Abstract
Diabetes increases the risk as well as the poor outcome of stroke. Matrix metalloprotease (MMP) activation disrupts blood-brain barrier integrity after cerebral ischemia. We have previously shown that type 2 diabetes promotes remodeling of middle cerebral arteries (MCA) characterized by increased media:lumen (M/L) ratio and MMP activity in an endothelin (ET)-1-dependent manner in the Goto-Kakizaki (GK) rat model. In the present study, we examined the effects of ET-1-mediated vascular remodeling on neurovascular damage following cerebral ischemic injury in GK rats 5 and 12 weeks after the onset of diabetes. The MCA structure, cerebral perfusion as well as infarct size, and hemorrhage were measured in control and diabetic rats subjected to transient MCA occlusion. M/L ratio was increased after 12 but not 5 weeks of diabetes. The baseline cerebral perfusion was lower and the infarct volume was smaller in diabetic rats at both age groups. The incidence of hemorrhagic transformation was higher after 5 weeks of diabetes as compared 12 weeks or the control groups. These findings provide evidence that ET-1 mediated cerebrovascular remodeling does not worsen the neurovascular damage of ischemic brain injury in diabetes. It is possible that this early remodeling response is compensatory in nature to regulate vascular tone and integrity especially when ischemia is layered on diabetic vascular disease.
Keywords: ET-1, remodeling, diabetes, cerebral ischemia, Goto-Kakizaki rats
Introduction
Diabetes raises the risk of experiencing heart disease and stroke by 2–4 fold. These life-threatening outcomes account for nearly 65% of all diabetes-related morbidity (ADA, 2009). Rather than merely an endocrine disorder, diabetes is increasingly described as a vascular disease. Thus, the diabetic vasculature becomes a key component in mediating these pathological processes.
Vascular endothelium plays a critical role in mediating vascular tone, permeability, coagulation, and smooth muscle growth. In cerebral circulation, changes of the endothelial function may be detrimental and could contribute to cerebrovascular disease. Studies have demonstrated endothelial damage in early diabetic process indicating that the endothelium could be an early attack target in diabetes (Tooke, 2000). Plasma ET-1 levels are elevated in both Type 1 and Type 2 diabetes as well as in experimental models of this disease (Collier et al., 1992; Haak et al., 1992; Takahashi et al., 1990; Takeda et al., 1991). ET-1 has been shown to mediate cerebrovascular dysfunction in animal models of diabetes (Alabadi et al., 2004; Harris et al., 2008; Matsumoto et al., 2004). We also found that diabetes promotes remodeling of middle cerebral arteries (MCA) characterized by increased media/lumen (M/L) ratio and matrix metalloprotease-2 (MMP-2) activity in an ET-1-dependent manner (Harris et al., 2005b). Since cerebral perfusion is related to the fourth power of vessel radius (Paulson et al., 1990), even a small decrease in lumen diameter may complicate perfusion under normal conditions and more so in ischemia/reperfusion injury. Whether and to what extent ET-1-mediated remodeling impacts the severity of acute cerebral ischemia in diabetes remains unknown.
Diabetes increases stroke risk and stroke related mortality, severity and rate of recurrent stroke (AHA, 2007; Barber et al., 2004; Kernan and Inzucchi, 2004; Leys et al., 2002; Luscher et al., 2003; Weimar et al., 2005). Several laboratories have demonstrated that MMP expression, mainly MMP-2 and MMP-9, increases after permanent or temporary focal cerebral ischemia (del Zoppo and Mabuchi, 2003; del Zoppo et al., 1998; Heo et al., 1999; Montaner et al., 2001; Romanic et al., 1997; Rosenberg et al., 1998). Studies, including our own, showed that ET-1 mediates MMPs activation and subsequent vascular remodeling (Harris et al., 2005b; Murray et al., 2004). In light of several reports that ET-1 overexpression leads to increased edema (Leung et al., 2009; Lo et al., 2005) and that ETA receptor antagonism reduces ischemic brain damage (Dawson et al., 1999; Gupta et al., 2005; Matsuo et al., 2001), we hypothesized that ET-1-mediated cerebrovascular remodeling augments neurovascular damage following cerebral ischemic injury in diabetes.
