Abstract
As neonatal resuscitation critically depends upon lung aeration at birth, knowledge of the progression of this process is required to guide ongoing care. We investigated whether expired CO2 (ECO2) levels indicate the degree of lung aeration immediately after birth in two animal models and in preterm infants. Lambs were delivered by caesarean section and ventilated from birth. In lambs, ECO2 levels were significantly (p<0.0001) related to tidal volumes and CO2 clearance/breath increased exponentially when tidal volumes were greater than 6 mL/kg. Preterm (28 days of gestation; term = 32 days) rabbits were also delivered by caesarean section and lung aeration was measured using phase contrast X-ray imaging. In rabbit kittens, ECO2 levels were closely related (p<0.001) to lung volumes at end-inflation and were first detected when ∼7% of the distal lung regions were aerated. ECO2 levels in preterm infants at birth also correlated with tidal volumes. In each infant, ECO2 levels increased to >10 mmHg 28 (median) (21–36) seconds before the heart rate increased above 100 beats per minute. These data demonstrate that ECO2 levels can indicate the relative degree of lung aeration after birth and can be used to clinically assess ventilation in the immediate newborn period.
Introduction
Infants, particularly premature infants, commonly suffer respiratory failure after birth and require breathing support because their airways are partially liquid-filled [1]. This restricts the onset of pulmonary gas exchange and delays the cardiovascular changes that underpin the transition to air breathing at birth [1]–[3]. An international consensus on neonatal resuscitation recommends the use of positive pressure ventilation if infants fail to initiate spontaneous breathing immediately after birth [4]. However, it is difficult to achieve a balance between providing adequate ventilation without causing lung damage [5].
Transcutaneous oxygen saturation (SpO2) and heart rate (HR) are important indicators of adequate ventilation in the delivery room [6], [7], which are further improved with respiratory function monitoring to measure gas flows and tidal volumes [8], [9]. However, these parameters provide little information on ventilation efficiency and the degree of gas exchange and provide limited feedback to guide clinical care when cardiorespiratory indicators fail to improve.
CO2 is produced in tissues as a by-product of oxidative metabolism, enters the blood and is eliminated from the body by diffusion across the alveolar epithelium before it is exhaled in the expired gas. As CO2 can only be present in expired gas if gas exchange has commenced, expired CO2 (ECO2) levels may indicate the degree and success of lung aeration and gas exchange. Indeed, colorimetric assessments of expired CO2 are commonly used to confirm correct endotracheal tube placement in newborn infants following intubation [10].
Low CO2 levels measured in arterial blood (PaCO2) upon arrival in the intensive care unit suggest that preterm infants are at risk of over-ventilation in the delivery room [11]. As a result, end-tidal CO2 levels have been used to estimate PaCO2 levels in newborn infants, with varying results [12],[13]. However, estimating PaCO2 levels from end-tidal CO2 values assumes that CO2 diffusion across the alveolar epithelium is not limited by low perfusion or gas exchange area [14]. Although a safe assumption in adults, low pulmonary perfusion and reduced gas exchange surface area caused by lung liquid retention, may limit gas exchange in newly born infants. Thus, rather than indicating PaCO2 levels, ECO2 levels may indicate the degree of aeration within the distal gas exchange regions of the lung immediately after birth. Our aim was to investigate whether ECO2 values indicate the degree of lung aeration immediately after birth and provide feedback on the success of pulmonary ventilation.
To investigate the relationship between ECO2 levels and the onset of ventilation, we measured ECO2 levels in lambs immediately after birth. To determine the relationship between lung aeration and ECO2 levels, we measured ECO2 levels in ventilated newborn rabbits during phase contrast (PC) X-ray imaging. PC X-ray imaging exploits the refractive index difference between air and water to generate high resolution images of the lung as it aerates after birth [15]–[20]. Lung gas volumes can be measured at any stage of a breath from the images [18], allowing corresponding measures of ECO2 levels and the volume of the preceding inflation. We also investigated whether measures of ECO2 are feasible in the delivery room and correlate with recordings of tidal volume during positive pressure ventilation in preterm infants.
Materials and Methods
Animal Experiments
The SPring-8 and/or Monash University’s Monash Medical Centre Committee A animal ethics committees approved all animal experiments. All imaging studies on rabbits were conducted in experimental hutch 3 of beam line 20B2, in the Biomedical Imaging Centre at the SPring-8 synchrotron in Japan [18], [21]–[23].
Lamb studies
Near term lambs at 139±1 (mean ± standard error of the mean; SEM) days of gestation (term = 147 days) were exposed by hysterotomy, had carotid artery and jugular vein catheters inserted and were intubated with a cuffed endotracheal tube (size 4.5 mm). Lambs were delivered by caesarean section, dried, weighed, and placed under a radiant heater to maintain body temperature, infused with dextrose 50 mg/ml (i.v.) and lightly sedated (alfaxane iv 5–15 mg/kg/h; Jurox, Auckland, New Zealand). All lambs (n = 9) were ventilated (Dräger Babylog 8000plus, Lübeck, Germany) with a peak inflating pressure (PIP) of 35 cmH2O, positive end expiratory pressure (PEEP) of 5 cmH2O and fraction of inspired oxygen (FiO2) of 0.21. Five lambs were ventilated at 60 inflations/min (inspiratory times 0.5 s) and 4 initially received five, 3 s inflations separated by a 1 s expiratory phase, before switching to 60 inflations/min. After 10 minutes of ventilation, all lambs received volume-guarantee ventilation for 20 min, with a set tidal volume (VT) of 8 mL/kg. Lambs were euthanized by an overdose of sodium pentobarbitone at the end of the experiment. Heart rate (HR), mean arterial pressure (BP), VT, PIP and ECO2 levels were measured continuously and recorded at 1000 Hz using Powerlab (ADInstruments, Sydney, Australia). ECO2 levels were measured using a Novametrix mainstream CO2 analyzer (Capnogard, Novametrix, Wallingford, CT, USA). Every inflation was analyzed over the first 30 s of ventilation, after which 3 inflations were analyzed every 30 s for the remainder of the experiment; ∼200 inflations were analyzed per animal. CO2 clearance per inflation (mL/kg) was calculated by integrating the product of the instantaneous gas flow and ECO2 concentration throughout expiration. Arterial blood samples were collected to measure partial pressure of oxygen (PaO2), PaCO2 and pH (ABL30, Radiometer, Copenhagen, Denmark).
