Abstract
The current study examined the neural correlates associated with local and global inhibitory processes used by bilinguals to resolve interference between competing responses. Two groups of participants completed both blocked and mixed picture naming tasks while undergoing functional magnetic resonance imaging (fMRI). One group first named a set of pictures in L1, and then named the same pictures in L2. The other group first named pictures in L2, and then in L1. After the blocked naming tasks, both groups performed a mixed language naming task (i.e., naming pictures in either language according to a cue). The comparison between the blocked and mixed naming tasks, collapsed across groups, was defined as the local switching effect, while the comparison between blocked naming in each language was defined as the global switching effect. Distinct patterns of neural activation were found for local inhibition as compared to global inhibition in bilingual word production. Specifically, the results suggest that the dorsal anterior cingulate cortex (ACC) and the supplementary motor area (SMA) play important roles in local inhibition, while the dorsal left frontal gyrus and parietal cortex are important for global inhibition.
Keywords: local inhibition, global inhibition, bilingualism, word production, fMRI
Introduction
Bilinguals are able to speak two or even more languages. A crucial feature of these bilinguals is that they typically make few mistakes when switching between their two languages. Thus, an important question is how bilinguals can select correct words in the correct language. The mechanism of bilingual language selection has become the focus of research interest in recent years. Overall, there are at least two viewpoints on lexical selection in language production. While there is agreement that words in both languages are activated in parallel when a bilingual intends to speak (e.g., Costa, 2005; Kroll et al., 2006), some researchers claim that only candidates in the target language are considered for selection (e.g., Costa & Caramazza, 1999; Costa et al., 2000; Costa et al., 1999). In contrast, others argue that candidates in both languages are activated, but there is an inhibitory mechanism to suppress the activation of the lexical representation of the nontarget language (e.g., Green, 1998; Hermans et al., 1998). De Groot and Christoffels (2006) further proposed that there are two types of inhibitory control involved in suppressing the unwanted language, i.e., global control and local control. Specifically, global control refers to the activation and/or inhibition of the complete language system, whereas local control refers to control exerted on a restricted set of memory representations, such as specific lexical items.
One method used to examine whether bilingual lexical selection is based on inhibition is the language switching paradigm or mixed language naming task. In this paradigm, participants are instructed to name pictures in either of their languages according to a cue. For example, Meuter and Allport (1999) asked bilinguals to name Arabic numbers either in their native language (L1) or in the second language (L2) according to the background color. A switch trial was defined as a trial in which the response language differed from that of the previous trial, while a nonswitch trial referred to a trial in which the response was in the same language as the previous trial. They found that naming times were slower on switch than on nonswitch trials, a difference taken to indicate the cost of switching. Critically, late bilinguals with a dominant L1 showed an asymmetry in switching cost, such that larger switch costs were observed for the L1 than the L2 (Meuter & Allport, 1999). These findings were interpreted as reflecting inhibitory effects required to overcome the activation of competitors from the nontarget language, and further suggested that the more dominant L1 is inhibited to a larger degree than the less dominant L2.
In subsequent language switching studies, there has been controversy regarding the interpretation of the asymmetric switch costs observed for the bilingual's two languages. Some studies with highly proficient bilinguals have not observed the asymmetry and have argued that highly proficient bilinguals do not require an inhibitory mechanism when producing words in one language alone (Costa & Santesteban, 2004; Costa et al., 2006). A more recent study also failed to observe the switch cost asymmetry for even less proficient bilinguals when the decision to switch between the two languages was made spontaneously (Gollan & Ferreira, 2009). A careful analysis of the Costa and Santesteban (2004) data also suggests that even when the switch cost asymmetry is absent, under the mixed language naming conditions in the switching paradigm, the L1 becomes slower to name than the L2, suggesting the presence of an inhibitory process. The mixed findings from behavioral studies suggest that the presence or absence of an asymmetrical switching cost may not provide unequivocal support for the inhibitory control hypothesis. In addition, the language switching task requires bilinguals to frequently switch between languages from trial to trial, which may only provide evidence for local inhibition of specific language representations and which may also have little bearing on the typical experience of a bilingual who would be unlikely to switch languages randomly and with such high frequency.
Past studies using the event-related potential (ERP) technique also shed light on the hypothesis that inhibition of the nontarget language is necessary to enable language selection during bilingual speech planning. In these ERP studies (Jackson et al., 2001; Christoffels et al., 2007; Verhoef et al., 2009), bilinguals performed the language switching task, while their brain electrical potentials were recorded. In these studies, switch trials produced significant N2 ERP effects, which are hypothesized to be related to inhibitory control (e.g., Falkenstein et al., 1999), and thus support the inhibitory control model. However, the pattern of results has not been consistent across studies. For example, Jackson et al. (2001) found a larger N2 ERP component only for switch trials in L2 relative to nonswitch trials in L2, but no such effect for L1, while Christoffels et al. (2007) observed a larger N2 for nonswitch trials in L1 relative switch trials in L1, but no such effect for L2. This raises the necessity of further studies to disentangle the discrepancies across these studies. Likewise, these language switching studies have primarily examined the issue of whether there is local inhibition in bilingual language production, operating over an immediate language switch. In a recent ERP study (Misra et al., under review), Chinese-English bilinguals named a set of pictures in L1 and then in L2 or in the reverse order, naming a set of pictures in L2 and then in L1. A greater negativity was produced by naming in the L1 after naming the same pictures in L2, even though the pictures were repeated after an entire block. In contrast, the expected priming, or facilitation effects, for repeated items were observed when naming in L2 after L1. These results suggest that there is persistent inhibition of the L1 when naming in the L2, which may operate at a more global level and may be different from the inhibitory processes revealed by the language switching paradigm.
Neuroimaging techniques such as functional magnetic resonance imaging (fMRI) and Positron Emission Topography (PET) have also recently been used to examine this issue of whether bilinguals require inhibition in word production to attempt to resolve the current controversies in the behavioral data (Abutalebi & Green, 2007). In contrast to behavioral studies, where the presence of inhibition must be inferred based on finding asymmetric switch costs or unexpected slowing of the L1, in neuroimaging studies the activation of neural areas observed to be required for other tasks requiring inhibition can be sought during bilingual language production tasks. If these areas are also activated in bilingual language production, it then provides evidence for the inhibitory account. Previous neuroimaging studies have revealed the neural mechanisms underlying cognitive control by comparing results of switch trials with those of non-switch trials. Most of these neuroimaging studies have provided evidence for inhibition even in highly proficient bilinguals, although results are somewhat mixed.
Price et al. (1999) first investigated the neural correlates of translation and the mechanism required to switch between languages with six proficient German-English bilinguals using PET. Participants were asked to translate or read visually presented words in German, English, or alternating between the two languages. Switch conditions relative to blocked word naming conditions increased activation in the left inferior frontal region and bilateral supramarginal gyri. Switching during translation also increased activity in bilateral ventral cerebellum and the left medial fusiform.
