Abstract
Background
Leptospirosis and human immunodeficiency virus (HIV) infection are prevalent in many areas, including northern Tanzania, yet little is known about their interaction.
Methods
We enrolled febrile inpatients at two hospitals in Moshi, Tanzania, over 1 year and performed HIV antibody testing and the microscopic agglutination test (MAT) for leptospirosis. Confirmed leptospirosis was defined as ≥four-fold rise in MAT titer between acute and convalescent serum samples, and probable leptospirosis was defined as any reciprocal MAT titer ≥800.
Results
Confirmed or probable leptospirosis was found in 70 (8.4%) of 831 participants with at least one serum sample tested. At total of 823 (99.0%) of 831 participants had HIV testing performed, and 203 (24.7%) were HIV infected. Among HIV-infected participants, 9 (4.4%) of 203 had confirmed or probable leptospirosis, whereas among HIV-uninfected participants 61 (9.8%) of 620 had leptospirosis. Leptospirosis was less prevalent among HIV-infected as compared to HIV-uninfected participants [odds ratio (OR) 0.43, p=0.019]. Among those with leptospirosis, HIV-infected patients more commonly presented with features of severe sepsis syndrome than HIV-uninfected patients, but differences were not statistically significant. Among HIV-infected patients, severe immunosuppression was not significantly different between those with and without leptospirosis (p=0.476). Among HIV-infected adolescents and adults, median CD4 percent and median CD4 count were higher among those with leptospirosis as compared to those with other etiologies of febrile illness, but differences in CD4 count did not reach statistical significance (p=0.015 and p=0.089, respectively).
Conclusions
Among febrile inpatients in northern Tanzania, leptospirosis was not more prevalent among HIV-infected patients. Although some indicators of leptospirosis severity were more common among HIV-infected patients, a statistically significant difference was not demonstrated. Among HIV-infected patients, those with leptospirosis were not more immunosuppressed relative to those with other etiologies of febrile illness.
Key Words: HIV infection, Leptospirosis, northern Tanzania
Introduction
Leptospirosis is a widespread, neglected, poverty-associated zoonosis transmitted via direct or indirect exposure to leptospire-contaminated animal urine. Leptospirosis often remains undetected in resource-limited settings due to lack of availability of diagnostics and a low clinical index of suspicion. We recently described leptospirosis as a common etiology of febrile illness among hospitalized patients in northern Tanzania (Biggs et al. 2011). In that study, among febrile inpatients tested for leptospirosis, confirmed or probable leptospirosis was found in 8.4%, and 24.7% of study participants were infected with human immunodeficiency virus (HIV). Despite the prevalence of both leptospirosis and HIV in many areas, including northern Tanzania, little is known about their relationship.
Leptospirosis infection in patients with HIV/acquired immunodeficiency syndrome (AIDS) has been described with varying clinical severity (Jones and Kim 2001, Ganoza et al. 2005). Leptospirosis most commonly presents as an undifferentiated febrile illness, but the clinical presentation ranges from asymptomatic seroconversion to severe, fatal illness. In the general population, severe leptospirosis is estimated to occur in about 5–10% of cases (Levett 2001). However, in one review of five cases of HIV and leptospirosis co-infection, all presented with one or more manifestations of severe leptospirosis (Jones and Kim 2001). The question thus arises whether those with HIV are at increased risk of infection with leptospirosis and whether the clinical course of leptospirosis is more severe in those infected with HIV. We describe the characteristics of HIV and leptospirosis co-infected patients hospitalized with febrile illness in northern Tanzania.
Materials and Methods
Setting
The study was conducted at Kilimanjaro Christian Medical Centre (KCMC) and Mawenzi Regional Hospital (MRH) in Moshi, Tanzania. Together, these two hospitals serve as the main providers of hospital care in the Moshi area.
Study procedures and participants
Study methods have been described in detail elsewhere (Crump et al. 2011a, Crump et al. 2011b). Briefly, as part of a comprehensive study of the etiology of febrile illness in northern Tanzania, we prospectively enrolled adult and pediatric inpatients at KCMC and adult inpatients at MRH from September 17, 2007, through August 31, 2008. Eligible patients were ≥13 years old with an oral temperature of ≥38.0°C or age ≥2 months to <13 years with a history of fever in the past 48 h, an axillary temperature of ≥37.5°C or a rectal temperature of ≥38.0°C. A study team member performed a standardized clinical history and physical examination on all participants. Within 24 h of hospital admission, blood was drawn for aerobic blood culture, complete blood count, examination for blood parasites, HIV antibody and RNA testing, and acute serum archiving. At the time of discharge, a standardized form was completed documenting in-hospital management and clinical outcome. Participants were asked to return 4–6 weeks after study enrollment to submit a convalescent serum sample. Acute and convalescent serum samples were sent to the United States Centers for Disease Control and Prevention (CDC) for serologic analysis for leptospirosis.
Laboratory methods
Leptospirosis laboratory diagnosis was made using the standard microscopic agglutination test (MAT) performed at the CDC using a panel of antigens from 20 different Leptospira serovars (Dikken and Kmety 1978). Methods have been previously described in detail (Biggs et al. 2011).
