Abstract
Insulin resistance involves decreased phosphorylation of insulin receptor substrate (IRS) proteins and/or Akt. In the vasculature, modulated Akt phosphorylation may cause impaired vasorelaxation via decreased eNOS activation. Diet-induced insulin resistance enhances endothelin-1(ET-1)-mediated vasoconstriction and prevents vasodilatation to insulin. Presently, we evaluated insulin-mediated vascular relaxation, assessed molecular markers of the insulin signaling pathway, and determined the involvement of ET-1 in response to insulin by using selective ETA or ETB receptor blockade in a lean model of type 2 diabetes. Dose-response curves to insulin (0.01–100 ng/ml) were generated with wire myograph, using thoracic aorta rings from control Wistar or diabetic Goto-Kakizaki (GK) rats (n=3–11). Maximum relaxation to insulin (Rmax) was significantly impaired and insulin sensitivity was decreased in the GK group. Preincubation with 1 uM BQ123 or BQ788 for ETA and ETB receptor blockade, respectively, exhibited improved insulin sensitivity. Immunoblotting for native and phosphorylated Akt and IRS-1 revealed a decrease in Akt activation in the GK group. In vivo hyperinsulinemic euglycemic clamp studies showed decreased glucose utilization in GK rats, indicative of insulin resistance. These findings provide evidence vascular insulin resistance occurs in a non-obese model of diabetes and both ET receptor subtypes are involved in vascular relaxation to insulin.
Introduction
Impaired relaxation in response to endothelium-derived factors such as NO as well as to insulin occurs in diabetes and contributes to vascular complications underlying increased risk of cardiovascular morbidity and mortality. Insulin resistance, an important component of type 2 diabetes, contributes to vascular dysfunction via altering vascular responsiveness to vasoactive factors. The potent vasoconstrictor ET-1 is chronically up-regulated in diabetes (Haak et al. 1992, Khan and Chakrabarti 2003). We and others have shown that ET-1 mediates vascular dysfunction and remodeling in type 2 diabetes (Chen et al. 2003, Ergul et al. 2004, Fukuda et al. 2005, Harris et al. 2005, Song and Ergul 2006). Miller et al. have reported that in a diet-induced model of insulin resistance, vasorelaxation to insulin is impaired due to augmented vasoconstriction by ET-1, which then offsets normal vasodilatation (Miller et al. 2002). Given the current thought that insulin resistance associated with obesity leads to the development of type 2 diabetes, it is also important to determine if vascular insulin resistance occurs in lean animals and whether ET-1 contributes to this impairment. We have shown that ET-1 causes enhanced constriction of mesenteric resistance and basilar arteries in Goto-Kakizaki (GK) rats, which is a lean, polygenic and spontaneous model of type 2 diabetes that do not develop hyperlipidemia or hypertension (Cheng et al. 2001, Janssen et al. 2004] allowing to study effects of mild/moderate hyperglycemia without the confounding factors usually associated with obesity-induced diabetes. While GK rats are reported to be insulin resistant based on conflicting reports of hyperinsulinemia {Witte, 2003 #4028, Sachidanandam et al. 2006), insulin-mediated vascular reactivity has not been studied. In addition, whether and to what extent ET-1 is involved in insulin response is yet to be determined. Lastly, in Zucker obese rats, vascular insulin signaling is blunted leading to a decreased vasodilatory response to insulin (Jiang et al. 1999). However, the extent to which vascular insulin signaling is affected in a lean model of diabetes is unknown.
Building on these past reports, we hypothesized that 1) insulin-mediated vascular relaxation is impaired in GK rats and this is partly due to suppressed phosphorylation of insulin receptor substrate (IRS) protein and Akt, and 2) endogenous ET-1 contributes to decreased insulin-mediated relaxation via the activation of ETA receptors.