Rabbit studies
Pregnant (28 days of gestation; term = 32 days) New Zealand white rabbits (n = 5) were anaesthetized (propofol; i.v.; 12 mg/kg bolus), intubated and anaesthesia was maintained by isoflourane inhalation (1.5–4%). Kittens (n = 17) were exposed by caesarean section, sedated (Nembutal; 0.1 mg, i.p.) and intubated before they were delivered and positioned upright in a prewarmed (37°C) water-filled plethysmograph (head out) located in the path of the X-ray beam [15]–[20]. Kittens were ventilated with a custom-built ventilator [23], synchronized with image acquisition, using air and a VT of 8 mL/kg (determined manually from the plethysmograph), PEEP of 8 cmH2O at 24 inflations/min for 7 minutes. Lung gas volumes (measured by plethysmography), airway pressures and a trigger signal indicating image acquisition were recorded digitally (Powerlab, ADInstruments; Sydney, Australia). Following the experiment, PIP and PEEP were varied to observe the resulting changes in ECO2 levels before kittens were killed with sodium pentobarbitone (>100 mg/kg; i.p.).
ECO2 levels were measured using a small mainstream CO2 analyzer placed in the expiratory limb of the ventilation circuit, immediately distal to the “Y” piece connecting to the endotracheal tube. Although small (∼1×1×0.5 cm3), the CO2 analyzer could not be incorporated into the “Y” piece due to its size and dead space volume (∼300 µL). As VT of these kittens is only 150–200 µL, we determined the response delay between exhalation and CO2 detection. The volume of an infused gas (containing CO2) required to detect 10%, 50% and 100% of the CO2 level in our system was 380, 780 and 1280 µL, respectively. Thus, to synchronize the changes in CO2 levels with other respiratory changes, the ECO2 recording was retrospectively time shifted by the time required to expire 780 µL.
PC X-ray imaging was performed as previously described [15]–[20]. The X-ray energy was 24 keV and kittens were located 3.0 m upstream of a fibre optics CCD camera (Hamamatsu, C9300-124F), which provided an effective pixel size of 16.2 µm and an active field of view of 32(H) × 32(W) mm2. Image acquisition was synchronized with ventilation such that 7 images, 300 ms apart, were acquired during each respiratory cycle with an exposure of 40 ms. An output trigger signal indicated on the physiological recording the precise timing of each image acquisition. The PC X-ray images were used to measure lung gas volumes, using a phase retrieval analysis [18], [22]. Lung gas volumes were calculated from each image to calculate functional residual capacity (FRC), dead space volumes of the lung [17], end-inflation gas volumes at which CO2 could be first detected in the expired air and to define the relationship between end-inflation gas volumes and ECO2. To determine the relationship between ECO2 levels and end-inflation lung volumes in the absence of other variables, such as the number of inflations required to achieve adequate lung aeration, the measured lung volumes were group and compared with the ECO2 level measured for each inflation.
Studies of Preterm Infants
All infants were born at The Royal Women’s Hospital, Melbourne, Australia, a tertiary perinatal centre where ∼ 6000 infants are delivered and more than 100 infants with a birth weight of <1000 g are admitted to the neonatal intensive care unit annually. The infants were enrolled in a randomized control trial comparing mask ventilation guided by either respiratory function monitoring or clinical assessment alone [9]. The trial was approved by The Royal Women’s Hospital Research and Ethics Committees and registered with Australian and New Zealand Clinical Trials Registry ACTRN12608000357358 [9]. Written consent was obtained before birth if the mother was not in established labour and if time permitted. Where this was not possible, a waiver of prospective consent was granted by the Research and Ethics Committees in accordance with Australian National Health and Medical Research Council guidelines and written parental consent was later obtained to use the data collected in the delivery room. Consent was sought from the parents of these infants, to use data obtained, as soon as possible after the birth.
Mask ventilation was provided with a size 00 round silicone face mask (Laerdal, Stavanger, Norway) connected to a T-piece device (Neopuff Infant Resuscitator, Fisher & Paykel Healthcare, Auckland, New Zealand), a continuous flow, pressure-limited device with a manometer and a PEEP valve. The default settings were a gas flow of 8 L/min, PIP of 30 cmH2O and PEEP of 5 cmH2O. A respiratory function monitor (Florian Acutronic Medical Systems AG, Hirzel, Switzerland), measured gas flow, airway pressure and inspiratory and expiratory VT by integrating the flow signal [8]. Continuous ECO2 levels were measured using a mainstream CO2 monitor (Capnogard, Novametrix, Wallingford, CT, USA) placed between the T-piece and facemask. Gas flow, VT, airway pressure and ECO2 levels were recorded at 200 Hz using a dedicated computer with Spectra software (Grove Medical, London, UK). In preterm infants the pressure, flow, VT and CO2 waveforms for each inflation were analyzed. If during facemask ventilation, the infant’s heart rate and oxygen saturations remained low, as determined by the attending clinician, the infant was intubated using a non-cuffed endotracheal tube. No matter whether the infant was intubated or ventilated via a facemask, the expired CO2 and VT values were included for analysis when the assessed leakage was <30%.
Statistical Analysis
Results from animal experiments are presented as mean ± SEM. Results from studies in infants are presented as mean (standard deviation) or median (interquartile range; IQR). To relate VT and end-inflation lung volumes with ECO2 values in lambs and rabbit kittens, maximum ECO2 values were clustered into groups based on the expired volume of the preceding inflation and a sigmoidal, 3 parameter, non-linear regression analysis performed. Similarly, to determine the relationship between CO2 clearance per breath and Vt, an exponential growth, 4 parameter non-linear regression analysis was performed. Differences with p<0.05 indicate a significant difference.
Results
Animal Experiments
Lamb studies
Compared to conventional ventilation (at 60 inflations/minute), initiating ventilation with five, 3 sec, inflations had no effect on any cardiorespiratory parameters examined. As a result, data from all lambs were combined to relate ECO2 to expired VT. Blood gas parameters measured at the beginning (fetal) and end of these experiments are presented in Table 1.