However, an fMRI study by Hernandez and colleagues (2000), which compared single and mixed-language picture naming with six early Spanish-English bilinguals, found increased activation of the left dorsolateral prefrontal cortex (DLPFC, BA 9/46) but not the supramarginal gyrus in language switching conditions relative to single-language processing. In another study, Hernandez et al. (2001) further explored whether similar neural correlates are involved in within and between language switching. In the within-language condition, six highly proficient and balanced English-Spanish bilinguals named pictures as either the actions or the objects depicted or switched between these two types of responses. In the between-language switching condition, participants named pictures of objects in English, in Spanish, or alternated between the two languages. The right dorsolateral prefrontal cortex was significantly more activated for the mixed-language condition relative to the blocked-language condition, while the comparison between the within-language mixed condition and blocked condition yielded no significant results even with a lower threshold. Hernandez et al. claimed that switching between languages involves increased general executive function whereas within-language switching may not depend on executive processing.
A criticism of the Hernandez et al. (2001) conclusion is that the limited number of participants or the covert naming task used in that study probably led to no increased activation in brain areas associated with executive control in within-language switching. Using a similar design, a more recent study (Abutalebi et al., 2008), examined overt picture naming in a group of 12 German-French bilinguals. Abutalebi et al. found activation of the left caudate and the anterior cingulate cortex (ACC) for switching between languages as compared to switching within a language, while activation in the left prefrontal cortex was found for both types of task switching. They proposed that the left prefrontal cortex is engaged in more general executive control, but that the left caudate and the ACC are more specific for language control.
Most of the previous studies have examined the performance of bilinguals whose two languages share the same script and might therefore be hypothesized to be more likely to compete because of their similarity. Wang et al. (2007) further investigated the neural substrates of language switching among Chinese-English bilinguals whose two languages use different scripts. During the experiment, participants were asked to silently name pictures in each of their two languages according to a visual cue. Several brain areas including the left medial frontal gyrus and left ACC showed increased activation when switching into L2, however, no regions related to executive control showed additional activation when switching into L1. They claimed that language switching involved both general executive regions and task-specific regions, but no regions dedicated to language switching were observed. This is consistent with the conclusions of previous studies (e.g., Hernandez et al., 2001) that the language switching effect is task but not language specific.
Neural evidence for inhibition during bilingual production also comes from neuroimaging studies using other tasks. In a study by Rodriguez-Fornells and colleagues (2005) addressing the neural inhibition of phonological interference from the non-target language, German-Spanish bilinguals and German monolinguals were asked to respond when the name of a picture started with a consonant but to withhold responding for names starting with a vowel. The materials were selected such that for half of the translation equivalents the German and Spanish names both started with a vowel or consonant, requiring the same response (congruent), or started differently (incongruent). Two regions, the DLPFC and the supplementary motor area (SMA), were shown to be associated with the contrast between incongruent and congruent trials in bilinguals when compared to monolinguals. According to Rodriguez-Fornells et al. (2005), these results indicated that the non-target language phonology was partly activated and that bilinguals recruited executive control processing mechanisms to negotiate the interference from the non-target name.
To summarize, a variety of regions, including the left inferior frontal region (Price et al., 1999; Abutalebi et al., 2008), bilateral supramarginal gyri (Price et al., 1999), the left dorsolateral prefrontal cortex (Hernandez et al., 2000; Rodriguez-Fornells et al., 2005), the right dorsolateral prefrontal cortex (Hernandez et al., 2001), the left caudate (Abutalebi et al., 2008), the left anterior cingulate cortex (Wang et al., 2007; Abutabeli et al., 2008), the left medial frontal gyrus (Abutalebi et al., 2007; Wang et al., 2007), and the supplementary motor area (Rodriguez-Fornells et al., 2005) have been observed to be involved in inhibition of lexical competition between a bilingual’s two languages in order to select the correct language (for reviews, see Abutabeli & Green, 2007; Rodriguez-Fornells et al., 2006).
The abovementioned studies have highlighted the advantage of using neuroimaging techniques to evaluate whether cognitive control is necessary for bilingual language processing. Most studies have found evidence that one or more areas believed to be involved in inhibitory control processes are activated during bilingual lexical selection, despite differences between tasks used for each study, the level of language proficiency of the bilinguals tested, the type of materials, and the similarity of the bilingual’s languages. However, most of the past studies have typically sought evidence for inhibition in situations in which a language switch occurred in a local context.
The current study aimed to examine lexical selection and inhibitory processes in conditions which might be expected to invoke local versus global inhibitory processes to enable a richer understanding of the generality of the proposed inhibitory mechanisms. By using a design similar to an ERP study we previously conducted (Misra et al., under review), we attempted to provide neural evidence for local and global inhibitory processes used by bilinguals to resolve interference between competing responses. We assume that bilinguals may use different levels of executive control, relying on distinct neural processes, to achieve a given goal in a given situation. Comparisons were made between local switches (i.e., switching between languages from trial to trial) and more global switches (i.e., switching between languages on successive blocks of trials). As in Wang et al. (2007) we were specifically interested in Chinese-English bilinguals whose languages use different scripts. However, Wang and colleagues used a silent naming task, which might have underestimated the neural activity related to production (e.g., Palmer et al., 2001). Movement artifacts, including head movements, can be problematic for studies using fMRI to evaluate overt speech production, but a carefully time-locked event-related (ER) design was used in the current study to obtain artifact-free images (Huang et al., 2001). Furthermore, while the Wang et al. study only evaluated neural correlates of local switching effects, the current study also aimed to examine the neural mechanism of global inhibition.
In the present experiment, two groups of Chinese-English bilinguals completed both blocked and mixed picture naming tasks. One group first named a set of pictures in L1, and then named the same pictures in L2. The other group first named pictures in L2, and then in L1. Following the blocked naming tasks, both groups performed a mixed language naming task (i.e., naming pictures in either language according to a cue). The comparison between the blocked and the mixed naming tasks, collapsed across groups, was operationalized as the local switching effect, with the switching effects calculated separately for each language. In addition, the comparison between blocked naming in each language was operationalized as the global switching effect.
The logic of our study is as follows:
In the mixed naming task, participants cannot select a response language until they see a cue, so both languages need to be kept active throughout the task. Therefore, as in previous studies, the results should reveal the neural mechanism of local inhibition in the switching effects. Relative to blocked naming, an increased activation in neural areas associated with cognitive control should be observed in the mixed naming condition.
In contrast, in the blocked naming task, participants are able to select a response language in advance. However, if both languages are always active, then even during blocked naming a global inhibitory process may be required to attenuate activation of the other language in order to complete naming pictures in the required language. To boost the probability that the non-target language label for each picture would be activated, our task involved repetition of the same pictures from one block to the other. The results of the blocked switching manipulation thus should reveal the neural mechanism of the global inhibition. Specifically, based on the results of our previous ERP and behavioral results (Misra et al., under review), we expected that in a typical block, naming pictures in L2 would activate the dominant L1, while naming pictures in L1 would not activate L2 to the same degree. Therefore, naming in L1 after L2 might lead to increased activation of brain areas related to executive control because of a need to overcome the L1 inhibition from the previous block. However, naming pictures in L2 after L1 might be predicted to show a different picture. Since L1 should be active regardless of the block configuration, additional inhibition should not be required to name in L2 after naming in L1.
Materials and Methods
Participants
Twenty four Chinese-English bilinguals participated in the present experiment. All of the participants had normal or corrected-to-normal vision and were free of neurological diseases. Participants were paid a small amount of money for their participation. They were randomly divided into two groups, 12 (6 male and 6 female) for each group. Group A first named a set of pictures in L1, and then named the same set of pictures in L2. Group B first named pictures in L2 and then in L1. After the blocked naming tasks, both groups performed the mixed naming task described below. All participants began to learn English at approximately age 12, and had no experience of studying abroad.
