Abstract
The biological underpinnings of borderline personality disorder (BPD) and its psychopathology including states of aversive tension and dissociation is poorly understood. Our goal was to examine transcriptional changes associated with states of tension or dissociation within individual patients in a pilot study. Dissociation is not only a critical symptom of BPD but has also been associated with higher risk for self-mutilation and depression. We conducted a whole blood gene expression profile analysis using quantitative PCR in 31 female inpatients with BPD. For each individual, two samples were drawn during a state of high tension and dissociation, while two samples were drawn at non-tension states. There was no association between gene expression and tension states. However, we could show that Interleukin-6 was positively correlated to dissociation scores, whereas Guanine nucleotide-binding protein G(s) subunit alpha isoforms, Mitogen-activated protein kinase 3 and 8, Guanine nucleotide-binding protein G(i) subunit alpha-2, Beta-arrestin-1 and 2, and Cyclic AMP-responsive element-binding protein were negatively correlated to dissociation. Our data point to a potential association of dissociation levels with the expression of genes involved in immune system regulation as well as cellular signalling/second-messenger systems. Major limitations of the study are the the possibly heterogeneous cell proportions in whole blood and the heterogeneous medication.
Introduction
Borderline Personality Disorder (BPD) affects about 3% of the general population [1] and is closely related to traumatic life experiences [2] and displays a high comorbidity with depressive illness [3]. Instability in interpersonal relationships and self-image as well as emotion dysregulation are core symptoms of BPD (American Psychiatric Association, 1994). Emotion dysregulation and traumatic remembrance are often accompanied by the experience of dissociation, which can be defined as an altered state of consciousness causing impairments in body awareness, perception, and memory. Dissociative states are very prevalent in BPD [4], [5] and are often triggered by emotional arousal or aversive tension [6], [7]. Dissociation in BPD has been associated with higher risk for self-mutilation and depression, also higher rate of reported childhood abuse was observed among patients who dissociated [8].
There is growing concern with currently available phenotypic tools for the assessment of BPD psychopathology. Therefore, our exploratory study aimed to identify objective blood marker profiles associated with specific disease states and to examine the use of such markers to improve our understanding of the underlying biology. We chose to study gene expression profiles since standardized processes exist that permit easy collection and quick and reliable preservation of gene transcripts in blood. We focused on genes that have been linked to depressive symptoms, given the more abundant literature and independent replication of these findings, and among these we preferentially focused on intracellular signalling factors, because a good correlation between gene and protein expression has been shown [9], [10]. We hypothesized that for a subset of the genes in our panel transcriptional differences could be related to the state of tension and dissociation in patients with BPD.
Psychiatric disorders such as BPD are associated with (neuro-) biological alterations, which affect the brain as well as the body [11]. For example, one of the most thoroughly examined biological alterations in psychiatric disorders such as depression or posttraumatic stress disorder (PTSD) is the dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which is likely to contribute to systemic manifestations of depressive illness and PTSD [12]. In addition, immune alterations and activation of the inflammatory response system can accompany depression [13]–[15]. One of the best studied cytokines in depression is interleukin (IL)-6 for which increased concentrations could be confirmed meta-analytically [14], [15]. Plasma-IL6-levels covaried negatively with hippocampal volumes [16], which is interesting in the context of reduced hippocampal volume being robustly associated with BPD [17]–[19]. In the CSF, high levels of IL-6 in patients with depression vs controls were reported with highest elevations in depressed patients with a recent violent suicide attempt [20]. In patients with BPD, immune dysregulation has not been as widely studied, but. increased IL-6 levels were found in the plasma of BPD patients [21], and IL-1ß levels increased significantly stronger after a glucose challenge in BPD patients as compared to healthy controls [22].
Besides cytokines, transcriptional factors have been reported to show alterations in mental disorders [23]–[25]. Cyclic AMP response element-binding protein (CREB) is a transcriptional target of the calcium signalling cascade. CREB has been reported to be a molecular marker for the response to antidepressants in neurons [26] and in patients with severe major depression [24]. Alterations of calcium signalling in the peripheral leukocytes have been reported in major depression [27]–[29]. PTSD patients had statistically lower levels of total CREB protein in lympho-monocytes than healthy control subjects [30].
Besides transcriptional factors, changes in other intra-cellular signalling pathways have been associated with multiple mental disorders. Extracellular signal-regulated kinases (ERKs) are a subfamily of the mitogen-activated protein kinases that are activated in neurons by a variety of extracellular signals, including various neurotransmitters [31]. Decreased mRNA and protein levels of ERK-1 and -2 as well as of upstream molecules of the ERK signalling cascade were found in the hippocampus and prefrontal cortex of suicide victims [32], [33]. The ERK MAP signalling pathways have been related to learning and memory as well as to PTSD psychopathology [34]. Another protein family involved in intra-cellular signalling, the ß-Arrestins, play an important role in G protein-coupled receptor desensitization. Mice chronically treated with corticosterone as a model of depressive states revealed reduced ß-Arrestin expression [9]. Furthermore, ß-Arrestin-1 levels were found to be elevated by antidepressants in rat cortex and hippocampus and reduced in leukocytes of depressed patients [35].
Interestingly, there is growing evidence that early adverse life experiences can modulate HPA activity and may potentiate abnormalities observed during an acute depressive episode [36]. In a similar vein, childhood abuse has been shown to increase secretion of IL-6 (but not other immune parameters) in subjects experiencing stressful situations later on in life [37].