Materials and methods
Animals
All experiments were performed on male control Wistar (Harlan, Indianapolis, IN) and diabetic Goto-Kakizaki (GK, in-house bred, derived from the Tampa colony) rats. GK rats became hyperglycemic around 6 weeks of age and all studies were performed 5 or 12 weeks after the onset of diabetes. Weight-matched Wistar rats were used as controls. The animals were housed at the Medical College of Georgia animal care facility, approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. During housing, water consumption, weight, and blood glucose and pressure measurements were performed twice weekly. Animals were housed in individual cages, maintained in a 12 hr light-dark cycle and fed standard rat chow and tap water ad libitum, until euthanasia. Glucose measurements were taken from the tail vein and measured on a commercially available glucose meter (AccuChek, Roche, Indianapolis, IN). Plasma ET-1 level was measured by enzyme-linked immunoassay as previously described (Sachidanandam et al., 2008).
Vascular structure
MCA was cannulated and pressure fixed in methanol-free 4% paraformaldehyde in phosphate buffered solution using the quick-transfer chamber (Living Systems Instrumentation, Burlington, VT), where vessels were maintained at a constant intraluminal pressure (80 mmHg) in calcium-free Krebs-HEPES buffer for 30 min and then stored in the same solution at 4°C. This procedure corrected for variations in vascular structure due to inconsistencies in manual perfusion. Vessels were embedded in paraffin, sectioned at 4 μm, and subjected to Masson trichrome staining. Images were captured and wall thickness, lumen, and outer diameter were measured from Masson stained cross sections using SPOT software (Diagnostic Instruments, Sterling Heights, MI).
Experimental cerebral ischemia
All animals were anesthetized with 2% isoflurane in 30% oxygen via inhalation. Focal cerebral ischemia was induced using the intraluminal suture MCAO model as previously reported (Ergul et al., 2007; Harris et al., 2005a). The right MCA was occluded with a 19–21 mm 4-0 surgical nylon filament, which was introduced from the external carotid artery lumen into the internal carotid artery to block the origin of the MCA. A significant drop in cerebral perfusion as measured by scanning laser Doppler imaging system (Perimed, North Royalton, OH) indicated successful occlusion of the MCA. The suture was removed after 3 hours of occlusion and measurement of cerebral perfusion was repeated to determine whether the flow was restored after reperfusion which appeared to be similar in both groups. The body temperature was maintained at 37.5°C by a rectal probe and heating pad system. At 24 h after occlusion, cerebral perfusion was evaluated with scanning laser Doppler again and animals were sacrificed. Brains were removed and immediately sliced in the coronal plane with 2 mm intervals. Section images were scanned before and after 2,3,5-triphenyltetrazolium chloride (TTC) staining of fresh slices. The infarct size and intracerebral hemorrhage was evaluated as previously described (Ergul et al., 2007). Hemorrhagic transformation (HT) was defined as the presence of macroscopic bleeding in coronal brain sections prior to staining in a binary fashion.
Measurement of cerebral perfusion
The cerebral perfusion was measured by the scanning laser Doppler imaging system (PeriScan PIM 3 System) (Cho et al., 2009). In brief, the top of skull was exposed by a median incision of the skin after the animal was anesthetized with 2% isoflurane inhalation. The laser beam was directed at the skull surface by a moving-mirror system in the scanner without tissue contact. The scanner was positioned to scan a 1.5 × 1.5 cm area (1600 detection points) covering the crosspoint of coronal and sagittal sutures. In this system, a built-in photo detector detects the reflected light from moving blood cells within 0.5 cm of the cortical surface and a color coded image is acquired based on the concentration and mean velocity of these blood cells using the LDPIwin software (Perimed, North Royalton, OH). The baseline perfusion is presented as the pixel intensity in the image (arbitrary units/mm2), whereas the perfusion after MCAO is shown as percentage decrease of baseline and the perfusion at 24 h after reperfusion is shown as percentage change of perfusion after MCAO.
