Abstract
Cerebrovascular complications make diabetic patients 2–6 times more susceptible to a stroke event and this risk is magnified in younger individuals and in patients with hypertension and complications in other vascular beds. In addition, when patients with diabetes and hyperglycemia experience an acute ischemic stroke they are more likely to die or be severely disabled and less likely to benefit from the one FDA-approved therapy, intravenous tissue plasminogen activator. Experimental stroke models have revealed that chronic hyperglycemia leads to deficits in cerebrovascular structure and function that may explain some of the clinical observations. Increased edema, neovascularization and protease expression as well as altered vascular reactivity and tone may be involved and point to potential therapeutic targets. Further study is needed to fully understand this complex disease state and the breadth of its manifestation in the cerebrovasculature.
Keywords: cerebral vasculature, diabetes, hemorrhage, ischemia, stroke, targets
Introduction
The steadily increasing prevalence of obesity in developed nations has contributed to the alarming rate of diagnoses of type 2 diabetes (T2D) and prediabetes, even in children.1 In addition, there is an equally alarming increase in the number of younger patients diagnosed with type 1 diabetes (T1D).2,3,4 Given the mortality and morbidity due to cardiovascular diseases (CVD) associated with diabetes, this increase in the incidence of diabetes will have an avalanche effect in health care. Hyperglycemia ravages all vascular beds in the human body, and in the brain, this has devastating consequences. A growing body of literature indicates that the cerebrovascular sequelae of diabetes may play an important role in the pathogenesis of cerebral complications of diabetes including stroke5,6 and other neurological diseases including Alzheimer’s Disease and Vascular Cognitive Impairment.7,8 Since diabetes is the fastest growing risk factor for stroke globally, this review will focus on stroke as a complication of diabetes and readers are referred to recent reviews for the neurodegenerative complications of diabetes.8,9,10,11 While traditionally stroke is considered a macrovascular complication of diabetes, there is increasing evidence that the microvasculature of the brain is severely affected in both forms of diabetes.7,8 In the following review, we will present the mechanisms and consequences of cerebrovascular damage due to diabetes, using both experimental and clinical evidence, and especially focus on the role of microvascular disease in stroke pathogenesis and outcomes in diabetes.
Diabetes and Stroke Incidence
Patients with diabetes mellitus are at markedly increased risk of death due to cerebrovascular disease, and this is true of both T1D and T2D.12 In fact, although T2D patients make up the vast majority of diabetic stroke (97% in the Nurses’ Health Study),13 the relative risk is actually greater in T1D, with more than 4 fold higher rates of stroke at all ages of the disease.13 This is magnified at younger ages, with T1D patients between 15 and 34 years of age having stroke rates more than 16 times that of the general population.14 Other vascular complications, in particular, diabetic nephropathy, predicted even greater risk of up to 75 times that of the stroke rate of aged matched controls.14 Nevertheless, most diabetic stroke occurs in T2D and most epidemiologic and prevention data reflects this bias.
A prospective population based study, with approximately 20 year follow-up, evaluated the effect of T2D alone on CVD in 13,105 subjects. The study showed an increased relative risk for developing stroke of 1.5 to 2 fold in men and 2 to 6.5 fold in women.15 This increased risk is seen even early after diagnosis. In a short term study conducted to evaluate 5-year risk of stroke in newly treated T2D patients, the relative risk of stroke was 2.1 (95% CI, 1.8 to 2.3) in the T2D group, compared to the general population. Additionally, younger patients (30 to 44 years old) had higher relative risk for stroke compared to older patients.16
Glucose Control and Stroke Risk
Although it is clear that diabetes increases the risk of stroke, it has proven difficult to determine whether controlling glucose effectively reduces this risk. The United Kingdom Prospective Diabetes Study (UKPDS) 33 was a randomized, prospective, multicenter trial that evaluated the effects of intensive glycemic control with insulin or sulphonylureas compared with standard glycemic control with diet on microvascular and macrovascular complications and stroke was included as an important macrovascular outcome. Although the investigators were successful in lowering glucose (target fasting blood glucose (FBG) of ≤ 6 mmol/L), and reducing some of the vascular complications like nephropathy and retinopathy, there was no significant reduction in stroke and myocardial infarction.17 However, in UKPDS 34, which aimed to achieve intensive glycemic control with metformin compared with conventional therapy (diet alone), the results were different. The study concluded that compared to intensive sulphonylureas or insulin control, intensive therapy with metformin (target FBG ≤ 6 mmol/L), led to a significantly greater risk reduction of developing any diabetes-related endpoint, defined as death due to macrovascular, such as MI and stroke, and microvascular complications, such as retinopathy, (P=0·0034), all-cause mortality (P=0·021), and stroke (P=0·032).18
Uncertainty regarding the effect of intensive glycemic control on decreasing CVD risk in T2D patients led to the launch of several longer term trials. The main objective of these trials was to compare the effects of intensive therapy with conventional therapy on CVD outcome in T2D patients. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, 10,251 patients received either intensive therapy (goal HbA1c <6%) or standard therapy (goal HbA1c 7 to 7.9%). Baseline characteristics included the following, mean age 62 years, mean duration of T2D of 10 years, and median HbA1c of 8.1%. At one year, the intensive group achieved HbA1c of 6.4%, and the conventional group achieved HbA1c of 7.5%. The rate of nonfatal stroke was not statistically significantly different in the intensive group compared to the conventional group (1.3% vs. 1.2%, respectively; P=0.74). The intensive group experienced more frequent hypoglycemia compared to the conventional group (P<0.001). Subjects were to be treated and followed for 4 to 8 years; however, the trial was terminated early, at 3.5 years. This was due to an increased mortality rate in the intensive group compared with conventional group (257 vs. 203 deaths; HR 1.22; 95% CI, 1.01 to 1.46; P=0.04).19 In the Action in Diabetes and Vascular Disease (ADVANCE) study, 11,140 patients were randomized to intensive therapy (goal HbA1c ≤ 6.5%) or standard therapy. Of note, participants had an average duration of T2D of 8 years and median baseline HbA1c of 7.2%. The intensive and standard groups achieved a median HbA1c of 6.3% and 7%, respectively. Results showed that combined microvascular and macrovascular primary endpoints were significantly reduced in the intensive therapy group compared to standard therapy (18.1% vs. 20%, respectively. HR, 0.90; 95% CI, 0.82 to 0.98; P = 0.01); this was primarily due to a 21% relative reduction in nephropathy (P=0.006). Therefore, there was a statistically significant reduction in microvascular complications (P=0.01). Reduction in macrovascular (stroke and MI) events were not statistically significant (P=0.32). There was no statistically significant increase in mortality rate between groups.20
Lastly, the Veterans Affairs Diabetes Trial (VADT) randomized 1791 patients with T2D to intensive glycemic control (goal HbA1c < 6%) or standard glycemic control. Baseline characteristics included a mean duration of T2D of 11.5 years and median HbA1c of 9.4%. Median HbA1c achieved in the intensive and standard groups were 6.9% and 8.5%, respectively. During a median 5.6 year follow-up period, the primary outcome of composite CVD events occurred in 235 patients in the intensive arm compared to 264 in standard group (HR, 0.88; 95% CI, 0.74 to 1.05; P=0.14). Although patients in the intensive group had lower stroke events compared to the standard group, it was not statistically significant (28 vs. 36 events, respectively; P = 0.32). The intensive group had more CVD deaths compared to the standard group (38 vs. 29), but the difference was not significant.21
The Diabetes Control and Complications Trial (DCCT), performed in T1D patients randomized 1141 patients to either intensive (achieving HbA1c of 7.4%) or conventional therapy (HbA1c of 9.1%) for a period of 6.5 years. In long term follow-up (mean of 17 years), early intensive glucose control resulted in a significant 57% reduction in the combination of nonfatal MI, stroke or cardiovascular death (p=0.02), despite the fact that the glycemic control did not remain different after the 6.5 years.22
In summary, it is likely that tight glycemic control reduces the risk of cerebrovascular disease and it takes many years of follow-up to demonstrate this benefit in patients.