Materials and methods
Animals
All experiments were performed on male Wistar (Harlan, Indianapolis, IN) and Goto-Kakizaki (in-house bred, derived from the Tampa colony) rats (Farese et al. 1994, Standaert et al. 2004). The animals were housed at the Medical College of Georgia animal care facility that is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. Animals were fed standard rat chow and tap water ad-libitum until sacrifice at 18 weeks of age. Animals were anesthesized and exsanguinated via cardiac puncture. Upon sacrifice, thoracic aorta were isolated without stretching, cleaned of any surrounding fat tissue under dissecting scope, cut into 2 mm-rings, and kept in oxygenated buffer until the reactivity experiments. Frozen aortic tissue from an additional set of animals were treated with ET receptor antagonists were also used for immunoblotting experiments. Treatment protocol for four weeks with highly selective antagonists for each receptor was as follows: ETA receptor blockade – Atrasentan (Abbott Labs) 5 mg/kg/day in drinking water and ETB receptor blockade – A-192621 (Abbott Labs) 30 mg/kg/day by oral gavage split into two daily doses or vehicle. Vehicle for the A-192621 consisted of 83% deionized water, 10% Polyethylene Glycol-400, 5% ethanol, and 2% Cremaphor EL. Tap water was used as vehicle for the Atrasentan.
Hyperinsulinemic-euglycemic clamp
Overnight fasted animals were placed under isoflourane anesthesia and placed on a heating pad to maintain body temperature. The carotid artery and femoral and jugular veins were exposed and cannulated with a PE 10 polyethylene tube. Glucose solution (100 mg/ml) and insulin (Novolin R, NovoNordisk, Clayton, NC, 1U/ml) were connected to the femoral and jugular line, respectively, and the carotid clamped with hemostats to be used for blood glucose sampling. Two baseline glucose readings were drawn and the insulin infusion was begun at a rate of 0.06U/hr. Once euglycemia (125 mg/dl) was achieved, glucose infusion was started at a rate of 1 mg/min. Blood glucose readings were taken at five minute intervals for a total of 100 minutes and glucose infusion rate was adjusted at each interval to maintain euglycemia.
Determination of vascular function
Isometric tension exerted by the vessels was recorded via a force transducer, using the wire-myograph technique (Danish Myo Technologies, Denmark). The myograph chambers were filled with Kreb’s buffer (NaCl 118.3, NaHCO3 25, KCl 4.7, MgSO .2, KH2PO4 1.2, CaCl2 1.5 and Dextrose 11.1 mM), gassed with 95% O2 and 5% CO2 and maintained at 37°C. Vessel segments were mounted in the chamber and adjusted to 2 g baseline tension. After stabilization, the vessels were challenged with 70 mM KCl and only those vessels that had 70% response above the baseline were considered viable. Vessels were preconstricted with norepinephrine to 70% of their resting diameter. Cumulative dose response curves to insulin (Novolin R, 0.01–100 ng/ml) were generated and the force generated was expressed as % change of baseline. Sensitivity (EC50) and maximum response (Rmax) values were calculated from the respective dose-response equations.
Immunoblotting protocol
Snap-frozen aortic tissue was homogenized in lysis buffer (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mmol/L sodium orthovanadate, 2.4 mM sodium flouride, 2.4 mM sodium deoxycholate, 1% Nonidet P-40, 20% SDS, 50mM Tris HCl, 0.1 mM EDTA, and 0.1 mM EGTA) and sonicated on ice. Samples were then rotated at 4°C for 2 hrs and centrifuged at 14,000 rpm for 10 min. Bradford assay was used to quantify the amount of protein in the supernatant. 50μg of protein was resolved using 10% SDS-PAGE gel electrophoresis and subsequently transferred to nitrocellulose membranes. The membranes were blocked for 1 hr at room temperature in 5% powdered skim milk, 0.1% bovine serum albumin in Tris-buffered saline containing 0.1% Tween 20 (T-TBS). Blots were incubated overnight at 4°C in a 1:1000 dilution of one of the following primary antibodies: total and phosphorylated Akt (Cell Signaling, Danvers, MA) and total (BD Biosciences, San Jose, CA) and phosphorylated IRS-1 (Y612) (Biosource, Camarillo, CA). Each membrane was subsequently probed with monoclonal β-actin antibody (Sigma, St. Louis, MO), to ensure equal loading of total protein. Blots were visualized using CHEMIGLOW West Substrate and the FluorChem HD2 system (Alpha Innotech, San Leandro, CA). Kaleidoscope prestained standards (BioRAD, Hercules, CA) were used to assess the specificity of the bands. Gel-Pro Analyzer software (Media Cybernetics, USA) was used to calculate band densitometry which is reported in arbitrary densitometry units.