Data analysis
The profile of physiological parameter changes over time was analyzed for group differences (control versus diabetic) using a repeated measures ANOVA in which the interaction of group by time was the test of interest. A Tukey adjustment was used for the post hoc comparison of the groups at each time point. Two-way ANOVA (disease and age) with a post-hoc Bonferroni analysis was done to compare cerebral perfusion, infarct size and HT between groups. Effects were considered statistically significant at p < 0.05. GraphPad Prism 5 software was used for all statistical analysis. Results are expressed as the means ± standard error (SEM).
Results
Animal data
Metabolic parameters after 5 or 12 weeks of diabetes and weight-matched control animals are summarized in Table 1. GK animals displayed significantly elevated blood glucose and plasma ET-1 levels at both age groups (p<0.0001).
Table 1.
Physiological parameters of control and diabetic animals.
| Wistar 5 wk (n=8) | GK 5 wk (n=8) | Wistar 12 wk (n=3) | GK 12 wk (n=7) | |
|---|---|---|---|---|
| BG (mg/dl) | 99 ± 4 | 155 ± 10* | 106 ± 5 | 181 ± 15* |
| BW (g) | 270 ± 5 | 257 ± 6 | 410 ± 12** | 391 ± 5** |
| Plasma ET-1 (fmol/ml) | 0.3 ± 0.01 | 0.6 ± 0.06* | 0.4 ± 0.02 | 1.4 ± 0.1* |
p<0.001 vs. Wistar,
p<0.001 vs. 5 wk after diabetes.
BG: blood glucose; BW: body weight.
Vascular structure
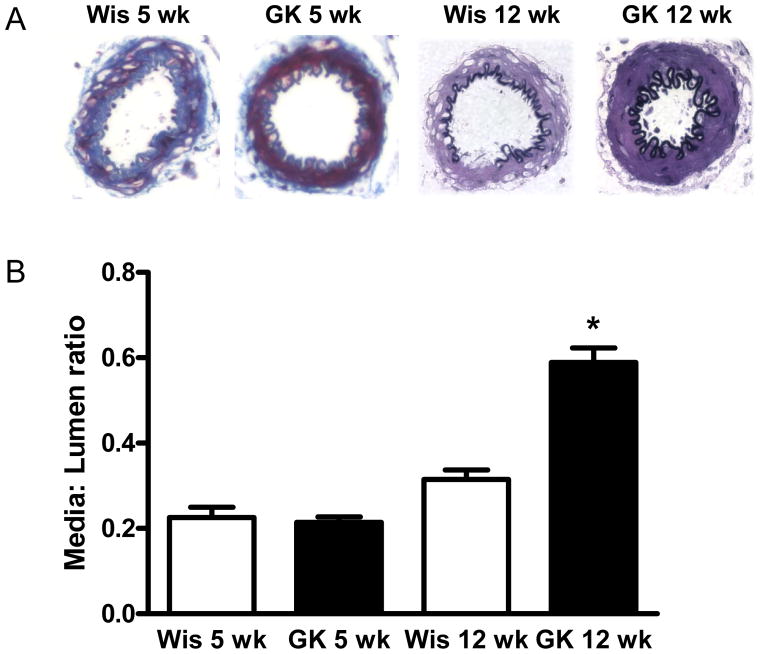
Masson staining of MCA cross sections showed that diabetic rats had medial thickening compared to controls after 12 but not 5 weeks of diabetes, although total vessel size was not different. This indicated encroachment of the media into the lumen, thereby causing higher M/L ratios (Fig 1B). Furthermore, increased collagen staining was seen in the media and adventitia in diabetes, as evidenced by deep purple staining by Masson staining in the cross-sections from GK rats (Fig 1A).