Blood Pressure and Stroke Risk
In patients with T2D, CV risk increases by 2–3 fold in patients with elevated systolic blood pressure.23 ACCORD Blood Pressure (BP) was a randomized, non-blinded, multi-center trial that evaluated the effects of intensive BP lowering on CVD risk in T2D patients. A total of 4,733 patients were randomly assigned to intensive BP therapy (goal SBP <120 mm Hg) or standard therapy (goal SBP < 140 mm Hg). Mean baseline systolic and diastolic BP were 139 mm Hg and 76 mm Hg, respectively. The primary endpoint was a composite of nonfatal stroke, MI, or death from CV events. Pre-specified secondary endpoints included fatal and nonfatal stroke. Mean systolic BP achieved in the intensive and standard groups were 119.3 m Hg and 133.5 mm Hg, respectively. Although there was no significant difference in the primary endpoint between the intensive and standard groups (1.87% vs. 2.09%, respectively; P=0.20), there was a significant difference in the intensive arm compared to standard arm in the annual rate of total stroke (0.32% vs. 0.53%; HR, 0.59; 95% CI, 0.39 to 0.89; P=0.01) and nonfatal stroke (0.30% vs. 0.47%; HR, 0.63; 95% CI, 0.41 to 0.96; P=0.03). Therefore, the data point to the importance of intensive BP control to reduce cerebrovascular complications of diabetes.24
Lipid Lowering and Stroke Risk
The most impressive results in reducing stroke risk in diabetes have been seen with the use of statin therapy. The Collaborative Atorvastatin Diabetes Study (CARDS) was a multicenter, randomized, placebo-controlled trial aimed to evaluate the effect of atorvastatin 10mg/day for primary prevention of major CV events in patients with T2D. The study randomized 2,838 patients to atorvastatin or placebo. Inclusion criteria included patients with low density lipoprotein cholesterol (LDL-C) levels ≤ 160 mg/dL. In the atorvastatin group, 83 patients had at least 1 major CVD event compared with 127 patients in the placebo group (P=0.001). When clinical outcomes were assessed separately, the rate of stroke was reduced by 48%. The authors concluded that atorvastatin is effective in reducing the risk of stroke in diabetic patients, even when the patient has normal LDL-C levels.25 Similarly, in the Heart Protection Study (HPS) trial, 5,963 diabetic patients and 14,573 non-diabetic patients with occlusive arterial disease were randomized to receive either simvastatin 40 mg or placebo. Results showed that simvastatin was associated with a 24% reduction in the occurrence of combined vascular event (95% CI 19–28, P < 0.0001). Among diabetic patients there was a significant 24% reduction in fatal or non-fatal stroke (P=0.01).26
The benefits of lipid lowering appear to be particularly evident with statin therapy. In the ACCORD Lipid trial, 5,518 patients, who were on simvastatin therapy, were randomized to receive either fenofibrate or placebo. Although reductions in cholesterol were achieved in all groups, the primary outcome was not significantly reduced in the fenofibrate group, as compared with placebo (2.2% vs. 2.4% respectively, P= 0.32). Similarly, no difference between the two groups with respect to stroke (P=0.48 in nonfatal stroke and P=0.80 in any stroke event) was seen. The authors concluded that combination therapy did not significantly reduce CV events, compared to simvastatin alone.27
In terms of primary prevention of cerebrovascular complications, treatment of diabetes requires a multi-factorial approach. Intensive approaches have been suggested, including tight glycemic control, hypertensive management, and reduction of LDL –C and all have been shown to reduce stroke risk in some studies.
Diabetes and Stroke outcome
Admission hyperglycemia is a well-established independent predictor of neurological worsening and poor outcome following stroke.28,29 Diabetic hyperglycemic ischemic stroke patients are at a 2-fold higher relative risk of 30-day mortality.30 Although there is a lack of evidence regarding optimal acute management of hyperglycemia, the American Stroke Association recommends managing hyperglycemia following stroke to a target glucose level of < 300 mg/dL.31 There is a lack of evidence supporting tight glycemic control in diabetic hyperglycemic ischemic stroke patients. In fact, there is evidence of a J-shaped curve describing the relationship between blood glucose and neurologic outcome after ischemic stroke. 32 In this study of more than 1,440 stroke patients in a single institution, admission blood glucose <3.7 and >7.3 mmol/L (<66 and >130 mg/dL) were associated with poor outcomes at 24 h and 12 months. The potential dangers of aggressive glucose control in acute stroke were also demonstrated in a 40 patient randomized controlled trial of glucose, potassium and insulin infusion in patients with blood glucose > 126 mg/dL.33 Although the investigators were successful in lowering serum glucose and brain lactate (measured by magnetic resonance imaging – MRI) within 6 hours, there was no effect on infarct growth at 7 days. It was somewhat alarming that 76% of the treated group became hypoglycemic during treatment. Large randomized clinical trials are planned to evaluate the safety and efficacy of modest glucose control in acute ischemic stroke patients. An important issue that remains to be resolved is the relative roles of acute hyperglycemia versus diabetes in stroke outcome. A meta-analysis suggests that patients that present with hyperglycemia and no history of diabetes suffer the most from acute ischemic stroke.30
Diabetes and Reperfusion Injury after Stroke
The importance of serum glucose in the complications of reperfusion therapy for acute ischemic stroke was first reported by Demchuk when 138 consecutive patients that received tissue plasminogen activator (rtPA) within 3 hours of the onset of stroke were studied.34 Symptomatic intracerebral hemorrhage (sICH), the dreaded complication of reperfusion therapy, occurred in 9% of patients and admission serum glucose was the only independent predictor of sICH in multivariate analysis. In patients with admission glucose > 11.2 mmol/L, the rate was more than 25%. This was replicated in the PROACT II study, where acute ischemic stroke patients were treated with intra-arterial prourokinase within 6 hours of onset of symptoms. In this study, patients with blood glucose > 200 mg/dL (11 mmol/L) experienced sICH at a rate of 36%.35
Other evidence supports the fact that increasing admission glucose leads to a loss of efficacy,36 and less safety.36, 37 There is also evidence that the damaging effects of high glucose may be particularly seen with reperfusion38 and elevated blood pressure.36 It is unclear whether the acute effects of hyperglycemia in the ischemic stroke patient are modulated by the duration and severity of preexisting diabetes. Since more than 70% of stroke patients with admission blood glucose values greater than 200 mg/dL (11 mmol/L) have diabetes,36 it is difficult to separate the two with regards to the occurrence sICH and dampened improvement after rtPA.