Table 1. Arterial blood gas parameters in lambs.
| Blood gasparameter (n = 9) | Fetal | 30 mins afterventilation onset |
| SaO2 (%) | 56.0±6.1 | 79.9±6.5 |
| pH | 7.22±0.02 | 7.22±0.04 |
| PaCO2 (mmHg) | 53.7±5.8 | 47.3±2.5 |
| PaO2 (mmHg) | 28.3±4.9 | 44.8±2 |
| Haemoglobin (g/dL) | 11.5±0.48 | 12.6±0.5 |
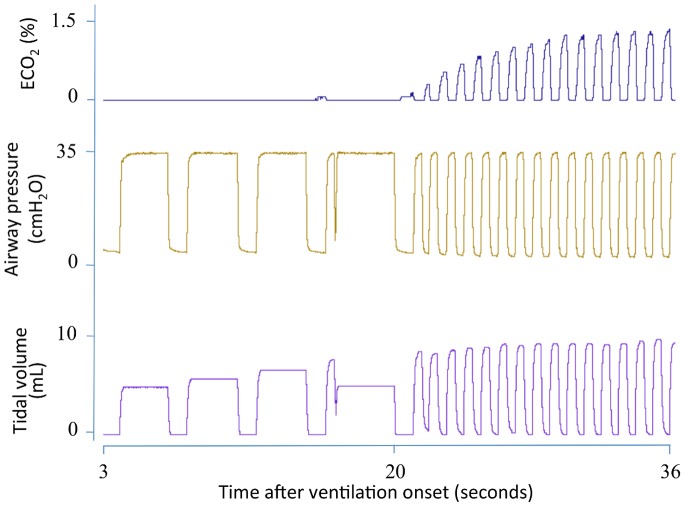
Figure 1 is a recording of the first 20 inflations in a lamb receiving 5 initial inflations of 3 s (first inflation not displayed) followed by ventilation at 60 inflations per minute. CO2 was not detected in the expired air of the first 3 inflations, despite achieving VT’s between 1.5 to 2 mL/kg, whereas both the 4th and 5th inflations (achieving >2 mL/kg) resulted in small increases in ECO2. With the next 15 inflations, at the same PIP, the ECO2 concentration rapidly increased (Fig. 1). Figure 2 shows the first 18 minutes of ventilation in a different lamb demonstrating the relationship between VT and maximum ECO2 levels.
Figure 1. Expired CO2 levels, airway pressure and tidal volumes in a newborn lamb immediately after birth.
Expired CO2 levels (ECO2) are demonstrated in the top panel, airway pressure in the middle panel and tidal volumes (VT) in the bottom panel. The lamb was resuscitated with five 3 second inflations (last 4 shown) followed by tidal ventilation. The first 3 inflations yielded no ECO2, despite achieving a VT of 5–7 mL, whereas the 4th and 5th inflations produced small increases in ECO2 levels. Subsequent inflations produced a gradual increase in both ECO2 levels and VT.
Figure 2. Expired CO2 levels and tidal volumes measured in a newborn lamb during the first 18 minutes after birth.
Expired CO2 levels (ECO2) are demonstrated in the top panel and tidal volumes (VT) in the bottom panel. Tidal volumes were increased at “A” and “B” by increasing the inflation pressure and then the ventilation mode was changed to volume guarantee at “C” to target a VT of 8 mL/kg. ECO2 levels and VT were closely associated.
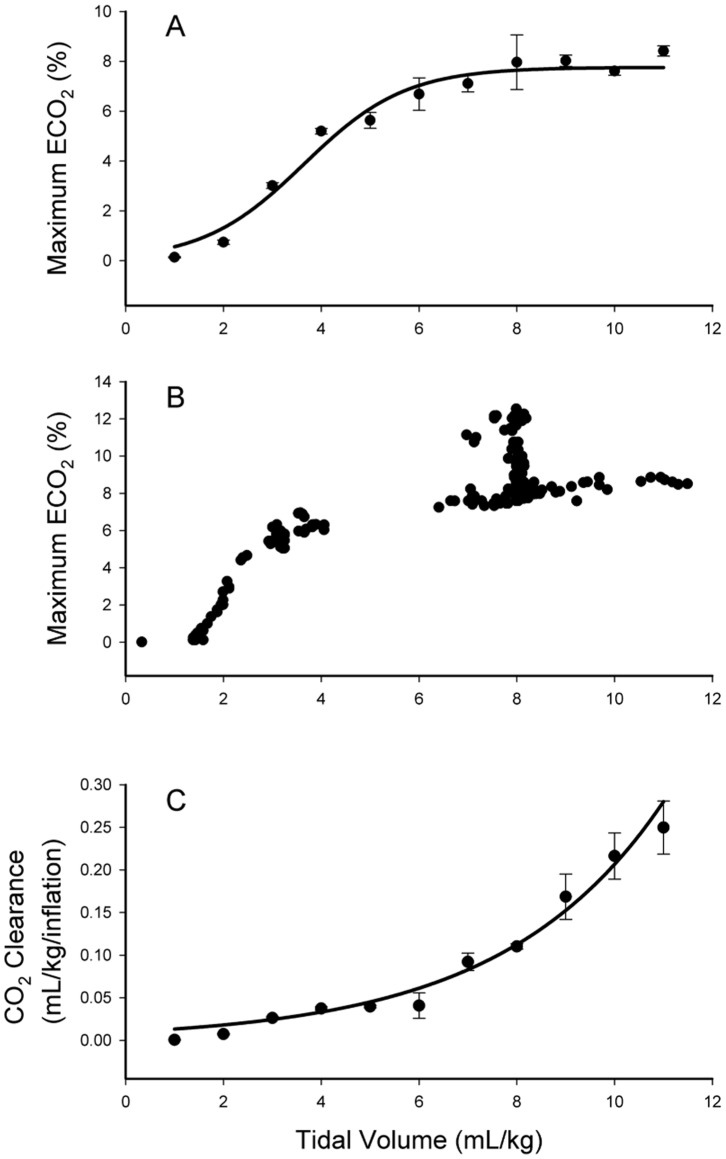
When data from all lambs were combined, maximum ECO2 levels were related to VT after ventilation onset, following a sigmoidal relationship (p<0.0001) (Fig. 3A). The upper inflection point of the curve occurred at ∼6 mL/kg, indicating that VT of at least 6 mL/kg were required to efficiently ventilate the lung. Data from an individual lamb are presented in Figure 3B, demonstrating the characteristic relationship between ECO2 levels and VT. At a VT of 8 mL/kg, the set VT after 10 min of ventilation, the ECO2 values tended to align vertically showing a large range at this volume. This reflects a gradual reduction in PaCO2 levels during the 20 mins of volume-controlled ventilation (decreased from 72.8 to 47.5 mmHg), resulting in a reduction in ECO2 levels for a set volume. CO2 clearance per inflation (Fig. 3C) was exponentially related (p<0.0001) to the VT, demonstrating a marked increase in CO2 clearance per breath at VT >6 mL/kg in these lambs. In comparison, at VT <6 mL/kg, the relationship between CO2 clearance and VT was reduced, such that a doubling in VT from 3 to 6 mL/kg had little effect on CO2 clearance; CO2 clearance per breath increased from 0.03±0.01 to 0.04±0.01 mL/kg/inflation.