Statistical analysis
EC50 and Rmax within-group differences were determined by one-way ANOVA with a post hoc Tukey test. Baseline Akt and IRS activation in Wistars vs. GKs was compared with a two-tailed Student’s t-test. Treatment effects of ET receptor antagonists in GK rats was analyzed by one-way ANOVA. Statistical significance was determined at p<0.05. GraphPad 5 software was used for all analyses.
Results
Animal data
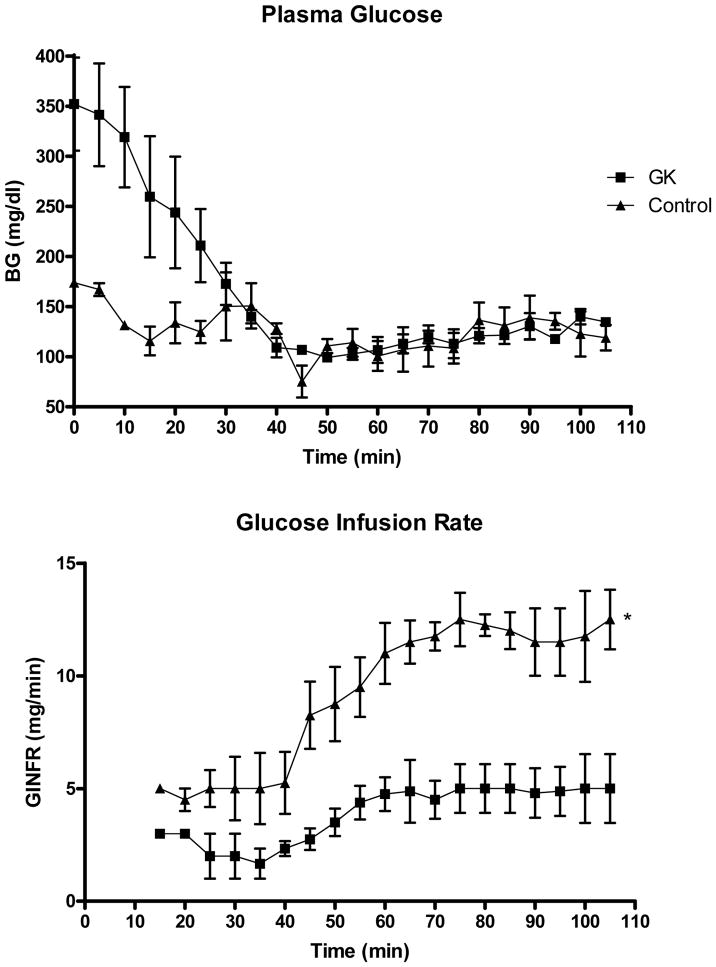
Metabolic parameters for control and diabetic (GK) animals are summarized in Table 1. Diabetic animals were significantly smaller than control animals. Glucose levels were lower in treated animals but this did not reach significance. While GK rats displayed lower insulin levels, hyperinsulinemic-euglycemic clamp studies demonstrated significantly impaired insulin sensitivity in the GK animals (Fig. 1). Insulin resistance was determined as a lesser requirement of glucose to maintain euglycemia at the same rate of insulin infusion as control animals.
TABLE 1.
Metabolic parameters
| Control (n=10) | GK (n=8) | GK + Atrasentan (n=8) | GK + A-192621 30 mg/kg (n=5) | |
|---|---|---|---|---|
| Weight | 555 ± 15 | 389 ± 17* | 357 ± 10 * | 322 ± 10 * |
| Glucose | 107 ± 3 | 209 ± 31* | 172 ± 17 * | 173 ± 31 * |
| Insulin | 2.7 ± 0.6 | 1.0 ± 0.3** | 0.7 ± 0.1 * | 0.6 ± 0.1* |
| ET-1 | 0.7 ± 0.1 | 1.5 ± 0.3* | 1.2 ± 0.1 * | 2.3 ± 0.3 *, *** |
|
| ||||
| MAP | 102 ± 1 | 108 ± 5 | 106 ± 3 | 116 ± 2*,*** |
p<0.05 vs Control,
p<0.0001 vs Control,
p<0.05 vs control or GK
Figure 1.