HIV-1 antibody testing was done on whole blood using both the Capillus HIV-1/HIV-2 (Trinity Biotech, PLC, Bray, Ireland) and Determine HIV-1/HIV-2 (Abbott Laboratories, Abbott Park, IL, USA) rapid HIV antibody tests. The Capillus test was replaced with the SD Bioline HIV-1/HIV-2 test (version 3.0; Standard Diagnostics, Kyonggi-do, Korea) on March 4, 2008, after a change in Tanzania Ministry of Health HIV testing guidelines. If rapid tests were discordant, the sample was tested using enzyme-linked immunosorbent assay (ELISA; Vironostika Uni- Form II plus O Ab; BioMérieux, Durham, NC). If the ELISA was negative, no further testing was done. If the ELISA was positive, a western blot (Genetic Systems HIV-1 Western blot kit; Bio-Rad, Hercules, CA) was done to confirm the result (Mayhood et al. 2008). HIV-1 RNA PCR was done using the Abbott m2000 system RealTime HIV-1 assay (Abbott Laboratories) (Crump et al. 2009, Scott et al. 2011). Early infant diagnosis for those aged <18 months was performed by HIV-1 RNA PCR. The CD4+ T lymphocyte count (CD4 count) and percent were measured using the FACSCalibur system (Becton Dickinson, Franklin Lakes, NJ).
Study definitions
Confirmed leptospirosis was defined as a ≥four-fold rise in the agglutination titer between acute and convalescent serum samples (Centers for Disease Control and Prevention 1997). Probable leptospirosis was defined as any single reciprocal MAT titer ≥800 among those not meeting the case definition for confirmed leptospirosis (Faine 1982, Levett 2001, World Health Organization 2003). Confirmed and probable cases were combined for analysis. Being negative for leptospirosis was defined in participants with paired serum samples as a lack of ≥four-fold rise in agglutination titer between samples and reciprocal titers of <800 in both samples. Negative for leptospirosis was defined for participants with a single serum sample available as a reciprocal MAT titer <800.
Severe immunosuppression was defined as an adult with a CD4 count <100 cells/μL, a child age <1 year with a CD4 percent <25%, a child age 1–3 years with a CD4 percent <20, or a child age >3 years with a CD4 percent <15% (World Health Organization 2006). Sepsis syndrome was defined as the presence of two or more of the following: temperature >38.3°C or <36°C, heart rate >90 beats/min, respiratory rate >20 breaths/min, white blood cell count >12,000 cells/μL or <4000 cells/μL (Bone et al. 1992, Levy et al. 2003, Dellinger et al. 2008). Criteria for severe sepsis syndrome were modified based on available study data. Severe sepsis syndrome was defined as sepsis syndrome with any of the following: Glasgow coma score <15, a platelet count <100,000 platelets/μL, or sepsis-induced hypotension (Bone et al. 1992, Levy et al. 2003, Dellinger et al. 2008). Sepsis induced hypotension was defined as either a systolic blood pressure <90 mmHg or mean arterial pressure (MAP) <70 mmHg (Dellinger et al. 2008). Variables using vital signs included only adolescents and adults given different normal reference ranges for infants and children. Thrombocytopenia was defined using locally established or verified hematologic reference ranges for children and adults (Saathoff et al. 2008, Buchanan et al. 2010).
Statistical analysis
Data were entered using the Cardiff Teleform system (Cardiff, Inc., Vista, CA) into an Access database (Microsoft Corp, Redmond, WA). Descriptive statistics are presented as proportions, medians, ranges, and interquartile ranges (IQR). The Fisher exact test was used to compare categorical data when any cell contained fewer than 10 observations. Wilcoxon rank sum was used to compare categorical and nonparametric continuous data. All p values are two sided and evaluated for statistical significance at the 0.05 significance level. All analyses were performed using STATA, version 10.1 (STATACorp, College Station, TX).
Research ethics
This study was approved by the KCMC Research Ethics Committee, the Tanzania National Institutes for Medical Research National Research Ethics Coordinating Committee, and the Institutional Review Boards of Duke University Medical Center and the CDC.