Fig 1.
The effect of the duration of diabetes on MCA morphology. MCA sections were analyzed for morphological changes and collagen deposition by Masson trichrome staining and representative images are given in panel A. 12 but not 5 weeks of diabetes induced significant collagen deposition and medial thickening. *p<0.001 vs Wistar or GK 12 wk.
Cerebral perfusion
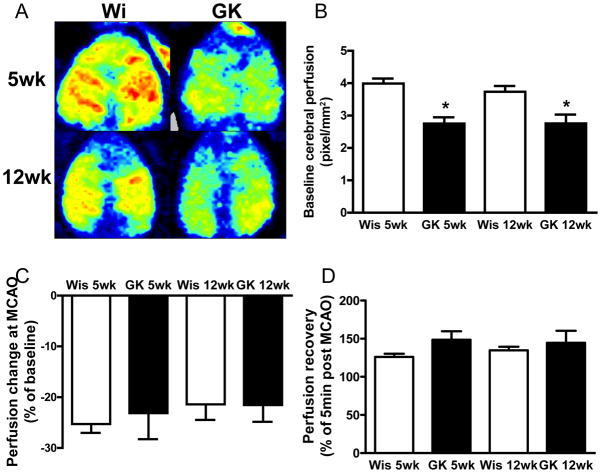
To determine whether and to what extent the cerebral vascular remodeling affects the blood supply to the brain, the baseline cerebral perfusion was measured with a scanning laser Doppler imaging system. Compared to the control groups, there was approximately a 25% decrease in cerebral perfusion in both age groups of diabetic rats (Fig 2B). However, the reduction in cerebral perfusion after MCAO (Fig 2C) and recovery after reperfusion (Fig 2D) was similar across the groups.
Fig 2.
Baseline cerebral perfusion was significantly lower in diabetic rats than that of control rats. Representative images of scanning laser Doppler imaging system (A) and summary of cerebral perfusion (B) in each group. Percent drop in perfusion during MCAO (C) and recovery after 24 h reperfusion (D) was similar across the groups. *p<0.05 vs. Wistar.
Infarct volume and hemorrhagic transformation
The average infarct size was smaller in diabetic animals than that of control group (p<0.0001) but age did not have an effect (Fig 3B). Duration of diabetes was important for the development of HT such that the incidence of bleeding was the highest in younger diabetic animals followed by older diabetic and then control rats (Fig 3C).
Fig 3.
Temporary MCAO causes hemorrhagic transformation after 5 but not 12 weeks of diabetes. Representative images of brain sections that show HT and infarct area as determined by TTC staining are shown in panel A. Summary of infarct volume of contralateral hemisphere and incidence of HT are given in panels B and C. *p<0.05 vs. Wistar.
Discussion
This study was designed to evaluate how diabetes-induced changes in the cerebrovascular structure influence the neurovascular outcomes of ischemia/reperfusion injury over the progression of the disease. Previously, we have made two important observations. First, we reported increased vascular damage as evidenced by greater incidence of hemorrhagic transformation following transient focal ischemia after 5 weeks of hyperglycemia in type 2 diabetic GK rats (Ergul et al., 2007). Although there was no difference in MCA morphology between control and diabetic animals, vascular remodeling process was initiated in these animals as evidenced by increased vascular MMP-2 activity. Second, we also found that diabetes promotes medial thickening and collagen deposition in the same vascular bed after 12 weeks of diabetes and this was significantly reduced when animals were treated with an ETA receptor antagonist (Harris et al., 2005b) suggesting an important role of ET-1 in diabetes-induced vascular remodeling. Building upon these past findings, the present study tested the hypothesis that ET-1-mediated thickening of the vessel wall augments neuronal and vascular ischemic damage in diabetes. Contrary to our hypothesis, this remodeling did not worsen the neurovascular damage after focal cerebral ischemia.