Participants in the two groups were closely matched in age, language proficiency level (measured by self assessments provided in a language history questionnaire and by performance on the Waters and Caplan (1996) Reading Span task conducted in English), and executive control (measured by performance of a Simon task). An independent-samples t test showed that there was no significant difference between the ages of the participants in each group (mean: 21.4 vs. 22.8 years), t (22) = −1.45, p > 0.1. According to the outcome of a three-way ANOVA on the self- rating scores (mean scores on a 10 point scale – Group A: L1 reading 8.8, writing 8.2, speaking 8.6, listening 8.8; L2 reading 6.8, writing 6.1, speaking 5.8, listening 6.3; Group B: L1 reading 7.8, writing 7.7, speaking 7.8, listening 8.1; L2 reading 5.9, writing 5.4, speaking 4.9, listening 5.8), both groups were more proficient in L1 than L2, F (1, 22) = 76.63, p < 0.001; but there was no significant difference in language proficiency between groups, F (1, 22) = 1.87, p > 0.1. In the reading span task, performance on sentence processing was measured along with sentence final word recall. There was no significant difference between how many words participants in each group recalled correctly (14.5% vs. 14.1%) or the correct judgment of sentence plausibility (36.8% vs.33.3%), t (22) < 11. In the Simon task, participants were instructed to judge whether a colored square was red or blue by pressing a button. The button for “blue” responses was on the left side of the response pad, while the button for “red” responses was on the right side. However, squares could appear on either side of the screen, leading to congruent trials (where the square of a given color appeared on the same side as the correct response button) and incongruent trials (where the square appeared on the side opposite to the appropriate response button). The difference between the incongruent trials and the congruent trials is termed the Simon Effect. There was no significant difference between the Simon effect for both groups in reaction time (RT: 40 ms vs. 43 ms), F (1, 22) < 1, or error rates (ER: 2.2% vs. 3.2%), F (1, 22) = 2.76, p > 0.1. These comparisons indicate that the two groups were well matched on both measures of language proficiency and cognitive control. All of the participants were L1 dominant bilinguals.
Materials
Seventy line drawings sampled from a wide range of semantic categories were selected as experimental stimuli. Another 10 pictures were used as practice items. A plus sign “+” presented on a white screen was used as the baseline. The ratio of pictures to baselines was: 5:4. The trial order was randomized, and item condition was counterbalanced across participants. Pictures were presented on either a red or blue background (see below).
Procedure
The experiment was carried out in an isolated room, free from external noises other than those associated with the fMRI scanning procedures. There were four runs: The first two runs were blocked naming, and the last two runs were mixed naming. Each run included the same set of pictures. In the blocked naming task, participants named pictures in L1 or L2 in separate runs. The order of the language presented first was counterbalanced such that Group A named pictures first in L1, while Group B named pictures first in L2. In the mixed naming runs, participants named successive pictures either in L1 or in L2 randomly according to the background color of the picture. The background color-language mapping was explained to participants verbally, and practice trials were used to verify that they understood the mapping approach. This mapping was counterbalanced across participants such that red could indicate either Chinese or English, depending on the participant. In the blocked naming condition, the background colors were kept the same as in the mixed naming condition. Thus, in the blocked naming condition the background color also alternated, but participants did not need to attend to the switches in color.
For each run, the same 70 pictures were used as the experimental stimuli, and another 56 plus signs ‘+’ were used as the baseline stimuli. Each picture or plus sign was presented for 1000 ms and then replaced by a blank screen for 2000 ms. Participants were instructed to name pictures as quickly and accurately as possible in a soft voice, but to remain silent when seeing a plus sign (i.e., baseline). Participants were instructed to say NO or BUZHIDAO depending on the language of output if they did not know a name. They were also instructed to minimize head, jaw and tongue movement when naming in the scanner. As in our previous studies (e.g., Misra et al., under review), participants were not pre-trained with the picture names to reduce repetition effects. Past studies using pre-training have assumed that the procedure will simply enhance performance but not differentially affect L1 and L2. Because it is well known that the less dominant language is likely to benefit more from repetition (e.g., Hernandez & Reyes, 2002), and because the goal of the present study was to examine language selection, we decided that it was better to tolerate some trials on which bilinguals would not know the picture’s name in L2 rather than to prime L2 in a way that would create bias. A short break was provided between runs.
Although participants made verbal responses to the pictures in the scanner, technical limitations did not allow these responses to be recorded. Therefore, behavioral data were collected again, outside of the scanner, about one month later, in a quiet, isolated room. After the behavioral picture naming experiment, participants completed the language history questionnaire, the Simon task, and the Reading Span task.
Data collection
All images were acquired using a 3T Siemens Sonata whole-body MRI scanner. Participants’ heads were secured to minimize movement. Functional scans were obtained using a single shot T2*-weighted gradient echo planar imaging (EPI) sequence. The following scan parameters were used: TR (repetition time) = 3000 ms, TE (echo time) = 30 ms, flip angle = 90°, field-of-view (FOV) = 200×200 mm2, matrix size = 64×64, and slice thickness/gap = 4 mm/0.8 mm. Thirty three contiguous axial slices were acquired to cover the whole brain at 128 time points. High-resolution, T1-weighted 3D images were also obtained. The following scan parameters were used: TR = 2530 ms, TE = 3.39 ms, flip angle = 7°, FOV = 256×256 mm2, matrix size =256×256, slice thickness = 1.33 mm, and number of slices = 128.
Imaging data analysis
Image processing and statistical analyses were performed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK) in conjunction with the ArtRepair software (Mazaika et al., 2007) implemented in MATLAB (The Mathworks Inc., Natick, Mass., USA). For each participant, the first two volumes in each run were discarded to allow magnetization to reach the equilibrium state. For the remaining functional images, slice timing correction was performed first to minimize differences in acquisition time between slices. The images were realigned to the first volume in the time series to correct for head motion. They were then normalized to the EPI template in SPM2, based on the Montreal Neurological Institute (MNI) stereotactic space, and then re-sampled with 2×2×2 mm spatial resolution. The images were spatially smoothed with a cubic Gaussian filter (8-mm full width at half-maximum).
To quantify the effect of head movement on the quality of the data, the data were inspected by using the ArtRepair toolbox for SPM2 (Mazaika et al., 2007), and the realignment parameters provided by the SPM2 motion correction procedure were examined before the general linear model (GLM) estimation. Of particular interest is the scan-to-scan (incremental) motion during the task (e.g., Barch et al., 1999), i.e., the change in position between two successive images. The movement parameters for extreme movements were also inspected by considering absolute movements, i.e., the displacement of a scan with respect to the first image in the time series. The criteria for inclusion were that a participant did not show absolute motion greater than the voxel size and incremental motion greater than 1 mm. All participants met the absolute motion inclusion criteria, but one participant did not meet the incremental motion inclusion criteria. As there were only 4 volumes with incremental motion greater than 1 mm, the data of this participant were kept in the following analyses after being corrected with ArtRepair. Individual volumes showing rapid inter-TR movements of greater than 0.5 mm were excluded via interpolation of the two nearest non-repaired volumes. Consequently, approximately 1.64% of total volumes for the 24 participants were corrected. Interpolated volumes were then partially de-weighted when first-level models were calculated on the repaired images (Mazaika et al., 2007).