Hyperinsulinemic-euglycemic clamp in diabetic (GK) and control (Wistar) rats. A. Plasma glucose measurements at 5 minute intervals demonstrate that euglycemia (~125 mg/dl) is maintained from approximately 40 minutes on. B. GK rats were less sensitive to insulin as indicated by decreased requirements for glucose during continuous, fixed insulin infusion. Mean ± SEM, n=4.
Insulin dose-response
Insulin-mediated relaxation was significantly blunted in GK rats (Rmax 69 ± 3% vs 98 ± 7%, p<0.05). Aortic rings from diabetic rats were also less sensitive to insulin with EC50 values of 5 ± 2 vs 26 ± 8 nM in control and GK rats, respectively (Fig. 2A). Incubation of aortic rings with 1 μM BQ-123 for 30 min prior to the addition of insulin improved maximum relaxation in GK rats (69 ± 3% vs 80 ± 10%, p<0.05) but it had no effect in the control group (98 ± 7% vs 98 ± 4%). ETA receptor antagonism also caused a dramatic leftward shift in dose response curve (EC50, 0.4 ± 0.3 nM), increasing insulin sensitivity in GK rats (Fig. 2B). Preincubation with BQ-788, on the other hand, partially restored the insulin-mediated vasorelaxation (Fig. 2C). While ETB antagonism had no significant effect on maximum relaxation (94 ± 7 and 78 ± 6% in control and GK rats, respectively), it caused a significant improvement of insulin sensitivity in the diabetic group (EC50, 4 ± 3 vs 26 ± 8 nM in untreated rings).
Figure 2.
Relaxation responses to insulin in aortic rings from diabetic (GK) and control (Wistar) rats. GK rats had impaired relaxation as compared to controls (A). Preincubation of rings with ETA receptor antagonist BQ-123 restored both maximum relaxation and improved sensitivity in GK rats (B) whereas ETB receptor blockade with BQ-788 improved only sensitivity (C). *EC50 p<0.05 vs C or GK + BQ-123 or GK + BQ-788, **Rmax p<0.05 vs C or GK + BQ-123, Mean ± SEM, n=4–9.
Insulin signaling
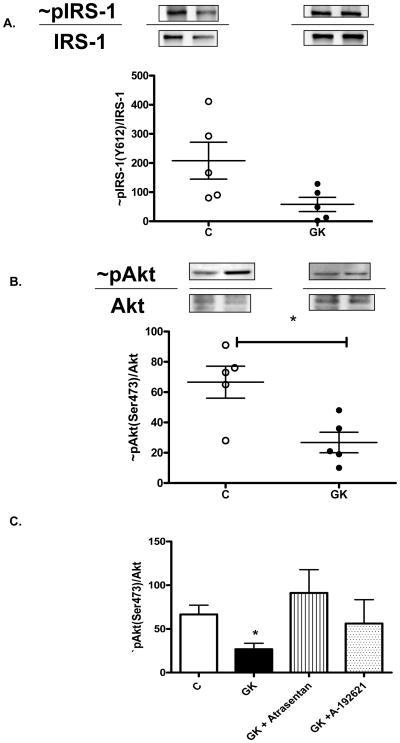
In order to determine the mechanistic basis of decreased insulin-mediated relaxation in diabetes, baseline levels of total and phosphorylated IRS-1 and Akt were assessed. Although there seemed to be an increase in total IRS-1 and Akt levels in GK rats, there was a trend for decreased IRS-1 activation (Fig. 3A) (p=0.058) and Akt phoshorylation was significantly blunted in GK rats (Fig. 3B). In diabetic rats treated with ETA receptor antagonist, Akt activation was restored to baseline levels but ETB blockade did not cause a significant change (Fig. 3C).
Figure 3.
IRS-1 (A) and Akt (B) phosphorylation in the aortic tissue of control and GK rats. A representative image of phosphorylated/total IRS-1 and Akt in control and diabetic groups are given on top and distribution of the ratio of phosphorylated to total protein is shown as scattered graph at the bottom. C) Comparison of Akt activation in control, GK and ET receptor antagonist treated GK animals. *p<0.05 vs C, n=4–8.
Discussion
There are three important findings of this study. First, attenuated IRS-1 and Akt phosphorylation leads to impaired insulin-mediated vascular relaxation in a lean model of type 2 diabetes. Second, endogenous ET-1 contributes to altered vascular insulin reactivity. Finally, both ETA and ETB receptors are involved in this response.
