco/endoplasmic reticulum Ca2+ (SERCA)-pumps: link to heart beats and calcium waves. Cell Calcium 25: 277-290. doi:10.1054/ceca.1999.0032. PubMed: 10456225. [DOI] [PubMed] [Google Scholar]
- 5. Misquitta CM, Sing A, Grover AK (1999) Control of sarcoplasmic/endoplasmic-reticulum Ca2+ pump expression in cardiac and smooth muscle. Biochem J 338(Pt 1): 167-173. doi:10.1042/0264-6021:3380167. PubMed: 9931313. [PMC free article] [PubMed] [Google Scholar]
- 6. Martin V Bredoux R, Corvazier E, Van Gorp R, Kovacs T et al (2002) Three novel sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) 3 isoforms. Expression, regulation, and function of the membranes of the SERCA3 family. J Biol Chem 277: 24442-24452 doi:10.1074/jbc.M202011200. PubMed: 11956212. [DOI] [PubMed]
- 7. Bobe R, Bredoux R, Corvazier E, Andersen JP, Clausen JD et al. (2004) Identification, expression, function, and localization of a novel (sixth) isoform of the human sarco/endoplasmic reticulum Ca2+ATPase 3 gene. J Biol Chem 279: 24297-24306. doi:10.1074/jbc.M314286200. PubMed: 15028735. [DOI] [PubMed] [Google Scholar]
- 8. Tavadia S, Authi KS, Hodgins MB, Munro CS (2004) Expression of the sarco/endoplasmic reticulum calcium ATPase Type 2 and 3 isoforms in normal skin and Darier's disease. Br J Dermatol 151: 440-445. doi:10.1111/j.1365-2133.2004.06130.x. PubMed: 15327552. [DOI] [PubMed] [Google Scholar]
- 9. Dally S, Monceau V, Corvazier E, Bredoux R, Raies A et al. (2009) Compartmentalized expression of three novel sarco/endoplasmic reticulum Ca2+ATPase 3 isoforms including the switch to ER stress, SERCA3f, in non-failing and failing human heart. Cell Calcium 45: 144-154. doi:10.1016/j.ceca.2008.08.002. PubMed: 18947868. [DOI] [PubMed] [Google Scholar]
- 10. Dode L, De Greef C, Mountian I, Attard M, Town MM et al. (1998) Structure of the human sarco/endoplasmic reticulum Ca2+-ATPase 3 gene. Promoter analysis and alternative splicing of the SERCA3 pre-mRNA. J Biol Chem 273: 13982-13994. doi:10.1074/jbc.273.22.13982. PubMed: 9593748. [DOI] [PubMed] [Google Scholar]
- 11. Maire ML, Lind KE, Jørgensen KE, Røigaard H, Møller JV (1978) Enzymatically active Ca2+ ATPase from sarcoplasmic reticulum membranes, solubilized by nonionic detergents. Role of lipid for aggregation of the protein. J Biol Chem 253: 7051-7060. PubMed: 151100. [PubMed] [Google Scholar]
- 12. MacLennan DH (1970) Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem 245: 4508-4518. PubMed: 4250726. [PubMed] [Google Scholar]
- 13. Olesen C, Picard M, Winther AM, Gyrup C, Morth JP et al. (2007) The structural basis of calcium transport by the calcium pump. Nature 450: 1036-1042. doi:10.1038/nature06418. PubMed: 18075584. [DOI] [PubMed] [Google Scholar]
- 14. Jensen AM, Sørensen TL, Olesen C, Møller JV, Nissen P (2006) Modulatory and catalytic modes of ATP binding by the calcium pump. EMBO J 25: 2305-2314. doi:10.1038/sj.emboj.7601135. PubMed: 16710301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sørensen TL, Møller JV, Nissen P (2004) Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 304: 1672-1675. doi:10.1126/science.1099366. PubMed: 15192230. [DOI] [PubMed] [Google Scholar]
- 16. Lenoir G, Menguy T, Corre F, Montigny C, Pedersen PA et al. (2002) Overproduction in yeast and rapid and efficient purification of the rabbit SERCA1a Ca(2+)-ATPase. Biochim Biophys Acta 1560: 67-83. doi:10.1016/S0005-2736(01)00458-8. PubMed: 11958776. [DOI] [PubMed] [Google Scholar]
- 17. Jidenko M, Lenoir G, Fuentes JM, le Maire M, Jaxel C (2006) Expression in yeast and purification of a membrane protein, SERCA1a, using a biotinylated acceptor domain. Protein Expr Purif 48: 32-42. doi:10.1016/j.pep.2006.03.001. PubMed: 16603381. [DOI] [PubMed] [Google Scholar]