Results
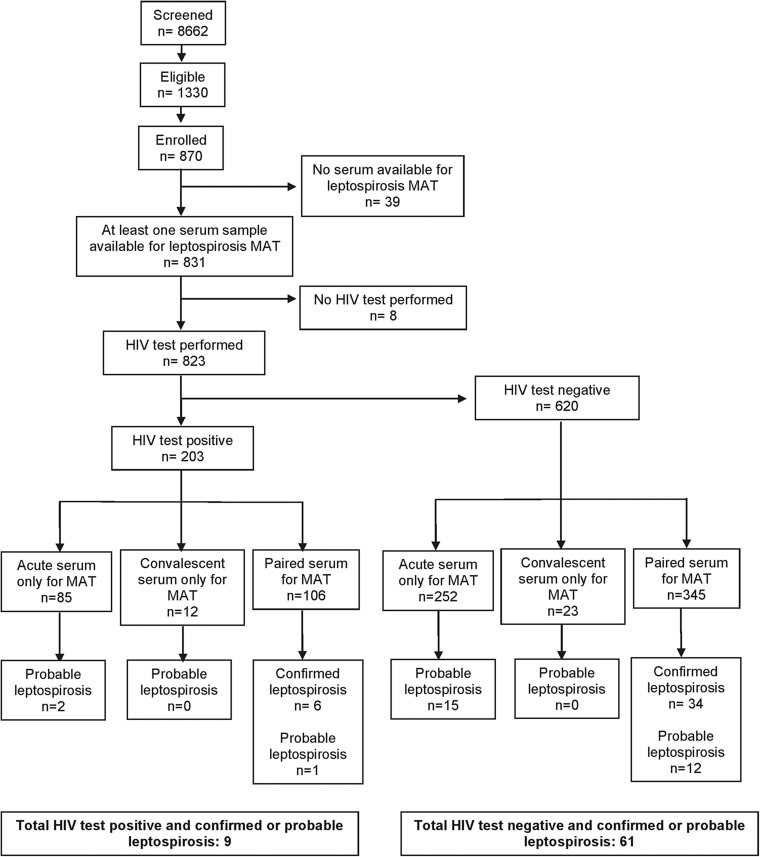
A total of 870 participants were enrolled in the study, and 831 (95.5%) had one or more serum samples tested for leptospirosis. Participants' characteristics have been described elsewhere (Biggs et al. 2011, Crump et al. 2011a, Crump et al. 2011b). A total of 453 participants had acute and convalescent (paired) serum tested for leptospirosis, of which 40 (8.8%) had confirmed leptospirosis. Of 791 patients with one or more serum samples tested for leptospirosis who did not have confirmed leptospirosis, 30 (3.8%) had probable leptospirosis. Overall 70 (8.4%) of 831 participants had confirmed or probable leptospirosis and 761 (91.6%) were negative for leptospirosis. Of the 831 participants with one or more serum samples tested for leptospirosis, 823 (99.0%) had HIV testing performed at the time of study enrollment, of which 203 (24.7%) were HIV infected. Among HIV-infected participants, 9 (4.4%) of 203 had confirmed or probable leptospirosis, and among HIV-uninfected participants 61 (9.8%) of 620 had leptospirosis (Fig. 1). Leptospirosis was less prevalent among those with HIV infection compared with those without HIV infection [odds ratio (OR) 0.43, p=0.019].
FIG. 1.
Flow chart indicating patients with leptospirosis and human immunodeficiency virus (HIV) infection. MAT, microscopic agglutination test.
Characteristics of the 9 patients with HIV and leptospirosis are shown in Table 1. The median age of those with HIV and leptospirosis was 31.4 (range 1.8, 58.5) years. Five (55.6%) of the nine with leptospirosis and HIV infection were male. CD4 counts and percents were available for eight of the nine patients with leptospirosis and HIV. The median (range) CD4 count of those with leptospirosis and HIV infection was 335 (86, 1105) cells/μL, and the median (range) CD4 percent was 20 (4, 45).
Table 1.
Characteristics of Patients with Leptospirosis and HIV Infection, Northern Tanzania, 2007–2008
| Cases | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 31.4 | 29.5 | 58.5 | 31.7 | 39.9 | 34.9 | 3.7 | 4.7 | 1.8 |
| Sex | Female | Female | Male | Male | Female | Male | Male | Male | Female |
| Leptospirosis confirmed or probable | Probable | Probable | Probable | Confirmed | Confirmed | Confirmed | Confirmed | Confirmed | Confirmed |
| Prior HIV test positivea | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes |
| CD4 (cells/μL) | 1105 | 203 | None | 86 | 98 | 484 | 313 | 1014 | 357 |
| CD4 % | 45 | 18 | None | 11 | 11 | 37 | 26 | 22 | 4 |
| Prescribed ART | Yes | No | No | Yes | No | Yes | Yes | Yes | Unknown |
| Prescribed SXT prophylaxisb | Yes | No | No | Yes | No | Yes | — | — | — |
| Died | No | Yes | Yes | No | No | No | No | No | No |
| Admitting diagnoses | Malaria | Meningitis, cerebral malaria | Urinary tract infection, malaria | Tuberculosis, malaria | Malaria | HIV, malaria | Pneumonia, otitis media | HIV/AIDS, pneumonia | HIV/AIDS, marasmus, malaria |
| Discharge diagnosesb | HIV, malaria | Meningitis | Severe malaria | Malaria | HIV, malaria | HIV, malaria | — | — | — |
| Concurrent infections | None | S. pneumoniae bacteremia | E. coli bacteremia | None | Salmonella Typhi bacteremia | None | S. pneumoniae bacteremia | None | None |
| Antibacterials during admission | None | Penicillin, chloramphenicol | Penicillin | None | SXT metronidazole | None | Ampicillin, gentamicin | Ampicillin, gentamicin | Ampicillin, gentamicin |
| Leptospirosis predominant serogroupc | Mini | Canicola | Mini | Celledoni | Australis | Australis | Ictero-hemorrhagiae, Mini | Mini | Ictero-hemorrhagiae, Mini |
| Highest MAT reciprocal titer | 1600 | 1600 | 800 | 800 | 400 | 400 | 200 | 200 | 200 |
Known to be HIV infected prior to study enrollment.