Such differences in life histories may not only affect the likelihood to develop a depressive disorder, but may also influence other trauma-related pathophysiology. One such study has described a specific profile of messenger RNA expression in peripheral blood mononuclear cells in subjects who experienced a trauma and later developed posttraumatic stress disorder [38]. Specifically, the authors showed that subjects who developed PTSD had elevated expression of genes indicating immune activation, while expression of a number of genes associated with transcription and intra-cellular signalling was decreased. Another recent study showed the influence of childhood maltreatment on the methylation extent of the BDNF gene [39], opening up the hypothesis that further genes could be affected by such epigenetic changes. Gene expression studies in psychiatry have often focused on differentiating patients from control subjects. However, expression may not only vary inter-individually, but also in relation to different disease states within the same individual. Such changes may be particularly relevant to measure treatment response within a given individual, and is less prone to potential confounds across individuals such as prior life experience. Transcriptional changes can occur within minutes to hours and thus could help understand the biology of behavioral and emotional changes.
Therefore, in the present study, we used a panel of 29 genes, selected based on their association with depressive states in humans. We elected to measure gene expression profiles in patients with BPD as they display high levels of fluctuations in affective states as well as in dissociation. Our patients participated in a standardized residential intensive psychotherapy program. Thus, life circumstances were kept more constant and controlled than is the case when studying outpatients.
Materials and Methods
Participants
31 female patients with Borderline Personality Disorder were included in this study. All patients were inpatients at the Department of Psychosomatic Medicine and Psychotherapy, Central Institute of Mental Health Mannheim, Germany. Diagnostic procedures were conducted by trained raters and comprised the International Personality Disorder Examination (IPDE [40], inter-rater reliability: κ = .77), and the Structured Clinical Interview for DSM-IV Axis-I (SCID-I [41], κ = .69). We excluded patients with alcohol or substance abuse within the past three months. Most patients were on psychotropic medication, which mainly consisted of SSRI, atypical neuroleptics and mood stabilizers. No patient was on benzodiazepines. An attempt was made to keep patients on constant medication throughout the study, however in 16 patients changes of medication with either adding or removing prescribed drug, increases or decreases of dosage were necessary. Table S1 lists medication for all participants at the tension and the non-tension state. We exluded patients with significant medical conditions, in particular those with immunological conditions.
Mean age was 27.47±7.95, mean weight 76.22±21.73 kg. 20 out of 31 patients were smokers. Comorbid diagnoses were as follows: MDD, current depressive episode (n = 16), MDD, currently remitted (4), Dysthymia (3), past alcohol abuse (5), past lcohol dependence (2), past sedatives abuse (3), past sedatives dependence (1), past cannabinoids abuse (1), past stimulants abuse (1), past multiple substance abuse (1), Panic disorder without agoraphobia (1), Panic disorder with agoraphobia (2), Social phobia (7), Specific phobia (1), Obsessive-compulsive disorder (2), Posttraumatic stress disorder (6), Anorexia nervosa (4), Bulimia nervosa (4), Eating Disorder NOS (1), Attention-deficit hyperactivity disorder (2).
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics committee of the University of Heidelberg. After explanation of the procedure, written informed consent was obtained. All participants had the capacity to consent, which was checked by a psychiatrist (C.S.).
Procedure
Blood samples for gene expression profiles were collected at two different states, a tension and a non-tension state, with one or two blood draws for each state, respectively. Blood was collected into PAXgeneTM blood RNA tubes, mixed by inversion several times, left at room temperature for 30 – 120 minutes, frozen at −20°C, and then transferred to −80°C for long term storage. RNA integrity is maintained for at least 5 years under such conditions.
For the tension state, patients were asked to signal to the research assistant during their stay in the hospital when they subjectively felt a high level of aversive inner tension. Level of tension was verified by assessment with the DSS (see below). The blood draws for the non-tension state were conducted approximately at the same time of the day (+/− 1 hour) as the tension blood draws. Tension Blood draws preceded non-tension blood draws in all cases. We included 91 observations from 31 subjects into the final analysis, as not in all participants, tension and non-tension stations could be sampled twice. One subject had no tension observation, nine subjects had one tension observation, and 21 had two tension observations. Nine subjects had no non-tension observation, one subject had one non-tension observation, and 21 had two non-tension observations. The study lasted for up to ten weeks.
Gene selection
A list of 29 transcripts was selected, based on multiple criteria including reported association with depressive disorders, assumed role in depressive pathophysiology, and technical feasibility (Table S2). Using stringent selection criteria from a methodological and technical point of view, we believe that changes in the selected genes could be indicative of a pathophysiological relevance, including changes occurring in the CNS [42].