The relative risk of cerebrovascular disease or stroke is 2 to 6-fold higher in diabetes (AHA, 2009; Leys et al., 2002). Diabetes also increases stroke related mortality, severity and rate of recurrent stroke (Kernan and Inzucchi, 2004; Luscher et al., 2003) and predicts early neurologic deterioration following ischemic stroke (Barber et al., 2004; Weimar et al., 2005). Patients with diabetes have an increased number of additional atherosclerotic risk factors including hypertension, obesity, and hyperlipidemia, moreover diabetes is a stroke risk factor independent of these comorbidities (Folsom et al., 1999; Stegmayr and Asplund, 1995). Experimental evidence from mainly streptozotocin (STZ)-induced short-term hyperglycemia showed increased infarct, edema and hemorrhage in hyperglycemic animals after reperfusion (de Courten-Myers et al., 1988; de Courten-Myers et al., 1992; de Courten-Myers et al., 1989; de Courten-Myers et al., 1990; Ergul et al., 2009; Kamada et al., 2007; Martini and Kent, 2007; Quast et al., 1997; Wagner et al., 1992). Marked blood-brain-barrier (BBB) disruption and edema was also reported in mild to transient severe hyperglycemia after temporary and permanent arterial occlusion (Ennis and Keep, 2007). In contrast to these studies, we recently reported relatively smaller infarct size but a greater incidence of hemorrhagic transformation in a lean model of type 2 diabetes (Ergul et al., 2007), and despite smaller infarcts functional outcome was worse in these animals most likely due to increased hemorrhage (Elewa et al., 2009). Diabetic GK animals exhibited increased neovascularization as evidenced by increased tortuosity, vascular density and collateral number which may be altogether contributing to smaller infarcts but increased bleeding (Li et al., 2010). Since these particular studies employed relatively younger animals shortly (5 weeks) after the spontaneous onset of diabetes, we hypothesized that neurovascular damage will be greater after 12 weeks of diabetes when there is significant remodeling with increased M/L ratio. Much to our surprise, infarct size was still smaller in the diabetic group and HT was less than that observed at 5-weeks of diabetes. Given that cerebral vessels have the autoregulatory properties to develop a state of tone that allows them to increase or decrease their diameter to regulate cerebral perfusion, it is possible that increased wall thickness is compensatory response to regulate vascular tone and integrity.
ET-1 is important for both function and structure of the cerebral blood vessels. Some studies, including our own, have shown ET-1 contributes to cerebrovascular dysfunction in animal models of diabetes (Alabadi et al., 2004; Harris et al., 2008; Matsumoto et al., 2004). In these studies, contractile response of basilar arteries to ET-1 was increased. Furthermore, endothelium-dependent relaxation was impaired and ET receptor antagonism restored the dilatory response. In the current study, we found significantly decreased cerebral perfusion after 5 and 12 weeks of diabetes in GK rats. Increased ET-1-mediated vasoconstriction may contribute to this finding (Harris et al., 2008). We have also shown that ETA antagonism restored the dysregulated MMP activity and prevented collagen deposition and medial thickening of MCAs (Harris et al., 2005b). In the current study, we found increased plasma ET-1 levels at the early stage of diabetes. Plasma and tissue MCA ET-1 increase was continuously seen in older animals, which was associated with increased MCA wall thickness and M/L ratio (Harris et al., 2005b). These results suggest that the ET system is dysregulated and contributes to the cerebrovascular remodeling in this animal model. ET-1 also plays an important role in ischemic brain injury as indicated by clinical and experimental studies that have shown increased ET-1 level after cerebral ischemic injury (Barone et al., 1994; Franceschini et al., 2001). Overexpression of ET-1 in endothelial cells and astrocytes causes larger infarct volume and increased cerebral edema (Leung et al., 2004; Lo et al., 2005). Several laboratories demonstrated that acute ETA receptor antagonism reduces ischemic brain damage and restores microvascular perfusion (Dawson et al., 1999; Gupta et al., 2005; Matsuo et al., 2001). When used in combination with tPA, blockade of ETA receptors also reduces hemorrhage (Zhang et al., 2008). In the present study, however, we found that rats that have an activated ET-1 system and develop ET-1-mediated medial thickening actually display less macroscopic bleeding. One likely explanation is that previous studies (Gupta et al., 2005; Matsuo et al., 2001; Zhang et al., 2008) used mainly healthy animals with the exception of one study that employed spontaneously hypertensive rats (Dawson et al., 1999). It is possible that preexisting vascular disease alters the relative role of ET-1 in ischemic injury. It also has to be noted that in the current study we did not employ acute ET receptor antagonism during ischemia/reperfusion injury but rather evaluated the neurovascular damage in rats that develop MCA remodeling in an ET-1-dependent manner. Further studies with acute use of selective or nonselective ET receptor antagonism during focal ischemia in the GK model are warranted.