Statistical analyses were performed by modeling different conditions (i.e., blocked naming in Chinese, mixed naming in Chinese, blocked naming in English, mixed naming in English) as explanatory variables within the context of the general linear model on a voxel-by-voxel basis. The analyses were performed individually for each participant. Using group analyses based on a random effects model, we first identified brain regions that showed a significant response to (a) mixed vs. blocked naming in Chinese and (b) mixed vs. blocked naming in English for all twenty-four participants.2 Only the clusters larger than 10 voxels (2×2×2 mm) activated above the height threshold of p < 0.05 (FWE corrected for multiple comparisons) were considered as significant. To detect the interaction effect between Language (L1/L2) and task (mixed naming/blocked naming), the mixed naming effect in the two languages was compared, i.e., [Chinese (mixed - blocked) vs. English (mixed - blocked)] and [English (mixed - blocked) vs. Chinese (mixed - blocked)]. To avoid false alarms from deactivations, the direct comparisons between languages were inclusively masked by activations in the first effect using a lenient uncorrected p < 0.001 threshold. Furthermore, to identify the global switching effect, we directly compared the activations across the entire brain of the participants in the different groups that named pictures in Chinese or English in different orders, using two-sample t-tests. Due to the reduced statistic power associated with the between-participant comparison, a more lenient threshold (p < 0.001 without further correction for clusters containing at least ten contiguous voxels) was used.
Results
Behavioral results
Participants’ responses during the behavioral data collection session were coded as correct using criteria that took into account the difficulty of producing an accurate name for items in both L1 and L2 without pre-training. Therefore, they were given credit for a correct response if they named the item correctly, if they used an appropriate category label for the item (e.g., naming a “coat” as “clothes”), or if they used a correct label with an L2 pronunciation error. Repetitions were also counted as correct as long as the name used fell into one of the previous categories. Response accuracy data for the 24 participants were analyzed with the assumption that similar accuracies would have been obtained in the original task. Reaction time data for five participants were not available due to equipment malfunction, but the timing data from those participants for whom it was available were also analyzed. The local and global switching effects were analyzed separately.
For the local switching effect, the 2 (task: mixed/blocked) * 2 (language: L1/L2) two-way ANOVAs on error rates found that the main effect of task was not significant, F (1, 23) < 1, but the main effect of language was significant, F (1, 23) = 28.99, p < 0.001. The interaction between task and language was also significant, F (1, 23) = 11.28, p < 0.005. Further paired t tests showed that mixed naming in L1 elicited more errors than blocked naming in L1 (5.24% vs. 2.5%), t (23) = 4.05, p < 0.005; however, there was no significant difference between blocked and mixed naming in L2 (14.76% vs. 13.15%), t (23) = 1.31, p > 0.2. The two-way ANOVAs on reaction times found that the main effect of task was significant, F (1, 18) = 10.25, p < 0.01, but the main effect of language was not significant, F (1, 18) = 2.88, p > 0.1. The interaction between the task and language was also significant, F (1, 18) = 122.47, p < 0.001. Further paired t tests showed that mixed naming in L1 was significantly slower than blocked naming in L1 (1145 ms vs. 929 ms, t (18) = 10.15, p < 0.001, however, mixed naming in L2 was faster than blocked naming in L2 (1034 ms vs. 1100 ms, t (18) = −2.12, p < 0.05. This result suggested that an asymmetric local switching effect was present.
The 2 (task order: first/second) * 2 (language: L1/L2) two-way ANOVAs on the global switching effects (Group A: L1 2.98% vs. L2 18.33%; Group B: L1 2.02% vs. L2 11.19%) found a significant main effect of language, F (1, 22) = 46.58, p < 0.001, suggesting that naming pictures in L2 elicited more errors. The main effect of task order trended towards significance, F (1, 22) = 3.33, p = 0.08. The interaction between language and task order was also marginally significant, F (1, 22) = 2.97, p = 0.099. The two-way ANOVAs on reaction times (Group A: L1 908 ms vs. L2 1083 ms; Group B: L1 952 ms vs. L2 1118 ms) only revealed a significant main effect of language, F (1, 17) = 33.81, p < 0.001, indicating that naming pictures in L1 was faster than naming pictures in L2.
Neuroimaging results: Local switching effect
The neuroimaging results for the local switching effect are summarized in Table 1 and Figures 1 and 2. Relative to blocked naming in Chinese (L1), mixed naming in L1 significantly activated frontal cortical areas including bilateral dorsal anterior cingulate gyri, and supplementary motor area (SMA), as well as the left precentral gyrus. Significant activations were also noted in posterior areas including bilateral cerebellum and several regions of the occipital lobe including bilateral superior occipital gyri, middle occipital gyri, inferior occipital gyri, fusiform gyri, lingual gyri, cuneus, and left precuneus. Additional areas of enhanced activation were noted in the parietal lobe in the left superior parietal gyrus and bilaterally in the postcentral gyri.
Table 1.
Brain activation in (1) mixed vs. blocked naming in Chinese and (2) mixed vs. blocked naming in English (p < 0.05, k > 10, FWE corrected).
| Comparison | Areas | BA | MNI coordinates (x, y, z) |
T value | ||
|---|---|---|---|---|---|---|
| Mixed vs. blocked naming in Chinese | L_SMA | 6/32 | −4 | 2 | 66 | 9.84 |
| R_SMA | 6 | 2 | 2 | 64 | 7.45 | |
| L_Anterior Cingulate Gyrus | 24/32 | −4 | 10 | 42 | 8.85 | |
| R_Anterior Cingulate Gyrus | 32 | 4 | 16 | 44 | 7.08 | |
| L_Precentral Gyrus | 6 | −50 | −2 | 44 | 8.39 | |
| L_Superior Occipital Gyrus | 17 | −8 | −98 | 5 | 12.43 | |
| R_Superior Occipital Gyrus | 17/18 | 22 | −99 | 7 | 9.90 | |
| L_Middle Occipital Gyrus | 17/18/19 | −16 | −100 | 0 | 12.96 | |
| R_Middle Occipital Gyrus | 18/19 | 32 | −90 | 15 | 8.55 | |
| L_Inferior Occipital Gyrus | 19/37 | −42 | −80 | −9 | 9.11 | |
| R_Inferior Occipital Gyrus | 18/19 | 31 | −80 | −9 | 8.68 | |
| L_Fusiform Gyrus | 18/19/37 | −24 | −76 | −12 | 12.61 | |
| R_Fusiform Gyrus | 18/19/37 | 36 | −55 | −19 | 10.21 | |
| L_Lingual Gyrus | 17/18/19 | −22 | −76 | −13 | 11.69 | |
| R_Lingual Gyrus | 17/18 | 10 | −82 | 0 | 15.63 | |
| L_Superior Parietal Gyrus | 7 | −16 | −72 | 52 | 7.95 | |
| L_Precuneus | 7 | −6 | −76 | 44 | 8.53 | |
| L_Cuneus | 19 | −8 | −79 | 42 | 7.02 | |
| R_Cuneus | 17 | 19 | −98 | 8 | 8.92 | |
| L_Postcentral Gyrus | 4/6/43/48 | −50 | −7 | 41 | 7.82 | |
| R_Postcentral Gyrus | 4 | 52 | −8 | 32 | 7.51 | |
| L_Cerebellum | −6 | −98 | 2 | 14.34 | ||
| R_Cerebellum | 12 | −91 | 2 | 12.80 | ||
| Mixed vs. blocked naming in English | L_Superior Medial Frontal Gyrus | 32 | −4 | 18 | 42 | 7.55 |
| L_SMA | 6/32 | 0 | 12 | 50 | 11.93 | |
| R_SMA | 6/32 | 2 | 1 | 66 | 9.64 | |
| L_Anterior Cingulate Gyrus | 24/32 | −4 | 14 | 44 | 8.96 | |
| R_Anterior Cingulate Gyrus | 32 | 7 | 18 | 41 | 7.50 | |
| L_Precentral Gyrus | 6 | −47 | −7 | 45 | 7.65 | |
| L_Superior Occipital Gyrus | 17 | −8 | −100 | 8 | 9.79 | |
| R_Superior Occipital Gyrus | 17/18 | 23 | −96 | 10 | 7.65 | |
| L_Middle Occipital Gyrus | 17/18 | −9 | −95 | 1 | 8.28 | |
| R_Middle Occipital Gyrus | 17/18 | 24 | −96 | 10 | 7.80 | |
| L_Inferior Occipital Gyrus | 18/19 | −25 | −81 | −11 | 9.50 | |
| R_Inferior Occipital Gyrus | 19 | 32 | −80 | −15 | 8.28 | |
| L_Fusiform Gyrus | 18/19/37 | −30 | −72 | −16 | 11.26 | |
| R_Fusiform Gyrus | 18/19/37 | 37 | −64 | −15 | 9.94 | |
| L_Lingual Gyrus | 17/18/19 | −26 | −80 | −12 | 9.30 | |
| R_Lingual Gyrus | 17/18 | 13 | −77 | −13 | 7.70 | |
| L_Cuneus | 17 | −6 | −99 | 13 | 8.38 | |
| R_Cuneus | 17/18 | 12 | −100 | 8 | 8.33 | |
| L_Postcentral Gyrus | 3/4/6/43/48 | −48 | −8 | 44 | 8.54 | |
| L_Cerebellum | −32 | −70 | −20 | 10.03 | ||
| R_Cerebellum | 26 | −62 | −26 | 12.37 | ||
Fig.1.