Figure 3. Relationship between expired CO2 levels at end-expiration and tidal volumes in lambs.
Rabbit studies
Plethysmograph recordings and PC X-ray images were acquired from 17 preterm (28 days of gestation) newborn rabbits mean weight of 28.9±1.5 g.
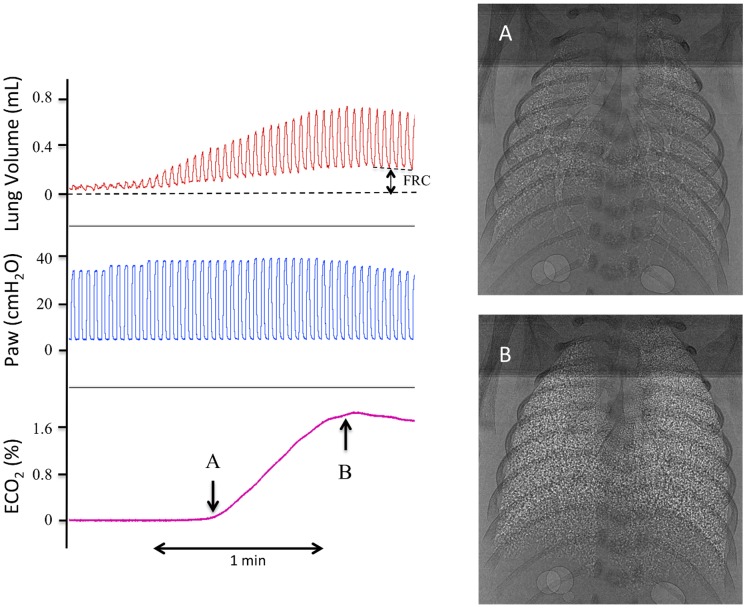
Increasing ECO2 levels were closely associated with increasing VT (Fig. 4) and relative aeration of the lung (see Movie S1), with the first appearance of gas in the distal airways almost exactly coinciding with the first detection of ECO2 (Fig. 4). The calculated dead space volume was 2.0±0.5 mL/kg and the end-inflation lung volume at which CO2 was first detected in expired gas was 3.4±0.3 mL/kg. The mean end-inflation lung gas volume (sum of FRC and VT) achieved was 20.1±1.5 mL/kg. The relationship between ECO2 levels and the immediate preceding end-inflation lung gas volume was highly significant (p<0.001; Fig. 5). Expressed as a percentage of the maximum ECO2 level achieved by each kitten, ECO2 levels increased from 0% at an end-inflation lung volume of 2.3±0.2 mL/kg to 40.8±2.5% at 8.0±0.6 mL/kg and to 89.6±2.8% at an end-inflation lung volume of 16.1±1.2 mL/kg (Fig. 5).
Figure 4. Lung gas volumes, airway pressures and expired CO2 levels in a ventilated newborn rabbit delivered preterm at 28 days of gestation.
Lung gas volumes are demonstrated in the top panel, airway pressures in the middle panel and expired CO2 (ECO2) levels in the bottom panel. Images A and B were acquired at the times indicated by the arrows. The increase in ECO2 levels closely followed the increase in end-inflation lung volumes. All of the corresponding phase contrast X-ray images have been compiled into Movie S1.
Figure 5. Relationship between expired CO2 levels and end-inflation lung gas volumes in ventilated newborn rabbits delivered preterm at 28 days of gestation.
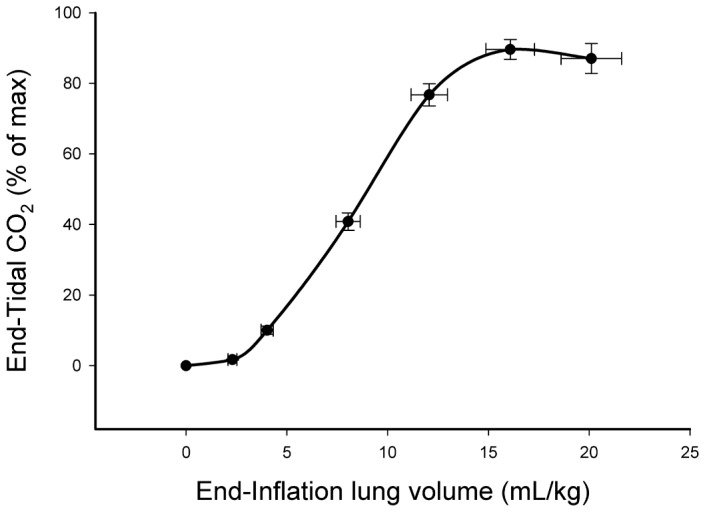
All data points are mean ± SEM and the relationship was highly significant (p<0.001), with an r2 value of 0.93.
To identify the relative contribution of VT and FRC (basal volumes at end-expiration; Fig. 6) to the relationship between end-inflation volumes and ECO2 levels, ventilation parameters were altered to determine the effect on ECO2 levels; 4 examples, from 4 different kittens, are displayed in figure 6. Increasing or decreasing end-inflation volumes always resulted in an increase or decrease in ECO2 levels, respectively (Fig. 6). The relationship between FRC and ECO2 levels was not significant (data not shown) as reductions in ECO2 were associated with both increases and decreases in FRC, depending on the associated VT and end-inflation lung volumes. Reductions in FRC resulted in increased ECO2 levels, when associated with increased VT (kittens 1 and 2), or reductions in ECO2 levels (kittens 3 and 4) when VT’s were reduced. VT was significantly (p<0.05) associated with ECO2 levels (data not shown), although increasing VT was not always associated with increasing ECO2 levels, particularly if end-inflation volumes were not altered. This occurred in kitten 3 (Fig. 6, first half of trace), where a reduction in FRC resulted in increased VT and reduced ECO2 levels without a change in end-inflation volumes. The changes in lung gas volumes caused by altering ventilation parameters in kitten 4 are displayed in Movie S2.