In conclusion, type 2 diabetic GK rats show elevated plasma and tissue ET-1 levels (Harris et al., 2005b), increased wall thickness and M/L ratio after 12 weeks of hyperglycemia. This early remodeling response may be compensatory in nature to regulate vascular tone and integrity especially when ischemia is layered on diabetic vascular disease.
Acknowledgments
This work was supported by grants from NIH (DK074385) and American Heart Association Established Investigator Award to Adviye Ergul.
References
- Alabadi JA, Miranda FJ, Llorens S, Centeno JM, Marrachelli VG, Alborch E. Mechanisms underlying diabetes enhancement of endothelin-1-induced contraction in rabbit basilar artery. Eur J Pharmacol. 2004;486:289–296. doi: 10.1016/j.ejphar.2004.01.005. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. National diabetes fact sheet. 2009 http://www.diabetes.org/diabetes-statistics.jsp.
- American Heart Association. Heart and stroke statistical update. 2009 www.americanheart.org.
- Barber M, Wright F, Stott DJ, Langhorne P. Predictors of early neurological deterioration after ischaemic stroke: a case-control study. Gerontology. 2004;50:102–109. doi: 10.1159/000075561. [DOI] [PubMed] [Google Scholar]
- Barone FC, Globus MY, Price WJ, White RF, Storer BL, Feuerstein GZ, Busto R, Ohlstein EH. Endothelin levels increase in rat focal and global ischemia. J Cereb Blood Flow Metab. 1994;14:337–342. doi: 10.1038/jcbfm.1994.41. [DOI] [PubMed] [Google Scholar]
- Cho JK, Moon DJ, Kim SG, Lee HG, Chung SP, Yoon CJ. Relationship between healing time and mean perfusion units of laser Doppler imaging (LDI) in pediatric burns. Burns. 2009;35:818–823. doi: 10.1016/j.burns.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Collier A, Leach JP, McLellan A, Jardine A, Morton JJ, Small M. Plasma endothelin-like immunoreactivity levels in IDDM patients with microalbuminuria. Diabetes Care. 1992;15:1038–1040. doi: 10.2337/diacare.15.8.1038. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Sugano H, McCarron RM, Hallenbeck JM, Spatz M. Endothelin receptor antagonist preserves microvascular perfusion and reduces ischemic brain damage following permanent focal ischemia. Neurochem Res. 1999;24:1499–1505. doi: 10.1023/a:1021139713026. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers G, Myers RE, Schoolfield L. Hyperglycemia enlarges infarct size in cerebrovascular occlusion in cats. Stroke. 1988;19:623–630. doi: 10.1161/01.str.19.5.623. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Holm P, DeVoe G, Schmitt G, Wagner KR, Myers RE. Hemorrhagic infarct conversion in experimental stroke. Ann Emerg Med. 1992;21:120–126. doi: 10.1016/s0196-0644(05)80144-1. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Wagner KR, Myers RE. Fatal strokes in hyperglycemic cats. Stroke. 1989;20:1707–1715. doi: 10.1161/01.str.20.12.1707. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Myers RE, Wagner KR. Effect of hyperglycemia on infarct size after cerebrovascular occlusion in cats. Stroke. 1990;21:357–358. doi: 10.1161/01.str.21.2.357. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, von Kummer R, Hamann GF. Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998;65:1–9. doi: 10.1136/jnnp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, Fagan SC. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330:532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis SR, Keep RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab. 2007;27:1573–1582. doi: 10.1038/sj.jcbfm.9600454. [DOI] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Li W, Elgebaly MM, Bruno A, Fagan SC. Hyperglycemia, diabetes and stroke: Focus on the cerebrovasculature. Vascul Pharmacol. 