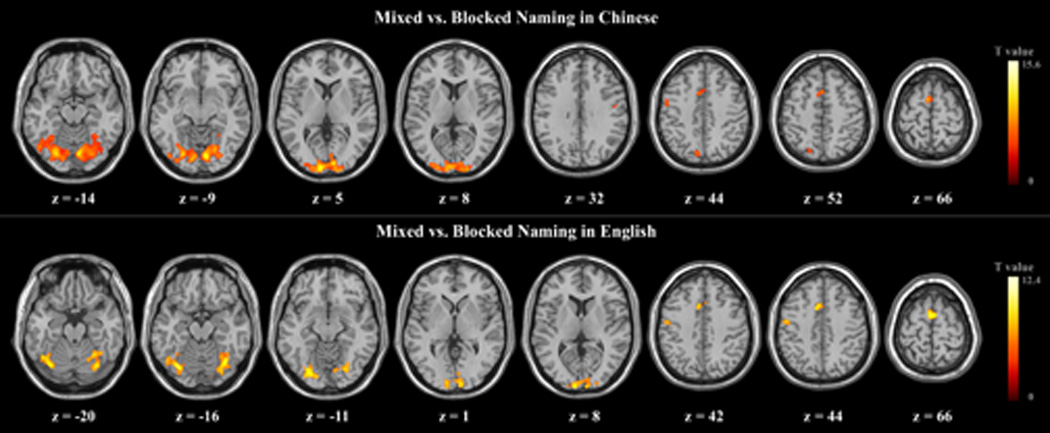
Group-averaged t-maps for mixed vs. blocked naming in Chinese (L1) and in English (L2).
Fig.2.
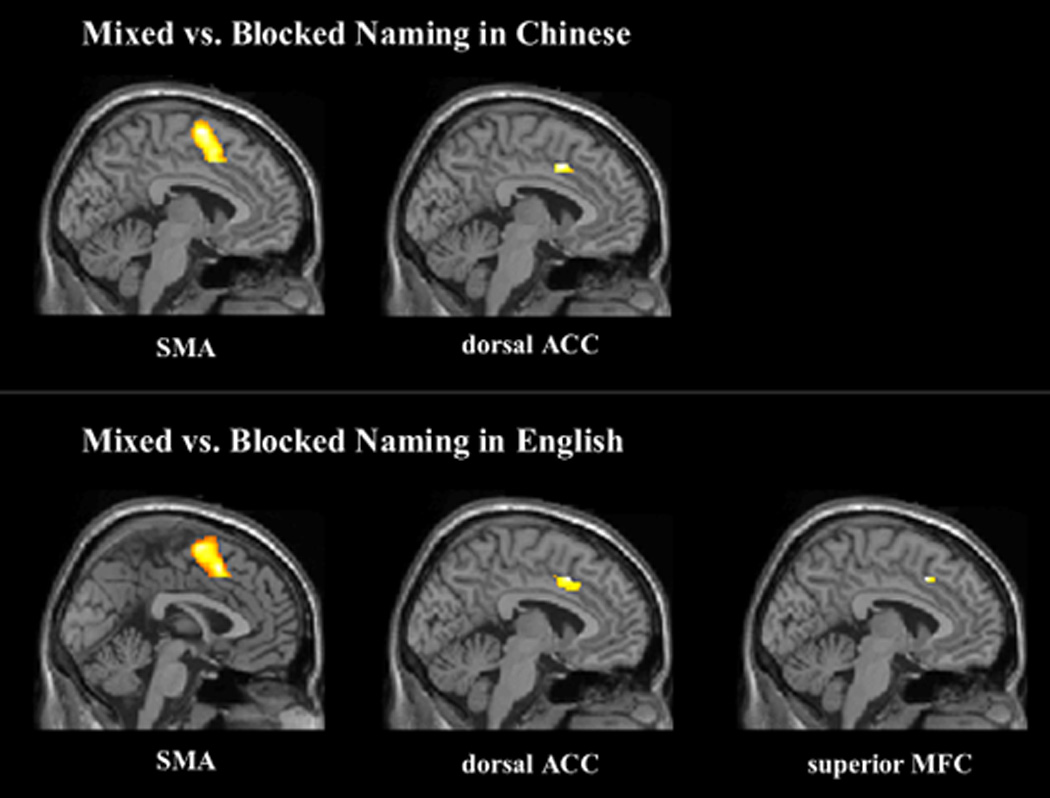
Activation in SMA and ACC for mixed vs. blocked naming in Chinese (L1) and activation in SMA, ACC and superior MFC for mixed vs. blocked naming in English (L2).
English (L2) mixed naming resulted in increased activation over L2 blocked naming in similar areas as observed for mixed versus blocked naming in L1. Specifically, enhanced activations for mixing naming were observed in frontal cortical areas including bilateral dorsal anterior cingulate gyri, and SMA, as well as in left precentral gyrus and superior medial frontal gyrus. Posterior activations were also again noted in bilateral cerebellum and bilaterally in occipital lobe regions including the cuneus, the superior occipital gyri, the middle occipital gyri, the inferior occipital gyri, the fusiform gyri, and the lingual gyri. Mixed vs. blocked naming in L2 also resulted in significant activation of left postcentral gyrus.
