Abstract
Excessive extracellular glutamate after traumatic brain injury (TBI) contributes to excitotoxic cell death and likely to post-traumatic epilepsy. Glutamate transport is the only known mechanism of extracellular glutamate clearance, and glutamate transporter 1 (GLT-1) is the major glutamate transporter of the mammalian brain. We tested, by immunoblot, in the rat lateral fluid percussion injury TBI model whether GLT-1 expression is depressed in the cortex after TBI, and whether GLT-1 expression after TBI is restored after treatment with ceftriaxone, a well-tolerated β-lactam antibiotic previously shown to enhance GLT-1 expression in noninjured animals. We then tested whether treatment with ceftriaxone mitigates the associated regional astrogliosis, as reflected by glial fibrillary acid protein (GFAP) expression, and also whether ceftriaxone treatment mitigates the severity of post-traumatic epilepsy. We found that 7 days after TBI, GLT-1 expression in the ipsilesional cortex was reduced by 29% (n=7/group; p<0.01), relative to the contralesional cortex. However, the loss of GLT-1 expression was reversed by treatment with ceftriaxone (200 mg/kg, daily, intraperitoneally). We found that ceftriaxone treatment also decreased the level of regional GFAP expression by 43% in the lesioned cortex, relative to control treatment with saline (n=7 per group; p<0.05), and, 12 weeks after injury, reduced cumulative post-traumatic seizure duration (n=6 rats in the ceftriaxone treatment group and n=5 rats in the saline control group; p<0.001). We cautiously conclude that our data suggest a potential role for ceftriaxone in treatment of epileptogenic TBI.
Key words: epilepsy, glia cell response to injury, immunoblots, recovery, traumatic brain injury
Introduction
Immediately after traumatic brain injury (TBI) and for hours to days after, rising extracellular glutamate concentration leads to the hyperexcitation of neurons resulting from excessive activation of Ca2+-permeable glutamate receptors, thereby causing excitotoxicity and cell death.1–3 Despite the critical role of acute glutamate-mediated pathophysiologic processes after TBI, there are no clinically available methods to remove excess glutamate in the acute and subacute periods after injury, when glutamate excitotoxicity is likely first to occur.
Glutamate, although the major excitatory neurotransmitter, is toxic to neurons even at low micromolar concentrations.4,5 Excitatory amino acid transporters (EAAT1–5 in humans) provide the only known endogenous mechanism for clearance of extracellular glutamate in mammals by transporting it into the intracellular space. Glutamate transporter 1 (GLT-1), the rodent analog of human EAAT2, provides the majority of glutamate clearance capacity in the rodent brain. GLT-1 is predominantly expressed in astrocytes, but it is also found in presynaptic neuronal terminals and oligodendrocytes.6–10 Published data show that TBI in rats results in an acute, 24-h reduction of GLT-1 messenger RNA (mRNA) and protein, and in rats with genetically decreased GLT-1 expression, TBI leads to increase in neuronal death.11–13 Yet, whether TBI causes a reduction in GLT-1 expression beyond the first 24 h after injury, and whether pharmacologically stimulated increase in GLT-1 expression after injury can mitigate neuronal damage, remains unknown.
β-lactam antibiotics, particularly ceftriaxone which exhibits excellent blood–brain barrier penetration, are potent stimulators of functional GLT-1 (EAAT2) expression in rodents as well as in human cultured astrocytes by yet incompletely known mechanisms.14,15 β-lactam neuroprotective capacity is supported by published data showing reduced neuronal death in rodent models of hypoxic-ischemic brain injury16,17 and behavioral improvement in a transgenic rodent model of Huntington's disease.18 Also, in the mouse amyotrophic lateral sclerosis (ALS) model, where glutamate-mediated excitotoxicity is contributory to the mechanisms of neuronal injury, ceftriaxone also delays neuronal loss and increases mouse survival. In this mouse model, ceftriaxone's effect on GLT-1 up-regulation was evident after 48 h of treatment and increased for the first week of treatment, which corresponds closely to the subacute time course of the initial excitotoxic phase of epileptogenesis after TBI.19,20
In the present study, we investigated a potential therapeutic effect of ceftriaxone in the well-established lateral fluid percussion injury (LFPI) rat TBI model.20,21 Specifically, we tested whether (1) GLT-1 expression is suppressed in lesioned brain region 1 week after TBI and whether ceftriaxone treatment (2) reverses post-traumatic GLT-1 suppression, (3) reduces post-traumatic astrogliosis, as reflected by glial fibrillary acidic protein (GFAP) expression, and (4) suppresses post-traumatic seizures.
Methods
Animals
Thirty-seven adult male Long-Evans rats (367±36 g, age 8–9 weeks, at time of surgery) were used for the present experiment. All procedures were approved by, and in accord with, the guidelines of the animal care and use committee at Boston Children's Hospital (Boston, MA) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of rats used in the present experiments.