- 18. Cardi D, Montigny C, Arnou B, Jidenko M, Marchal E et al. (2010) Heterologous expression and affinity purification of eukaryotic membrane proteins in view of functional and structural studies: The example of the sarcoplasmic reticulum Ca(2+)-ATPase. Methods Mol Biol 601: 247-267. doi:10.1007/978-1-60761-344-2_15. PubMed: 20099150. [DOI] [PubMed] [Google Scholar]
- 19. Lompre AM, de la Bastie D, Boheler KR, Schwartz K (1989) Characterization and expression of the rat heart sarcoplasmic reticulum Ca2+-ATPase mRNA. FEBS Lett 249: 35-41. doi:10.1016/0014-5793(89)80010-9. PubMed: 2542094. [DOI] [PubMed] [Google Scholar]
- 20. Hovnanian A (2007) SERCA pumps and human diseases. Subcell Biochem 45: 337-363. doi:10.1007/978-1-4020-6191-2_12. PubMed: 18193643. [DOI] [PubMed] [Google Scholar]
- 21. Enouf J, Bredoux R, Papp B, Djaffar I, Lompré AM et al. (1992) Human platelets express the SERCA2-b isoform of Ca(2+)-transport ATPase. Biochem J 286(Pt 1): 135-140. PubMed: 1387787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dally S, Bredoux R, Corvazier E, Andersen JP, Clausen JD et al. (2006) Ca2+-ATPases in non-failing and failing heart: evidence for a novel cardiac sarco/endoplasmic reticulum Ca2+-ATPase 2 isoform (SERCA2c). Biochem J 395: 249-258. doi:10.1042/BJ20051427. PubMed: 16402920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gélébart P, Martin V Enouf J, Papp B (2003) Identification of a new SERCA2 splice variant regulated during monocytic differentiation. Biochem Biophys Res Commun 303: 676-684. doi:10.1016/S0006-291X(03)00405-4. PubMed: 12659872. [DOI] [PubMed] [Google Scholar]
- 24. Vandecaetsbeek I, Trekels M, De Maeyer M, Ceulemans H, Lescrinier E et al. (2009) Structural basis for the high Ca2+ affinity of the ubiquitous SERCA2b Ca2+ pump. Proc Natl Acad Sci U S A 106: 18533-18538. doi:10.1073/pnas.0906797106. PubMed: 19846779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vandecaetsbeek I, Raeymaekers L, Wuytack F, Vangheluwe P (2009) Factors controlling the activity of the SERCA2a pump in the normal and failing heart. Biofactors 35: 484-499. doi:10.1002/biof.63. PubMed: 19904717. [DOI] [PubMed] [Google Scholar]
- 26. Mercadier JJ, Lompré AM, Duc P, Boheler KR, Fraysse JB et al. (1990) Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest 85: 305-309. doi:10.1172/JCI114429. PubMed: 2136864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M (1993) Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 72: 463-469. doi:10.1161/01.RES.72.2.463. PubMed: 8418995. [DOI] [PubMed] [Google Scholar]
- 28. Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C et al. (1995) Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92: 778-784. doi:10.1161/01.CIR.92.4.778. PubMed: 7641356. [DOI] [PubMed] [Google Scholar]
- 29. Burge SM, Wilkinson JD (1992) Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol 27: 40-50. [DOI] [PubMed] [Google Scholar]
- 30. Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S et al. (1999) Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet 21: 271-277. doi:10.1038/6784. PubMed: 10080178. [DOI] [PubMed] [Google Scholar]
- 31. Strugatsky D, Gottschalk KE, Goldshleger R, Bibi E, Karlish SJ (2003) Expression of Na+,K+-ATPase in Pichia pastoris: analysis of wild type and D369N mutant proteins by Fe2+-catalyzed oxidative cleavage and molecular modeling. J Biol Chem 278: 46064-46073. doi:10.1074/jbc.M308303200. PubMed: 12949069. [DOI] [PubMed] [Google Scholar]