For the interaction between language and task, the two contrasts, i.e., [Chinese (mixed - blocked) vs. English (mixed - blocked)] and [English (mixed - blocked) vs. Chinese (mixed - blocked)], revealed no significant differences in any brain areas, suggesting that no significant interaction was found.
Neuroimaging results: Global switching effect
The neuroimaging results for the global switching effect are shown in Table 2 and Figure 3. Compared with naming pictures first in L1, naming pictures in L1 after naming pictures in L2 led to activation of the right postcentral gyrus and a series of left hemisphere areas including the middle frontal gyrus, the middle temporal gyrus, the precuneus, the inferior parietal gyrus, and the angular gyrus. In contrast, relative to naming pictures first in L2, naming pictures in L2 after naming pictures in L1 merely increased the activity of the right cuneus/precuneus.
Table 2.
Brain activation for direct comparison of two groups: (1) Chinese (L1) second vs. Chinese first and (2) English (L2) second vs. English first (p < 0.001, k > 10, uncorrected)
| Comparison | Areas | BA | MNI coordinates (x, y, z) |
T value | ||
|---|---|---|---|---|---|---|
| Chinese second vs. Chinese first | L_Middle frontal Gyrus | 10/45/46/47 | −36 | 46 | 8 | 5.53 |
| L_Middle Temporal Gyrus | 37 | −58 | −58 | 14 | 3.66 | |
| L_Precuneus | 7/19 | −12 | −70 | 36 | 3.67 | |
| R_Postcentral Gyrus | 3/4 | 38 | −34 | 66 | 4.27 | |
| L_Inferior Parietal Gyrus | 7/39/40 | −42 | −60 | 52 | 5.41 | |
| L_Angular Gyrus | 39/40 | −42 | −54 | 46 | 4.28 | |
| English second vs. English first | R_Cuneus/Precuneus | 23 | 18 | −66 | 24 | 4.45 |
Fig.3.

Direct comparison between groups: Activation network for Chinese (L1) second vs. Chinese first and Right cuneus/precuneus activation for English (L2) second vs. English first.
Discussion
Bilinguals commonly use only one of their two languages to communicate with others at a given time. However, previous behavioral and neuroimaging studies have provided converging evidence that both languages are activated during bilingual language production and that bilinguals need to inhibit the interference from activation of the nontarget language, especially when they speak their non-dominant language (for a review, see Kroll et al., 2008). Recent fMRI studies have attempted to identify the neural mechanism for bilingual inhibitory process. However, these studies have mainly evaluated inhibitory mechanisms using the language switching paradigm. While there are circumstances, such as when translating between languages, in which bilinguals must fluidly switch back and forth between languages, most interactions do not require this type of switching. It is not yet clear whether bilinguals allocate different levels of neural inhibition when their languages are used in distinct conversational contexts. It is therefore important to identify whether different types of neural inhibition are necessary for bilinguals to reach different goals.
The current study was designed to distinguish between the neural networks involved in local and global inhibition in bilingual word production. Specifically, local inhibition was investigated with the local switching effect (i.e., switching languages in the context of a mixed naming condition), whereas global inhibition was examined with the global switching effect (i.e., switching languages across naming blocks). As summarized and discussed below, distinct networks were observed to be engaged in these two contexts.
The neural basis of local inhibition in bilingual word production
In the mixed naming task, a visual cue instructed participants to name pictures in either L1 or L2. In the blocked naming task, participants named pictures in one of two languages in the entire block, although the same pictures were presented across blocks. Consistent with previous studies (e.g., Meuter & Allport, 1999), the behavioral results showed a significant asymmetric switching cost with a larger switching cost in the dominant L1, which further suggests the dominant L1 is inhibited to a larger degree when less proficient bilinguals produce words in their L2.
More critically, the fMRI results demonstrated that switching into L1 or L2 engages a largely similar neural network. Specifically, switching into either language in the mixed naming condition, as opposed to blocked naming, was associated with increased activation of the bilateral dorsal anterior cingulate (ACC) gyri, the bilateral supplementary motor area (SMA), left precentral gyrus, left postcentral gyri, bilateral cerebellum, bilateral superior occipital gyri, bilateral middle occipital gyri, bilateral inferior occipital gyri, bilateral fusiform gyri, bilateral cuneus, and bilateral lingual gyri. Switching into naming in L1 was also associated with increased activation of the left superior parietal gyrus, left precuneus, and right postcentral gyrus, while switching into naming in L2 engaged increased activation of the left medial superior frontal gyrus. However, direct contrasts between the two directions of switching revealed no significant differences in their neural substrates.
Increased activity of posterior regions such as occipital gyri, and cuneus may be due to the fact that participants needed to pay more visual attention to the background colors in the mixed picture naming condition, as the background color was a visual cue for the language of naming. However, in the blocked naming condition, although pictures were also presented against different background colors, the cues were redundant with the language of the blocked task so that participants did not need to consciously process the background colors. In addition, increased activation in lingual gyri, and fusiform gyri may be due to the fact that lexical candidates in both languages are activated for selection in the mixed naming condition, while enhanced activation during the mixing condition in the bilateral cerebellum (e.g., Ackermann et al., 1998) and postcentral gyri (Hillis et al., 2004) may have been related to articulatory processing. These results suggest that the switch trials may have required more effort to generate the picture names in the target language. These are not the main foci of the current study, so we will not discuss these results in detail.
Importantly, there was a significant difference between mixed and blocked naming in several regions related to attentional control in the frontal and parietal lobes. Specifically, the left medial superior frontal gyrus, bilateral SMAs, left precentral gyrus, the dorsal anterior cingulate gyri, the left precuneus, and the left superior parietal gyrus were involved in mixed naming relative to blocked naming. Activation of the SMA and precentral gyrus has been reported to be related to motor control and is reliably found in the go/nogo task (Talati & Hirsch, 2005). One possible reason for enhanced activation in these areas for switch trials in the current study, regardless of language, is that words in two languages are activated to a larger extent in the mixed naming task compared with the blocked naming task, and that bilinguals need to select the correct words in the target language by inhibiting the non-target verbal response in the mixed naming task. However, it is unclear why switching into L2 (English) engaged more anterior areas of the attentional network such as the left superior medial frontal gyrus, while switching into L1 (Chinese) involved in more activation in the posterior areas of the attentional network such as the left precuneus and left superior parietal gyrus. The precuneus, in the posterior region of the medial parietal cortex, may play an important role in higher cognitive functions such as episodic memory retrieval (for a review, see Cavanna & Trimble, 2006). Activation of the left superior parietal gyrus was previously found when participants switched between two manual responses (Rushworth et al., 2001) and when participants switched between verbal fluency tasks (Gurd et al., 2002). These results suggest that language proficiency (L2 vs. L1) or language similarity (English vs. Chinese) might modulate engagement of the frontal and parietal attention networks in bilingual language production although direct contrasts between the two directions of switching effects in the present study revealed no significant differences. Further studies may determine which factors play a role.
Enhanced activation in the dorsal ACC was also observed for switching versus blocked naming in both languages. There is some controversy about whether the ACC is related to conflict detection or error detection, or whether these two functions might be processed separately in distinct subdivisions in ACC (for reviews, see Bush et al., 2000; Rushworth et al., 2004). Typically, activation of a more dorsal level in the ACC has been found in cognitive tasks that involve response selection or conflict monitoring. For instance, in a study using the counting Stroop paradigm, participants were asked to report the number of words on the screen while ignoring word meaning (Bush et al., 1998). Compared with neutral trials that contained common animal words, the interference trials consisting of number words that were incongruent with the correct response (e.g., “two” written three times) increased the activation of the dorsal ACC. In the current experiment, the mixed naming task may involve more competition for response selection, leading to increased activation in bilateral dorsal ACC in mixed compared with blocked naming.