LFPI
Rats (n=21) were anesthetized using 2–4% isoflurane vapor by nosecone and mounted on a stereotactic frame (Stoelting Co., Wood Dale, IL). During anesthesia, normothermia was maintained with a 40°C exothermic chemical warming pad. A 4-mm craniectomy was made over the left posterior cortex, anteriolateral to the lambda without crossing the sutures, with the lateral edge of the craniectomy adjacent to the lateral ridge (Fig. 1). The dura was examined to confirm its integrity. A length of plastic tubing was fitted to the male connector of the fluid-percussion device (AmScien Instruments, Richmond VA), and the connection was made airtight using polytetrafluoroethylene (PTFE) tape. The free end of the tubing was attached to a 1000-μL pipette tip that had been cut to leave a 4-mm aperture on its free end, again made airtight using PTFE tape. The tubing was then filled with sterile saline at room temperature, and the tip was positioned over the exposed dura using the micromanipulator arm of the frame, such that the edges of the pipette tip fit tightly over the skull. A percussion wave of 2.3±0.1 atm (atmospheres) was delivered to induce moderate TBI. During the TBI procedure, each rat's respiratory rate was monitored by visual inspection, and epochs of apnea were recorded. Sham control animals (n=7) underwent the craniectomy without LFPI. After each verum or sham TBI surgery, animals were observed continuously until they were sternal and were then placed in their home cages with ad libitum access to food and water. Opioid analgesia (buprenorphine HCl; 0.1–0.3 mg/kg) was administered subcutaneously (s.c.) every 8–12 h for 48 h postoperatively. Animals were observed twice-daily for the first postoperative week and daily thereafter until their endpoints.
FIG. 1.
Location of injury site and skull screw electrodes. A 4-mm craniectomy is represented by the circle with crosshair. Solid black circles indicate the electroencephalography recording electrode positions over the right olfactory bulb (reference electrode) and over the anterior perilesional cortex.
Ceftriaxone and saline treatment
Rats were divided into three experimental groups as follows: (1) “saline-sham” treated with daily saline injections for 7 days after sham TBI surgery; (2) “saline-TBI” treated with daily saline injections for 7 days after verum TBI surgery; and (3) “ceftriaxone-TBI” treated with (200 mg/kg) ceftriaxone daily intraperitoneal (i.p.) injections for 7 days after verum TBI surgery.22,23 Ceftriaxone (or saline) treatment was limited to 7 days. Per experimental group, cortical tissue was harvested separately from the ipsilesional (left) and contralesional (right) hemisphere.
Rats were divided into two groups (n=7 per group) as follows: (1) the ceftriaxone-TBI group treated with an i.p. injections of ceftriaxone at 200 mg/kg per day, whereas (2) the saline-TBI and (3) the saline-sham surgery (saline-sham) groups were given saline as a control condition. The first injection occurred 30 min post-TBI and continued once per 24 h for 7 consecutive days thereafter.
Cortex protein extraction
Rats were sacrificed 7 days after TBI by decapitation. The left and right neocortex was separated from the remaining brain. Per side, the posterior two thirds of the cortex, corresponding to the ipsi- and contralesional regions, was retained for immunoblot analysis. Tissue was flash-frozen and stored at −80°C until use. Dissected posterior cortical tissue from rats 7 days post-TBI was homogenized, on ice, by 25 strokes of a dounce homogenizer in homogenization buffer (20 mL/g tissue mass) containing Tissue Protein Extraction Reagent (T-PER; ThermoScientific, Rockford, IL) and cOmplete Mini Protease Inhibitor tablets (1 tablet per 10 mL of T-PER; Roche, Mannheim, Germany). Samples were vortexed for 15 sec before centrifugation at 13,000 rpm at 4°C and stored at −20° C. Sample concentration was determined using a colorimetric protein assay kit (Bio-Rad, Hercules, CA).
Immunoblot analysis
Aliquots of brain homogenate were mixed with SDS-Sample buffer (Boston BioProducts, Ashland, MA), separated on 4–20% Precise Tris-HEPES Gels (ThermoScientific), and then transferred to polyvinylidene fluoride membranes (BioRad) by electroblotting. Blots were incubated with primary antibodies (Abs; rabbit anti-nGLT-16, rabbit anti-GFAP; AB5804; Millipore, Billerica, MA) and mouse anti-β-actin (A5316; Sigma-Aldrich Co., Natick, MA) overnight at 4°C in 5% nonfat milk, Tris-buffered saline (TBS; Boston BioProducts), and 0.1% Triton X-100 (Sigma-Aldrich) and then washed three times with TBST (TBS and Tween 20) buffer, incubated for 1 h with the appropriate secondary Abs, anti-mouse immunoglobulin G horseradish peroxidase (IgG HRP) conjugate, or anti-rabbit IgG HRP conjugate (172-1011 and 172-1019; Bio-Rad), and washed three times with TBST. Immunoreactive proteins were detected by SuperSignal West Pico Chemiluminescent Substrate (ThermoScientific), captured using the LAS-3000 imaging system (Fujifilm, Tokyo, Japan), and quantified using MultiGauge software (Fujifilm). Sequential images with progressively increased exposure times were obtained per sample, and blots with saturated bands were excluded from densitometry. GLT-1 and GFAP absorbance levels were normalized to each samples' respective level of β-actin as a loading control. The total amount of GLT-1 protein was determined as a sum of both monomer and dimer GLT-1 bands.