Data not collected for infants and children.
Defined as the serogroup for the reacting serovar with the highest MAT titer.
HIV, human immunodeficiency virus; ART, antiretroviral therapy; SXT, trimethoprim-sulfamethoxazole; MAT, microscopic agglutination test.
Comparison of HIV-infected participants with and without leptospirosis
Among those with CD4 count data, three (37.5%) of eight HIV-infected patients with leptospirosis met the definition of severe immunosuppression, and 102 (54.3%) of 188 HIV-infected patients with a febrile illness not due to leptospirosis met the definition of severe immunosuppression. There was no significant difference in severe immunosuppression between these groups (p=0.476; Table 2). Comparison of severe immunosuppression using only confirmed leptospirosis cases compared with leptospirosis negative cases with paired sera, also did not show any significant difference (p=1.000). Among HIV-infected adolescents and adults, median CD4 percent and median CD4 count were higher among those with leptospirosis compared with those with febrile illness not due to leptospirosis, although differences in CD4 count did not reach statistical significance (p=0.015 and p=0.089, respectively). Of 152 participants with antiretroviral therapy (ART) use documented, five (62.5%) of eight HIV-infected patients with leptospirosis reported taking ART prior to study enrollment as compared to 55 (38.2%) of 144 HIV-infected patients without leptospirosis (p=0.265).
Table 2.
Characteristics of HIV-Infected Patients with and without Leptospirosis, Northern Tanzania, 2007–2008
| HIV-infected with leptospirosis n=9 | HIV-infected without leptospirosis n=194 | Odds ratio | p value | |
|---|---|---|---|---|
| Median age (range) years | 31.4 (1.8, 58.5) | 34.5 (0.2, 73.0) | — | 0.287 |
| Median CD4 (range) cells/μLa | 335 (86, 1105) | 123 (1, 2316) | — | 0.034 |
| Median CD4 (range) cells/μL, for adults and adolescents onlyb | 203 (86, 1105) | 99 (1, 813) | — | 0.089 |
| Median CD4 percent (range)c | 20 (4,45) | 9 (0,56) | — | 0.019 |
| Median CD4 percent (range), for adults and adolescents onlyd | 18 (11, 45) | 8 (0, 43) | — | 0.015 |
| Severe immunosuppressiona | 3/8 (37.5%) | 102/188 (54.3%) | 0.52 | 0.476 |
| Prescribed ART | 5/8 (62.5%) | 55/144 (38.2%) | 2.70 | 0.265 |
| Prescribed SXT prophylaxise | 3/6 (50.0%) | 48/143 (33.6%) | 1.98 | 0.412 |
n=8 for HIV-infected with leptospirosis; n=188 for HIV-infected without leptospirosis.
n=5 for HIV-infected with leptospirosis; n=142 for HIV-infected without leptospirosis.
n=8 for HIV-infected with leptospirosis; n=180 for HIV-infected without leptospirosis.
n=5 for HIV-infected with leptospirosis; n=136 for HIV-infected without leptospirosis.
n=6 for HIV-infected with leptospirosis; n=143 for HIV-infected without leptospirosis.
HIV, human immunodeficiency virus; ART, antiretroviral therapy; SXT, trimethoprim-sulfamethoxazole.
Leptospirosis manifestations in HIV-infected and HIV-uninfected patients
Symptoms in HIV-infected patients with leptospirosis were similar to those in HIV-uninfected patients with leptospirosis with the exception of cough being reported more frequently in HIV-infected patients (OR 8.55, p=0.031) (Table 3). On presentation, HIV-infected adults and adolescents with leptospirosis had a higher median heart rate, lower median systolic and diastolic blood pressures, and lower median MAP than HIV-uninfected patients with leptospirosis. However, these differences did not reach statistical significance. Other presenting vital signs, including temperature, respiratory rate, and oxygen saturation, were not significantly different between those with leptospirosis with and without HIV infection. Sepsis syndrome was no different between those with leptospirosis with and without HIV (p=1.000). Severe sepsis syndrome was more common in HIV-infected patients with leptospirosis, however, this was not statistically significant (p=0.174). No recorded physical examination findings differentiated HIV-infected and HIV-uninfected patients than HIV-uninfected patients with leptospirosis. There was no difference in thrombocytopenia between the two groups (p=1.000). Among patients who had a chest radiograph performed, five (71.4%) of seven HIV-infected patients with leptospirosis had a documented pulmonary infiltrate compared to five (35.1%) of 37 HIV-uninfected patients with leptospirosis (p=0.103). The length of hospital stay in HIV-infected patients with leptospirosis was 4 days (IQR 3,7); the length of stay in HIV-uninfected patients with leptospirosis was 5 days (IQR 4,9), (p=0.176).