Multiple lines of evidence suggest that ET-1 contributes to insulin resistance and vascular dysfunction. At a cellular level, chronic exposure to ET-1 blunts insulin signaling leading to decreased insulin sensitivity (Ishibashi et al. 2001, Jiang et al. 1999). ET-1 infusion for 5 days is sufficient to cause peripheral insulin resistance and furthermore this effect is mediated by decreased insulin signaling in the muscle (Wilkes et al. 2003). In the Zucker fatty rat model, chronic endothelin receptor antagonism improves glucose tolerance and insulin sensitivity (Berthiaume et al. 2005a, Berthiaume et al. 2005b). Chronic blockade of ETA receptor subtype for 7 months has been reported to decrease plasma insulin levels, suggesting improvement of insulin sensitivity in the GK model of type 2 diabetes (Balsiger et al. 2002). From a clinical perspective, plasma ET-1 levels are elevated in diabetic patients who oftentimes present with insulin resistance (Takahashi et al. 1990). When given to healthy individuals, exogenous ET-1 induces insulin resistance and this is associated with reduced glucose uptake in skeletal muscle without a change in blood flow, suggesting metabolic effects independent of regulation of vascular reactivity (Ottosson-Seeberger et al. 1997). Moreover, Ferri et al. reported that there is a negative correlation between plasma ET-1 levels and total glucose uptake in obese patients with type 2 diabetes (Ferri et al. 1995). Two recent clinical studies demonstrated that endogenous ET-1 contributes to insulin resistance in obese individuals (Ahlborg et al. 2007, Lteif et al. 2007). Collectively these past reports provide very important information about the role of ET-1 in insulin sensitivity in obesity. Since metabolic effects of insulin depend on delivery of insulin to the tissues, flow and vascular function are equally important in insulin sensitivity. Our findings extend these studies to the vascular level and provide additional support that endogenous ET-1 impairs insulin-mediated relaxation and it does so in a lean model of type 2 diabetes.
It is well known that two G-protein coupled receptors, ETA and ETB, mediate the many actions of ET-1. While ETA, localized mainly on vascular smooth muscle cells (VSMC) of blood vessels, is responsible for the vasocontractile response to ET-1, ETB receptors located on endothelial cells mediate vasodilatation via the release of relaxing factors while ETB receptors on vascular smooth muscle cells (VSMC (ETB)) exert vasoconstriction (Battistini et al. 2006, Elgebaly et al. 2007). Miller et al. showed that in diet-induced insulin resistance, ETA receptors mediate the inhibitory effect of ET-1 on insulin-mediated relaxation (Miller et al. 2002). In obese and insulin resistant individuals, ETA receptor antagonism appears to improve leg blood flow and subsequent glucose extraction (Lteif et al. 2007). In this study, ETB receptor blockade improved insulin sensitivity but it did not affect maximum relaxation. Endothelial ETB receptors promote relaxation whereas smooth muscle ETA and ETB mediate contraction. BQ-788 blocks both endothelial and smooth muscle ETB receptors therefore endothelial- ETB-mediated relaxation is lost. Because ETA receptors are still functional one would expect the relaxation to get worse if endothelial ETB receptors are balancing the ETA-mediated contraction. In contrast we see improvement of sensitivity. One possible explanation is that smooth muscle ETA and ETB receptors work together to cause contraction to offset insulin-mediated relaxation and upon blockade with BQ-788, in the absence of functional smooth muscle ETB receptors, sensitivity is improved yet maximum relaxation is not changed.