Total RNA isolation
Extraction of RNA from PAXgene tubes was performed using the PAXgene Blood RNA MDx kit (#762431) run on a Qiagen Biorobot Universal instrument according to the manufacturer's protocol. To ensure that the RNA samples were free of contaminating genomic DNA, a second DNase digestion was added to the standard Qiagen protocol. In our experience, this reduces the level of DNA in the samples such that it accounts for less than 0.5% of the signal generated by the downstream qPCR analysis. As such, it is unnecessary to design the qPCR primers used for gene expression profiling to span intron//exon junctions. After isolation, an aliquot of the RNA was analyzed by capillary electrophoresis on a Caliper GX Lab Chip instrument to assess quality and quantity, The RNA concentrations derived from this analysis were used to normalize the RNA r to a final concentration of 13 ng/ul by the addition of water. The samples were stored at −80°C.
cDNA synthesis and qPCR assays
Reverse transcription of 0.5 ug of total RNA into cDNA was accomplished using Superscript II (Life Technologies, # 18064–014) according to the manufacturer's protocol. Following the reaction, the cDNA was desalted using a Multiscreen PCR96 filter plate (Millipore # LSKMPCR10) and the cDNA eluted in 100 ul of water. The cDNA was quantified using the Quant-IT oligreen spectrophotometric assay (Quant-It oligreen ssDNA reagent, Life Technologies #07582). Following quantitation, all cDNA samples were normalized to a concentration of 2.5 ng/ul by the addition of water and stored at −20°C. Quantitative polymerase-chain reaction (qPCR) is preferred over whole genome array as it reliably quantifies transcription changes of small but physiologically relevant magnitude. This is in line with other recent work showing that small but quantitative changes can be used to better characterize psychiatric patient populations [43]. For qPCR, 0.5 ng of cDNA was assayed in a 5 ul reaction volume on an Applied Biosystems 7900HT Fast Real Time PCR instrument using Taqman style primers and probes (Table S3). Because the expression level of IL-6 and SERT are relatively low, the qPCR assays were conducted with 5 ng of cDNA per well to increase the signal. The qPCR master mix was BrilliantII FAST (Agilent # 600845) using the reaction conditions, cycling times and temperatures recommended by the manufacturer. Duplicate plates were run for each assay. Cycle thresholds were set manually for each run and the raw Ct values from the duplicate plates were averaged for analysis. Each assay plate also contained negative control wells (water) and 4 wells of cDNA derived from RNA extracted and pooled from the blood of 10 healthy control individuals. This served as the reference cDNA (see below).
Normalization of gene expression and copy number determination
Averaged expression values from the seven normalization transcripts (Table S4) were used to normalize the raw gene expression data for each well. The seven normalization transcripts were selected based on their stable expression pattern in human blood as determined by the Genorm software program. The stability testing included healthy controls, PTSD patients, depressed patients, and depressed patients receiving treatment with duloxetine or escitalopram, indicating that the normalization process is not impacted by these medications. The relative gene expression in each well, including the reference cDNA, was determined by subtracting the average Ct for the transcript of interest from the average Ct of the seven normalization transcripts (the 2−delta Ct method). Next, setting the relative expression level of the reference cDNA at 100%, all other samples were expressed as a percentage of the reference. Finally, these percentages were converted to copies per ng of cDNA by multiplying this percentage by the number of copies of each transcript contained in the reference cDNA. The copy number determinations for the reference cDNA were derived separately by comparing the expression level in the reference to a standard curve of synthetic DNA for each transcript of interest.
Psychometric measures
Immediately after each blood draw, patients filled in a scale measuring aversive tension and dissociation (Dissoziations-Spannungs-Skala, DSS [44]. This scale has 21 items assessing different aspects of dissociation (e.g. depersonalisation, derealisation, and reduced sensory perception) as well as one item for the assessment of aversive inner tension (tension item). Each week during the course of the study, patients filled in the Borderline-Symptom-List (BSL, [45]) to assess the overall severity of BPD pathology.
Data analysis
The purpose of the data analysis was to establish links between the biology as described by the gene expression levels and the severity of BPD and the severity of tension and dissociation states. This can basically be done in two different ways. One approach is to calculate a predictive marker that given expression levels predict symptom severity. The alternative is to calculate how the expression levels will change due to symptom severity. The two approaches are similar but the first method might identify only the genes with the strongest association with disease state. The latter method will identify all genes with a significant association with the disease. Here the ambition was to understand the biology and therefore the latter approach was prefered. Hence, the expression levels were used as response variables and the symptom scores as explanatory variables in the analysis. With this approach we explicitly calculated what genes/biological processes were associated with the disease states.
BSL and DSS scores represent different time resolution of the disease progression. The overall progression is represented by the BSL and the acute state is represented using the DSS. The BSL score taken once weekly does not necessarily coincide in time with the occasions where blood and BSL scores were collected. The nearest BSL rating before the blood sampling occasion was assigned to the observation representing the long term progression of the disorder.
Four variables describing the disease status were of potential interest. The quantitative BSL score, the DSS total score, the DSS tension sub-score and a categoricalindicator for tension/non-tension state. Data are limited and the explanatory variables are correlated and describe different aspects of the same disorder. We analysed the association between the variables and expression level one by one. Each approach involved one mixed effect model for each gene with expression level as dependent variable.
One of the disease markers plus the subject identity was used as explanatory variables in each approach. We analyzed the following groups of explanatory variables for each gene: (1) subject identities and BSL score, (2) subject identities and tension/non-tension state, (3) subject identities and DSS tension item score, (4) Subject identities and DSS total score. Mixed effect models were chosen for the analysis to account for within-subject correlation between measurements. The subject identities were incorporated as a random effect and all other variables were treated as fixed factors. In order to compensate for the non-symmetric distribution of the gene expressions levels, logarithmic expression values were chosen. The analysis was performed using “proc mixed” in SAS version 9.2. Four mixed effect models were built in this way. The only difference between them was the choice of disease score used as explanatory variable. All 91 non-missing observations from the 31 subjects went into the model. No imputation of missing data was performed.