EEG telemetry unit implantation
Saline- (n=5) and ceftriaxone-treated (n=6) rats that had undergone LFPI were implanted with wireless electroencephalography (EEG) telemetry units 12 weeks after injury. In addition, to calibrate our automated seizure detection protocol, 5 naïve animals, which experienced neither surgery nor drug injection (weight matched to the 12-week post-TBI group), were also implanted with wireless EEG telemetry units (A3019D; Open Source Instruments Inc., Waltham, MA). Rats were anesthetized using 2–4% isoflurane vapor and mounted on a stereotactic frame (Stoelting). To implant the transmitter, an s.c. pocket was made along the animals' dorsum, above the scapulae, in which the transmitter would be located. Two burr holes, 1 mm in diameter, were drilled over the right olfactory bulb and 2 mm anterior to the TBI craniectomy, into which were placed the telemetry units' skull screw epidural electrodes. Once in place, the skull screws were covered with dental cement (Dentsply International Inc., Milford, DE).
EEG recording
EEG was obtained by epidural recording from stainless steel skull screw electrodes (1.1 mm in diameter; PN B002SG89QQ; Antrin Miniature Specialties, Fallbrook, CA). All EEG data were recorded differentially with the reference electrode placed over the right olfactory bulb, and the active electrode was placed into the bone on the most anterior margin of the craniotomy site (Fig. 1). EEG electrodes were connected to an s.c. transmitter, an implantable telemetric sensor for monitoring EEG. It is a small, battery-powered sensor (volume=2.4 mL; weight=4.2 g; width=23 mm diameter; height=7 mm) with an operating life of 18 weeks and sampling at 512 samples/sec. EEG transmitters were implanted in the s.c. space above the rats' scapulae. Antennas to pick up radiofrequency (RF) transmissions from the sensors were inside the Faraday case with the rats to isolate the sensors from ambient RF interference. Appropriate electronics received, decoded, and transferred unmodified signals over the internet (Open Source Instruments).
After acquiring data, the power in frequency bands ranging from 4 to 160 Hz was analyzed for seizures. Automated seizure detection relied on metrics that utilize the Fourier transform of the signal and also an array of nonlinear EEG features (event power, transient power, high-frequency power, oscillation contour, asymmetry, and intermittency) of the signal.24 A library of reference events was built by marking seizures and nonseizure artifacts by visually inspecting several hours of EEG signals from each animal. After building an event and artifact library, the detection software used the previously derived metrics to compare the raw signals with the library reference events. A spike cluster was defined as one or more sharp deflections of voltage exceeding background activity and lasting at least 1 sec. Per clinical convention, a seizure was defined as a continuous run of such spikes lasting at least 10 sec. Epileptiform EEG signal events within 5 sec of separation from one another were thus automatically classified as seizures. For quality control purposes, automatically detected seizures were confirmed by visual inspection.
To validate the automated seizure detection, a group of uninjured naïve rats (n=5) was implanted with the same transmitters and monitored 12 weeks postimplantation. The same seizure detection routine was applied to the EEG data acquired from the naïve group to test whether the software could differentiate the prolonged post-traumatic seizures from the naturally occurring high-voltage rhythmic spikes in rats, which are a common confounder in rodent encephalography.24,25
Statistical analysis
Data from duplicate blots of nGLT-1 or GFAP absorbance levels normalized to their respective level of β-actin were used to determine the relative expression level of protein per sample. Values from multiple immunoblots were compared to a standard sample common to each blot and normalized accordingly. To control for environmental factors, saline and ceftriaxone control animals were generated in pairs, and homogenates from paired animals were run on the same gel. Accordingly, comparisons between paired saline-treated and ceftriaxone-treated rats, as well as between the left and right hemispheres in an individual rat, were made by paired test, either the Student's t-test or Wilcoxon's signed-rank test. Rats that underwent sham TBI surgery were generated as a separate cohort, and thus data from this group were compared to verum TBI data by unpaired t-test or Mann-Whitney's test, as appropriate. One-way analysis of variance (ANOVA) was used to compare GLT-1 expression in multiple cortical samples. Two-way ANOVA was used to test for contribution of injury and treatment condition to GLT-1 expression as well as to test for contribution of treatment condition and seizure duration to cumulative seizure counts. All data were analyzed using GraphPad Prism statistical software (version 5.01; GraphPad Software Inc., La Jolla, CA), with significance level defined as p<0.05.