- 32. André N, Cherouati N, Prual C, Steffan T, Zeder-Lutz G et al. (2006) Enhancing functional production of G protein-coupled receptors in Pichia pastoris to levels required for structural studies via a single expression screen. Protein Sci 15: 1115-1126. doi:10.1110/ps.062098206. PubMed: 16597836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newstead S, Kim H, von Heijne G, Iwata S, Drew D (2007) High-throughput fluorescent-based optimization of eukaryotic membrane protein overexpression and purification in Saccharomyces cerevisiae . Proc Natl Acad Sci U S A 104: 13936-13941. doi:10.1073/pnas.0704546104. PubMed: 17709746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen DC, Yang BC, Kuo TT (1992) One-step transformation of yeast in stationary phase. Curr Genet 21: 83-84. doi:10.1007/BF00318659. PubMed: 1735128. [DOI] [PubMed] [Google Scholar]
- 35. Drew D, Newstead S, Sonoda Y, Kim H, von Heijne G et al. (2008) GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae . Nat Protoc 3: 784-798. doi:10.1038/nprot.2008.44. PubMed: 18451787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image Processing with ImageJ. Biophotonics Int, 11: 36-42. [Google Scholar]
- 37. Pullman ME, Penefsky HS, Datta A, Racker E (1960) Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem 235: 3322-3329. PubMed: 13738472. [PubMed] [Google Scholar]
- 38. Nørby JG (1988) Coupled assay of Na+,K+-ATPase activity. Methods Enzymol 156: 116-119. doi:10.1016/0076-6879(88)56014-7. PubMed: 2835597. [DOI] [PubMed] [Google Scholar]
- 39. Vandecaetsbeek I, Christensen SB, Liu H, Van Veldhoven PP, Waelkens E et al. (2011) Thapsigargin affinity purification of intracellular P(2A)-type Ca(2+) ATPases. Biochim Biophys Acta 1813: 1118-1127. doi:10.1016/j.bbamcr.2010.12.020. PubMed: 21215281. [DOI] [PubMed] [Google Scholar]
- 40. Donald M, Bers. (2010) CWPaRN (2010) A Practical Guide to the Preparation of Ca2+ Buffers. Methods Cell Biol 99: 1-26. PubMed: 21035681. [DOI] [PubMed] [Google Scholar]
- 41. Miras R, Cuillel M, Catty P, Guillain F, Mintz E (2001) Purification of heterologous sarcoplasmic reticulum Ca2+-ATPase Serca1a allowing phosphoenzyme and Ca2+-affinity measurements. Protein Expr Purif 22: 299-306. doi:10.1006/prep.2001.1436. PubMed: 11437606. [DOI] [PubMed] [Google Scholar]
- 42. Stolz J, Darnhofer-Demar B, Sauer N (1995) Rapid purification of a functionally active plant sucrose carrier from transgenic yeast using a bacterial biotin acceptor domain. FEBS Lett 377: 167-171. doi:10.1016/0014-5793(95)01333-4. PubMed: 8543043. [DOI] [PubMed] [Google Scholar]
- 43. Magro M, Faralli A, Baratella D, Bertipaglia I, Giannetti S et al. (2012) Avidin Functionalized Maghemite Nanoparticles and their Application for Recombinant Human Biotinyl-SERCA Purification. Langmuir 28: 15392-15401. doi:10.1021/la303148u. PubMed: 23057670. [DOI] [PubMed] [Google Scholar]
- 44. Bornhorst JA, Falke JJ (2000) Purification of Proteins Using Polyhistidine Affinity Tags. Methods Enzymol: 245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Block H, Maertens B, Spriestersbach A, Brinker N, Kubicek J (2009) Immobilized-Metal Affinity Chromatography (IMAC): A Review. Methods Enzymol, 463: 439–73. PubMed: 19892187. [DOI] [PubMed] [Google Scholar]
- 46. Chaga G, Hopp J, Nelson P (1999) Immobilized metal ion affinity chromatography on Co2+-carboxymethylaspartate-agarose Superflow, as demonstrated by one-step purification of lactate dehydrogenase from chicken breast muscle. Biotechnol Appl Biochem 29(Pt 1): 19-24. PubMed: 9889081. [PubMed] [Google Scholar]
- 47. Waldo GS, Standish BM, Berendzen J, Terwilliger TC (1999) Rapid protein-folding assay using green fluorescent protein. Nat Biotechnol 17: 691-695. PubMed: 10404163. [DOI] [PubMed] [Google Scholar]
- 48. Kawate T, Gouaux E (2006) Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14: 673-681. PubMed: 16615909. [DOI] [PubMed] [Google Scholar]