The number of genes is similar to the number of patients studied. Repeating the analysis of significant regression coefficients for 29 genes will overestimate the number of genes associated with BPD. One traditional approach avoiding this is using Bonferroni correction for multiple testing which will give a shorter list of significantly associated genes. At a specified level of significance all associated genes on the list will be correct findings. Alternatives exist that will allow a few false findings among the associated genes. The benefit is an increased sensitivity to genes associated with BPD. To identify groups of genes that interacts with BPD we chose to use false discover rate, FDR, as selection criteria. The actual calculations follow the Benjamini-Hochberg procedure for false discovery rates. We were ready to accept genes with a false discovery rate below 20% due to the exploratory nature of the study. Among the genes identified at a FDR of 0.20 there is expected to be 20% of false findings. Hence, by using false discovery rate as a criterion we are accepting to have some errors in the list of affected genes instead of potentially not finding a significant group of affected genes using the more conservative Bonferroni correction method.
Results
DSS total score and tension score
Figure 1 shows distribution of DSS total scores and tension scores. A better separation between groups was found for tension scores (y-axis) than for total scores (x-axis). Hence, the tension score was assumed to be better at capturing the state than the total score.
Figure 1. DSS total scores and tension sub-scores for tension and non-tension state.
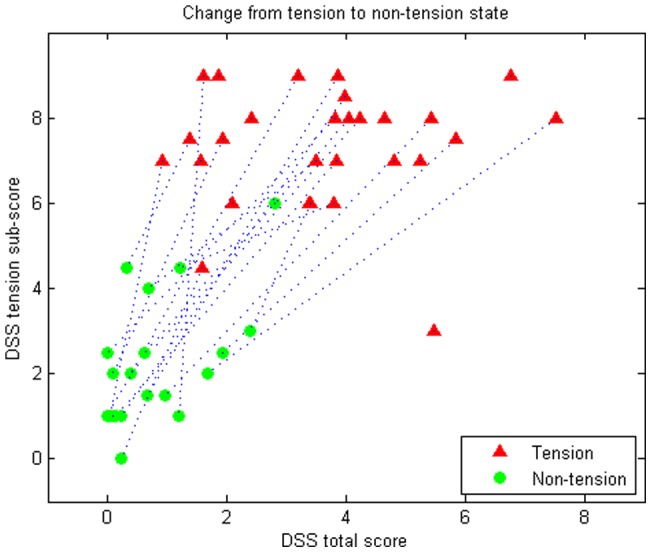
Scores are averages over the two observations in each state. The dashed line represents the patients and the two states of each patient.
Gene expression analyses
No significant effects were found at gene expression level for BSL score, Tension/Non-tension state, and DSS tension item at a FDR of 20% (see Tables S5, S6, and S7). However, 14 genes showed a significant correlation with DSS total score at a FDR of 20%.
Guanine nucleotide-binding protein G(s) subunit alpha isoforms short (GNAS), Interleukin-6 (IL6), Interleukin-1B (IL1B),Mitogen-activated protein kinases 1,3, and 8 (MAPK1, MAPK3, MAPK8), Guanine nucleotide-binding proten G(i) subunit alpha-2 (GNAI2), Beta-arrestin-1 (ARRB1), Beta-arrestin-2 (ARRB2), Cyclic AMP-responsive element-binding protein (CREB1), Glucocorticoid receptor (NR3C1), Regulator of G-protein signaling 2 (RGS2), Synaptic vesicular amine transporter (SLC18A24), and Prolylendopeptidase (PREP) (see Table 1). All of these genes with the exception of IL-6 showed a negative correlation, i.e. reduced expression with increasing DSS total scores, while IL-6 expression was higher with increasing DSS total scores.
Table 1. Gene expression level associated with DSS total score sorted by FDR.
| Gene | Estimated regression coefficients | FDR | p-value |
| GNAS | −0.05223 | 0.11065 | 0.0103 |
| IL6 | 0.05219 | 0.11065 | 0.0119 |
| MAPK3 | −0.04340 | 0.11065 | 0.0135 |
| GNAI2 | −0.04864 | 0.11065 | 0.0153 |
| ARRB2 | −0.05073 | 0.11439 | 0.0242 |
| CREB1 | −0.03976 | 0.11439 | 0.0289 |
| ARRB1 | −0.03937 | 0.11439 | 0.0307 |
| MAPK8 | −0.03770 | 0.11439 | 0.0316 |
| IL1B | −0.04611 | 0.14406 | 0.0480 |
| NR3C1 | −0.03488 | 0.14406 | 0.0506 |
| RGS2 | −0.04235 | 0.14406 | 0.0546 |
| SLC18A2 | −0.03917 | 0.15303 | 0.0633 |
| PREP | −0.02603 | 0.16873 | 0.0756 |
| MAPK1 | −0.02939 | 0.19587 | 0.0946 |
| ODC1 | −0.03759 | 0.20242 | 0.1047 |
| SLC6A4 | −0.04805 | 0.26513 | 0.1463 |
| S100A10 | −0.01472 | 0.42987 | 0.2520 |
| MAPK14 | −0.02145 | 0.45085 | 0.2798 |
| CD8A | 0.02818 | 0.47601 | 0.3120 |
| DPP4 | −0.01444 | 0.47601 | 0.3283 |
| NR3C2 | −0.01975 | 0.59230 | 0.4312 |
| DUSP1 | −0.02013 | 0.59230 | 0.4493 |
| ADA | 0.01993 | 0.60730 | 0.4817 |
| P2RX7 | −0.01231 | 0.66572 | 0.5509 |
| IDO1 | −0.01393 | 0.74882 | 0.6455 |
| CD8B | 0.005458 | 0.79109 | 0.7209 |
| TSPO | −0.01115 | 0.79109 | 0.7365 |
| IL8 | −0.00725 | 0.81301 | 0.7850 |
| ATF2 | 0.002497 | 0.82935 | 0.8294 |
Discussion
To our knowledge, this is the first study investigating longitudinal intra-individual gene expression profiles in blood in patients with borderline personality disorder. We recognize that this is a small study and thus needs replication of the data in a larger study. On the other hand, our design to compare longitudinal intraindividual changes in gene expression during different emotional states in BPD is in line with the suggestion that within-subject comparisons may provide better insights into the pathophysiology than cross-sectional comparisons [23]–[25]. One recent study reported intraindividual comparisons in BPD found that changes in BDNF methylation were influenced by symptoms of depression [39]. This finding together with an earlier study showing that dissociation in BPD is associated with higher risk for depression [8] are in line with our findings of an association between transcriptional changes of genes linked to depressive symptoms and dissociation scores in BDP.