The abovementioned areas such as the dorsal ACC and the SMA have also been reported to be active in previous studies on lexical selection in bilingual language production (Abutabeli et al., 2008; Rodriguez et al., 2005; Wang et al., 2007), and further suggest that cognitive control is necessary for bilinguals to select the correct language in contexts in which they must make a decision between languages frequently. However, we did not observe activation of the left caudate and the supramarginal gyrus, which have been found in some other studies of language switching (e.g., Abutalebi et al., 2008). One possibility is that different types of switching effects were compared in different studies. Most previous studies (e.g., Price et al., 1999) have only compared neural activity for switching trials and nonswitching trials collapsed across languages, rather than separating the two languages for analysis. Wang et al. (2007) evaluated the two directions of switching effects separately, and only found activation of the right supramarginal gyrus in switching for L1, but no activation of the left caudate. Another possible reason that we did not find activation in the left caudate in our study is that the left caudate may be more likely to be activated in bilinguals who speak two similar languages. In other words, it may be easier to switch between two languages of different scripts such as Chinese and English, as opposed to languages such as Spanish and English, resulting in no significant activation of these brain areas. In addition, most previous studies used a covert naming task, rather than overt naming, and presented a visual cue prior to or after the picture, rather than simultaneously with picture onset. These task differences may have also impacted the observed results. Further studies will be required to investigate these issues directly.
The neural basis of global inhibition in bilingual word production
In the blocked naming task, participants named pictures in one of their two languages in the same block, but the order of the language of naming differed. One group of participants first named pictures in L1 and then in L2, while the other group first named pictures in L2 and then in L1. Behaviorally, there was no significant interaction between task order and language, but our findings revealed different patterns of brain activation when switching into L1 after a block of naming in L2 as compared to switching into L2 after an L1 naming block.
Compared with naming pictures in L1 first, naming pictures in L1 after naming pictures in L2 resulted in activation of a network of brain regions including left middle frontal gyrus, the left temporal gyrus, the left precuneus, the left inferior parietal gyrus, the left angular gyrus, and the right postcentral gyrus. Many prior studies have provided evidence for the role of these brain regions in cognitive control.
The DLPFC, which encompasses the middle frontal gyrus, has been associated with interference suppression in tasks such as the flanker task (Bunge et al., 2002), and is recruited during maintenance of task-relevant information (for review, see Miller & Cohen, 2001). The activation of this area when naming in L1 after a block of naming in L2 suggests its role in overcoming inhibition of L1 that is required in the previous L2 naming block to maintain a goal through the entire experimental session (e.g., naming pictures in L1 only).
Activation of the temporal and parietal cortices is often associated with visual attention. For example, in a task where participants were required to switch attention between local or global features of hierarchically organized letters (e.g., a large letter h consisting of small letters s), the temporal-parietal areas were activated (Fink et al., 1997). Left inferior parietal gyrus has also been observed to be activated in tasks requiring interference suppression, such as the flanker task (Bunge et al., 2002), and in cases where visual attentional set shifts are required, such as in the attention-switching paradigm (Rushworth et al., 2001). In the current blocked naming tasks, switching languages between blocks was endogenously driven. The activation of left middle temporal gyrus, left inferior parietal gyrus, and left precuneus might therefore be related to selecting the appropriate language to name the pictures.
Increased activation in the right postcentral gyrus may be due to recruitment of additional resources for articulatory processes when naming in L1 after L2. However, the role of the left angular gyrus during this task is less clear. Previously, this region has been hypothesized to be related to mapping visual inputs onto phonological representations (Horwitz et al., 1998). However, its role in language production has not been well addressed. The present finding that this area was activated in response to the block order effect in L1 might simply suggest that naming pictures in L1 after naming pictures in L2 recruits more processing resources, but further studies are needed to clarify the role of this region in language production.
The above results from the global switching effect for blocked naming in L1 suggest that when bilinguals speak the L2 over an extended block, there is inhibition of the L1 that is persistent to the next block, which lead to activation of the neural correlated associated with cognitive control. In contrast, naming a block of pictures in L2 after naming the same pictures in L1 enhanced only the activation of the right cuneus/precuneus relative to naming the same set of pictures first in L2. This result may be due to participants’ awareness of picture repetitions, as this area is related to visual processing.
The dorsal ACC was not differentially activated for either comparison in our blocked naming analysis. This result is consistent with previous findings that the ACC is activated only when participants need to make a decision between changing response sets or in situations with response conflicts (for a review, see Rushworth et al., 2004). In contrast, during the mixed naming task, in which participants needed to frequently select which language to produce, dorsal ACC activations were observed.
The general pattern of differential inhibition for the L1 relative to the L2 converges closely with the results obtained by Misra et al. (under review) when examining similar conditions using ERPs. That is, during blocked language naming, participants need to inhibit the other language in order to maintain the same goal through the experiment. For naming in the L2, the presence of a previous block of trials in L1 may have posed only minimal additional interference over the general situation of naming in one’s non-dominant language. However, if completing a block of trials in which the L2 name had been retrieved required inhibition of the L1, this may have lead to apparent interference when retrieving the L1 label in a subsequent block.
In summary, the present study attempted to investigate whether unbalanced bilinguals exhibited a neural dissociation in cognitive control in situations requiring rapid, frequent language switches, as opposed to situations which require sustained processing in one language versus the other. To our knowledge, the present findings are the first to demonstrate the neural correlates of a functional dissociation between two levels of inhibition in bilingual speakers. We found distinct patterns of neural activation for local inhibition as compared to global inhibition in bilingual word production. Specifically, our results suggest that the dorsal ACC and the SMA play important roles in local inhibition, while the dorsal left frontal gyrus and parietal cortex are important for global inhibition. However, it is still unknown whether these effects were specific for bilinguals or induced by the task requirements. In future studies it will be important to recruit monolinguals as a control group to exclude the latter possibility.
Research Highlights.
Neural evidence was found for bilingual inhibition in word production.
The dorsal ACC and SMA play important roles in local inhibition.
The dorsal left frontal and parietal cortex are important for global inhibition.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Number 30600179) to Taomei Guo. The writing of this article was supported in part by NIH Grant R01-HD053146 to Judith F. Kroll, Taomei Guo, and Maya Misra, and by NSF Grant OISE-0968369 from the Partnerships for International Research and Education (PIRE) Program to Judith F. Kroll. We thank Wenping You, Jingjing Guo, Min Chen, and Xiujun Li for their help with collecting data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note that these very low accuracy scores are a consequence of completing this challenging task in one’s L2. While reading span is generally considered a measure of working memory (see e.g., Waters & Caplan, 1996), in this context it is more accurately described as an L2 proficiency measure.
To identify whether the order in which a given language was named in the blocked sessions affects the results of local switching effect, we performed two-sample t-tests between the two groups (i.e., Group A (mixed vs. blocked naming) - Group B (mixed vs. blocked naming) for each language. The results revealed that no areas showed significant activation to the order effect in either Chinese or English naming, at the height threshold of p < 0.05 (corrected). Thus, the local switching effect was measured by comparing the blocked naming and mixed naming tasks collapsed across the two groups.