- 49. Sørensen TL, Olesen C, Jensen AM, Møller JV, Nissen P (2006) Crystals of sarcoplasmic reticulum Ca(2+)-ATPase. J Biotechnol 124: 704-716. PubMed: 16597471. [DOI] [PubMed] [Google Scholar]
- 50. Jidenko M, Nielsen RC, Sørensen TL, Møller JV, le Maire M et al. (2005) Crystallization of a mammalian membrane protein overexpressed in Saccharomyces cerevisiae . Proc Natl Acad Sci U S A 102: 11687-11691. PubMed: 16087876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. le Maire M, Arnou B, Olesen C, Georgin D, Ebel C et al. (2008) Gel chromatography and analytical ultracentrifugation to determine the extent of detergent binding and aggregation, and Stokes radius of membrane proteins using sarcoplasmic reticulum Ca2+-ATPase as an example. Nat Protoc 3: 1782-1795. PubMed: 18974737. [DOI] [PubMed] [Google Scholar]
- 52. Slotboom DJ, Duurkens RH, Olieman K, Erkens GB (2008) Static light scattering to characterize membrane proteins in detergent solution. Methods 46: 73-82. PubMed: 18625320. [DOI] [PubMed] [Google Scholar]
- 53. Strop P, Brunger AT (2005) Refractive index-based determination of detergent concentration and its application to the study of membrane proteins. Protein Sci 14: 2207-2211. PubMed: 16046633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Menguy T, Chenevois S, Guillain F, le Maire M, Falson P et al. (1998) Ligand binding to macromolecules or micelles: use of centrifugal ultrafiltration to measure low-affinity binding. Anal Biochem 264: 141-148. PubMed: 9866675. [DOI] [PubMed] [Google Scholar]
- 55. Kiianitsa K, Solinger JA, Heyer WD (2003) NADH-coupled microplate photometric assay for kinetic studies of ATP-hydrolyzing enzymes with low and high specific activities. Anal Biochem 321: 266-271. PubMed: 14511695. [DOI] [PubMed] [Google Scholar]
- 56. de Foresta B, Henao F, Champeil P (1992) Kinetic characterization of the perturbation by dodecylmaltoside of sarcoplasmic reticulum Ca(2+)-ATPase. Eur J Biochem 209: 1023-1034. PubMed: 1425684. [DOI] [PubMed] [Google Scholar]
- 57. Dode L, Andersen JP, Leslie N, Dhitavat J, Vilsen B et al. (2003). Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J Biol Chem 278: 47877-47889. [DOI] [PubMed] [Google Scholar]
- 58. Lund S, Orlowski S, de Foresta B, Champeil P, le Maire M et al. (1989) Detergent structure and associated lipid as determinants in the stabilization of solubilized Ca2+-ATPase from sarcoplasmic reticulum. J Biol Chem 264: 4907-4915. PubMed: 2522447. [PubMed] [Google Scholar]
- 59. Vilsen B, Andersen JP (1992) Deduced amino acid sequence and E1-E2 equilibrium of the sarcoplasmic reticulum Ca(2+)-ATPase of frog skeletal muscle. Comparison with the Ca(2+)-ATPase of rabbit fast twitch muscle. FEBS Lett 306: 213-218. [DOI] [PubMed] [Google Scholar]
- 60. Zhang Z, Lewis D, Strock C, Inesi G, Nakasako M et al. (2000) Detailed characterization of the cooperative mechanism of Ca(2+) binding and catalytic activation in the Ca(2+) transport (SERCA) ATPase. Biochemistry 39: 8758-8767. PubMed: 10913287. [DOI] [PubMed] [Google Scholar]
- 61. Tepsic V, Ristic V, Ristic D, Vasiljevic N, Pecelj-Gec M (1998) Heart phospholipid content and fatty acid composition in the rat after feeding different lipid supplemented diets. Physiol Res 47: 413-418. PubMed: 10453748. [PubMed] [Google Scholar]
- 62. Waku K, Uda Y, Nakazawa Y (1971) Lipid composition in rabbit sarcoplasmic reticulum and occurrence of alkyl ether phospholipids. J Biochem 69: 483-491. PubMed: 4323968. [PubMed] [Google Scholar]
- 63. Møller JV, Nissen P, Sørensen TL, le Maire M (2005) Transport mechanism of the sarcoplasmic reticulum Ca2+ -ATPase pump. Curr Opin Struct Biol 15: 387-393. PubMed: 16009548. [DOI] [PubMed] [Google Scholar]