Table 3.
Clinical Characteristics of HIV-Infected and HIV-Uninfected Patients with Leptospirosis, Northern Tanzania, 2007–2008
| HIV-infected with leptospirosis n=9 | HIV-uninfected with leptospirosis n=61 | Odds ratio | p value | |
|---|---|---|---|---|
| Median age, (range) years | 31.4 (1.8, 58.5) | 22.0 (0.4,78) | 0.951 | |
| Number ≤13 years old | 3 (33.3%) | 24 (40.0%) | 1.00 | |
| Symptoms and signs | ||||
| Rigorsa | 5 (83.3%) | 30 (81.1%) | 1.17 | 1.000 |
| Headachea | 3 (50%) | 24 (64.9%) | 0.54 | 0.655 |
| Neck stiffness | 1 (11.1%) | 3 (4.9%) | 2.42 | 0.431 |
| Photophobiaa | 0 (0.0%) | 5 (13.5%) | — | 1.000 |
| Cough | 8 (88.9%) | 29 (48.3%) | 8.55 | 0.031 |
| Difficulty breathing | 5 (55.6%) | 22 (36.1%) | 2.22 | 0.292 |
| Hemoptysisa | 0 (0.0%) | 1 (2.7%) | — | 1.000 |
| Vomiting | 2 (22.2%) | 20 (32.8%) | 0.59 | 0.709 |
| Diarrhea | 2 (22.2%) | 7 (11.7%) | 2.16 | 0.333 |
| Jaundice | 0 (0.0%) | 3 (5.1%) | — | 1.000 |
| Days since onset of illness, median (IQR) days | 7 (3,14) | 7 (3,14) | 0.797 | |
| Temperature, median (IQR) °C | 38.9 (38.2, 39.2) | 38.5 (38.0, 39.1) | 0.765 | |
| Heart rate, median (IQR) beats/minutea | 111.5 (96.0, 116.0) | 92.0 (84.0, 111.0) | 0.156 | |
| Systolic blood pressure, median (IQR) mmHga | 99 (90.0,118.0) | 113 (105.0, 123.0) | 0.161 | |
| Diastolic blood pressure, median (IQR) mmHga | 55.5 (54.0,71.0) | 70 (63.0, 80.0) | 0.155 | |
| MAP, median (IQR) mmHga | 69.8 (65.0, 88.7) | 82.7 (77.3, 95.3) | 0.136 | |
| Respiratory rate, median (IQR) breaths/minutea | 25.5 (24.0, 27.0) | 24.0 (21.0, 30.0) | 0.846 | |
| Oxygen saturation, median (IQR) percentb | 96.0 (88.0, 98.0) | 97.0 (94.0, 98.0) | 0.314 | |
| Hepatomegaly | 0 (0.0%) | 12 (19.7%) | — | 0.314 |
| Crepitations/crackles | 4 (44.4%) | 20 (32.8%) | 1.64 | 0.481 |
| Infiltrate on chest radiographc | 5 (71.4%) | 13 (35.1%) | 4.62 | 0.103 |
| Thrombocytopenia | 2 (22.2%) | 18 (30.5%) | 0.65 | 1.000 |
| Sepsisa | 5 (83.3%) | 31 (83.8%) | 0.97 | 1.000 |
| Severe sepsisa | 4 (66.7%) | 12 (32.4%) | 4.2 | 0.174 |
| Died | 2 (22.2%) | 3 (5.0%) | 5.43 | 0.124 |
| Concurrent bacterial bloodstream infection | 4 (44.4%) | 5 (8.2%) | 8.96 | 0.013 |
| Length of hospital admission, median (IQR) days | 4 (3,7) | 5 (4,9) | 0.176 |
Data available for adults and adolescents only; leptospirosis and HIV infection, n=6; leptospirosis without HIV infection, n=37.
Leptospirosis and HIV infection, n=7.
Leptospirosis and HIV infection, n=7; leptospirosis without HIV infection, n=37.
HIV, human immunodeficiency virus; IQR, interquartile range; MAP, mean arterial pressure.
Mortality comparisons were limited since, by definition, it was only possible to become a confirmed leptospirosis case if a patient survived to provide a convalescent serum sample. Therfore, all of those who died had probable leptospirosis. Two (66.7%) of three HIV-infected patients with probable leptospirosis died during admission compared to three (11.5%) of 26 HIV-uninfected patients with probable leptospirosis (p=0.068). One HIV-uninfected patient with probable leptospirosis did not have death data available. Both HIV-infected patients with leptospirosis that died had concurrent bacterial bloodstream infections compared with one (33.3%) of three HIV-uninfected patients with leptospirosis that died. Overall, four (44.4%) of the nine HIV-infected patients with leptospirosis had concurrent bacterial bloodstream infections compared with five (8.2%) of 61 HIV-uninfected patients with leptospirosis (p=0.013). The concurrent bloodstream pathogens in the HIV-infected patients with leptospirosis were Streptococcus pneumoniae in two patients, Salmonella serotype Typhi in one patient, and Escherichia coli in one patient.