2009 doi: 10.1016/j.vph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom AR, Rasmussen ML, Chambless LE, Goward G, Cooper LS, Schmidt MI, Heiss G. Prospective associations of fasting insulin, body fat distribution, and diabetes with rate of ischemic stroke. Diabetes Care. 1999;22:1077–1083. doi: 10.2337/diacare.22.7.1077. [DOI] [PubMed] [Google Scholar]
- Franceschini R, Gandolfo C, Cataldi A, Del Sette M, Rolandi A, Corsini G, Rolandi E, Barreca T. Twenty-four-hour endothelin-1 secretory pattern in stroke patients. Biomed Pharmacother. 2001;55:272–276. doi: 10.1016/s0753-3322(01)00059-2. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Briyal S, Sharma U, Jagannathan NR, Gulati A. Effect of endothelin antagonist (TAK-044) on cerebral ischemic volume, oxidative stress markers and neurobehavioral parameters in the middle cerebral artery occlusion model of stroke in rats. Life Sci. 2005;77:15–27. doi: 10.1016/j.lfs.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Haak T, Jungmann E, Felber A, Hillmann U, Usadel KH. Increased plasma levels of endothelin in diabetic patients with hypertension. Am J Hypertens. 1992;5:161–166. doi: 10.1093/ajh/5.3.161. [DOI] [PubMed] [Google Scholar]
- Harris AK, Elgebaly MM, Li W, Sachidanandam K, Ergul A. Effect of chronic endothelin receptor antagonism on cerebrovascular function in type 2 diabetes. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1213–1219. doi: 10.1152/ajpregu.00885.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Ergul A, Kozak A, Machado LS, Johnson MH, Fagan SC. Effect of neutrophil depletion on gelatinase expression, edema formation and hemorrhagic transformation after focal ischemic stroke. BMC Neurosci. 2005a;6:49. doi: 10.1186/1471-2202-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK, Hutchinson JR, Sachidanandam K, Johnson MH, Dorrance AM, Stepp DW, Fagan SC, Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005b;54:2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koizol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernan WN, Inzucchi SE. Type 2 diabetes mellitus and insulin resistance: Stroke prevention and management. Curr Treat Options Neurol. 2004;6:443–450. doi: 10.1007/s11940-004-0002-y. [DOI] [PubMed] [Google Scholar]
- Leung JW, Chung SS, Chung SK. Endothelial endothelin-1 over-expression using receptor tyrosine kinase tie-1 promoter leads to more severe vascular permeability and blood brain barrier breakdown after transient middle cerebral artery occlusion. Brain Res. 2009;1266:121–129. doi: 10.1016/j.brainres.2009.01.070. [DOI] [PubMed] [Google Scholar]
- Leung JW, Ho MC, Lo AC, Chung SS, Chung SK. Endothelial cell-specific over-expression of endothelin-1 leads to more severe cerebral damage following transient middle cerebral artery occlusion. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S293–300. doi: 10.1097/01.fjc.0000166277.70538.b0. [DOI] [PubMed] [Google Scholar]