Stroke Types in Diabetes
Since the etiology of ischemic stroke may affect prognosis, outcome and management of the disease, the landmark Trial of Org 10172 in Acute Stroke Treatment (TOAST)39 classified stroke into five subtypes: 1) large artery atherosclerosis, 2) cardioembolism, 3) small vessel occlusion (lacunar), 4) stroke of other etiology and 5) stroke of undetermined etiology. The same study found that hyperglycemia worsened outcome in non-lacunar stroke but not in lacunar stroke.40 Evidence also suggests that lacunar infarcts resulting from occlusion of a small penetrating arteriole are more common in diabetic patients.40,41 However, when one looks at the recent clinical trials, Glucose Regulation in Acute Ischemic Stroke patients (GRASP) and Treatment of Hyperglycemia in Ischemic Stroke (THIS), only 15–29% of the patients had lacunar whereas the rest had non-lacunar strokes.42,43 It also has to be noted that silent infarcts may be more common in diabetic patients contributing to greater incidence of cognitive impairment.44,45 Clearly, there is a compelling need to further our understanding of micro and macrovascular complications with relevance to stroke pathogenesis and management.
Summary of Clinical Evidence
Diabetic patients are at 2–6 fold increased risk of stroke, compared to their age-matched counterparts, and the highest relative risks occur in young, T1D diabetics. In addition, hyperglycemia at the time of acute ischemic stroke is associated with increased death and dependency. Acute hyperglycemia, especially > 200 mg/dL, increases the risk of severe intracerebral hemorrhage after treatment with reperfusion therapy and decreases the likelihood of neurologic recovery. Although trials are ongoing to test the efficacy of glucose control in the acute stroke period, the risk of hypoglycemia is an ever present threat. Understanding the mechanisms and consequences of cerebrovascular damage due to diabetes, using experimental models, may assist in the design of therapeutic strategies that are vascular protective.
Cerebrovascular Complications of Diabetes: Evidence from Experimental Studies
Undoubtedly, regulation of cerebrovascular function and structure is very important for proper cerebral perfusion and neuronal function. Vascular health is not only important for maintenance of cerebral blood flow to provide nutrients and remove metabolites from this highly metabolically active organ but also for structural and functional stability of the blood brain barrier (BBB). Therefore, micro and macrovascular disease of the brain can have profound effects on neurologic function in diabetes especially when a secondary injury is superimposed on this existing pathology. In this section, we will first summarize diabetes-induced changes in cerebrovascular function and structure and then discuss how these alterations can influence stroke outcomes in experimental diabetes.
Cerebrovascular Structure
In the clinical studies above, stroke is considered a macrovascular complication of diabetes due to accelerated atherosclerosis and carotid artery disease.46 However, there is emerging evidence that the cerebral microvasculature is affected by the disease and the brain has been recognized as a target organ for microvascular complications of diabetes.47,9 Therefore, we will mainly focus on diabetes induced changes in the cerebral microvessels. Numerous studies, mostly using streptozotocin (STZ)-induced T1D model, have reported that there is thickening of the cerebral microvascular basement membrane characterized by collagen deposition and amorphous nodules described as “cotton tufts.”48,49,50,51,52,53 The widening of the basement membrane compromises the integrity of adjacent vascular smooth muscle cells, pericytes and astrocytic end feet that sit on the basement membrane and serve as a functional bridge between the vasculature and neuronal cells of the brain.51 There is diffuse swelling of the astrocytic endfeet50,51 and vascular smooth muscle mitochondria and endoplasmic reticulum.54 There is also degeneration of the endothelium.54 It has to be noted that these changes are similar to what have been reported in the retinal microvasculature and as such, it has been proposed that retinal microvascular image analysis can be used as a potential screening tool for cerebrovascular disease.55
In addition to these ultrastructural alterations, there is significant vascular remodeling as early as 4 weeks after the STZ induction of diabetes (blood glucose levels over 300 mg/dl) when vessels become tortuous.51 Interestingly, we have found that in a mild and lean model of T2D, namely the Goto-Kakizaki (GK) rats, there is extensive vascular remodeling characterized by increased tortuosity, collateral numbers and collateral size.56,57 These changes occur 5–6 weeks after the spontaneous onset of diabetes in this model that has average blood glucose levels around 150–180 mg/dl. Moreover, these changes were accompanied by parallel increases in matrix metalloprotease (MMP)-2 and -9. Both glycemic control with metformin, one of the most commonly used oral hypoglycemic agents for the treatment of diabetes, and MMP inhibition with minocycline, started at the onset of diabetes, prevented these vascular changes. 56,57 In an earlier study, we reported that a longer duration of diabetes (~10–12 weeks) also caused significant extracellular matrix deposition and increased wall thickness of middle cerebral arteries in an endothelin (ET-1)-dependent manner.58 Glycemic control with metformin or ET receptor antagonism prevented this remodeling response.59,60 While numerous growth factors can be involved in cerebrovascular remodeling of the micro and macrovasculature in diabetes, one potential target is ET-1, the most potent vasoconstrictor with proliferative properties. The role of ET-1 has been recently reviewed as it plays a very important role in vascular complications of diabetes.61 Microvessels in the brain are unique in that, through tight junctions between endothelial cells, they form a horizontal layer of stability which is reinforced vertically by the strong interaction between astrocytic end-feet and basal lamina.62,63 Altogether, this unique dynamic structure allows the communication within the endothelial cells, glial cells and neurons of the neurovascular unit and defines the integrity of the blood brain barrier (BBB). Changes in vessel structure in diabetes ultimately affect BBB permeability. Studies reported increased BBB permeability to albumin as early as 2 weeks after the induction of T1D.64 While Mayhan et al did not find evidence for increased FITC-labeled albumin leakage in their STZ model, 64 Hawkins et al reported increased BBB permeability and altered tight junctions 2 weeks after STZ-induced diabetes.65 As an underlying mechanism, authors reported decreases in protein levels of tight junction proteins, occludin and zona occludens, with parallel increases in MMP levels. Insulin replacement started 1 week after the diabetes induction prevented BBB leakage. Another group found similar decreases in occludin but not zona occludens levels.66 We have extended these observations to T2D and reported increased permeability in the GK model after 5–6 weeks of diabetes.57 Increases in BBB permeability was associated with concurrent increases in MMP-2 and 9, a decrease in occludin, but no change in claudin-5 or collagen IV which is the major matrix protein in the basement membrane of the microvasculature.57
Not only the structure, but also the density of the vasculature can change with diabetes. Numerous studies have reported decreased vessel density and angiogenesis in the peripheral vasculature.67,68,69 On the other hand, it is well established that excessive pathological angiogenesis leads to retinopathy in diabetes.67,70 The effect of diabetes on brain microvascular density is not clear. We have reported increased neovascularization in T2D GK rats.57 As reported above, these animals also displayed increased tortuosity and collateral numbers which indicate arteriogenesis. Furthermore, the overall vessel density in the brain was greater in diabetic animals as compared to controls57 and our most recent results suggest augmented angiogenesis in this model (unpublished results). Interestingly, Beaquis et al reported decreased number of microvessels in the dentate gyrus of the hippocampus in the same model of diabetes.71 These intriguing results strongly support the idea that the effect of the type of diabetes and the degree and duration of hyperglycemia on spatial and temporal regulation of cerebral angiogenesis needs to be further studied.
Cerebrovascular Function
Vessel diameter, a primary determinant of stroke outcome, is regulated by several pathways that ensure sufficient blood flow under normal physiological conditions. These include nitric oxide production by nitric oxide synthase, activation of potassium channels leading to the hyperpolarization of vascular smooth muscle, and beta-adrenergic stimulation of adenylate cyclase.72 Diabetes induces alterations in these dilator pathways that lead to impaired reactivity and contribute to the pathogenesis of stroke.50 Studies investigating T1D effects on the vasculature using STZ-induced models predominate the literature; however, there are some reports in genetic models of T2D.