Figure 6. Lung gas volumes, airway pressures and expired CO2 levels in 4 ventilated newborn rabbits delivered preterm at 28 days of gestation.
Lung gas volumes are demonstrated in the top panel, airway pressures in the middle panel and expired CO2 (ECO2) levels in the bottom panel. Functional residual capacity (FRC) and end-inflation lung volumes (EILV) are indicated on the lung volume trace. Changes in ECO2 levels closely followed changes in end-inflation lung volumes caused by altering ventilation parameters. The corresponding phase contrast X-ray images, compiled into a movie, for kitten 4 are displayed in Movie S2.
Preterm Infant Studies
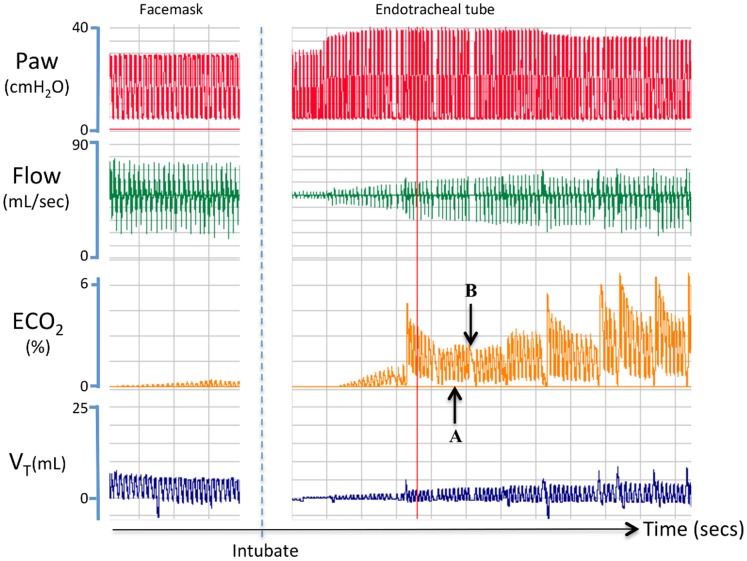
Demographics of the ten preterm infants included in the study are presented in table 2. Mask ventilation started at a median (IQR) time of 46 (37–60) seconds after birth. Time to achieve an ECO2 of >10 mmHg (1.3%) was 92 (46–150) seconds. Median (IQR) time for heart rate to exceed 100 beats/min was 126 (96–160) seconds and followed the increase in ECO2 levels to >10 mmHg by 28 (21–36) seconds (Table 3). Data recorded from a preterm infant during mask ventilation, followed by intubation, is presented in figure 7. During mask ventilation, despite an apparent good VT with no leak, the infant remained bradycardic (68–72 beats/min) and very little ECO2 could be detected. Following intubation, the same PIP resulted in a markedly lower VT, necessitating an increase in PIP (to 40 cmH2O). The resulting increase in VT was associated with increasing ECO2 levels, despite initially measuring smaller VT’s than during mask ventilation (Fig. 7). The measured HR and SpO2 values at intubation were 67 beats/min and 22%, respectively. Following restoration of PPV, an increase in PIP and a resulting increase in VT, ECO2 levels markedly increase, which was followed by an increase in the infant’s heart rate; increased from 70 beats/min at “A” to 100 beats/min at “B”.
Table 2. Demographics of included preterm infants.
| Preterm infants (n = 10) | |
| Birth weight (gram)* | 902 (287) |
| Gestational age (weeks)* | 27 (2) |
| Male | 5 (50%) |
| Apgar Score at 1 Minutes# | 3 (2–4) |
| Apgar Score at 5 Minutes# | 6 (5–6) |
| Infants Intubated | 6 (60%) |
| Antenatal Steroids | 10 (100%) |
| Caesarean Section | 9 (90%) |
Values are numbers (percentage) unless indicated,
mean (standard deviation), #median (interquartile range).
Table 3. Parameters of ECO2, HR at start of mask ventilation in preterm infants.
| Preterm infants (n = 10) | |
| Time from birth to start mask ventilation (seconds) | 51 (27–91) |
| Time to ECO2>10 mmHg (seconds) | 96 (46–150) |
| Time to HR>100 beats/min (seconds) | 126 (96–160) |
| Time between ECO2>10 mmHg and HR>100/min (seconds) | 28 (21–36) |
| Speed of HR change (bpm/second) | 45 (14–97) |
Values are median (interquartile range).
Figure 7. Airway pressure, gas flow, expired CO2 levels and tidal volumes measured in a preterm infant during positive pressure ventilation.
This preterm infant received positive pressure ventilation that was initially applied via facemask and then followed by intubation. Before intubation, little expired CO2 (ECO2) could be detected, despite a good tidal volume (VT) and no detectable facemask leak. Following intubation, ECO2 levels rapidly increased with increasing VT and preceded the increase in heart rate, which increased from 75 beats/min at “A” to 100 beats/min at “B”.
Valid ECO2 and VT data from all infants and inflations analyzed are presented in figure 8A. A significant (p<0.001) relationship between VT and ECO2 levels (n = 10 babies, 517 breaths) was observed with the maximum ECO2 value increasing, on average, 0.15% with each 1 mL/kg increase in VT. As the relationship between ECO2 and VT appeared to differ between infants, the relationship for each individual infant has been plotted in figures 8B (n = 5 infants) and 8C (n = 5 infants). All 5 infants in figure 8B showed a strong relationship between ECO2 values and VT.
Figure 8. Maximum expired CO2 levels against tidal volume in preterm infants.