Results
GLT-1 expression is suppressed 7 days after TBI
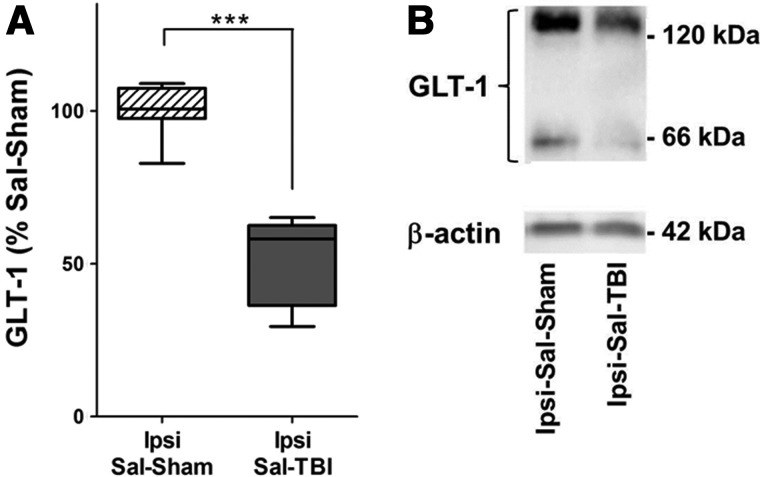
We initially compared GLT-1 expression in the lesioned (left) cortex from the saline-TBI group to GLT-1 expression in the lesioned (left) cortex saline-sham group (Fig. 2) and found that TBI resulted in a 48% decrease in GLT-1 expression (n=7 per group; Mann-Whitney's test, p=0.0006). Subsequently, using each rat in the saline-TBI group as an internal control, we compared GLT-1 expression in the ipsilesional (left) cortex with the contralesional (right) cortex (Fig. 3) and found also a 29% decrease in GLT-1 expression in the lesioned cortex after TBI (n=7 per group; paired t-test, p<0.01). In contrast, there was no difference between the hemispheres in the sham-TBI group.
FIG. 2.
Regional GLT-1 expression is suppressed 7 days after TBI. (A) GLT-1 expression in ipsi-lesional cortex homogenates from the saline-TBI group (Ipsi-Sal-TBI; n=7) is significantly reduced by 48%, compared to homogenates from the uninjured control saline-sham (Ipsi-Sal-Sham) group (n=7). Boxes indicate median and first and third quartile. Tukey's error bar is indicated by top and bottom whiskers. ***p<0.001, Mann-Whitney's test. (B) Representative immunoblot of GLT-1 expression 7 days after sham lesion or after TBI, respectively. GLT-1, glutamate receptor 1; TBI, traumatic brain injury; Ipsi, ipsilesional; Sal, saline.
FIG. 3.
GLT-1 expression is restored in the lesioned cortex and enhanced in the contralesional cortex with ceftriaxone treatment after TBI. (A) Bars indicate GLT-1 expression in four sample groups: ipsi- and contralesional cortex samples in the saline-TBI condition (Ipsi-Sal-TBI and Contra-Sal-TBI, correspondingly; n=7 rats) and ipsi- and contralesional cortex samples in the ceftriaxone-TBI condition (Ipsi-Cef-TBI and Contra-Cef-TBI, correspondingly; n=7 rats). Repeated-measures one-way analysis of variance reveals a significant group effect (F(3,24)=8.86; p<0.001). Tukey's post-hoc multiple comparisons show significantly lower ipsilesional values in the saline-TBI condition, relative to either hemisphere in the ceftratriaxone-TBI condition. The graph also shows a trend toward GLT-1 overexpression with ceftriaxone treatment (a 29 % increase in the Contra-Cef-TBI group, relative to matched saline control; paired t-test, p=0.07). A separate within-subject comparison of GLT-1 expression in two hemispheres in the saline-TBI condition reveals 29% lower GLT-1 expression in the ipsilesional cortex, relative to the contralesional cortex (paired t-test, p<0.01). Error bars indicate standard error of the mean. *p<0.05; **p<0.01; ***p<0.001. (B) Representative immunoblot of GLT-1 expression 7 days after TBI in saline- and ceftriaxone-treated animals. GLT-1, glutamate receptor 1; TBI, traumatic brain injury; Ipsi, ipsilesional; Sal, saline; Contra, contralesional; Cef, ceftriaxone.
Ceftriaxone treatment for 7 days after TBI restores GLT-1 expression in the lesioned cortex
We next compared separately GLT-1 expression in four groups of homogenates derived from (1) the ipsilesional (left) and (2) contralesional (right) cortex in the saline-TBI condition and the (3) ipsilesional (left) and (4) contralesional (right) cortex in the ceftriaxone-TBI condition. For purposes of analysis, we designated GLT-1 expression in untreated and uninjured right cortex in the saline-TBI group as control (i.e., Contra-Sal-TBI=100%). Repeated-measures one-way ANOVA revealed a significant group effect of ceftriaxone treatment on GLT-1 expression (F(3,24)=8.86; p<0.001), with Tukey's post-hoc multiple comparisons showing a significant reduction in ipsilesional GLT-1 expression in the saline-TBI group, relative to GLT-1 expression in either the ipsi- or contralesional cortex in the ceftriaxone-TBI condition (p<0.05 and p<0.001, respectively; Fig. 3A).