- 64. Dalton KA, Pilot JD, Mall S, East JM, Lee AG (1999) Anionic phospholipids decrease the rate of slippage on the Ca(2+)-ATPase of sarcoplasmic reticulum. Biochem J 342(Pt 2): 431-438. PubMed: 10455031. [PMC free article] [PubMed] [Google Scholar]
- 65. Lee AG, East JM (1998) The effects of phospholipid structure on the function of a calcium pump. Biochem Soc Trans 26: 359-365. PubMed: 9765879. [DOI] [PubMed] [Google Scholar]
- 66. Verboomen H, Wuytack F, De Smedt H, Himpens B, Casteels R (1992) Functional difference between SERCA2a and SERCA2b Ca2+ pumps and their modulation by phospholamban. Biochem J 286(Pt 2): 591-595. PubMed: 1326945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Verboomen H, Wuytack F, Van den Bosch L, Mertens L, Casteels R (1994) The functional importance of the extreme C-terminal tail in the gene 2 organellar Ca(2+)-transport ATPase (SERCA2a/b). Biochem J 303(Pt 3): 979-984. PubMed: 7980471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kho C, Lee A, Jeong D, Oh JG, Chaanine AH et al. (2011) SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477: 601-605. PubMed: 21900893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yeh ET (2009) SUMOylation and De-SUMOylation: wrestling with life’s processes. J Biol Chem 284: 8223-8227. PubMed: 19008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Girish V, Vijayalakshmi A (2004) Affordable image analysis using NIH Image/ImageJ. Indian J Cancer 41: 47 PubMed: 15105580. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Purification optimisation: two Ni-NTA reverse binding steps for hSERCA2a-GFP-His8. Coomassie stained 4-12% Tris-Glycine SDS PAGE gel: M- protein ladder, E- eluted protein, D- sample after cleavage with TEV protease and dialysis, R1- after first Ni-NTA reverse binding, R2- after second Ni-NTA reverse binding.
(TIF)
Comparison of SEC purification profile of hSERCA2a-His10 and hSERCA2a-GFP-His8. A. SEC profile for hSERCA2a-His10. B. SEC profile for hSERCA-GFP-His8. Affinity purification was performed using Talon resin. The purification protocol did not involve washing the membranes prior to solubilisation. The protein was concentrated with 50KDa cut-off filter concentrators prior to SEC purification.
(TIF)
Detergent screening for hSERCA2a-GFP-His8. A. Fluorescence size exclusion chromatography profile for hSERCA2a-GFP-His8 solubilised in the presence of 2% w/v cholesterol hemisuccinate salt and 1% w/v FC12 or 1% w/v β-DDM. The same volume of solubilised hSERCA2-GFP-His8 was loaded onto a Superose 6 10/300 column for each detergent screen. The eluted fractions were analysed using a SpectraMax spectrophotometer, as described in Materials and Methods. Aggregation peak corresponds to the void volume. B. HPLC-SEC profile for large scale purification of hSERCA2a-GFP-His, in the presence of FC12 and hemisuccinate salt cholesterol.
(TIF)
Tetra-detector analysis of purified hSERCA2a before and after SEC. The construct used for this analysis was hSERCA2a-GFP-His8. A. Protein sample analysis before SEC. The profile obtained is comparable to the SEC profiles obtained for hSERCA2a. The data shows two UV peaks after the void volume, the first peak (at 13.83mL) corresponds to hSERCA2a monomer and the second peak (at 16.31mL) presence a refractive index trace, indicating excess detergent micelles. B. Protein sample analysis after SEC. The protein fraction eluted from SEC, corresponding to the monomer form of hSERCA2a, was run on the Tetra-detector. The result indicates that the concentrated detergent micelles are separated and eliminated during SEC. The refractive index peak corresponds to the protein-detergent complex.
(TIF)