References
- Abutalebi J, Green D. Bilingual language production: The neurocognition of language representation and control. J. Neurolinguistics. 2007;20:242–275. [Google Scholar]
- Abutalebi J, Annoni JM, Zimine I, Pegna AJ, Seghier ML, Lee-Jahnke H, Lazeyras F, Cappa SF, Khateb A. Language control and lexical competition in bilinguals: An event-related fMRI study. Cere. Cortex. 2008;18:1496–1505. doi: 10.1093/cercor/bhm182. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Wildgruber D, Daumc I, Grodd W. Does the cerebellum contribute to cognitive aspects of speech production? A functional magnetic resonance imaging (fMRI) study in humans. Neurosci. Lett. 1998;247:187–190. doi: 10.1016/s0304-3940(98)00328-0. [DOI] [PubMed] [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: Empirical investigations of problems and potential solutions. Neuroimage. 1999;10:642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting Stroop: An interference task specialized for functional neuroimaging-validation study with functional MRI. Hum. Brain Mapp. 1998;6:270–282. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Christoffels IK, Firk C, Schiller NO. Bilingual language control: An event-related brain potential study. Brain Res. 2007;1147:192–208. doi: 10.1016/j.brainres.2007.01.137. [DOI] [PubMed] [Google Scholar]
- Costa A. Lexical access in bilingual production. In: Kroll JF, De Groot AMB, editors. Handbook of bilingualism: Psycholinguistic approaches. NY: Oxford University Press; 2005. pp. 308–325. [Google Scholar]
- Costa A, Caramazza A. Is lexical selection in bilingual speech production language specific? Further evidence from Spanish-English and English-Spanish bilinguals. Bilingualism Lang. Cogn. 1999;2:231–244. [Google Scholar]
- Costa A, Miozzo M, Caramazza A. Lexical selection in bilinguals: Do words in the bilingual’s two lexicons compete for selection? J. Mem. Lang. 1999;41:365–397. [Google Scholar]
- Costa A, Caramazza A, Sebastián-Gallés N. The cognate facilitation effect: Implications for the models of lexical access. J. Exp. Psychol. Learn. Mem. Cogn. 2000;26:1283–1296. doi: 10.1037//0278-7393.26.5.1283. [DOI] [PubMed] [Google Scholar]
- Costa A, Santesteban M, Ivanova I. How do highly proficient bilinguals control their lexicalization process? Inhibitory and language-specific selection mechanisms are both functional. J. Exp. Psychol. Learn. Mem. Cogn. 2006;32:1057–1074. doi: 10.1037/0278-7393.32.5.1057. [DOI] [PubMed] [Google Scholar]
- De Groot AMB, Christoffels IK. Language control in bilinguals: Monolingual tasks and simultaneous interpreting. Bilingualism Lang. Cogn. 2006;9:189–201. [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RSJ, Dolan RJ. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120:1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- Gollan TH, Ferreria VS. Should I stay or should I switch? A cost-benefit analysis of voluntary language switching in young and aging bilinguals. J. Exp. Psychol. Learn. Mem. Cogn. 2009;35:640–665. doi: 10.1037/a0014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DW. Mental control of the bilingual lexico-semantic system. Bilingualism Lang. Cogn. 1998;1:67–81. [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Marshall JC, Fink GR. Posterior parietal cortex is implicated in continous switching between verbal fluency tasks: An fMRI study with clinical implications. Brain. 2002;125:1024–1038. doi: 10.1093/brain/awf093. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Martinez A, Kohnert K. In Search of the language switch: An fMRI study of picture naming in Spanish-English bilinguals. Brain Lang. 2000;73:421–431. doi: 10.1006/brln.1999.2278. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: An fMRI Study. NeuroImage. 2001;14:510–520. doi: 10.1006/nimg.2001.0810. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Reyes I. Within- and Between-Language Priming Differ: Evidence From Repetition of Pictures in Spanish-English Bilinguals. J. Exp. Psychol. Learn. Mem. Cogn. 2002;28:726–734. doi: 10.1037//0278-7393.28.4.726. [DOI] [PubMed] [Google Scholar]
- Hermans D, Bongaerts T, de Bot K, Schreuder R. Producing words in a foreign language: Can speakers prevent interference from their first language? Bilingualism Lang. Cogn. 1998;1:213–229. [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Pro. Natl. Acad. Sci. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Carr TH, Cao Y. Comparing cortical activations for silent and overt speech using event-related fMRI. Hum. Brain Mapp. 2001;15:39–53. doi: 10.1002/hbm.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson GM, Swainson R, Cunnington R, Jackson SR. ERP correlates of executive control during repeated language switching. Bilingualism Lang. Cogn. 2001;4:169–178. [Google Scholar]
- Kroll JF, Bobb SC, Misra M, Guo TM. Language selection in bilingual speech: Evidence for inhibitory processes. Acta Psychologica. 2008;128:416–430. doi: 10.1016/j.actpsy.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JF, Bobb S, Wodniecka Z. Language selectivity is the exception, not the rule: Arguments against a fixed locus of language selection in bilingual speech. Bilingualism Lang. Cogn. 2006;9:119–135. [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, Reiss A, Glover G. Artifact repair for fMRI data from high motion clinical participants. Poster presented at 13th Annual Meeting of the Organization for Human Brain Mapping; Chicago, IL. 2007. [Google Scholar]
- Meuter RFI, Allport A. Bilingual language switching in naming: Asymmetrical costs of language selection. J. Mem. Lang. 1999;40:25–40. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Ann. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Misra M, Guo T, Bobb SC, Kroll JF. Electrophysiological evidence for global inhibition in bilingual word production. under review. [Google Scholar]
- Price CJ, Green D, von Studnitz RA. Functional imaging study of translation and language switching. Brain. 1999;122:2221–2236. doi: 10.1093/brain/122.12.2221. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Van der Lugt A, Rotte M, Britti B, Heinze HJ, Munte TF. Second language interferes with word production in fluent bilinguals: Brain potential and functional imaging evidence. J. Cogn. Neurosci. 2005;17:422–433. doi: 10.1162/0898929053279559. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, De Diego Balaguer R, Munte TF. Executive control in bilingual language processing. Lang. Learn. 2006;56:133–190. [Google Scholar]
- Rushworth MFS, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. J. Neurosci. 2001;21:5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Talati A, Hirsch J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “ when,” and “where” related information: An fMRI study. J. Cogn. Neurosci. 2005;17:981–993. doi: 10.1162/0898929054475226. [DOI] [PubMed] [Google Scholar]
- Verhoef K, Roelofs A, Chwilla DJ. Role of inhibition in language switching: Evidence from event-related brain potentials in overt picture naming. Cognition. 2009;110:84–99. doi: 10.1016/j.cognition.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xue G, Chen C, Xue F, Dong Q. Neural bases of asymmetric language switching in second-language learners: An ER-fMRI study. NeuroImage. 2007;35:862–870. doi: 10.1016/j.neuroimage.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Waters GS, Caplan D. The measurement of verbal working memory capacity and its relation to reading comprehension. Q. J. Exp. Psychol. 1996;49A:51–79. doi: 10.1080/713755607. [DOI] [PubMed] [Google Scholar]