Endothelium-dependent relaxation is attenuated in patients as well as experimental animal models of diabetes.50,73,74,75,76 Its cause is multifactorial and has been attributed to increased oxidative stress,74, 77,78,79 disturbances in NO synthesis and production, 72, 75,80 impairment of vascular smooth muscle ion channels,81,82,83 and inhibition of Rho-kinase activity.74
The duration of the disease influences vascular reactivity to vasoactive agents as well as vessel function. Endothelial-dependent dilator responses of cerebral arterioles to acetylcholine are impaired after 5–6 weeks of diabetes.84 There are conflicting reports of vascular responses to 5-hydroxytryptamine (5-HT). Initial reports suggest that responses to 5-HT are not affected within 10 weeks of diabetes,85,86 whereas Van Buren, et al reported enhanced sensitivity to 5-HT in short-term diabetes (4 weeks) and decreased sensitivity in long-term diabetes (40 weeks).87 We have shown that after 12–14 weeks of diabetes, pressurized MCAs isolated from T2D rats have reduced myogenic tone compared to those taken from rats that were exposed to 4–6 weeks of diabetes. Furthermore, we have reported that treatment with oral metformin starting at the onset of diabetes restored myogenic tone in 18 week-old rats.59
Vascular responses in the cerebral macro- and microvasculature are differentially affected by diabetes. Acetylcholine-induced dilation in basilar arteries (BAs) from rats is impaired after 8–12 weeks of diabetes and is reversed by insulin treatment.88 Constrictor responses in these vessels to norepinephrine (NE) and potassium ions were not different from non-diabetic counterparts.85 In an STZ model, BA responses to the vasoconstrictors endothelin-1, angiotensin II, arginine vasopressin, and thromboxane are unaffected by 12–16 weeks of diabetes and are unaltered by L-NMMA, suggesting that these responses are independent of nitric oxide synthesis or release. 86,89 Large-conductance Ca2+-activated K+ (BKCa) channels are downregulated in STZ-treated rats fed a high fructose diet, consequently increasing vascular tone and blood pressure in this model. We have reported that BAs from a rodent model of T2D have heightened sensitivity to endothelin-1 that is abolished by chronic ETA receptor blockade.90 In posterior cerebral arteries, long-term diabetes (20–32 weeks) decreases dilation to histamine and mediates increased myogenic tone in diabetic BBZDR/Wor rats.91 In middle cerebral arteries (MCAs) isolated from alloxan-treated rabbits, contractile responses to 5-HT and histamine were similar to non-diabetic controls. Furthermore, there were no differences in dilator responses to acetylcholine. Vessels from diabetic rabbits did, however, exhibit non-endothelium dependent diminished contractile responses to noradrenaline and neuropeptide Y.92 MCAs from STZ-treated rats display increased vasoconstriction resulting from decreased sensitivity to ATP-sensitive K+ channel openers such as pinacidil and levcromakalim in an endothelium-dependent manner.82 We have reported that MCAs from T1D diabetic rats have heightened sensitivity to endothelin-1 that is abolished by chronic ETA receptor blockade.90 Cerebral arterioles develop endothelial dysfunction sooner than cerebral macrovessels such as the basilar artery87 and extracranial vessels such as the carotid artery.84 Dilator responses to acetylcholine, adenosine 5′-diphosphate, and beta-adrenergic receptor activation are reduced in cerebral arteries,72,75,79 whereas contractile responses to norepinephrine, 5-HT, and prostaglandin F2 alpha are similar non-diabetic controls.93 NOS-dependent reactivity in cerebral arterioles is impaired, in part, by the generation of excess free radicals, particularly superoxide. Angiotensin II has been implicated in the attenuation of eNOS-dependent reactivity in cerebral arteries – an effect that was alleviated by losartan-mediated reductions in superoxide production.94 Treatment with enalapril,80 apocynin,79 tempol,72 chronic ETA receptor blockade,95 and poly (ADP-ribose) polymerase (PARP) inhibition72 can also rescue NOS-dependent reactivity by reducing oxidative stress, underlining the multifactorial nature of cerebrovascular dysfunction in diabetes.
Effect of Diabetes on Stroke Injury and Functional Outcomes in Experimental Models
While the clinical studies reviewed above support that the risk of having stroke is increased and the outcome is poorer in patients with diabetes, the underlying mechanisms are still not fully understood. Until recently, experimental studies have approached this problem in general as “hyperglycemic” but not “diabetic” stroke. As recently reviewed, numerous studies have shown increased infarct size, edema and hemorrhage in hyperglycemic animals as compared to normal counterparts.5,96. In these studies, hyperglycemia was induced either by glucose injection prior to stroke or with STZ a few days prior to stroke surgery. Our knowledge on the effects of diabetes on stroke outcomes is relatively limited compared to acute elevations in blood glucose. In the following paragraphs, we will summarize the recent literature on stroke research first in T2D and then in T1D.
It was reported that cerebral ischemia causes greater infarction size in the db/db mouse, a genetic model of T2D.97,98 These mice also showed a delayed and blunted inflammatory response after ischemic injury which led the investigators to conclude that diabetes may worsen stroke damage by impairing the healing process mediated by inflammatory cells.98 The same group recently reported that pretreatment with the peroxisome proliferator-activated receptor (PPAR)-γ agonist, darglitazone, for one week normalizes the acute inflammatory response in the ob/ob mice and significantly reduces the infarct size to levels lower than that seen in the wild-type animals treated with the same agent.99 Interestingly, it was also reported that infarct sizes are greater in the male but not female db/db mice100 and this difference was not explained by differences in regional cerebral blood flow or high energy reserves. These findings, along with emerging evidence that there are gender differences in stroke pathophysiology and outcomes, call for special attention to the effects of diabetes on cerebrovascular complications.101, 102,103 Another study reported that subacute use of PPAR-γ agonists, rosiglitazone and pioglitazone, also confer neuroprotection in db/db mice when given either at 4 hours before ischemia or 6 hours after reperfusion without changing the blood glucose levels in these animals. These results strongly suggest that PPAR-γ agonists have direct neuroprotective actions and the greater neuronal injury observed in the severely diabetic db/db model is not related to blood glucose levels at the time of the stroke.104 The finding that pretreatment with these agents for 3 weeks prior to stroke normalized the blood glucose levels and significantly improved the neuroprotection compared to animals treated subacutely suggests that chronic hyperglycemia contributes to greater neuronal injury in diabetes. A recent study provided evidence that telmisartan, an angiotensin II type 1 receptor blocker, reduced ischemic brain damage in another T2D model, KK-Ay mice, and co-treatment with a PPAR-γ antagonist prevented this protective effect.105 Collectively these studies suggest that while chronic use of PPAR-γ agonists can be beneficial from a prevention perspective, acute use of these agents in stroke and especially in diabetic stroke may also be an effective strategy to reduce the burden of the disease.