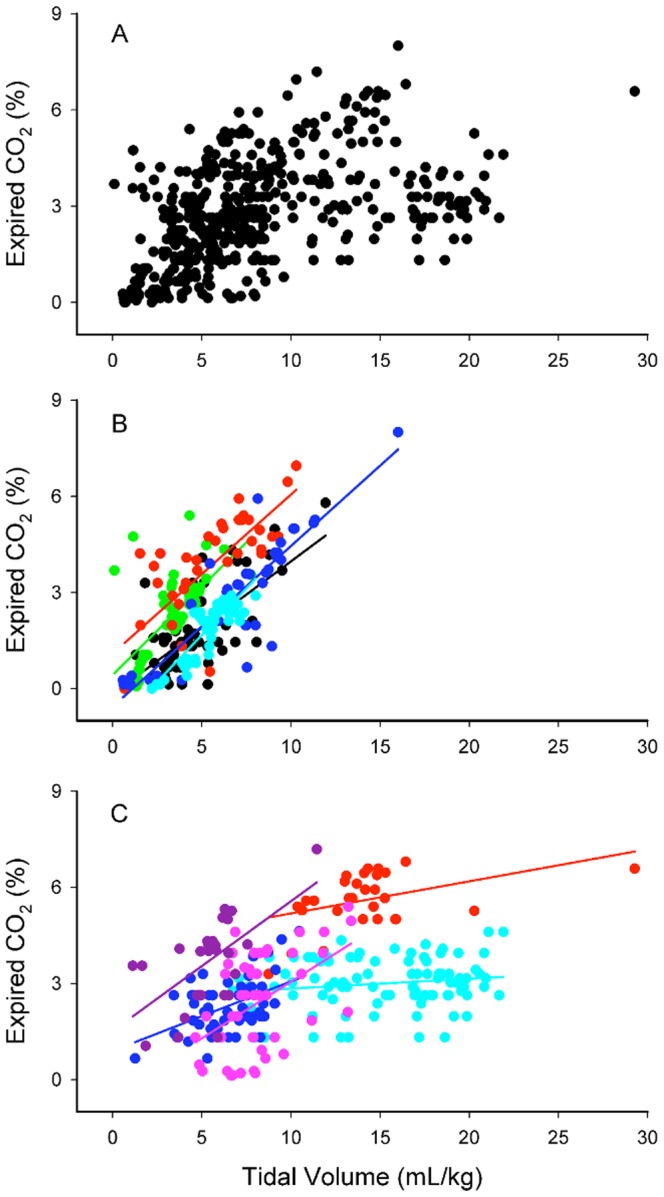
A demonstrates data from all preterm infants studied (A; n = 10 infants and 517 breaths). The individual relationships between ECO2 and VT for each infant are displayed in (B) and (C) for clarity.
Discussion
We have demonstrated that ECO2 levels can provide important information to guide resuscitation immediately after birth when respiratory support fails to improve cardiorespiratory parameters in preterm infants. In the absence of mask leak, an inability to detect ECO2 indicates that gas has not penetrated down into the distal gas-exchange structures of the lung to allow gas exchange to commence. Increasing ECO2 levels in subsequent inflations indicate increasing aeration of distal gas exchange regions. An increase in ECO2 levels with increasing lung aeration was most closely associated with end-inflation lung volumes, was associated to a lesser degree with VT and was not directly associated with FRC. These data confirm that ECO2 levels can indicate relative lung aeration immediately after birth but do not provide an accurate measure of PaCO2 levels. This is most likely due to limitations in gas exchange surface area and pulmonary perfusion [14].
As CO2 is highly soluble and the gas exchange surface area is large in a mature lung, the primary factors regulating end-tidal CO2 levels are the CO2 partial pressure gradient between alveolar gas and blood and the rate of pulmonary perfusion. As such, end-tidal and mixed venous CO2 levels can be used to estimate pulmonary perfusion and cardiac output [14]. In the neonate immediately after birth, although both pulmonary perfusion and CO2 partial pressure gradients likely influence ECO2 levels once gas exchange commences, our data indicate that the degree of lung aeration at end inflation (when gas volumes and gas exchange surface area are maximal) is the predominant factor. As such, extrapolations of PaCO2 values from ECO2 levels are problematic and large differences in lung aeration likely explain the large variability and difficulty in establishing a close relationship between PaCO2 and end-tidal CO2 levels in newborn infants [12], [13]. Indeed, low ECO2 levels are more likely to reflect poor lung aeration rather than over-ventilation during the immediate newborn period. Based on our data, determining whether a low ECO2 is due to poor lung aeration or over-ventilation can be determined by simply altering VT; an increasing ECO2 with a small increase in VT indicates improving lung aeration and possible under-ventilation. However, following the immediate newborn period, after the lung has fully aerated, numerous other factors may contribute to altered ECO2 levels. In particular, low pulmonary blood flow, due to poor cardiac output or pulmonary hypertension resulting in left-to-right shunting through the ductus arterious, as well as alveolar gas trapping may reduce ECO2 levels.
In lamb studies, increasing VT was closely associated with increasing ECO2 levels during lung aeration, following a sigmoidal shaped curve. As PEEP was constant, end-inflation lung volumes are mainly determined by VT, which explains the close relationship between VT and ECO2 levels. After a VT of ∼2 mL/kg, ECO2 levels increased linearly, indicating that at least 2 mL/kg of gas is required to initially penetrate into distal gas exchange regions. Although this largely reflects the dead space volume of the lung, it likely under-estimates this volume because many medium to small conducting airways, and the alveoli they supply, probably remained liquid-filled, as lung aeration is usually heterogeneous at birth [15], [16], [19]. After a VT of ∼6 mL/kg, it appeared that increasing VT only caused small increases in ECO2 levels, but care should be used in interpreting these data. As for rabbit kittens (Fig. 5), altering VT in lambs changed ECO2 levels on an inflation-by-inflation basis (Fig. 2) even at higher VT levels. However, a decrease in PaCO2 levels with time reduced the arterial/alveolar CO2 gradient causing a vertical shift in the relationship (Fig. 3B). This likely explains the flattening of the curve at higher VT levels as larger volumes were mostly attained later in the experiment when PaCO2 levels were decreasing. Figure 3B, which shows data from a single animal, clearly demonstrates this concept. After 10 mins, when the VT was held constant at 8 mL/kg, initially the maximum ECO2 was high but gradually decreased as the PaCO2 decreased (from 72.8 to 47.5 mmHg), causing a vertical shift in the data points.
It is interesting that CO2 clearance per inflation increased exponentially with increasing VT; this measure is different to the maximum ECO2 value as it is also depends upon the duration of the expiratory gas flow. As a result, increasing VT had little effect on CO2 clearance below a VT of ∼6 mL/kg. Presumably, this is because expiratory gas flows are shorter and the dead space volume had a relatively greater impact on CO2 clearance at lower volumes, which became much less significant after ∼6 mL/kg. Although the precise factors regulating the relationship between CO2 clearance and VT are unclear, as the inflation rate was kept constant it is interesting that the relationship was exponential and not linear. This likely results from the simultaneous contribution of other factors, in addition to increased surface area, to the efficiency of CO2 clearance. Simultaneous increases in pulmonary blood flow with lung aeration [3], [24], must greatly increase the efficiency of CO2 clearance, thereby contributing to the exponential relationship.