Using each rat as an internal control, we also found that, relative to the contralesional (right) hemisphere, TBI led to significant reduction of GLT-1 expression in the lesioned (left) cortex in the saline-TBI group (n=7 left-right cortex pairs; paired t-test, p<0.001). In contrast, in the ceftriaxone-TBI condition, GLT-1 expression in the ipsilesional cortex was essentially equal to control level [105.1%; paired t-test, not significant (n.s.)] and 33% increased relative to the ipsilesional (left) cortex in the saline-TBI condition (n=7 per group; paired t-test, p<0.02; Fig. 3A). The difference in GLT-1 expression between hemispheres was not apparent by direct comparison of the ipsi- and contralesional cortex in the ceftriaxone-TBI condition (n=7 left-right cortex pairs; paired t-test, n.s.). However, cumulative GLT-1 expression (averaged between hemispheres) in the ceftriaxone-TBI condition was significantly (37%) greater than in the saline-TBI control (n=7 per group, paired t-test, p<0.009). The net contribution of ceftriaxone treatment to GLT-1 expression increase in all rats is further supported by two-way ANOVA of our data, arranged in a 2×2 matrix (by hemisphere and treatment by condition), which reveals a significant contribution of treatment condition to GLT-1 expression (F(1,24)=5.375; p<0.03)
Increase in regional GFAP after TBI is reduced with ceftriaxone treatment
Saline-sham and saline-TBI groups' lesioned cortex homogenates were compared (Fig. 4A) to reveal an expected 192% increase in GFAP expression in injured rats, compared to sham (n=7 per group; Mann-Whitney's test, p<0.02). In addition, saline-TBI and ceftriaxone-TBI lesioned (left) cortex homogenates were compared (Fig. 4B) and a 43% decrease in GFAP expression was found in the ceftriaxone-treated group, compared to the saline control group (n=7 per group; Wilcoxon's signed-rank test; p<0.04).
FIG. 4.
Increase in regional GFAP after TBI is reduced with ceftriaxone treatment. (A) Saline-sham (Ipsi-Sal-Sham) and saline-TBI (Ipsi-Sal-TBI) groups' (n=7 per group) ipsilesional cortex homogenates were compared to reveal a 192% increase in GFAP expression in TBI rats, relative to sham. (B) A comparison of Ipsi-Sal-TBI and Ipsi-Cef-TBI groups' (n=7 per group) lesioned cortex homogenates shows a 43% decrease in GFAP expression in the ceftriaxone-treated group, compared to saline control. Boxes indicate median and first and third quartile. Tukey's error bar is indicated by top and bottom whiskers. A single outlier value is indicated, beyond Tukey's error range by the solid circle. *p<0.05, Mann-Whitney's and Wilcoxon's signed-rank tests. (C) Representative immunoblot of GFAP expression in saline-sham and saline-TBI groups. (D) Representative immunoblot of GFAP expression in saline-TBI and ceftriaxone-TBI animals. GFAP, glial fibrillary acidic protein; TBI, traumatic brain injury; Ipsi, ipsilesional; Sal, saline; Cef, ceftriaxone.
Reduction of post-traumatic seizures after ceftriaxone treatment
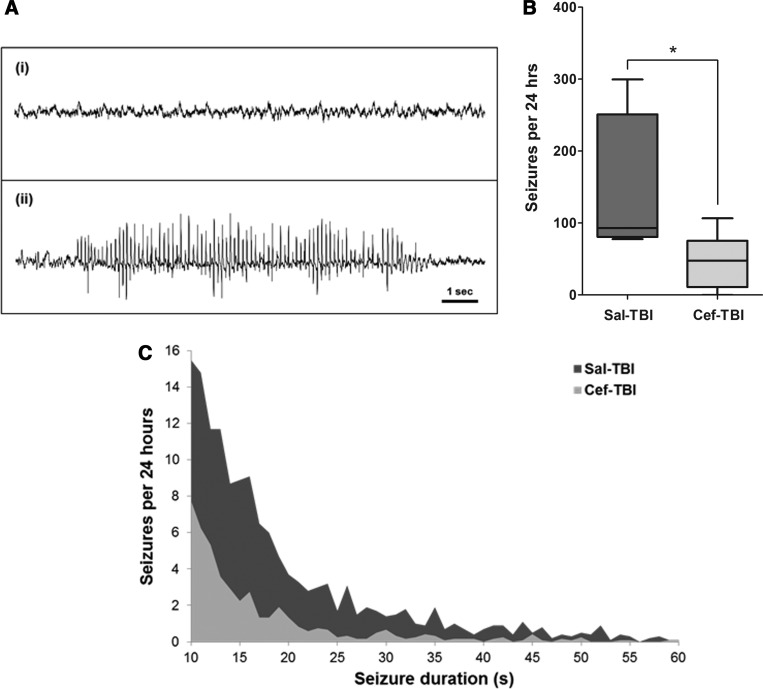
Seizure frequency per 24 h of EEG recording (seizures defined as runs of spikes longer than 10 sec; Fig. 5A) was reduced by 69% to 47±15 (mean±standard error) seizures in the ceftriaxone-TBI group, relative to 151±44 seizures in saline-treated rats (n=6 ceftriaxone-TBI, n=5 saline-TBI; Mann-Whitney's test, p=0.03; Fig. 5B). The comparison of seizures per 24 h in the saline-TBI and ceftriaxone-TBI groups revealed also that the saline-treated group had significantly longer, more frequent seizures than the ceftriaxone-treated group. Correspondingly, there was a 19% reduction in mean seizure duration in the ceftriaxone-TBI group to 18.47±0.58 sec, relative to 22.72±0.47 sec in saline-TBI rats (unpaired t-test, p<0.001).