It has been long recognized that not all insulin-regulated processes and tissues become resistant to insulin action. Alterations in the abundance of insulin receptor and glucose transporter (GLUT) proteins alone are not sufficient to explain insulin resistance. It is accepted that impairment of the intrinsic tyrosine kinase activity of the insulin receptor and/or downstream signaling pathways play a very important role in the development of insulin resistance in a pathway- and tissue-specific manner (Biddinger and Kahn 2006, Hotamisligil 2006). Binding of insulin to its receptor autophosphorylates the receptor which then stimulates a cascade of downstream phosphorylation events, including tyrosine phosphorylation of substrate proteins such as insulin receptor substrate (IRS-1 and -2) (Saltiel and Kahn 2001, White 2003) and phosphorylation of serine/threonine kinase Akt (Saltiel and Kahn 2001). This kinase then activates glycogen synthase kinase 3 (GSK 3) stimulating glycogen synthesis, GLUT-4 promoting glucose uptake and endothelial nitric oxide synthase (eNOS) resulting in vasodilatation. Thus, in this study, we used basal IRS-1 and Akt phosphorylation as molecular markers of insulin signaling. Decreased Akt activation was associated with impaired vasorelaxation of aortic rings in the GK model of diabetes. There was also a trend for decreased tyrosine phosphorylation of IRS-1. Immunoblotting of aortic tissue from Wistars and GKs chronically treated with Atrasentan revealed an increase in Akt phosphorylation providing further evidence that support our contractility studies which showed significant improvement of insulin-mediated relaxation in the presence of ETA receptor blockade. Animals treated with ETB receptor antagonist A192621 showed a trend for increased Akt activation which was short of generating an improvement of maximum relaxation to insulin.
In conclusion, this study demonstrates that vascular insulin sensitivity is decreased in a mild and nonobese model of Type 2 diabetes. Endogenous ET-1 contributes to impaired relaxation in the aorta by activation of both ETA and ETB receptors, as evidenced by improved sensitivity to insulin in the presence of acute ETA or ETB receptor antagonism. Thus, ET receptor blockade may prove beneficial in diabetes-induced vascular disease, not only by affecting vascular structure, but also improving flow and thereby insulin delivery to the target organs.
Acknowledgments
This work was supported by grants from NIH (DK074385), Philip Morris Inc and Philip Morris International to Adviye Ergul.
References
- Ahlborg G, Shemyakin A, Bohm F, Gonon A, Pernow J. Dual endothelin receptor blockade acutely improves insulin sensitivity in obese patients with insulin resistance and coronary artery disease. Diabetes Care. 2007;30 (3):591–596. doi: 10.2337/dc06-1978. [DOI] [PubMed] [Google Scholar]
- Balsiger B, Rickenbacher A, Boden PJ, Biecker E, Tsui J, Dashwood M, Reichen J, Shaw SG. Endothelin A-receptor blockade in experimental diabetes improves glucose balance and gastrointestinal function. Clin Sci (Lond) 2002;103(Suppl 48):430S–433S. doi: 10.1042/CS103S430S. [DOI] [PubMed] [Google Scholar]
- Battistini B, Berthiaume N, Kelland NF, Webb DJ, Kohan DE. Profile of past and current clinical trials involving endothelin receptor antagonists: the novel “-sentan” class of drug. Exp Biol Med (Maywood) 2006;231(6):653–695. [PubMed] [Google Scholar]
- Berthiaume N, Carlson CJ, Rondinone CM, Zinker BA. Endothelin antagonism improves hepatic insulin sensitivity associated with insulin signaling in Zucker fatty rats. Metabolism. 2005a;54(11):1515–1523. doi: 10.1016/j.metabol.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Berthiaume N, Wessale JL, Opgenorth TJ, Zinker BA. Metabolic responses with endothelin antagonism in a model of insulin resistance. Metabolism. 2005b;54(6):735–740. doi: 10.1016/j.metabol.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Chen S, Mukherjee S, Chakraborty C, Chakrabarti S. High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-kappa B and AP-1. Am J Physiol Cell Physiol. 2003;284(2):C263–272. doi: 10.1152/ajpcell.00192.2002. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Vaskonen T, Tikkanen I, Nurminen K, Ruskoaho H, Vapaatalo H, Muller D, Park JK, Luft FC, Mervaala EMA. Endothelial dysfunction and salt-sensitive hypertension in spontaneously diabetic Goto-Kakizaki rats. Hypertension. 2001;37:433–439. doi: 10.1161/01.hyp.37.2.433. [DOI] [PubMed] [Google Scholar]