Five (55.6%) of nine HIV-infected patients with leptospirosis were treated with penicillin or ampicillin, three (33.3%) received no antibacterials, and one (11.1%) received antibacterials not typically recommended for treatment of leptospirosis. Among 60 HIV-uninfected patients with treatment data available, 30 (50%) received penicillin, ampicillin, or ceftriaxone; two (3.3%) received chloramphenicol; 18 (30.0%) received no antibacterial; and 10 (16.7%) received other antibacterials not typically recommended for treatment of leptospirosis.
Discussion
Among febrile hospitalized patients in northern Tanzania, leptospirosis was not more prevalent among those with HIV infection as compared to those without HIV infection. Those with HIV and leptospirosis were not more severely immunocompromised than other HIV-infected study participants. Differences in some markers of disease severity between HIV-infected and HIV-uninfected patients were observed but did not reach statistical significance. Our ability to demonstrate statistically significant differences in disease severity may have been limited by relatively small numbers.
Literature on leptospirosis and HIV co-infection is sparse and limited to case reports. To our knowledge, there are eight published case reports of leptospirosis and HIV co-infection (Da Silva et al. 1990, Neves Ede et al. 1994, Jones and Kim 2001, Ganoza et al. 2005, Guerra et al. 2008, Kumar et al. 2010). Jones and Kim (2001) described a patient with a CD4 count of 16 cells/μL and severe, fulminant leptospirosis. They also reviewed four additional cases of HIV and leptospirosis co-infection, and noted that all had one or more severe manifestation of leptospirosis, and two of the four with available CD4 counts had CD4 counts <100 cells/μL. Despite the presence of severe leptospirosis, none of these patients died. Ganoza et al. (2005) report one case of leptospirosis and HIV co-infection in Peru presenting with an acute self-resolving febrile illness and asserted that the range of leptospirosis manifestations in HIV-infected patients likely reflected the variation in leptospirosis disease seen in HIV-uninfected populations. It is not possible to draw conclusions about the severity of leptospirosis manifestations in HIV-infected patients based on published case reports because of the possibility of reporting bias. In this paper, we contribute nine cases of leptospirosis and HIV co-infection and compare clinical manifestations of leptospirosis among HIV-infected and HIV-uninfected patients in the same study population.
Reported indicators of leptospirosis severity include respiratory symptoms, thrombocytopenia, decreased urine output, elevated serum creatinine, and elevated bilirubin or jaundice (Dupont et al. 1997, Tantitanawat and Tanjatham 2003, Spichler et al. 2008). Respiratory symptoms, including shortness of breath and infiltrates on chest radiograph, were common among HIV-infected patients with leptospirosis in our study, but only cough was significantly different between HIV-infected and HIV-uninfected patients. There was no difference in shortness of breath, hemoptysis, respiratory rate, or oxygen saturation. Thrombocytopenia was also not different between HIV-infected and HIV-uninfected patients with leptospirosis. Jaundice was observed in only three patients with leptospirosis, and all were HIV-uninfected. We observed lower systolic and diastolic blood pressures, lower MAPs, and higher heart rates among HIV-infected patients with leptospirosis as compared to HIV-uninfected patients, but these differences did not reach statistical significance. Severe sepsis syndrome and death also occurred in a greater proportion of HIV-infected patients, but no statistical difference was observed. Urine output, serum creatinine, and bilirubin were not measured in this study.
Concomitant bacterial bloodstream infection was significantly more common among HIV-infected patients with leptospirosis compared with HIV-uninfected patients with leptospirosis in our study. This difference may, in part, be attributable to the known increased risk for invasive pneumococcal disease among those with HIV infection (Janoff et al. 1992), as the etiology of two of the four bloodstream infections was Streptococcus pneumoniae. However, we cannot determine from our data whether the presence of one bacterial infection predisposed to the other among HIV-infected patients. The apparent co-occurrence of leptospirosis and bacterial bloodstream infection with another organism may reflect true co-infection or MAT cross-reactivity. Although MAT cross-reactivity in the setting of other infections has been reported (Weyant et al. 1999, Bajani et al. 2003), co-infection with Leptospira spp. and other bacteria demonstrated by culture of both organisms has also been reported. Culture of both Leptospira spp. and Salmonella serotype Typhi from the same febrile patient was reported in one African study of co-infection (Parker et al. 2007). A case report of an adolescent with leptospirosis followed by nontyphoidal Salmonella osteomyelitis raised the question of whether a leptospirosis-induced vasculitis affecting the gut mucosa may predispose to bacterial translocation (Osebold 2008). Both of the HIV-infected patients with leptospirosis in our study who died had concomitant bloodstream infection with another organism, making it impossible to attribute their deaths to severe leptospirosis alone. Additionally, the presence of concomitant bloodstream infection in a higher proportion of HIV-infected patients with leptospirosis complicates interpretation of markers of disease severity because, similarly, it is not possible to attribute observed differences to leptospirosis alone.