FIG. 5.
Ceftriaxone reduces both seizure frequency per 24 h and total ictal time after TBI. (A) Representative EEG traces: (i) sample baseline EEG without spikes; (ii) sample EEG shows a characteristic automatically detected seizure with spiking activity outlasting 10 sec. (B) The number of seizures per 24 h of EEG recording in the saline-TBI control group (151±44) was significantly reduced to 47±15 seizures in the ceftriaxone-TBI group (n=5 saline; n=6 ceftriaxone). A seizure was defined as runs of spikes 10 sec or longer. *p<0.05, Mann-Whitney's test. (C) A comparison of seizure counts and durations demonstrates an overall reduction in the cumulative ictal time in the ceftriaxone-TBI group, relative to the sham-TBI group. Treatment effect is confirmed by two-way analysis of variance (F(1,194)=27.53; p<0.0001). The figure illustrates a representative area under the curve plots for seizure durations of 10–60 sec. TBI, traumatic brain injury; EEG, electroencephalography; Sal, saline; Cef, ceftriaxone.
The area under the curve (?AUC) describing the product of seizure frequency and seizure duration also demonstrated lower cumulative post-traumatic seizure activity in the ceftriaxone-TBI group. A comparison of average seizure frequency as a function of treatment condition and seizure duration (range, 10–204 sec) by two-way ANOVA confirmed a significant treatment effect (F(1,194)=27.53; p<0.0001). The expected contribution of seizure duration to seizure counts was also confirmed (F(194,194)=4.87; p<0.0001). Representative AUCs for the seizure duration range of 10–60 sec are shown in Figure 5C.
Consistent with the published data, the naïve (no injection and no TBI) group of nonepileptic rats that we used to calibrate the seizure detection algorithm also had spikes on EEG, a common confounder in rodent encephalography where naturally occurring, sharply contoured rhythmic EEG activity is routinely detected in nonepileptic rats.24,25 However, as predicted, the naturally occurring runs of spikes in the naïve rat data were fewer in number, and overwhelmingly (in >92% of instances) were shorter than 10 sec, and which enabled us to set a duration threshold for automated detection of epileptic seizures.
Discussion
TBI leads to a decrease of GLT-1 expression in the injured cortex lasting for at least 7 days
This is the first demonstration that a sustained regional reduction in cortical GLT-1 protein expression results from TBI in rats. Previous studies showed that TBI in rats results in an acute, 24-h reduction of both GLT-1 mRNA and protein, and infusion of GLT-1 antisense mRNA in rats leads to increased neuronal death after ischemic brain injury.11–13 We now demonstrate that GLT-1 suppression in the lesioned cortex persists for at least 7 days in Long-Evans rats and perhaps well into the post-traumatic recovery period. These data indicate a potential link between GLT-1 depression and acquired post-traumatic epileptogenesis. Such coupling between GLT-1 reduction and epileptogenesis is suggested by the published finding that mice deficient in GLT-1 exhibit spontaneous epileptic seizures,26 but has not been previously demonstrated in an acquired epilepsy model.
Ceftriaxone treatment restores GLT-1 expression in the injured cortex
Another novel finding in our data is that 1 week of ceftriaxone treatment leads to normalization of GLT-1 expression in the lesioned cortex. Our results imply that GLT-1 expression is modifiable after injury and point to a plausible TBI treatment strategy where reduced GLT-1 expression may be restored by ceftriaxone or related agents. However, because ceftriaxone induces a global increase in GLT-1 expression, the term “normalization” may be applicable only to injured cortex where pathologically low GLT-1 expression levels are restored to approximately normal values. We thus note that, as in uninjured rodents,19 GLT-1 expression in the contralesional cortex showed a trend toward enhancement by ceftriaxone treatment. Whether this has any clinical relevance is uncertain, but given the overall favorable neurologic profile of ceftriaxone in clinical practice, adverse sequelae of ceftriaxone-mediated GLT-1 expression to above baseline seem unlikely.
We also find that the comparison of ipsi- and contralesional GLT-1 expression in saline-treated rats shows a significant decline in the injured cortex, but a similar comparison in ceftriaxone-treated rats does not reach statistical significance. This underscores that GLT-1 expression is disproportionately increased in the injured cortex. Confirmation of this finding will be necessary as will be an explicit test of the mechanisms by which GLT-1 expression may be easier to modulate by ceftriaxone in acutely injured cortical tissue. However, there are plausible mechanisms for a differential effect in injured and uninjured cortex: perhaps a ceiling effect of GLT-1 up-regulation in uninjured cortex or perhaps an increased capacity for GLT-1 up-regulation after injury resulting from astrocyte and microglia (which also express GLT-1) proliferation in the injured tissue.27–29 Of course, these hypotheses are speculative and will require experiments beyond the scope of this report.