- Elgebaly MM, Portik-Dobos V, Sachidanandam K, Rychly D, Malcom D, Johnson MH, Ergul A. Differential effects of ET(A) and ET(B) receptor antagonism on oxidative stress in type 2 diabetes. Vasc Pharmacol. 2007;47 (2–3):125–130. doi: 10.1016/j.vph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ergul A, Schultz Johansen J, Stromhaug C, Harris AK, Hutchinson J, Tawfik A, Rahimi A, Rhim E, Wells B, Caldwell RW, Anstadt MP. Vascular dysfunction of venous bypass conduits is mediated by reactive oxygen species in diabetes: Role of endothelin-1. J Pharmacol Exp Ther. 2004;313:70–77. doi: 10.1124/jpet.104.078105. [DOI] [PubMed] [Google Scholar]
- Farese RV, Standaert ML, Yamada K, Huang LC, Zhang C, Cooper DR, Wang Z, Yang Y, Suzuki S, Toyota T, et al. Insulin-induced activation of glycerol-3-phosphate acyltransferase by a chiro-inositol-containing insulin mediator is defective in adipocytes of insulin-resistant, type II diabetic, Goto-Kakizaki rats. Proc Natl Acad Sci U S A. 1994;91(23):11040–11044. doi: 10.1073/pnas.91.23.11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri C, Bellini C, Desideri G, Di Francesco L, Baldoncini R, Santucci A, De Mattia G. Plasma endothelin-1 levels in obese hypertensive and normotensive men. Diabetes. 1995;44(4):431–436. doi: 10.2337/diab.44.4.431. [DOI] [PubMed] [Google Scholar]
- Fukuda G, Khan ZA, Barbin YP, Farhangkhoee H, Tilton RG, Chakrabarti S. Endothelin-mediated remodeling in aortas of diabetic rats. Diabetes Metab Res Rev. 2005;21(4):367–375. doi: 10.1002/dmrr.527. [DOI] [PubMed] [Google Scholar]
- Haak T, Jungmann E, Felber A, Hillmann U, Usadel KH. Increased plasma levels of endothelin in diabetic patients with hypertension. Am J Hypertens. 1992;5:161–166. doi: 10.1093/ajh/5.3.161. [DOI] [PubMed] [Google Scholar]
- Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54(9):2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Ishibashi KI, Imamura T, Sharma PM, Huang J, Ugi S, Olefsky JM. Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes. J Clin Invest. 2001;107(9):1193–1202. doi: 10.1172/JCI11753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen U, Vassiliadou A, Riley SG, Phillips AO, Floege J. The quest for a model of type II diabetes with nephropathy: the Goto Kakizaki rat. J Nephrol. 2004;17(6):769–773. [PubMed] [Google Scholar]
- Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104(4):447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZA, Chakrabarti S. Endothelins in chronic diabetic complications. Can J Physiol Pharmacol. 2003;81(6):622–634. doi: 10.1139/y03-053. [DOI] [PubMed] [Google Scholar]
- Lteif A, Vaishnava P, Baron AD, Mather KJ. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes. 2007;56(3):728–734. doi: 10.2337/db06-1406. [DOI] [PubMed] [Google Scholar]
- Miller AW, Tulbert C, Puskar M, Busija DW. Enhanced endothelin activity prevents vasodilation to insulin in insulin resistance. Hypertension. 2002;40(1):78–82. doi: 10.1161/01.hyp.0000022806.87281.62. [DOI] [PubMed] [Google Scholar]
- Ottosson-Seeberger A, Lundberg JM, Alvestrand A, Ahlborg G. Exogenous endothelin-1 causes peripheral insulin resistance in healthy humans. Acta Physiol Scand. 1997;161(2):211–220. doi: 10.1046/j.1365-201X.1997.00212.x. [DOI] [PubMed] [Google Scholar]
- Sachidanandam K, Harris A, Hutchinson J, Ergul A. Microvascular versus macrovascular dysfunction in type 2 diabetes: differences in contractile responses to endothelin-1. Exp Biol Med (Maywood) 2006;231(6):1016–1021. [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Song W, Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: Relation to vessel size. Cardiovasc Diabetol. 2006;5(1):3. doi: 10.1186/1475-2840-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert ML, Sajan MP, Miura A, Kanoh Y, Chen HC, Farese RV, Jr, Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279(24):24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ghatei MA, Lam HC, O’Halloran DJ, Bloom SR. Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia. 1990;33:306–310. doi: 10.1007/BF00403325. [DOI] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302(5651):1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Wilkes JJ, Hevener A, Olefsky J. Chronic endothelin-1 treatment leads to insulin resistance in vivo. Diabetes. 2003;52(8):1904–1909. doi: 10.2337/diabetes.52.8.1904. [DOI] [PubMed] [Google Scholar]