Our ischemic brain injury studies in GK rats, a mild and lean model of T2D as discussed above, yielded very interesting results. In this model, ischemic injury induced by a 3 hour occlusion of the middle cerebral artery followed by 21 hours reperfusion caused the development of smaller infarcts mainly localized to the striatum as compared to control animals.56 Furthermore, there was greater hemorrhage formation, an important complication of reperfusion, as detailed in the clinical section above. Despite the fact that these rats had smaller infarctions, the neurologic deficit was greater in diabetic rats suggesting that the infarct size is not the sole determinant of poor outcomes. As discussed under “cerebrovascular structure” above, these animals displayed extensive cerebrovascular remodeling and neovascularization.57 In the absence of reperfusion, there was no hemorrhage. It is highly likely that increased neovascularization may have spared the cortex from infarction but these newly remodeled vessels were susceptible to reperfusion injury and bleeding causing hemorrhagic transformation of the infarct. In a follow-up study, we reported that prevention of vascular remodeling either by chronic glycemic control with metformin or MMP inhibition with minocycline decreased hemorrhage and improved functional outcomes106 emphasizing the importance of vascular protection in diabetic stroke. Moreover, acute atorvastatin treatment given at the beginning of reperfusion, almost abolished hemorrhagic transformation in this model and this finding suggests that, in addition to chronic glycemic control, acute interventions can be beneficial in reducing neurovascular injury after stroke in high risk groups.107
The obese Zucker rat is a commonly used model of metabolic syndrome. It was reported that ischemic brain injury results in significantly greater neuronal damage in this model and this is associated with inward remodeling of the cerebrovasculature. It has to be noted that Zucker rats have dyslipidemia and slightly elevated blood pressure but blood glucose is within the normal range.108 Blood pressure control prevents cerebrovascular remodeling and improves stroke severity in this model. These findings highlight the differences in stroke injury in different models of diabetes and metabolic syndrome and emphasize the compelling need for additional studies investigating the complex interaction of hyperglycemia, high blood pressure and dyslipidemia in experimental stroke research.
Use of models of T1D allows investigators to directly study the effects of hyperglycemia. Towards this end, STZ-induced diabetes with blood glucose above 350 mg/dl is the most commonly used model in experimental stroke studies. Rizk et al reported that temporary MCA occlusion for 2 hours caused greater infarction as well as apoptotic response in the sensory-motor cortex and the CA1 and CA3 regions of the hippocampus in diabetic animals.109 High dose insulin replacement (12 U/kg) which reduced blood glucose levels to 250 mg/dl as compared to 502 mg/dl in the untreated group was able to reduce lesion size and apoptosis. Interestingly, acute insulin treatment at the same dose given 30 min before stroke had no effect on blood glucose (517 mg/dl) but reduced injury significantly, suggesting that insulin may have other protective effects. In addition, acute administration of insulin like growth factor (IGF-1) 30 min prior or 2 h after MCA occlusion reduced infarct size and apoptosis in this model without any effect on blood glucose.110 Taken together, these studies suggest that IGF-1signaling may be promoting the neuroprotective properties of insulin. Muranyi et al also suggested that the activation of the apoptotic pathway to a greater degree may be responsible for exaggerated brain damage in diabetes.111 Reduced fibrinolytic activity has been proposed to be another player in greater ischemic damage in diabetes. An interesting study reported that T1D reduces neuroserpin levels, which inhibits endogenous tPA and activation of microglia after ischemic injury and this is associated with greater ischemic lesion size in diabetic animals. It was postulated that a decrease in this protein can cause greater activation of microglia and as well as leakage of tPA into the brain parenchyma worsening neuronal damage.112 On the other hand, another report demonstrated that diabetes downregulates tPA in rat brain capillaries and this impairs restoration of blood flow after ischemia leading to augmented brain injury.113 In summary, the effect of diabetes on ischemic brain injury is quite complex and depends on the duration (acute vs chronic), severity and presence of other confounding factors (hyperglycemia alone vs metabolic syndrome). It is clear that gender differences in ischemic brain injury in diabetes have not been adequately explored. Equally important, most studies to date focused on mostly neuronal injury but vascular complications of ischemic injury in diabetes need to be explored to develop novel therapeutic strategies.
Conclusion
The brain is a site of both macrovascular and microvascular complications of diabetes. Microvessel structural and functional changes due to acute and chronic hyperglycemia lead to increased incidence and worsened outcomes from stroke in diabetic patients. Acute hyperglycemia also reduces both the safety and efficacy of reperfusion therapy in acute ischemic stroke patients. Lessons learned from experimental models of acute, chronic, mild and severe diabetes help explain some, but not all of these clinical observations. More investigations need to be performed to identify novel targets for therapeutic intervention so that the consequences of cerebrovascular complications can be mitigated in patients with diabetes.
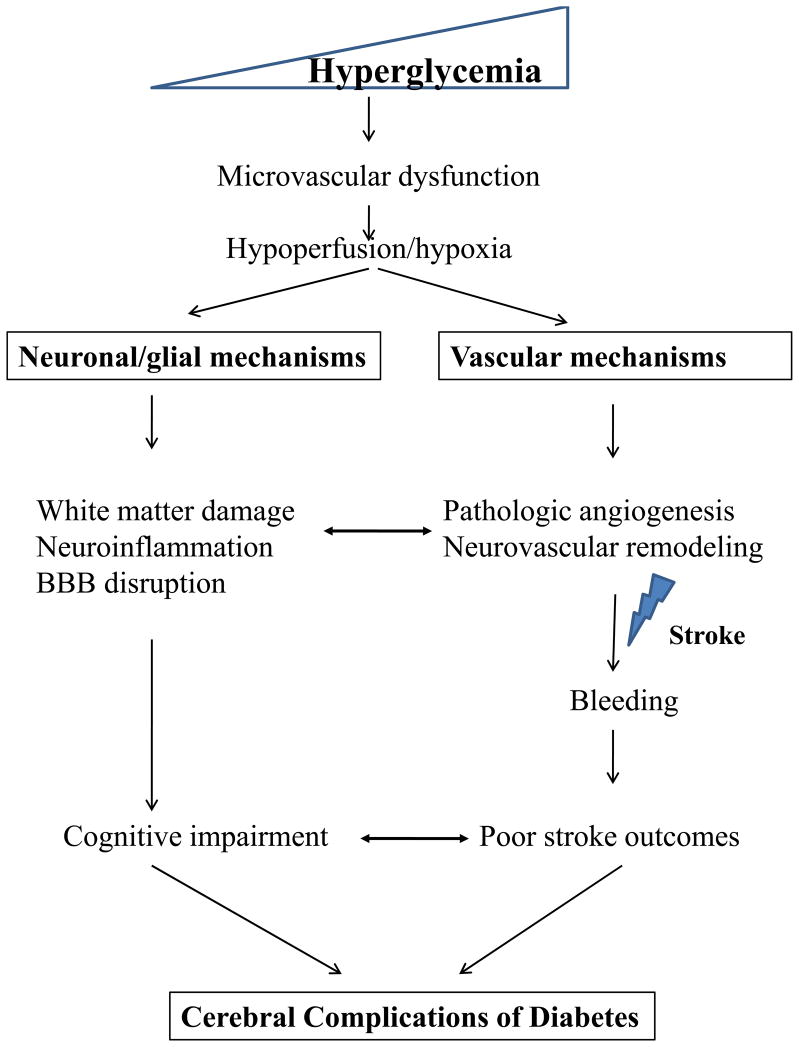
Fig. 1. Cerebral Complications of Diabetes.
Hyperglycemia leads to microvascular dysfunction, triggering both neuronal/glial and vascular injury pathways. While pathologic neurovascular remodeling increases the risk of bleeding after ischemic stroke and reperfusion, cell damage to neurons and glial cells also play a part in blood brain barrier disruption and cognitive impairment
Table 1.