We intraindividually compared patients in two different states of the disease and analyzed gene expression in relation to acute states, in this case tension and dissociation. With a relatively liberal statistical threshold due to the exploratory nature of our study, we found no effect of acute tension or overall symptom severity on gene expression profiles. However, the expression of several genes, including those involved in immune system activation, HPA axis regulation as well as intracellular signalling/second-messenger systems were associated with the level of dissociation, one of the most prominent psychopathological features of the disorder and closely related to states of tension. While the genes were selected mostly based on their association with depressive states, the shared symptomatology between depressive states and emotional disturbances in borderline personality disorders and the risk for depression in patients with greater dissociation could point to shared biological underpinnings. This is in line with the notion that biological research into psychiatric disorders may have to cross diagnostic boundaries to advance our understanding of the pathophysiology of complex clinical presentations [46]. Thus the selected genes and the reported changes may be of relevance beyond BPD. Our data also add to the growing literature on relevant correlations between protein and gene expression for factors involved in intracellular processes [9], [10] as well as the relevance of blood markers for CNS disorders [42], [47].
Dissociation is characterized by a disintegration of perception, consciousness, identity, and memory (DSM-IV-TR). Dissociative symptoms are part of the diagnostic criteria in BPD and several studies revealed frequent dissociative states in BPD [5], [8], [48]. Stiglmayr and coworkers [6] demonstrated dissociative states to be significantly associated with every-day distress in patients with BPD. The neurobiological underpinnings of dissociation are mostly unknown. Neuroimaging studies point to decreased activity in the amygdala in conjunction with increased medial prefrontal/anterior cingulate activity [49], [50].
The genetic background of dissociation is also an area which lacks sufficient research. Twin studies revealed a significant influence of genetic factors on the phenotypic expression of dissociation [51] [52]. Koenen and colleagues [53] found that genetic polymorphisms of FKBP5, which is involved in glucocorticoid receptor regulation, explains up to 14% of the variance in dissociation of children during and after accidents. BDNF polymorphisms were associated with lower dissociation scores [54]; this study also revealed a gene-environment interaction between the Catechol-O-methyltransferase (COMT) val158met-polymorphism and traumatic life events: For the val/val-genotype, greater traumatic experience was associated with increasing dissociation scores, while the met/met-genotype showed an opposite picture. For the serotonin transporter gene, an interaction of the short variant and traumatic neglect was found in the generation of dissociative symptoms [55].
There have been several investigations of the HPA axis in BPD. Dexamethasone suppression has been found to be neither particularly sensitive nor specific for BPD [56]–[58]. Lieb and coworkers [59] collected salivary cortisol during every-day life conditions in patients with BPD and found significantly higher salivary cortisol levels than in healthy controls. Nater and coworkers [60] reported cortisol hyporeactivity to a psycho-social stressor. Taken together, these data suggest that in BPD blood markers may be changed under both stable as well as stress conditions. In contrast to the HPA axis, immune dysregulation has not been as widely studied in BPD. One study found increased IL-6 levels [21], while IL-1ß levels increased significantly stronger after a glucose challenge in BPD patients as compared to healthy subjects [22]. In the current study, IL-6 gene expression was the only transcript to show a positive correlation with acute dissociation levels at an FDR-level of 11%. Thus, our data add to the growing literature on correlations between protein and gene expression, at least for some genes, and also provide insights into the pathophysiology of specific disease states.
Besides immune system regulation genes, we found genes involved in signalling/second-messenger systems as well as genes related to transcriptional factors to be potentially related to dissociation in BPD. Changes in expression of beta-Arrestin-1 and -2, mitogen-activated protein kinase 3 as well as extracellular signal-regulated kinase 1 were negatively correlated to dissociation scores at FDR-levels between 11 and 11.5%. It is of interest in this context that decreased ERK-1-levels were also found in the hippocampus and prefrontal cortex of suicide victims [32], [33]. IL-6 was found to be elevated in the CSF of suicide attempters [20]. The suicide rate of patients with BPD is very high with approximately 10% of all patients committing suicide [61]. Further research is needed to disentangle the interaction between dissociation and disturbances of signalling/second-messenger systems in patients with BPD and/or suicidal behavior.