- Leys D, Deplanque D, Mounier-Vehier C, Mackowiak-Cordoliani MA, Lucas C, Bordet R. Stroke prevention: management of modifiable vascular risk factors. J Neurol. 2002;249:507–517. doi: 10.1007/s004150200057. [DOI] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Chen AY, Hung VK, Yaw LP, Fung MK, Ho MC, Tsang MC, Chung SS, Chung SK. Endothelin-1 overexpression leads to further water accumulation and brain edema after middle cerebral artery occlusion via aquaporin 4 expression in astrocytic end-feet. J Cereb Blood Flow Metab. 2005;25:998–1011. doi: 10.1038/sj.jcbfm.9600108. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–451. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yoshiyama S, Kobayashi T, Kamata K. Mechanisms underlying enhanced contractile response to endothelin-1 in diabetic rat basilar artery. Peptides. 2004;25:1985–1994. doi: 10.1016/j.peptides.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Mihara S, Ninomiya M, Fujimoto M. Protective effect of endothelin type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke. 2001;32:2143–2148. doi: 10.1161/hs0901.94259. [DOI] [PubMed] [Google Scholar]
- Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, Monasterio J. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2767. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- Murray DB, Gardner JD, Brower GL, Janicki JS. Endothelin-1 mediates cardiac mast cell degranulation, matrix metalloproteinase activation, and myocardial remodeling in rats. Am J Physiol Heart Circ Physiol. 2004;287:H2295–2299. doi: 10.1152/ajpheart.00048.2004. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM, Hillman GR, Kent TA. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab. 1997;17:553–559. doi: 10.1097/00004647-199705000-00009. [DOI] [PubMed] [Google Scholar]
- Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone F. Matrix metalloproteinase expression increases after cerebral focal ischemia. Stroke. 1997;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- Sachidanandam K, Elgebaly MM, Harris AK, Hutchinson JR, Mezzetti EM, Portik-Dobos V, Ergul A. Effect of chronic and selective endothelin receptor antagonism on microvascular function in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2008;294:H2743–2749. doi: 10.1152/ajpheart.91487.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayr B, Asplund K. Diabetes as a risk factor for stroke. A population perspective. Diabetologia. 1995;38:1061–1068. doi: 10.1007/BF00402176. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Ghatei MA, Lam HC, O’Halloran DJ, Bloom SR. Elevated plasma endothelin in patients with diabetes mellitus. Diabetologia. 1990;33:306–310. doi: 10.1007/BF00403325. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Miyamori I, Yoneda T, Takeda R. Production of endothelin-1 from the mesenteric arteries of streptozotocin induced diabetic rats. Life Sci. 1991;48:2553–2556. doi: 10.1016/0024-3205(91)90611-e. [DOI] [PubMed] [Google Scholar]
- Tooke JE. Possible pathophysiological mechanisms for diabetic angiopathy in type 2 diabetes. J Diabetes Complications. 2000;14:197–200. doi: 10.1016/s1056-8727(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Kleinholz M, de Courten-Myers GM, Myers RE. Hyperglycemic versus normoglycemic stroke: topography of brain metabolites, intracellular pH, and infarct size. J Cereb Blood Flow Metab. 1992;12:213–222. doi: 10.1038/jcbfm.1992.31. [DOI] [PubMed] [Google Scholar]
- Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, Diener HC. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. 2005;62:393–397. doi: 10.1001/archneur.62.3.393. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang C, Zhang L, Roberts C, Lu M, Kapke A, Cui Y, Ninomiya M, Nagafuji T, Albala B, Zhang ZG, Chopp M. Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke. Stroke. 2008;39:2830–2836. doi: 10.1161/STROKEAHA.108.515684. [DOI] [PMC free article] [PubMed] [Google Scholar]