Cerebrovascular Complications of Diabetes: Key Findings
| Findings | References | |
|---|---|---|
| Clinical | ||
| Chronic | 2–6 x increased incidence | 5, 6, 13, 14 |
| Increased mortality | 12 | |
| Decreased event rate with glycemic control | 18, 20, 22 | |
| BP lowering decreases risk | 24 | |
| Statin therapy decreases risk | 25, 26 | |
| Acute | Increased mortality | 30 |
| Worsened outcome | 28, 29, 32, 33 | |
| Increased hemorrhage after reperfusion therapy | 34 – 38 | |
|
| ||
| Experimental | ||
| Chronic | ||
| Structure | Thick basement membrane | 48 – 53 |
| Swollen astrocytic endfeet | 50, 51 | |
| Degeneration of endothelium | 54 | |
| Increased remodeling | 51, 56, 57 | |
| Increased neovascularization | 57 | |
| Increased BBB permeability | 57, 64, 65 | |
| Increased MMP2/9 | 56, 57 | |
| Function | Decreased vasodilation | 50, 72 – 90 |
| Decreased myogenic tone | 59, 82, 91 | |
| Acute | Increased infarct size | 5, 56, 96 – 98, 100 – 103 |
| Increased edema | 5, 96 | |
| Increased reperfusion injury/hemorrhage | 5, 56, 96 | |
BP = Blood pressure; BBB = Blood brain barrier; MMP= Matrix metalloprotease
Acknowledgments
Supported, in part, by RO1 NS0063965 (SCF), R21 NS070239 (AE), AHA Established Investigator Award 0740002N (AE) and VA Merit Review (SCF, AE) funding.
References
- 1.American Diabetes Association. [Accessed on March 30, 2011];National diabetes fact sheet. Available from http://www.diabetes.org/diabetes-statistics.jsp.
- 2.Ehehalt S, Blumenstock G, Willasch AM, Hub R, Ranke MB, Neu A. Continuous rise in incidence of childhood Type 1 diabetes in Germany. Diabet Med. 2008;25(6):755–7. doi: 10.1111/j.1464-5491.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Krishna P, Reddy SC, Gurappa M, Aravind SR, Munichoodappa C. Incidence of type 1 diabetes mellitus and associated complications among children and young adults: results from Karnataka Diabetes Registry 1995–2008. J Indian Med Assoc. 2008;106(11):708–11. [PubMed] [Google Scholar]
- 4.Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355(9207):873–6. [PubMed] [Google Scholar]
- 5.Ergul A, Li W, Elgebaly MM, Bruno A, Fagan SC. Hyperglycemia, diabetes and stroke: Focus on the cerebrovasculature. Vascul Pharmacol. 2009;51:44–49. doi: 10.1016/j.vph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick MT, Muir KW, Gray CS, Walters MR. Management of hyperglycemia in acute stroke: how, when, and for whom? Stroke. 2008;39(7):2177–85. doi: 10.1161/STROKEAHA.107.496646. [DOI] [PubMed] [Google Scholar]
- 7.Huber JD. Diabetes, cognitive function, and the blood-brain barrier. Curr Pharm Des. 2008;14(16):1594–600. doi: 10.2174/138161208784705441. [DOI] [PubMed] [Google Scholar]
- 8.Sima AA. Encephalopathies: the emerging diabetic complications. Acta Diabetol. 2010;47(4):279–93. doi: 10.1007/s00592-010-0218-0. [DOI] [PubMed] [Google Scholar]
- 9.Stiles MC, Seaquist ER. Cerebral structural and functional changes in type 1 diabetes. Minerva Med. 2010;101(2):105–14. [PubMed] [Google Scholar]
- 10.Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes. 2008;57(11):3083–9. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29(4):494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laing SP, Swerdlow AJ, Carpenter LM, Slater SD, Burden AC, Botha JL, Morris AD, Waugh NR, Gatling W, Gale EA, Patterson CC, Qiao Z, Keen H. Mortality from cerebrovascular disease in a cohort of 23 000 patients with insulin-treated diabetes. Stroke. 2003;34(2):418–21. doi: 10.1161/01.str.0000053843.03997.35. [DOI] [PubMed] [Google Scholar]
- 13.Janghorbani M, Hu FB, Willett WC, Li TY, Manson JE, Logroscino G, Rexrode KM. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses’ Health Study. Diabetes Care. 2007;30(7):1730–5. doi: 10.2337/dc06-2363. [DOI] [PubMed] [Google Scholar]
- 14.Sundquist K, Li X. Type 1 diabetes as a risk factor for stroke in men and women aged 15–49: a nationwide study from Sweden. Diabet Med. 2006;23(11):1261–7. doi: 10.1111/j.1464-5491.2006.01959.x. [DOI] [PubMed] [Google Scholar]
- 15.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164(13):1422–6. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 16.Jeerakathil T, Johnson JA, Simpson SH, Majumdar SR. Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: a population-based cohort study. Stroke. 2007;38(6):1739–43. doi: 10.1161/STROKEAHA.106.481390. [DOI] [PubMed] [Google Scholar]
- 17.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):83753. [PubMed] [Google Scholar]
- 18.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- 19.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 21.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 22.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu G, Sarti C, Jousilahti P, Peltonen M, Qiao Q, Antikainen R, Tuomilehto J. The impact of history of hypertension and type 2 diabetes at baseline on the incidence of stroke and stroke mortality. Stroke. 2005;36(12):2538–43. doi: 10.1161/01.STR.0000190894.30964.75. [DOI] [PubMed] [Google Scholar]
- 24.Cushman WC, Evans GW, Byington RP, Goff DC, Jr, Grimm RH, Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 26.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–16. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JF, Robinson GM, French JM, O’Connell JE, Alberti KG, Gray CS. Prevalence of admission hyperglycaemia across clinical subtypes of acute stroke. Lancet. 1999;353(9150):376–7. doi: 10.1016/s0140-6736(05)74948-5. [DOI] [PubMed] [Google Scholar]
- 29.Lindsberg PJ, Roine RO. Hyperglycemia in acute stroke. Stroke. 2004;35(2):363–4. doi: 10.1161/01.STR.0000115297.92132.84. [DOI] [PubMed] [Google Scholar]
- 30.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32(10):2426–32. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 31.Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology, Intervention Council and the Atherosclerotic Peripheral Vascular Disease, Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 32.Ntaios G, Egli M, Faouzi M, Michel P. J–shaped association between serum glucose and functional outcome in acute ischemic stroke. Stroke. 2010;41(10):2366–70. doi: 10.1161/STROKEAHA.110.592170. [DOI] [PubMed] [Google Scholar]
- 33.McCormick M, Hadley D, McLean JR, Macfarlane JA, Condon B, Muir KW. Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol. 2010;67(5):570–8. doi: 10.1002/ana.21983. [DOI] [PubMed] [Google Scholar]
- 34.Demchuk AM, Morgenstern LB, Krieger DW, Linda Chi T, Hu W, Wein TH, Hardy RJ, Grotta JC, Buchan AM. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30(1):34–9. doi: 10.1161/01.str.30.1.34. [DOI] [PubMed] [Google Scholar]
- 35.Kase CS, Furlan AJ, Wechsler LR, Higashida RT, Rowley HA, Hart RG, Molinari GF, Frederick LS, Roberts HC, Gebel JM, Sila CA, Schulz GA, Roberts RS, Gent M. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57(9):1603–10. doi: 10.1212/wnl.57.9.1603. [DOI] [PubMed] [Google Scholar]
- 36.Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, Lyden PD, Broderick JP, Kwiatkowski TG, Fineberg SE. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59(5):669–74. doi: 10.1212/wnl.59.5.669. [DOI] [PubMed] [Google Scholar]