Ceftriaxone treatment reduces post-traumatic astrogliosis and ameliorates post-traumatic epilepsy
In agreement with our hypothesis, ceftriaxone treatment ameliorates both seizure frequency and seizure duration in the LFPI post-traumatic epilepsy model. These data support ceftriaxone as a safe prophylactic treatment after TBI, which may mitigate post-traumatic seizures.30 Although this does not affect our main finding of seizure reduction with ceftriaxone treatment, this underscores the variability in seizure expression among rodent TBI models, which complicates translational post-traumatic epilepsy research in general. Relevant to our experiment, strain differences (Long-Evans rats may be more seizure prone than other strains31), craniotomy size, and other factors, such as choice of anesthetic, postoperative analgesia, differences in EEG recording techniques, and sensitivities of seizure detection algorithms, distinguish our model from those in past published reports.
It is likely, in our estimate, that the antiepileptogenic effect of ceftriaxone is related to enhanced glutamate clearance that follows increased GLT-1 expression. However, an alternative explanation may relate to our finding of reduced regional GFAP expression, which may be consequent to the GLT-1 up-regulation, but alternatively may be a direct ceftriaxone effect. Because astrogliosis and glial dysfunction contribute to epileptogenesis, reduced seizure severity in our data may, in part, reflect reduced gliotic changes in the injured cortex.32,33 We note that the post-traumatic increase in GFAP expression correlates with a decrease in GLT-1 expression in our data, and vise versa: A decrease in GFAP with ceftriaxone treatment corresponds to increased GLT-1. Thus, the changes in expressions of GFAP and GLT-1 are inversely related, but the causal mechanism of this relationship is not yet clear and should be further investigated. Also, the contribution of additional mechanisms related to glutamate homeostasis that can be modulated by ceftriaxone, such as the cystine-glutamate exchange,15 will require evaluation in follow-up to the present experiments.
Our data from the rat LFPI post-traumatic epilepsy model suggest a utility of ceftriaxone treatment as a neuroprotective and antiepileptogenic therapeutic intervention. In addition, our finding of reduced GFAP indicates a net reduction of injury severity in the ceftriaxone-treated rats and points to a plausible neuroprotective effect, as previously suggested by data from other rodent disease models, such as stroke and ALS.16,19 Although in support of our overall hypothesis that GLT-1 up-regulation by ceftriaxone mitigates glutamate-mediated excitotoxicity, conclusive evidence that GLT-1 restoration after TBI is causally related to reduced gliosis and reduced post-traumatic seizure counts will have to be obtained in studies beyond the scope of this report. We thus also anticipate to study whether individual GLT-1 isoforms are differentially depressed after injury and which isoform may be modulated preferentially by ceftriaxone.11 Similarly, more work needs to be done to better characterize ceftriaxone's effect on GFAP and GLT-1 for periods longer than just 7 days after TBI. Further histopathologic analysis will also be required to detect any neuroprotective effect attributed to ceftriaxone treatment. We cautiously conclude that our data provide evidence that ceftriaxone offers antigliotic and -epileptogenic benefits after TBI, potentially by restoring GLT-1 expression and function; however, follow-up studies are warranted.
Acknowledgments and Author Disclosure Statement
This work was supported by the Department of Defense (DoD) Defense Medical Research and Development Program Basic Research Award (PT090716; to A.R. and A.Y.K.), Citizens United for Research in Epilepsy (CURE; to A.R.), and NS066019 (to P.A.R.). The authors thank Drs. Roman Gersner and David Zurakowski for their advice related to statistical analysis of EEG data.
References
- 1.Goforth P.B. Ren J. Schwartz B.S. Satin L.S. Excitatory synaptic transmission and network activity are depressed following mechanical injury in cortical neurons. J. Neurophysiol. 2011;105:2350–2363. doi: 10.1152/jn.00467.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo P. Fei F. Zhang L. Qu Y. Fei Z. The role of glutamate receptors in traumatic brain injury: implications for postsynaptic density in pathophysiology. Brain Res. Bull. 2011;85:313–320. doi: 10.1016/j.brainresbull.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Andriessen T.M. Jacobs B. Vos P.E. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J. Cell. Mol. Med. 2010;14:2381–2392. doi: 10.1111/j.1582-4934.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg P.A. Aizenman E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocyte-poor cultures of rat cerebral cortex. Neurosci. Lett. 1989;103:162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg P.A. Amin S. Leitner M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J. Neurosci. 1992;12:56–61. doi: 10.1523/JNEUROSCI.12-01-00056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W. Mahadomrongkul V. Berger U.V. Bassan M. DeSilva T. Tanaka K. Irwin N. Aoki C. Rosenberg P.A. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J. Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furness D.N. Dehnes Y. Akhtar A.Q. Rossi D.J. Hamann M. Grutle N.J. Gundersen V. Holmseth S. Lehre K.P. Ullensvang K. Wojewodzic M. Zhou Y. Attwell D. Danbolt N.C. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2) Neuroscience. 2008;157:80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melone M. Bellesi M. Conti F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia. 2009;57:108–117. doi: 10.1002/glia.20744. [DOI] [PubMed] [Google Scholar]