Cyclic AMP response element-binding protein was also negatively related to dissociation scores. Alterations in CREB metabolism has been related to depression [24], [27]–[29]. In a recent study, PTSD patients (who also often show elevated dissociation scores) had statistically lower levels of total CREB protein than healthy control subjects [30].
Overall, our data on blood gene expression changes are in good agreement with reported protein changes in psychiatric patients with depressive symptoms. Specifically, we found a negative association between selected cellular signalling molecules and dissociation and a positive association for IL-6, similar to published protein data in depression and related disease states by showing [9], [13]–[15].
Limitations of our study include the fact that medication was not held constant in all patients and a relatively small sample size. And while many published studies on transcriptional profiles in psychiatric disorders are small and different in terms of design and gene selection and thus do not permit a direct comparison of results, medication, whenever examined, had no clear impact on the results [23], [62].
Other state-dependent symptoms besides dissociation, e.g. anxiety, which were not assessed in our study, may have confounded our results. Since in most patients, several weeks lay between the assessments of the tension and the non-tension states, other factors, e.g. subclinical infection, may have influenced the change of immune system-related gene expression. On the other hand, careful diagnostic procedures were conducted and all patients were inpatients at the same unit. Also, intraindividual comparison as in our study can be considered a way to assess emotional states and reduce confounds of other interindividual variables [23]. In addition to the intraindividual comparison, we also collected blood samples twice for each disease state, and used the average of the same disease state, thereby further reducing the impact of possible confounds. As further strength can be considered that, unlike genetics and proteomics data, the RNA infrastructure [63] may better capture also the effect of epigenetic changes that have recently been reported in psychiatric disorders, such as BPD [62], [64] .
Overall, this pilot study gave some preliminary hints at alterations in gene expression related to dissociative psychopathology in patients with BPD. Our data point to a potential association between gene expression involved in immune regulation as well as signalling/second-messenger systems and the level of dissociation in patient with BPD. Our findings, while preliminary, suggest that further research into molecular alterations in blood associated with specific disease states such as dissociation in BPD may improve our understanding of the underlying pathophysiology. In view of the shared molecular pathophysiology between psychiatric disorders such insights may be applicable to similar disease states in multiple psychiatric disorders.
Supporting Information
List of medication for all participants at the tension and the non-tension state.
(DOCX)
List of analyzed genes.
(DOCX)
Targets and primers.
(DOCX)
Normalization transcripts.
(DOCX)
Gene expression level associated with BSL score sorted by FDR.
(DOCX)
Gene expression level associated with Tension/Non-tension state sorted by FDR.
(DOCX)
Gene expression level associated with DSS tension item sorted by FDR.
(DOCX)
Acknowledgments
We thank Marija Gligorijevic for help with data assessment.
Funding Statement
The authors have no support or funding to report.
References
- 1. Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ (2010) Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. J Pers Disord 24: 412–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zanarini MC (2000) Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am 23: 89–101. [DOI] [PubMed] [Google Scholar]
- 3. Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, et al. (1998) Axis I comorbidity of borderline personality disorder. Am J Psychiatry 155: 1733–1739. [DOI] [PubMed] [Google Scholar]
- 4. Zanarini MC, Ruser T, Frankenburg FR, Hennen J (2000) The dissociative experiences of borderline patients. Compr Psychiatry 41: 223–227. [DOI] [PubMed] [Google Scholar]
- 5. Stiglmayr CE, Shapiro DA, Stieglitz RD, Limberger MF, Bohus M (2001) Experience of aversive tension and dissociation in female patients with borderline personality disorder – a controlled study. J Psychiatr Res 35: 111–118. [DOI] [PubMed] [Google Scholar]
- 6. Stiglmayr CE, Ebner-Priemer UW, Bretz J, Behm R, Mohse M, et al. (2008) Dissociative symptoms are positively related to stress in borderline personality disorder. Acta psychiatrica Scandinavica 117: 139–147. [DOI] [PubMed] [Google Scholar]
- 7. Ludascher P, Bohus M, Lieb K, Philipsen A, Jochims A, et al. (2007) Elevated pain thresholds correlate with dissociation and aversive arousal in patients with borderline personality disorder. Psychiatry research 149: 291–296. [DOI] [PubMed] [Google Scholar]
- 8. Brodsky BS, Cloitre M, Dulit RA (1995) Relationship of dissociation to self-mutilation and childhood abuse in borderline personality disorder. Am J Psychiatry 152: 1788–1792. [DOI] [PubMed] [Google Scholar]
- 9. David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, et al. (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gry M, Rimini R, Stromberg S, Asplund A, Ponten F, et al. (2009) Correlations between RNA and protein expression profiles in 23 human cell lines. BMC Genomics 10: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold PW, Chrousos GP (2002) Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 7: 254–275. [DOI] [PubMed] [Google Scholar]