This study has a number of limitations that may have influenced our findings. Despite this being the largest study of leptospirosis and HIV co-infection that we are aware of, the number of patients with leptospirosis and HIV was relatively small. Additionally, some data were not available for infants and children, resulting in reduced numbers for analysis. Small numbers may have influenced our ability to demonstrate statistical significance in analyses. Additionally, we did not specify a severity of illness score a priori. We did not measure creatinine, urine output, or bilirubin, all of which have been previously described as markers of leptospirosis severity or predictors of mortality in leptospirosis (Dupont et al. 1997, Tantitanawat and Tanjatham 2003, Spichler et al. 2008). Furthermore, our definition of severe sepsis syndrome was limited to only the markers measured, which could have resulted in missed cases. This study included only hospitalized patients, so there is likely bias toward more severe disease. Furthermore, estimating true mortality in leptospirosis cases is difficult given that both acute and convalescent serum must be collected for serologic confirmation of the diagnosis. We could have missed cases of leptospirosis in patients who died before a convalescent serum sample could be collected. We used a conservative MAT value cutoff for probable leptospirosis, which is appropriate for endemic settings, but again could have resulted in missed cases among those who had lower initial antibody titers and died before a convalescent sample was collected. Additionally, the performance of MAT in the setting of severe immune suppression is not known; however, previous case reports have shown high MAT titers in patients with severe immunosuppression due to HIV/AIDS (Jones and Kim 2001, Ganoza et al. 2005).
Conclusions
Among febrile inpatients in northern Tanzania, leptospirosis was not more prevalent among those with HIV infection, and infection with leptospirosis was not associated with increased HIV-related immunosuppression. While leptospirosis is a common cause of febrile illness among hospitalized patients in northern Tanzania, among HIV-infected patients leptospirosis was relatively less common as compared to other etiologies of febrile illness. Although differences in some markers of disease severity were observed between HIV-infected and HIV-uninfected patients with leptospirosis, these did not reach statistical significance, possibly due to small numbers. Studies with increased case numbers, using direct methods of leptospirosis diagnosis, such as culture or nucleic acid amplification testing, and including additional markers of leptospirosis disease severity in populations in which HIV and leptospirosis are prevalent, would help better define the relationship between HIV and leptospirosis.
Acknowledgments
We thank Ahaz T. Kulanga for providing administrative support to this study; Pilli M. Chambo, Beata V. Kyara, Beatus A. Massawe, Anna D. Mtei, Godfrey S. Mushi, Lillian E. Ngowi, Flora M. Nkya, and Winfrida H. Shirima for reviewing and enrolling study participants; Gertrude I. Kessy, Janeth U. Kimaro, Bona K. Shirima, and Edward Singo for managing participant follow-up; and Evaline M. Ndosi and Enock J. Kessy for their assistance in data entry. We are grateful to the leadership, clinicians, and patients of KCMC and MRH for their contributions to this research.
This research was supported by an International Studies on AIDS Associated Co-infections (ISAAC) award, a United States National Institutes of Health (NIH)-funded program (U01 AI062563). This work was funded in part by US National Institutes of Health grant R01TW009237 as part of the joint NIH-NSF Ecology of Infectious Disease program and the UK Economic and Social Research Council and Biotechnology and Biological Sciences Research Council. This publication was made possible with help from the Duke University Center for AIDS Research (CFAR), an NIH-funded program (2P30 AI064518). Authors received support from the NIH Fogarty International Center AIDS International Training and Research Program D43 PA-03-018 (J.A.C., V.P.M.), the Duke Clinical Trials Unit and Clinical Research Sites U01 AI069484 (J.A.C., V.P.M.), and NIAID-AI007392 (H.M.B.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services or the Centers for Disease Control and Prevention.