PC X-ray imaging of ventilated newborn rabbits allowed direct numerical comparisons between the degree of lung aeration and ECO2 levels. Consistent with data obtained from lambs, we found that end-inflation lung gas volumes directly related to ECO2 levels in the immediate newborn period, both during and after lung aeration. This is clearly evident in Movie S1 with the increase in ECO2 levels almost exactly coinciding with the first appearance of gas in the distal airways (see Fig. 4). The end-inflation gas volume of the lung at which ECO2 was first detected in preterm rabbit kittens was 3.4±0.3 mL/kg. Following subtraction of the calculated dead space volume, this indicates that ECO2 can be detected when only ∼7% (∼1.4 mL/kg) of the distal gas exchange regions of the lung are aerated.
Due to the small size (28.9±1.5 g) and VT (∼0.2 mL) of preterm rabbits, it was not possible to incorporate a CO2 analyzer into the “Y” piece that connects to the ET tube to measure inflation-by-inflation changes in ECO2 levels. Instead, the CO2 analyzer (a main stream CO2 analyzer) was placed in the expiratory limb of the circuit, immediately downstream of the “Y” piece. As our ventilator does not use a bias gas flow [23], mainly expired gas enters this limb of the circuit. However, the combined dead space of the “Y” piece and CO2 analyzer (∼350 µL) was usually greater than the kitten’s VT. As a result, the ECO2 curve represents a smoothed diluted average of ECO2 levels across an entire inflation and the detection was slightly delayed. The delay was adjusted by time-shifting the ECO2 curve by the time required to expire 780 µL in preceding inflations (see Methods). Despite these limitations, we found that changes in ECO2 levels were extraordinarily sensitive to changes in end-inflation lung gas volumes (Fig. 6).
The parameter primarily regulating ECO2 levels in newborn rabbits was end-inflation lung volumes. Of the two components that comprise end-inflation lung volumes (FRC and VT; Fig. 6), only VT was significantly related to ECO2 levels, as decreases in FRC were associated with both increases (Fig. 6, kitten 1) and decreases (Fig. 6, kittens 3 and 4) in ECO2 levels. When increases in ECO2 levels were associated with a decrease in FRC (Fig. 6, kitten 1), this was most likely due to a corresponding increase in VT and an associated increase in CO2 clearance (Fig. 3). On the other hand, decreases in ECO2 levels were always associated with a decrease in FRC when end-inflation lung volumes (Fig. 6, kittens 1–4) also decreased. Movie S2 clearly demonstrates the effects of changing ventilation strategy on lung aeration, with the loss of PEEP and the decrease in VT causing the basal lobes to either collapse or re-fill with lung liquid. In contrast, the apical lobes, particularly the right apical lobe (upper right of image), remained well ventilated and was possibly over-expanded (indicated by bulging between ribs), despite the remainder of the lung virtually collapsing at end-expiration.
Consistent with our findings in both lambs and rabbit kittens, we found that ECO2 levels were significantly associated with VT in preterm infants, with some infants showing a strong relationship between these parameters (Fig. 8B). The large variability in the entire data set was likely due to large differences in ventilation success in these infants, as previously observed [8], as well as the effect that facemask or endotracheal leak will have on the measured ECO2 level [25]. For instance, in Figure 7 the initial mask ventilation failed to increase ECO2 levels and the infant’s heart rate remained low, despite good VT’s and no apparent mask leak. However, following intubation, smaller VT’s resulted in larger ECO2 levels, indicating that each inflation resulted in better lung aeration. This suggestion is consistent with the finding of a gradual increase in SpO2 and a rapid increase in HR (from 70 to 100 bpm between A and B) following intubation. In all preterm infants, we found that ECO2 levels increased above 10 mmHg approximately 28 (21–36) seconds before the HR increased above 100 beats/min. Thus, increasing ECO2 levels not only indicate the success and degree of lung aeration, they may also predict an impending increase in HR, although this is only preliminary data in a small number of infants (n = 10). It is not known why mask ventilation failed in the infant displayed in Figure 7, despite the measurement of good VT’s. However, it is possible that initially, when the airways are partially liquid filled, gas flows and volumes measured at the facemask do not necessarily reflect the flow of gas through the glottis and into the airways.
This study investigated whether ECO2 levels indicate the degree of lung aeration and provide useful feedback information when cardiorespiratory parameters fail to improve in response to assisted ventilation in the delivery room. We found that ECO2 levels are closely associated with end-inflation lung volumes during the immediate newborn period, preceding the increase in HR by ∼20 secs. Our data indicate that the surface area available for gas exchange is a major determinant of ECO2 levels during the newborn period. Although pulmonary perfusion and the concentration gradient for CO2 diffusion must also determine ECO2 levels at this time, the close relationship between end-inflation lung volumes and ECO2 levels indicates that ECO2 levels provide a good indication of aeration in gas exchange regions of the lung. As a result, ECO2 levels are unlikely to provide a reliable indication of PaCO2 levels in the immediate newborn period.
Supporting Information
Phase contrast X-ray movie of the increase in lung aeration in a newborn rabbit kitten (28 days of gestation). The appearance of gas in the distal gas exchange units of the lung coincided with the increase in ECO2 (see Figure 4).
(MP4)
Phase contrast X-ray movie of a newborn rabbit kitten (28 days of gestation) demonstrating the changes in lung aeration when ventilation parameters are altered. The changes in ventilation parameters are demonstrated in Figure 6, Kitten 4.