- 12.Gold PW, Gabry KE, Yasuda MR, Chrousos GP (2002) Divergent endocrine abnormalities in melancholic and atypical depression: clinical and pathophysiologic implications. Endocrinol Metab Clin North Am 31: 37–62, vi. [DOI] [PubMed]
- 13. Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71: 171–186. [DOI] [PubMed] [Google Scholar]
- 15. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, et al. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67: 446–457. [DOI] [PubMed] [Google Scholar]
- 16. Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR (2008) Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry 64: 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J (2003) Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res 122: 193–198. [DOI] [PubMed] [Google Scholar]
- 18. Schmahl C, Berne K, Krause A, Kleindienst N, Valerius G, et al. (2009) Hippocampus and amygdala volumes in patients with borderline personality disorder with or without posttraumatic stress disorder. J Psychiatry Neurosci 34: 289–295. [PMC free article] [PubMed] [Google Scholar]
- 19. Nunes PM, Wenzel A, Borges KT, Porto CR, Caminha RM, et al. (2009) Volumes of the hippocampus and amygdala in patients with borderline personality disorder: a meta-analysis. J Pers Disord 23: 333–345. [DOI] [PubMed] [Google Scholar]
- 20. Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, et al. (2009) Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry 66: 287–292. [DOI] [PubMed] [Google Scholar]
- 21. Kahl KG, Bens S, Ziegler K, Rudolf S, Dibbelt L, et al. (2006) Cortisol, the cortisol-dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biol Psychiatry 59: 667–671. [DOI] [PubMed] [Google Scholar]
- 22. Westling S, Ahren B, Traskman-Bendz L, Brundin L (2011) Increased IL-1beta reactivity upon a glucose challenge in patients with deliberate self-harm. Acta Psychiatr Scand 124: 301–306. [DOI] [PubMed] [Google Scholar]
- 23. Munkholm K, Vinberg M, Berk M, Kessing LV (2012) State-related alterations of gene expression in bipolar disorder: a systematic review. Bipolar Disord 14: 684–696. [DOI] [PubMed] [Google Scholar]
- 24. Belzeaux R, Formisano-Treziny C, Loundou A, Boyer L, Gabert J, et al. (2010) Clinical variations modulate patterns of gene expression and define blood biomarkers in major depression. J Psychiatr Res 44: 1205–1213. [DOI] [PubMed] [Google Scholar]
- 25. Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Treziny C, et al. (2012) Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry 2: e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blendy JA (2006) The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59: 1144–1150. [DOI] [PubMed] [Google Scholar]
- 27. Bohus M, Forstner U, Kiefer C, Gebicke-Harter P, Timmer J, et al. (1996) Increased sensitivity of the inositol-phospholipid system in neutrophils from patients with acute major depressive episodes. Psychiatry Res 65: 45–51. [DOI] [PubMed] [Google Scholar]
- 28. Iga J, Ueno S, Yamauchi K, Numata S, Motoki I, et al. (2006) Gene expression and association analysis of LIM (PDLIM5) in major depression. Neurosci Lett 400: 203–207. [DOI] [PubMed] [Google Scholar]
- 29. Vollmayr B, Sulger J, Gabriel P, Aldenhoff JB (1995) Mitogen stimulated rise of intracellular calcium concentration in single T lymphocytes from patients with major depression is reduced. Prog Neuropsychopharmacol Biol Psychiatry 19: 1263–1273. [DOI] [PubMed] [Google Scholar]
- 30.Martini C, Da Pozzo E, Carmassi C, Cuboni S, Trincavelli ML, et al.. (2011) Cyclic adenosine monophosphate responsive element binding protein in post-traumatic stress disorder. World J Biol Psychiatry. [DOI] [PubMed]
- 31. Lewis TS, Shapiro PS, Ahn NG (1998) Signal transduction through MAP kinase cascades. Adv Cancer Res 74: 49–139. [DOI] [PubMed] [Google Scholar]
- 32. Dwivedi Y, Rizavi HS, Conley RR, Pandey GN (2006) ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry 11: 86–98. [DOI] [PubMed] [Google Scholar]
- 33. Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, et al. (2001) Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem 77: 916–928. [DOI] [PubMed] [Google Scholar]
- 34. Trollope AF, Gutierrez-Mecinas M, Mifsud KR, Collins A, Saunderson EA, et al. (2012) Stress, epigenetic control of gene expression and memory formation. Exp Neurol 233: 3–11. [DOI] [PubMed] [Google Scholar]
- 35. Avissar S, Matuzany-Ruban A, Tzukert K, Schreiber G (2004) Beta-arrestin-1 levels: reduced in leukocytes of patients with depression and elevated by antidepressants in rat brain. Am J Psychiatry 161: 2066–2072. [DOI] [PubMed] [Google Scholar]
- 36. Heim C, Plotsky PM, Nemeroff CB (2004) Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 29: 641–648. [DOI] [PubMed] [Google Scholar]
- 37. Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK (2012) Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, et al.. (2005) Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Mol Psychiatry 10: 500–513, 425. [DOI] [PubMed]
- 39. Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, et al. (2013) Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl Psychiatry 3: e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loranger AW (1999) International Personality Disorder Examination (IPDE): DSM-IV and ICD-10 modules. Odessa: Psychological Assessment Resources.
- 41.First MB, Spitzer RL, Gibbon M, Williams JBW (1995) Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (SCID-I/P). New York: American Psychiatric Press.