References
- Bajani MD. Ashford DA. Bragg SL. Woods CW, et al. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J Clin Microbiol. 2003;41:803–809. doi: 10.1128/JCM.41.2.803-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggs HM. Bui DM. Galloway RL. Stoddard RA, et al. Leptospirosis among hospitalized febrile patients in northern Tanzania. Am J Trop Med Hygiene. 2011;85:275–281. doi: 10.4269/ajtmh.2011.11-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone RC. Balk RA. Cerra FB. Dellinger RP, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Buchanan AM. Muro FJ. Gratz J. Crump JA, et al. Establishment of haematological and immunological reference values for healthy Tanzania children in the Kilimanjaro Region. Trop Med Int Health. 2010;15:1011–1021. doi: 10.1111/j.1365-3156.2010.02585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep. 1997;46(RR-10):1–55. [PubMed] [Google Scholar]
- Crump JA. Ramadhani HO. Morrissey AB. Saganda W, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011a;52:341–348. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA. Ramadhani HO. Morrissey AB. Msuya LJ, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop Med Int Health. 2011b;16:830–837. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA. Scott LE. Msuya E. Morrissey AB, et al. Evaluation of the Abbott m2000rt RealTime HIV-1 assay with manual sample preparation compared with the ROCHE COBAS AmpliPrep/AMPLICOR HIV-1 MONITOR 1.5 using specimens from East Africa. J Virolog Methods. 2009;162:218–222. doi: 10.1016/j.jviromet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva MV. Batista L. Camargo ED. Leitao PA, et al. Leptospirosis in patients with anti-HIV antibodies: Report of 2 cases. Rev Soc Bras Med Trop. 1990;23:229–231. doi: 10.1590/s0037-86821990000400009. [DOI] [PubMed] [Google Scholar]
- Dellinger RP. Levy MM. Carlet JM. Bion J, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- Dikken H. Kmety E. Serological typing methods of leptospires. In: Bergan T., editor; Norris J. R., editor. Methods in Microbiology. London: Academic Press; 1978. [Google Scholar]
- Dupont H. Dupont-Perdrizet D. Perie JL. Zehner-Hansen S, et al. Leptospirosis: Prognostic factors associated with mortality. Clin Infect Dis. 1997;25:720–724. doi: 10.1086/513767. [DOI] [PubMed] [Google Scholar]
- Faine S. Guidelines for the Control of Leptospirosis. Geneva: World Health Organization; 1982. [Google Scholar]
- Ganoza CA. Segura ER. Swancutt MA. Gotuzzo E, et al. Brief Report: Mild self-resolving acute leptospirosis in an HIV-infected patient in the Peruvian Amazon. Am J Trop Med Hygiene. 2005;73:67–68. [PMC free article] [PubMed] [Google Scholar]
- Guerra B. Schneider T. Luge E. Draeger A, et al. Detection and characterization of Leptospira interrogans isolates from pet rats belonging to a human immunodeficiency virus-positive patient with leptospirosis. J Med Microbiol. 2008;57:133–135. doi: 10.1099/jmm.0.47452-0. [DOI] [PubMed] [Google Scholar]
- Jones S. Kim T. Fulminant leptospirosis in a patient with human immunodeficiency virus infection: Case report and review of the literature. Clin Infect Dis. 2001;33:e31–33. doi: 10.1086/322645. [DOI] [PubMed] [Google Scholar]
- Janoff EN. Breiman RF. Daley CL. Hopewell PC. Pneumococcal disease during HIV infection: Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–324. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- Kumar AB. Majumdar B. Goru R. Tewari R, et al. A case of complete heart block in a patient with HIV and leptospirosis. Kardiol Pol. 2010;68:562–563. [PubMed] [Google Scholar]
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MM. Fink MP. Marshall JC. Abraham E, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Mayhood MK. Afwamba IA. Odhiambo CO. Ndanu E, et al. Validation, performance under field conditions, and cost-effectiveness of Capillus HIV-1/HIV-2 and Determine HIV-1/2 rapid human immunodeficiency virus antibody assays using sequential and parallel testing algorithms in Tanzania. J Clin Microbiol. 2008;46:3946–3951. doi: 10.1128/JCM.01045-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves Ede S. Pereira MM. Galhardo MC. Caroli A, et al. Leptospirosis patient with AIDS: The first case reported. Rev Soc Bras Med Trop. 1994;27:39–42. doi: 10.1590/s0037-86821994000100008. [DOI] [PubMed] [Google Scholar]
- Osebold WR. Systemic leptospirosis followed by salmonella vertebral osteomyelitis without sickling or immunosuppression. Spine. 2008;33:55–61. doi: 10.1097/BRS.0b013e3181604708. [DOI] [PubMed] [Google Scholar]
- Parker TM. Murray CK. Richards AL. Samir A, et al. Concurrent infections in acute febrile illness patients in Egypt. Am J Trop Med Hygiene. 2007;77:390–392. [PubMed] [Google Scholar]
- Saathoff E. Schneider P. Kleinfeldt V. Geiss S, et al. Laboratory reference values for healthy adults from southern Tanzania. Trop Med Int Health. 2008;13:612–625. doi: 10.1111/j.1365-3156.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- Scott L E. Crump JA. Msuya E. Morrissey AB, et al. Abbott RealTime HIV-1 m2000rt viral load testing: Manual extraction versus the automated m2000sp extraction. J Virolog Methods. 2011;172:78–80. doi: 10.1016/j.jviromet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spichler AS. Vilaca PJ. Athanazio DA. Albuquerque JO, et al. Predictors of lethality in severe leptospirosis in urban Brazil. Am J Trop Med Hygiene. 2008;79:911–914. [PMC free article] [PubMed] [Google Scholar]
- Tantitanawat S. Tanjatham S. Prognostic factors associated with severe leptospirosis. J Med Assn Thailand. 2003;86:925–931. [PubMed] [Google Scholar]
- Weyant RS. Bragg SL. Kaufmann AF. Leptospira and Leptonema. In: Murray PR, editor; Baron EJ, editor; Pfaller MA, editor; Tenover FC, editor; Yolken RH, editor. Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology; 1999. [Google Scholar]
- World Health Organization. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. Geneva: World Health Organization; 2003. [Google Scholar]
- World Health Organization. Antiretroviral Therapy of HIV Infection in Infants and Children in Resource-Limited Settings: Towards Universal Access: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2006. [Google Scholar]