- 37.Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32(4):617–22. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator--treated patients. Stroke. 2003;34(5):1235–41. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- 39.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 40.Bruno A, Biller J, Adams HP, Jr, Clarke WR, Woolson RF, Williams LS, Hansen MD. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurology. 1999;52(2):280–4. doi: 10.1212/wnl.52.2.280. [DOI] [PubMed] [Google Scholar]
- 41.Tuttolomondo A, Pinto A, Salemi G, Di Raimondo D, Di Sciacca R, Fernandez P, Ragonese P, Savettieri G, Licata G. Diabetic and non-diabetic subjects with ischemic stroke: differences, subtype distribution and outcome. Nutr Metab Cardiovasc Dis. 2008;18(2):152–7. doi: 10.1016/j.numecd.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39(2):384–9. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- 43.Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. Glucose Regulation in Acute Stroke Patients (GRASP) Trial. A Randomized Pilot Trial. Stroke. 2009 doi: 10.1161/STROKEAHA.109.561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Putaala J, Kurkinen M, Tarvos V, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts and leukoaraiosis in young adults with first-ever ischemic stroke. Neurology. 2009;72(21):1823–9. doi: 10.1212/WNL.0b013e3181a711df. [DOI] [PubMed] [Google Scholar]
- 45.Eguchi K, Kario K, Shimada K. Greater impact of coexistence of hypertension and diabetes on silent cerebral infarcts. Stroke. 2003;34(10):2471–4. doi: 10.1161/01.STR.0000089684.41902.CD. [DOI] [PubMed] [Google Scholar]
- 46.Association, A. H. Heart and stroke statistical update [Google Scholar]
- 47.Frier BM. Cognitive functioning in type 1 diabetes: the Diabetes Control and Complications Trial (DCCT) revisited. Diabetologia. 2011;54(2):233–6. doi: 10.1007/s00125-010-1983-6. [DOI] [PubMed] [Google Scholar]
- 48.Bohlen HG, Niggl BA. Arteriolar anatomical and functional abnormalities in juvenile mice with genetic or streptozotocin-induced diabetes mellitus. Circ Res. 1979;45(3):390–6. doi: 10.1161/01.res.45.3.390. [DOI] [PubMed] [Google Scholar]
- 49.Bohlen HG, Niggl BA. Adult microvascular disturbances as a result of juvenile onset diabetes in Db/Db mice. Blood Vessels. 1979;16(5):269–76. doi: 10.1159/000158215. [DOI] [PubMed] [Google Scholar]
- 50.Mayhan WG. Cerebral circulation during diabetes mellitus. Pharmacol Ther. 1993;57(2–3):377–91. doi: 10.1016/0163-7258(93)90062-i. [DOI] [PubMed] [Google Scholar]
- 51.McCuskey PA, McCuskey RS. In vivo and electron microscopic study of the development of cerebral diabetic microangiography. Microcirc Endothelium Lymphatics. 1984;1(2):221–44. [PubMed] [Google Scholar]
- 52.Mukai N, Hori S, Pomeroy M. Cerebral lesions in rats with streptozotocin-induced diabetes. Acta Neuropathol. 1980;51(1):79–84. doi: 10.1007/BF00688853. [DOI] [PubMed] [Google Scholar]
- 53.Tomassoni D, Bellagamba G, Postacchini D, Venarucci D, Amenta F. Cerebrovascular and brain microanatomy in spontaneously hypertensive rats with streptozotocin-induced diabetes. Clin Exp Hypertens. 2004;26(4):305–21. doi: 10.1081/ceh-120034136. [DOI] [PubMed] [Google Scholar]
- 54.Moore SA, Bohlen HG, Miller BG, Evan AP. Cellular and vessel wall morphology of cerebral cortical arterioles after short-term diabetes in adult rats. Blood Vessels. 1985;22(6):265–77. doi: 10.1159/000158613. [DOI] [PubMed] [Google Scholar]
- 55.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206(4):319–48. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive Cerebral Neovascularization in a Model of Type 2 Diabetes: Relevance to Focal Cerebral Ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54(9):2638–44. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- 59.Kelly-Cobbs A, Elgebaly MM, Li W, Ergul A. Pressure-independent cerebrovascular remodelling and changes in myogenic reactivity in diabetic Goto-Kakizaki rat in response to glycaemic control. Acta Physiol (Oxf) 2010 doi: 10.1111/j.1748-1716.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly-Cobbs AI, Harris AK, Elgebaly MM, Li W, Sachidanandam K, Portik-Dobos V, Johnson M, Ergul A. Endothelial EndothelinB Receptor-mediated Prevention of Cerebrovascular Remodeling is Attenuated in Diabetes Due to Upregulation of Smooth Muscle Endothelin Receptors. J Pharmacol Exp Ther. 2010 doi: 10.1124/jpet.110.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ergul A. Endothelin-1 and diabetic complications: Focus on the vasculature. Pharmacol Res. 2011 doi: 10.1016/j.phrs.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354(6):553–5. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 63.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23(8):879–94. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 64.Stauber WT, Ong SH, McCuskey RS. Selective extravascular escape of albumin into the cerebral cortex of the diabetic rat. Diabetes. 1981;30(6):500–3. doi: 10.2337/diab.30.6.500. [DOI] [PubMed] [Google Scholar]
- 65.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50(1):202–11. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- 66.Chehade JM, Haas MJ, Mooradian AD. Diabetes-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1) expression. Neurochem Res. 2002;27(3):249–52. doi: 10.1023/a:1014892706696. [DOI] [PubMed] [Google Scholar]
- 67.Silvestre JS, Levy BI. Molecular basis of angiopathy in diabetes mellitus. Circ Res. 2006;98(1):4–6. doi: 10.1161/01.RES.0000200396.90220.41. [DOI] [PubMed] [Google Scholar]
- 68.Silvestre JS, Levy BI, Tedgui A. Mechanisms of angiogenesis and remodelling of the microvasculature. Cardiovasc Res. 2008;78(2):201–2. doi: 10.1093/cvr/cvn070. [DOI] [PubMed] [Google Scholar]
- 69.Silvestre JS, Mallat Z, Tedgui A, Levy BI. Post-ischaemic neovascularization and inflammation. Cardiovasc Res. 2008;78(2):2429. doi: 10.1093/cvr/cvn027. [DOI] [PubMed] [Google Scholar]
- 70.Caldwell RB, Bartoli M, Behzadian MA, El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI, Caldwell RW. Vascular endothelial growth factor and diabetic retinopathy: role of oxidative stress. Curr Drug Targets. 2005;6(4):511–24. doi: 10.2174/1389450054021981. [DOI] [PubMed] [Google Scholar]
- 71.Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol. 2010;222(1):125–34. doi: 10.1016/j.expneurol.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 72.Arrick DM, Sharpe GM, Sun H, Mayhan WG. nNOS-dependent reactivity of cerebral arterioles in Type 1 diabetes. Brain Res. 2007;1184:365–71. doi: 10.1016/j.brainres.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Sacca L, Ferrannini E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55(4):1133–40. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 74.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke. 2005;36(2):342–7. doi: 10.1161/01.STR.0000152952.42730.92. [DOI] [PubMed] [Google Scholar]
- 75.Schwaninger RM, Sun H, Mayhan WG. Impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type II diabetic rats. Life Sci. 2003;73(26):3415–25. doi: 10.1016/j.lfs.2003.06.029. [DOI] [PubMed] [Google Scholar]
- 76.Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol Metab. 1998;83(6):1946–52. doi: 10.1210/jcem.83.6.4907. [DOI] [PubMed] [Google Scholar]