- 9.Berger U.V. DeSilva T.M. Chen W. Rosenberg P.A. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J. Comp. Neurol. 2005;492:78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeSilva T.M. Kabakov A.Y. Goldhoff P.E. Volpe J.J. Rosenberg P.A. Regulation of glutamate transport in developing rat oligodendrocytes. J. Neurosci. 2009;29:7898–7908. doi: 10.1523/JNEUROSCI.6129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi J.H. Pow D.V. Hazell A.S. Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia. 2005;49:121–133. doi: 10.1002/glia.20099. [DOI] [PubMed] [Google Scholar]
- 12.Yi J.H. Hazell A.S. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem, Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Rao V.L. Dogan A. Todd K.G. Bowen K.K. Kim B.T. Rothstein J.D. Dempsey R.J. Antisense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J. Neurosci. 2001;21:1876–1883. doi: 10.1523/JNEUROSCI.21-06-01876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S.G. Su Z.Z. Emdad L. Gupta P. Sarkar D. Borjabad A. Volsky D.J. Fisher P.B. Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J. Biol. Chem. 2008;283:13116–13123. doi: 10.1074/jbc.M707697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewerenz J. Albrecht P. Tien M.L. Henke N. Karumbayaram S. Kornblum H.I. Wiedau-Pazos M. Schubert D. Maher P. Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J. Neurochem. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- 16.Lai P.C. Huang Y.T. Wu C.C. Lai C.J. Wang P.J. Chiu T.H. Ceftriaxone attenuates hypoxic-ischemic brain injury in neonatal rats. J. Biomed. Sci. 2011;18:69. doi: 10.1186/1423-0127-18-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu K. Lee S.T. Sinn D.I. Ko S.Y. Kim E.H. Kim J.M. Kim S.J. Park D.K. Jung K.H. Song E.C. Lee S.K. Kim M. Roh J.K. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 18.Miller B.R. Dorner J.L. Shou M. Sari Y. Barton S.J. Sengelaub D.R. Kennedy R.T. Rebec G.V. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington's disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothstein J.D. Patel S. Regan M.R. Haenggeli C. Huang Y.H. Bergles D.E. Jin L. Dykes Hoberg M. Vidensky S. Chung D.S. Toan S.V. Bruijn L.I. Su Z.Z. Gupta P. Fisher P.B. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 20.D'Ambrosio R. Fairbanks J.P. Fender J.S. Born D.E. Doyle D.L. Miller J.W. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–314. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntosh T.K. Vink R. Noble L. Yamakami I. Fernyak S. Soares H. Faden A.L. Traumatic brain injury in the rat: characterization of a lateral fluid-percussion model. Neuroscience. 1989;28:233–244. doi: 10.1016/0306-4522(89)90247-9. [DOI] [PubMed] [Google Scholar]
- 22.Gasparovic C. Yeo R. Mannell M. Ling J. Elgie R. Phillips J. Doezema D. Mayer A.R. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: an 1H-magnetic resonance spectroscopy study. J. Neurotrauma. 2009;26:1635–1643. doi: 10.1089/neu.2009.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sari Y. Smith K.D. Ali P.K. Rebec G.V. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J. Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw F.Z. Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J. Neurophysiol. 2004;91:63–77. doi: 10.1152/jn.00487.2003. [DOI] [PubMed] [Google Scholar]
- 25.Shaw F.Z. 7-12 Hz high-voltage rhythmic spike discharges in rats evaluated by antiepileptic drugs and flicker stimulation. J. Neurophysiol. 2007;97:238–247. doi: 10.1152/jn.00340.2006. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K. Watase K. Manabe T. Yamada K. Watanabe M. Takahashi K. Iwama H. Nishikawa T. Ichihara N. Kikuchi T. Okuyama S. Kawashima N. Hori S. Takimoto M. Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 27.Gao X. Chen J. Moderate traumatic brain injury promotes neural precursor proliferation without increasing neurogenesis in the adult hippocampus. Exp. Neurol. 2012;239:38–48. doi: 10.1016/j.expneurol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grady M.S. Charleston J.S. Maris D. Witgen B.M. Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: analysis by stereological estimation. J. Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- 29.Chirumamilla S. Sun D. Bullock M.R. Colello R.J. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- 30.Curia G. Levitt M. Fender J.S. Miller J.W. Ojemann J. D'Ambrosio R. Impact of injury location and severity on posttraumatic epilepsy in the rat: role of frontal neocortex. Cereb. Cortex. 2011;21:1574–1592. doi: 10.1093/cercor/bhq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntyre D.C. Gilby K.L. Genetically seizure-prone or seizure-resistant phenotypes and their associated behavioral comorbidities. Epilepsia. 2007;48(Suppl 9):30–32. doi: 10.1111/j.1528-1167.2007.01398.x. [DOI] [PubMed] [Google Scholar]
- 32.Seifert G. Carmignoto G. Steinhauser C. Astrocyte dysfunction in epilepsy. Brain Res. Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Li T. Lytle N. Lan J.Q. Sandau U.S. Boison D. Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis? Glia. 2012;60:83–95. doi: 10.1002/glia.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]