(MP4)
Funding Statement
This research is supported by Australian Research Council (ARC), Australian National Health and Medical Research Council (NHMRC #491103) and the Victorian Government’s Operational Infrastructure Support Program. Authors acknowledge travel funding provided by the International Synchrotron Access Program (ISAP) managed by the Australian Synchrotron and funded by the Australian Government. The authors also gratefully acknowledge support provided by the SPring-8 synchrotron facility (Japan), granted by the SPring-8 Program Review Committee (proposal nos. 2010B0022). GMS acknowledges funds by the Laerdal Foundation for Acute Medicine. MLS was a recipient of an Australian NHMRC Postgraduate Scholarship, MJK was a recipient of an ARC Australian Research Fellowship and PGD and SBH are recipients of Australia NHMRC Research Fellowships. ABtP was a recipient of a Veni-grant, The Netherlands Organisation for Health Research and Development (ZonMw), part of the Innovational Research Incentives Scheme Veni-Vidi-Vici (project nos. 91612027). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. te Pas AB, Davis PG, Hooper SB, Morley CJ (2008) From liquid to air: breathing after birth. Journal of Pediatrics 152: 607–611. [DOI] [PubMed] [Google Scholar]
- 2. Iwamoto HS, Teitel D, Rudolph AM (1987) Effects of Birth-Related Events on Blood-Flow Distribution. Pediatric Research 22: 634–640. [DOI] [PubMed] [Google Scholar]
- 3. Teitel DF, Iwamoto HS, Rudolph AM (1990) Changes in the pulmonary circulation during birth-related events. Pediatric Research 27: 372–378. [DOI] [PubMed] [Google Scholar]
- 4. Perlman JM, Wyllie J, Kattwinkel J, Atkins DL, Chameides L, et al. (2010) Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics 126: e1319–1344. [DOI] [PubMed] [Google Scholar]
- 5. Jobe AH (2011) The new bronchopulmonary dysplasia. Curr Opin Pediatr 23: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, et al. (2010) Defining the reference range for oxygen saturation for infants after birth. Pediatrics 125: e1340–1347. [DOI] [PubMed] [Google Scholar]
- 7. Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, et al. (2010) Changes in heart rate in the first minutes after birth. Arch Dis Child Fetal Neonatal Ed 95: F177–181. [DOI] [PubMed] [Google Scholar]
- 8. Schmolzer GM, Kamlin OC, Dawson JA, te Pas AB, Morley CJ, et al. (2010) Respiratory monitoring of neonatal resuscitation. Arch Dis Child Fetal Neonatal Ed 95: F295–303. [DOI] [PubMed] [Google Scholar]
- 9.Schmolzer GM, Morley CJ, Wong C, Dawson JA, Kamlin CO, et al.. (2012) Respiratory function monitor guidance of mask ventilation in the delivery room: a feasibility study. J Pediatr 160: 377–381 e372. [DOI] [PubMed]
- 10. Finer NN, Rich WD (2004) Neonatal resuscitation: raising the bar. Curr Opin Pediatr 16: 157–162. [DOI] [PubMed] [Google Scholar]
- 11. Tracy M, Downe L, Holberton J (2004) How safe is intermittent positive pressure ventilation in preterm babies ventilated from delivery to newborn intensive care unit? Arch Dis Child Fetal Neonatal Ed 89: F84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu CH, Chou HC, Hsieh WS, Chen WK, Huang PY, et al. (2003) Good estimation of arterial carbon dioxide by end-tidal carbon dioxide monitoring in the neonatal intensive care unit. Pediatr Pulmonol 35: 292–295. [DOI] [PubMed] [Google Scholar]
- 13. Tingay DG, Stewart MJ, Morley CJ (2005) Monitoring of end tidal carbon dioxide and transcutaneous carbon dioxide during neonatal transport. Arch Dis Child Fetal Neonatal Ed 90: F523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trillo G, von Planta M, Kette F (1994) ETCO2 monitoring during low flow states: clinical aims and limits. Resuscitation 27: 1–8. [DOI] [PubMed] [Google Scholar]
- 15. te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, et al. (2009) Establishing functional residual capacity at birth: the effect of sustained inflation and positive end expiratory pressure in a preterm rabbit model. Pediatric Research 65: 537–541. [DOI] [PubMed] [Google Scholar]
- 16. Siew ML, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, et al. (2009) Inspiration regulates the rate and temporal pattern of lung liquid clearance and lung aeration at birth. JApplPhysiol 106: 1888–1895. [DOI] [PubMed] [Google Scholar]
- 17. Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, et al. (2009) Positive end expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. JApplPhysiol 106: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 18. Kitchen MJ, Lewis RA, Morgan MJ, Wallace MJ, Siew MLL, et al. (2008) Dynamic measures of lung air volume using phase contrast X-ray imaging. PhysMedBiol 53: 6065–6077. [DOI] [PubMed] [Google Scholar]
- 19. Hooper SB, Kitchen MJ, Wallace MJ, Yagi N, Uesugi K, et al. (2007) Imaging lung aeration and lung liquid clearance at birth. FASEB J 21: 3329–3337. [DOI] [PubMed] [Google Scholar]
- 20. Hooper SB, Kitchen MJ, Siew ML, Lewis RA, Fouras A, et al. (2009) Imaging lung aeration and lung liquid clearance at birth using phase contrast X-ray imaging. ClinExpPharmacolPhysiol 36: 117–125. [DOI] [PubMed] [Google Scholar]
- 21. te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, et al. (2009) Effect of sustained inflation length on establishing functional residual capacity at birth in ventilated premature rabbits. Pediatric Research 66: 295–300. [DOI] [PubMed] [Google Scholar]
- 22. Siew ML, Te Pas AB, Wallace MJ, Kitchen MJ, Islam MS, et al. (2011) Surfactant increases the uniformity of lung aeration at birth in ventilated preterm rabbits. Pediatr Res 70: 50–55. [DOI] [PubMed] [Google Scholar]
- 23. Kitchen MJ, Habib A, Fouras A, Dubsky S, Lewis RA, et al. (2010) A new design for high stability pressure-controlled ventilation for small animal lung imaging. Journal of Instrumentation 5: T02002. [Google Scholar]
- 24. Iwamoto HS, Teitel DF, Rudolph AM (1993) Effects of lung distension and spontaneous fetal breathing on hemodynamics in sheep. Pediatric Research 33: 639–644. [DOI] [PubMed] [Google Scholar]
- 25. Schmalisch G, Al-Gaaf S, Proquitte H, Roehr CC (2012) Effect of endotracheal tube leak on capnographic measurements in a ventilated neonatal lung model. Physiol Meas 33: 1631–1641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phase contrast X-ray movie of the increase in lung aeration in a newborn rabbit kitten (28 days of gestation). The appearance of gas in the distal gas exchange units of the lung coincided with the increase in ECO2 (see Figure 4).
(MP4)
Phase contrast X-ray movie of a newborn rabbit kitten (28 days of gestation) demonstrating the changes in lung aeration when ventilation parameters are altered. The changes in ventilation parameters are demonstrated in Figure 6, Kitten 4.
(MP4)