- 42. Sullivan PF, Fan C, Perou CM (2006) Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet 141B: 261–268. [DOI] [PubMed] [Google Scholar]
- 43. Pajer K, Andrus BM, Gardner W, Lourie A, Strange B, et al. (2012) Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Transl Psychiatry 2: e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stiglmayr CE, Braakmann D, Haaf B, Stieglitz RD, Bohus M (2003) [Development and characteristics of Dissociation-Tension-Scale acute (DSS-Akute)]. Psychother Psychosom Med Psychol 53: 287–294. [DOI] [PubMed] [Google Scholar]
- 45. Bohus M, Limberger MF, Frank U, Chapman AL, Kuhler T, et al. (2007) Psychometric properties of the Borderline Symptom List (BSL). Psychopathology 40: 126–132. [DOI] [PubMed] [Google Scholar]
- 46. Sullivan PF, Daly MJ, O'Donovan M (2012) Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 13: 537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le-Niculescu H, Kurian SM, Yehyawi N, Dike C, Patel SD, et al. (2009) Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry 14: 156–174. [DOI] [PubMed] [Google Scholar]
- 48. Ebner-Priemer UW, Badeck S, Beckmann C, Wagner A, Feige B, et al. (2005) Affective dysregulation and dissociative experience in female patients with borderline personality disorder: a startle response study. J Psychiatr Res 39: 85–92. [DOI] [PubMed] [Google Scholar]
- 49.Krause-Utz A, Oei NY, Niedtfeld I, Bohus M, Spinhoven P, et al.. (2012) Influence of emotional distraction on working memory performance in borderline personality disorder. Psychol Med: 1–12. [DOI] [PubMed]
- 50. Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, et al. (2010) Emotion modulation in PTSD: Clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry 167: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jang KL, Paris J, Zweig-Frank H, Livesley WJ (1998) Twin study of dissociative experience. The Journal of nervous and mental disease 186: 345–351. [DOI] [PubMed] [Google Scholar]
- 52. Becker-Blease KA, Deater-Deckard K, Eley T, Freyd JJ, Stevenson J, et al. (2004) A genetic analysis of individual differences in dissociative behaviors in childhood and adolescence. Journal of child psychology and psychiatry, and allied disciplines 45: 522–532. [DOI] [PubMed] [Google Scholar]
- 53. Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, et al. (2005) Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular psychiatry 10: 1058–1059. [DOI] [PubMed] [Google Scholar]
- 54. Savitz JB, van der Merwe L, Newman TK, Solms M, Stein DJ, et al. (2008) The relationship between childhood abuse and dissociation. Is it influenced by catechol-O-methyltransferase (COMT) activity? The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum 11: 149–161. [DOI] [PubMed] [Google Scholar]
- 55. Lochner C, Seedat S, Hemmings SM, Moolman-Smook JC, Kidd M, et al. (2007) Investigating the possible effects of trauma experiences and 5-HTT on the dissociative experiences of patients with OCD using path analysis and multiple regression. Neuropsychobiology 56: 6–13. [DOI] [PubMed] [Google Scholar]
- 56. Lahmeyer HW, Val E, Gaviria FM, Prasad RB, Pandey GN, et al. (1988) EEG sleep, lithium transport, dexamethasone suppression, and monoamine oxidase activity in borderline personality disorder. Psychiatry Res 25: 19–30. [DOI] [PubMed] [Google Scholar]
- 57. Korzekwa M, Steiner M, Links P, Eppel A (1991) The dexamethasone suppression test in borderlines: is it useful? Can J Psychiatry 36: 26–28. [DOI] [PubMed] [Google Scholar]
- 58. De la Fuente JM, Mendlewicz J (1996) TRH stimulation and dexamethasone suppression in borderline personality disorder. Biol Psychiatry 40: 412–418. [DOI] [PubMed] [Google Scholar]
- 59. Lieb K, Rexhausen JE, Kahl KG, Schweiger U, Philipsen A, et al. (2004) Increased diurnal salivary cortisol in women with borderline personality disorder. J Psychiatr Res 38: 559–565. [DOI] [PubMed] [Google Scholar]
- 60. Nater UM, Bohus M, Abbruzzese E, Ditzen B, Gaab J, et al. (2010) Increased psychological and attenuated cortisol and alpha-amylase responses to acute psychosocial stress in female patients with borderline personality disorder. Psychoneuroendocrinology 35: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 61. Black DW, Blum N, Pfohl B, Hale N (2004) Suicidal behavior in borderline personality disorder: prevalence, risk factors, prediction, and prevention. J Pers Disord 18: 226–239. [DOI] [PubMed] [Google Scholar]
- 62. Higuchi F, Uchida S, Yamagata H, Otsuki K, Hobara T, et al. (2011) State-dependent changes in the expression of DNA methyltransferases in mood disorder patients. J Psychiatr Res 45: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 63. Collins LJ, Penny D (2009) The RNA infrastructure: dark matter of the eukaryotic cell? Trends Genet 25: 120–128. [DOI] [PubMed] [Google Scholar]
- 64. Dammann G, Teschler S, Haag T, Altmuller F, Tuczek F, et al. (2011) Increased DNA methylation of neuropsychiatric genes occurs in borderline personality disorder. Epigenetics 6: 1454–1462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of medication for all participants at the tension and the non-tension state.
(DOCX)
List of analyzed genes.
(DOCX)
Targets and primers.
(DOCX)
Normalization transcripts.
(DOCX)
Gene expression level associated with BSL score sorted by FDR.
(DOCX)
Gene expression level associated with Tension/Non-tension state sorted by FDR.
(DOCX)
Gene expression level associated with DSS tension item sorted by FDR.
(DOCX)