- 77.Erdos B, Snipes JA, Tulbert CD, Katakam P, Miller AW, Busija DW. Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am J Physiol Heart Circ Physiol. 2006;290(3):H1264–70. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- 78.Erdos B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53(5):1352–9. doi: 10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- 79.Mayhan WG, Arrick DM, Sharpe GM, Patel KP, Sun H. Inhibition of NAD(P)H oxidase alleviates impaired NOS–dependent responses of pial arterioles in type 1 diabetes mellitus. Microcirculation. 2006;13(7):567–75. doi: 10.1080/10739680600885194. [DOI] [PubMed] [Google Scholar]
- 80.Trauernicht AK, Sun H, Patel KP, Mayhan WG. Enalapril prevents impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in diabetic rats. Stroke. 2003;34(11):2698–703. doi: 10.1161/01.STR.0000092121.62649.DC. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y, Zhang HT, Su XL, Deng XL, Yuan BX, Zhang W, Wang XF, Yang YB. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovasc Res. 2010;7(2):75–84. doi: 10.2174/156720210791184925. [DOI] [PubMed] [Google Scholar]
- 82.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81(6):996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]
- 83.Mayhan WG, Mayhan JF, Sun H, Patel KP. In vivo properties of potassium channels in cerebral blood vessels during diabetes mellitus. Microcirculation. 2004;11(7):605–13. doi: 10.1080/10739680490503410. [DOI] [PubMed] [Google Scholar]
- 84.Kitayama J, Faraci FM, Gunnett CA, Heistad DD. Impairment of dilator responses of cerebral arterioles during diabetes mellitus: role of inducible NO synthase. Stroke. 2006;37(8):2129–33. doi: 10.1161/01.STR.0000231654.79017.df. [DOI] [PubMed] [Google Scholar]
- 85.Abiru T, Kamata K, Miyata N, Kasuya Y. Differences in vascular responses to vasoactive agents of basilar artery and aorta from rabbits with alloxan-induced diabetes. Can J Physiol Pharmacol. 1990;68(7):882–8. doi: 10.1139/y90-134. [DOI] [PubMed] [Google Scholar]
- 86.Mayhan WG. Effect of diabetes mellitus on responses of the basilar artery in rats to products released by platelets. Stroke. 1992;23(10):1499–503. doi: 10.1161/01.str.23.10.1499. [DOI] [PubMed] [Google Scholar]
- 87.Van Buren T, Vleeming W, Krutzen MM, Van de Kuil T, Gispen WH, De Wildt DJ. Vascular responses of isolated mesenteric resistance and basilar arteries from short- and long-term diabetic rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358(6):663–70. doi: 10.1007/pl00005309. [DOI] [PubMed] [Google Scholar]
- 88.Mayhan WG, Trauernicht AK, Irvine SD. Insulin reverses impaired acetylcholine-induced dilatation of the rat basilar artery during diabetes mellitus. Brain Res. 2001;893(1–2):195–201. doi: 10.1016/s0006-8993(00)03314-x. [DOI] [PubMed] [Google Scholar]
- 89.Mayhan WG. Constrictor responses of the rat basilar artery during diabetes mellitus. Brain Res. 1998;783(2):326–31. doi: 10.1016/s0006-8993(97)01387-5. [DOI] [PubMed] [Google Scholar]
- 90.Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1213–9. doi: 10.1152/ajpregu.00885.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. Eur J Pharmacol. 2008;579(1–3):298–307. doi: 10.1016/j.ejphar.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andersson D, Brunkwall J, Bergqvist D, Edvinsson L. Diminished contractile responses to neuropeptide Y of arteries from diabetic rabbits. J Auton Nerv Syst. 1992;37(3):215–22. doi: 10.1016/0165-1838(92)90043-g. [DOI] [PubMed] [Google Scholar]
- 93.Rosenblum WI, Levasseur JE. Microvascular responses of intermediate-size arterioles on the cerebral surface of diabetic mice. Microvasc Res. 1984;28(3):368–72. doi: 10.1016/0026-2862(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 94.Arrick DM, Sharpe GM, Sun H, Mayhan WG. Losartan improves impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type 1 diabetic rats. Brain Res. 2008;1209:128–35. doi: 10.1016/j.brainres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arrick DM, Mayhan WG. Inhibition of endothelin-1 receptors improves impaired nitric oxide synthase-dependent dilation of cerebral arterioles in type-1 diabetic rats. Microcirculation. 2010;17(6):439–46. doi: 10.1111/j.1549-8719.2010.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27(3):435–51. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- 97.Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, Vannucci SJ. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. J Clin Invest. 2004;113(1):85–95. doi: 10.1172/JCI200418336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab. 2007;27(4):710–8. doi: 10.1038/sj.jcbfm.9600382. [DOI] [PubMed] [Google Scholar]
- 99.Kumari R, Willing LB, Patel SD, Krady JK, Zavadoski WJ, Gibbs EM, Vannucci SJ, Simpson IA. The PPAR-gamma agonist, darglitazone, restores acute inflammatory responses to cerebral hypoxia-ischemia in the diabetic ob/ob mouse. J Cereb Blood Flow Metab. 2010;30(2):352–60. doi: 10.1038/jcbfm.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21(1):52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- 101.Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29(4):670–4. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu F, Li Z, Li J, Siegel C, Yuan R, McCullough LD. Sex differences in caspase activation after stroke. Stroke. 2009;40(5):1842–8. doi: 10.1161/STROKEAHA.108.538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turtzo LC, McCullough LD. Sex differences in stroke. Cerebrovasc Dis. 2008;26(5):462–74. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101(1):41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- 105.Iwanami J, Mogi M, Tsukuda K, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator–activated receptor-gamma activation in diabetic mice. J Hypertens. 28(8):1730–7. doi: 10.1097/HJH.0b013e32833a551a. [DOI] [PubMed] [Google Scholar]
- 106.Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, Fagan SC, Ergul A. Vascular protection in diabetic stroke: role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab. 2010;30(12):1928–38. doi: 10.1038/jcbfm.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, Fagan SC. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330(2):532–40. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodeling, and severity of stroke in the Zucker rat. Hypertension. 2009;53(2):381–6. doi: 10.1161/HYPERTENSIONAHA.108.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res. 2006;1096(1):204–12. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 110.Rizk NN, Myatt-Jones J, Rafols J, Dunbar JC. Insulin like growth factor-1 (IGF-1) decreases ischemia-reperfusion induced apoptosis and necrosis in diabetic rats. Endocrine. 2007;31(1):66–71. doi: 10.1007/s12020-007-0012-0. [DOI] [PubMed] [Google Scholar]
- 111.Muranyi M, Fujioka M, He Q, Han A, Yong G, Csiszar K, Li PA. Diabetes activates cell death pathway after transient focal cerebral ischemia. Diabetes. 2003;52(2):481–6. doi: 10.2337/diabetes.52.2.481. [DOI] [PubMed] [Google Scholar]
- 112.Liang W, Chuan-Zhen L, Qiang D, Jian Q, Hui-Min R, Bao-Guo X. Reductions in mRNA of the neuroprotective agent, neuroserpin, after cerebral ischemia/reperfusion in diabetic rats. Brain Res. 2004;1015(1–2):175–80. doi: 10.1016/j.brainres.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 113.Kittaka M, Wang L, Sun N, Schreiber SS, Seeds NW, Fisher M, Zlokovic BV. Brain capillary tissue plasminogen activator in a diabetes stroke model. Stroke. 1996;27(4):712–9. doi: 10.1161/01.str.27.4.712. [DOI] [PubMed] [Google Scholar]